Abstract
Aim:
To determine profiles of non-chlamydia conjunctival bacteria and their antimicrobial susceptibility from adults who underwent trachomatous trichiasis surgery in rural areas of Ethiopia.
Materials and Methods:
A cross-sectional study was conducted in rural districts in West Gojjam administrative zone. Conjunctival swabs were collected during surgery and transported using Stuart transport broth (Oxoid, UK). Antibiotic susceptibility of conjunctival isolates was determined using the Kirby-Bauer disc-diffusion method.
Results:
Non-chlamydia pathogenic bacteria were recovered from conjunctiva of 438 (31%) participants before treatment. The isolated conjunctival bacteria were Staphylococcus aureus, coagulase-negative Staphylococci, Streptococcus group (A, C, F and G), Enterococci, Streptococcus pneumoniae, Moraxella spp., Escherichia coli, Citrobacter spp., Proteus spp., Klebsiella spp., Pseudomonas spp. and Enterobacter spp. Overall, resistance rates of 57.8% to azithromycin and 68.5% to chloramphenicol were found. However, 86-94.4% sensitivity was demonstrated to ciprofloxacin and norfloxacin. Moderate sensitivity rates (61.8-78.4%) were observed to ceftriaxone, tetracycline and cotrimoxazole.
Conclusion:
Fluoroquinolones that have activity against the majority of bacterial isolates were potent at in vitro. However, unacceptably high levels of resistance to azithromycin and chloramphenicol in rural community indicated a need for further study and antimicrobial resistance surveillance.
Keywords: Azithromycin, chloramphenicol, fluoroquinolone, non-chlamydia conjunctival bacteria, Ethiopia
A number of studies have demonstrated that arrays of non-chlamydia bacterial pathogens promote inflammation of conjunctival lining of eyelids (trichiasis).[1,2] Topical antibacterial agents such as azithromycin, chloramphenicol, tetracycline and norfloxacin have been prescribed to minimize the risk of conjunctival infection following surgery.[3] Mass azithromycin treatment has been reducing this infection.[4] The World Health Organization (WHO) has begun worldwide program to eradicate blinding trachoma by the year 2020 using mass distribution azithromycin.[5] However, there is a concern that large-scale distribution of azithromycin may lead to the spread of azithromycin-resistant bacterial flora.[6] Therefore, the study was aimed at determining the ocular pathogens and trends of antimicrobial resistance particularly to azithromycin, fluoroquinolon and chloramphenicol in rural community.
Materials and Methods
Conjunctival swabs were collected from adult patients, who underwent trachomatous trichiasis surgery before treatment in rural communities in West Gojjam, Ethiopia from November, 2009 to February, 2010. Conjunctival swabs were collected using sterile Dacron swab during surgery by ophthalmic nurse and physician. Eye swabs were transported using Stuart transport broth (Oxoid, UK) to the Bahir Dar Regional Health Research Laboratory within 24 hours.
Conjunctival swabs were inoculated onto 5% sheep's blood agar, mannitol salt agar and MacConkey agar plates (Oxoid). The plates were incubated at 37°C aerobically (MacConkey agar, mannitol salt agar) and in an atmosphere of 5% carbon dioxide (5% sheep's blood agar). Urea 40% broth, Simmons citrate, motility-indole medium, Triple Sugar Iron Agar (TSI) and Kligler's Iron Agar (KIA) (Oxoid) were used for differentiation of the Enterobacteriaceae. Phenotypic bacterial identification was made manually in accordance with guidelines of the microbiology standards using colony appearance on culture, Gram staining, biochemical and serology methods.[7]
Morphologically identical 4 to 6 bacterial colonies from overnight culture were suspended in 5 ml Mueller-Hinton broth and incubated for 4 hours at 37°C. Turbidity of the broth culture was equilibrated to match 0.5 McFarland standards. Antimicrobial susceptibility test were performed on Mueller-Hinton agar and 5% sheep's blood plus Mueller-Hinton agar for Streptococcus spp. using disk diffusion method.[7] Ciprofloxacin (5 μg), ceftriaxone (30 μg), norfloxacin (10 μg), chloramphenicol (30 μg), gentamicin (10 μg), tetracycline (30 μg), azithromycin (15 μg), cotrimoxazole (25 μg) ampicillin (10 μg) and amoxycillin (10 μg) (Oxoid) were used. Resistance and susceptibility data were interpreted according to National Committee for Clinical Laboratory Standards.[8] Reference strains E. coli ATCC 25922 and S. aureus ATCC 25923 were used as controls. Ethical approval was secured from Research Ethics Committee of Bahir Dar University. Individual verbal consent was obtained from each participant to take conjunctival swab before treatment.
Results
Non-chlamydia pathogenic bacteria were recovered from conjunctiva of 438 (31%) participants. Bacterial species isolated from adults with trichiasis include S. aureus, coagulase-negative Staphylococci, Streptococcus group (A, C, F and G), Enterococci, S. pneumoniae, Moraxella spp., E. coli, Citrobacter spp., Proteus spp., Klebsiella spp., Pseudomonas spp. and Enterobacter spp.
Table 1 shows antimicrobial susceptibility patterns of Gram-positive and negative bacteria. Overall, the highest level of resistance was displayed to chloramphenicol, followed by azithromycin, cotrimoxazole, tetracycline and ceftriaxone. However, the lowest levels of resistance were found to ciprofloxacin, norfloxacin and gentamicin [Fig. 1]. All Gram-negative bacteria tested for antimicrobial susceptibility except Moraxella spp. were susceptible to ciprofloxacin. Azithromycin was tested against 420 bacterial isolates and 243 (57.8%) showed resistance. Overall, azithromycin-resistant bacteria revealed higher levels of resistance to other class of antimicrobials than azithromycin-sensitive pathogenic bacteria [Fig. 2].
Table 1.
Antimicrobial resistance patterns of non-chlamydia bacteria from conjunctival swabs in rural community, Ethiopia, 2010
Figure 1.

Susceptibility and resistance profile of non-chlamydia bacteria isolated from conjunctival swabs from rural community, Ethiopia, 2010
Figure 2.
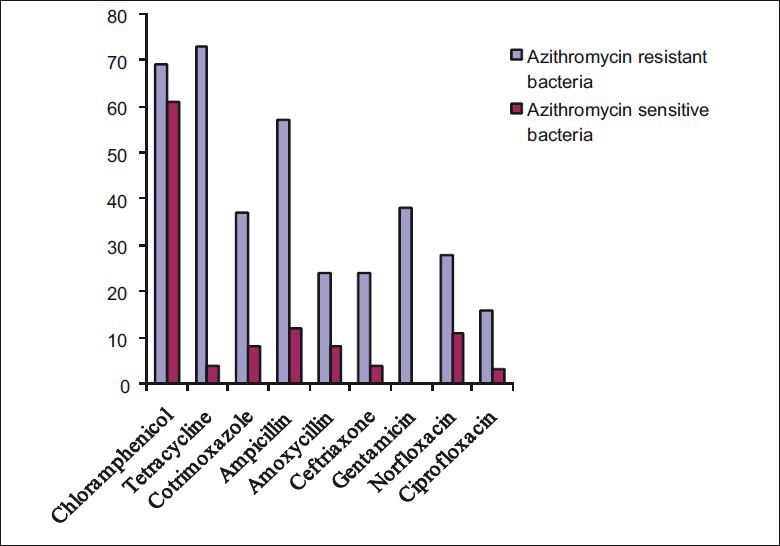
Antimicrobial resistance patterns of azithromycin resistant and sensitive bacteria against other class of antimicrobials resistance, Ethiopia, 2010
From Gram-positive bacteria, S. aureus, coagulase-negative Staphylococci and Streptococcus spp. demonstrated high levels of resistance (>80%) to chloramphenicol. Enterococci showed high levels of resistance (72-100%) to tetracycline, azithromycin and ampicillin. Both S. aureus and coagulase-negative Staphylococci revealed 66-80% resistance to ampicillin and 57-66% to amoxycillin [Table 1]. For Gram-negative bacteria, high levels of resistance were found to cotrimoxazole, chloramphenicol and azithromycin.
Discussion
The non-chlamydia conjunctival bacteria isolated in this study were S. aureus, coaugulase negative Staphylococci spp., Streptococcus spp., S. pneumoniae, Proteus spp., E. coli, Klebsiella spp., Citrobacter spp., Pseudomonas spp., Enterobacter spp. and Moraxella spp. This is result is in agreement with the findings of other studies in Africa.[1,2] However, Chern et al.[9] from Nepal found that the most frequently isolated non-chlamydia bacteria were S. pneumoniae, Hemophillus influenza and Moraxella spp and with less frequency of Gram-negative rods such as E. coli.
We speculated low level of antimicrobial resistance patterns for bacterial isolates from rural communities than clinical isolates. However, this study demonstrated high azithromycin resistance (57.8 %) compared to clinical isolates reported from Turkey and USA.[10,11] However, Mendes et al.[12] from Brazil reported 8-36% azithromycin resistance for Gram-positive cocci.
In this study, at baseline higher rate of azithromycin-(28%) resistant S. pneumoniae were detected compared to other studies. Leach et al.[13] reported 1.9 % S. pneumoniae azithromycin resistance at baseline from Australia. However, following azithromycin treatment, significantly higher azithromycin-resistant S. pneumoniae were detected. A study conducted in Australia found 55% azithromycin-resistant S. pneumoniae after 2 -3 weeks and in Nepal 42.8% resistant rate after 2 weeks.[13,14]
The possible explanation for high resistance to azithromycin in the present study at baseline could be the selective pressure of azithromycin mass treatment. Since, mass community-wide distributions of azithromycin have been practiced in Ethiopia since 2003.[15] Thus this might enable rapid expansion of resistant strains through cross-transmission. However, Hong et al.[16] reported non-azithromycin-resistant C. trchomatis in Ethiopia.
Regarding chloramphenicol, very high levels of resistance to the majority of bacterial isolates from rural communities were observed [Fig. 1]. Specifically, S. aureus, Streptococcus spp., Proteus spp., Klebsiella spp. and Citrobacter spp. revealed higher resistance levels compared to their respective clinical isolates from previous studies from Ethiopia.[17,18] Chloramphenicol-resistant bacteria might be acquired through cross-contamination from close contact with domestic animals. This can be supported by a study conducted in Nigeria indicating that antimicrobial-resistant Staphylococci from animals were associated with the cause of wound infections in outpatients.[19] Moreover, in developing countries, people with close contact with domestic animals have chance of acquiring resistant microbes from animals.[20] High level of chloramphenicol resistance in rural communities could be due to indiscriminate use of antibiotics by rural communities.[21] However, further systematic surveillance need to be carried out to elucidate this concern.
Fluoroquinolones are critically important antimicrobials in treatment of human infections. Overall, lowest levels of resistance were observed to ciprofloxacin and norfloxacin. From all tested bacteria S. aureus and Moraxella spp. displayed highest fluoroquinolone resistance. Abera et al.[22] found high ciprofloxacin resistance levels to methicillin-resistant S. aureus (MRSA) at local hospital near the present study area. Currently, fluoroquinolones particularly ciprofloxacin is prudent for empirical treatments of community infections in rural communities.
Enterococci become an increasingly important cause of nosocomial infections most frequently in urinary tract infections, endocarditis and wound infections. In this study, Enterococci from conjunctival swabs showed high levels of resistance (72-100%) to ampicillin, azithromycin and tetracycline. This conforms to the results of clinical isolates from other study in Jimma hospital, Ethiopia.[18] However, Simonsen et al.[23] indicated that no ampicillin resistance to E. faecalis and 48.8% ampicillin resistance to E. faecium from hospitals in Norway.
In conclusion, unacceptable levels of azithromycin and chloramphenicol resistance to an array of non-chlamydia conjunctival bacteria from rural communities were observed. However, ciprofloxacin, norfloxacin and gentamicin would be prudent for empirical treatment of community-acquired infections. This preliminary finding indicated a need for further study and antimicrobial resistance surveillances.
Acknowledgements
We acknowledge Bahir Dar University for financial support and microbiology department staff of Bahir-Dar Regional Health Research Laboratory for facilitating the study. We would also like to thank all the participants who cooperated with us in this study.
Footnotes
Source of Support: Supported by a grant from Bahir Dar University
Conflict of Interest: Nil.
References
- 1.Burton MJ, Kinteh F, Jallow O, Sillah A, Bah M, Faye M, et al. A randomised controlled trial of azithromycin following surgery for trachomatous trichiasis in the Gambia. Br J Ophthalmol. 2005;89:1282–8. doi: 10.1136/bjo.2004.062489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Burton MJ, Bowman RJ, Faal H, Aryee EN, Ikumapayi UN, Alexander ND, et al. The Long-Term Natural History of Trachomatous Trichiasis in the Gambia. Invest OphthalmicVis Sci. 2006;47:847–2. doi: 10.1167/iovs.05-0714. [DOI] [PubMed] [Google Scholar]
- 3.Miller I, Wittreich M, Cook JM, Vogel TR. The safety and efficacy of topical norfloxacin compared with chloramphenicol for the treatment of external ocular bacterial infections. Eye (Lond) 1992;6:111–4. doi: 10.1038/eye.1992.23. [DOI] [PubMed] [Google Scholar]
- 4.Schachter J, West SK, Mabey D, Dawson Cr, Bobo L, Bailey R, et al. Azithromycin in control of trachoma. Lancet. 1999;354:630–5. doi: 10.1016/S0140-6736(98)12387-5. [DOI] [PubMed] [Google Scholar]
- 5.Geneva: WHO; 1997. World Health Organization. Report of the first meeting of the WHO alliance for the global elimination of trachoma. [Google Scholar]
- 6.Emerson PM, Ngondi J, Biru E, Graves PM, Ejigsemahu Y, Gebre T, et al. Integrating an NTD with One of ‘The Big Three’: Combined Malaria and Trachoma Survey in Amhara Region of Ethiopia. PLoS Negl Trop Dis. 2008;2(3):e197. doi: 10.1371/journal.pntd.0000197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.2nd ed. Geneva: WHO; 2003. Basic Laboratory Procedures in Clinical Bacteriology; pp. 37–50. [Google Scholar]
- 8.Wayne A, editor. National Committee for Clinical Laboratory Standards 1999; Clinical Laboratory Standards Institute (formerly NCCLS). Performance standards for antimicrobial susceptibility testing, 9th information supplement. Approved standard M100-S9. [Google Scholar]
- 9.Chern KC, Shrestha SK, Cevallos V, Dhami HL, Tiwari P, Chern L, et al. Alterations in the conjunctival bacterial flora following a single dose of azithromycin in a trachoma endemic area. Br J Ophthalmol. 1999;83:1332–5. doi: 10.1136/bjo.83.12.1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Adaleti R, Nakipoglu Y, Ceran N, Tasdemir C, Kaya F, Tasdemir S. Prevalence of phenotypic resistance of Staphylococcus aureus isolates to macrolide, lincosamide, streptogramin B, ketolid and linezolid antibiotics in Turkey. Braz J Infect Dis. 2010;14:11–4. doi: 10.1590/s1413-86702010000100003. [DOI] [PubMed] [Google Scholar]
- 11.Ta CN, He L, Mino H, Kaspar De. In vitro antibiotic susceptibility preoperative normal conjunctival bacteria. Eye (Lond) 2009;23:559–60. doi: 10.1038/eye.2008.65. [DOI] [PubMed] [Google Scholar]
- 12.Mendes CFM, Sinto SI, Oplustil CP resist net Brazil group. In vitro susceptibility of gram positive Cocci isolated from Skin and Respiratory tract to Azithromycin and twelve other antimicrobial agents. Braz J Infect Dis. 2001;5:269–76. doi: 10.1590/s1413-86702001000500005. [DOI] [PubMed] [Google Scholar]
- 13.Leach AJ, Shelby-James TM, Mayo M, Gratten M, Laming AC, Currie BJ, et al. A prospective study of the impact of community-based azithromycin treatment of trachoma on carriage and resistance of Streptococcus pneumoniae. Clin Infect Dis. 1997;24:356–62. doi: 10.1093/clinids/24.3.356. [DOI] [PubMed] [Google Scholar]
- 14.Gaynor BD, Holbrook KA, Whitcher JP, Holm SO, Jha HC, Chaudhary JSP, et al. Community treatment with azithromycin for trachoma is not associated with antibiotic resistance in Streptococcus pneumoniae at 1 year. Br J Ophthalmol. 2003;87:147–8. doi: 10.1136/bjo.87.2.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Emerson PM, Ngondi J, Biru E, Graves PM, Ejigsemahu Y, Gebre T, et al. Integrating an NTD with One of ‘The Big Three’: Combined Malaria and Trachoma Survey in Amhara Region of Ethiopia. PLoS Negl Trop Dis. 2008;2:e197. doi: 10.1371/journal.pntd.0000197. doi: 10.1371/journal.pntd.0000197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hong KC, Schachter J, Moncada J, Zhou Z, House J, Lietman TM. Lack of macrolide resistance in Chlamydia trachomatis after mass azithromycin distributions for trachoma. Emerg Infect Dis. 2009;15:1088–90. doi: 10.3201/eid1507.081563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Abera B, Biadeglegne F. Antimicrobial resistance patterns of Staphylococcus aureus and Proteus spp. isolated from otitis media at Bahir Dar regional laboratory, North West Ethiopia. Ethiop Med J. 2009;47:271–6. [PubMed] [Google Scholar]
- 18.Gebre-Sealssie S. Antimicrobial resistance patterns of clinical bacterial isolates in South West Ethiopia. Ethiop Med J. 2007;45:363–70. [PubMed] [Google Scholar]
- 19.Kolawole DO, Shittu AO. Unusual recovery of animal staphylococci from septic wounds of hospital patients in Ile-Ife, Nigeria. Lett Appl Microbiol. 1997;24:87–90. doi: 10.1046/j.1472-765x.1997.00337.x. [DOI] [PubMed] [Google Scholar]
- 20.Simango C, Rukure G. Potential sources of Campylobacter species in the homes of farm workers in Zimbabwe. J Trop Med Hyg. 1991;94:388–2. [PubMed] [Google Scholar]
- 21.Okeke IN, Laxminarayan R, Bhutta ZA, Duse AGG, Jenkins P, O’Brien TF, et al. Antimicrobial resistance in developing countries. Part I: Recent trends and current status. Review: Lancet Infect Dis. 2005;5:481–3. doi: 10.1016/S1473-3099(05)70189-4. [DOI] [PubMed] [Google Scholar]
- 22.Abera B, Alem A, Bezabih B. Methicillin- resistant strains of Staphylococcus aureus and coagulase-negative staphylococus from clinical isolates at Felege Hiwot refferal hospital, North West Ethiopia. Ethiop Med J. 2008;46:149–4. [PubMed] [Google Scholar]
- 23.Simonsen GS, Smabrekke L, Monnet DL, Sørensen TL, Møller JK, Kristinsson KG, et al. Prevalence of resistance to ampicillin, gentamicin and vancomycin in Enterococcus faecalis and Enterococcus faecium isolates from clinical specimens and use of antimicrobials in five Nordic hospitals. J Antimicrob Chemother. 2003;51:323–1. doi: 10.1093/jac/dkg052. [DOI] [PubMed] [Google Scholar]


