Abstract
In this retrospective case series, we report the spectrum and outcomes of aggressive posterior retinopathy of prematurity (APROP) in infants ≥1500 g birth weight. Twenty-nine eyes of 15 infants are included. All infants were referred from level I or II nurseries, received supplemental unmonitored oxygen for prolonged duration (>1 week) and had multiple systemic co-morbidities. Of the 29 eyes, 10 (34.5%) had zone 1 and 19 (65.5%) had posterior zone 2 disease. Twenty-five (86.2%) eyes had flat neovascularization and 4 (13.8%) eyes had brush like proliferation. We noticed large vascular loops in 10 (34.5%) eyes. After confluent laser photocoagulation, 22 (75.9%) eyes had a favorable outcome. The study concludes that APROP in heavier (≥1500 g birth weight) premature infants occurs mostly in posterior zone 2 with flat neovascularization and atypical features like large vascular loops. Supplemental unmonitored oxygen for prolonged duration and multiple systemic co-morbidities could be a contributing factor.
Keywords: Aggressive posterior retinopathy of prematurity, APROP, atypical ROP, developing country, heavier infants, neonatal care, oxygen induced retinopathy, retinopathy of prematurity, ROP, zone 1 ROP
Aggressive posterior retinopathy of prematurity (APROP) is characterized by severe plus disease, flat neovascularization in zone 1 or posterior zone 2, intraretinal shunting, hemorrhages, and a rapid progression to retinal detachment.[1] Despite early laser photocoagulation, the favorable outcome rates for APROP vary from 71% to 84%.[2,3,4] In contrast, the favorable outcome rate for classical staged retinopathy of prematurity (ROP) is more than 90%.[5] APROP generally occurs in extremely premature and low birth weight infants.[1,2] However, recent studies also report APROP in heavier and more mature infants.[3,4,6,7] The present study reports the clinical features and outcome of APROP in infants ≥1500 g birth weight.
Materials and Methods
After institutional review board approval, we did a retrospective chart review of infants ≥1500 g birth weight and treated for APROP between January 2006 and December 2009. The neonatal intensive care unit at our institute is a level III nursery, which also caters infants born at other centers (outborn).[8]
APROP was diagnosed in accordance with the international classification of ROP[1] and documented by detailed retinal drawings and Retcam (Clarity MSI, Pleasanton, California, USA). We completed a chart review that retrieved various parameters including birth weight, gestational age, setting of birth (inborn/outborn), level of nursery care (Level I nursey: Basic level nursery, Level II: Specialty services like mechanical ventilation, Level III Sub-specialty care including sustained life support),[8] neonatal illnesses, and oxygenation. We excluded infants with hydrocephalus, as this condition leads to an exaggerated birth weight. Characteristics of ROP including the zone, pattern of neovascularization, and atypical features were noted.
All infants underwent confluent laser (less than half burn width apart) application with either a diode laser or 532 nm laser delivered through the indirect ophthalmoscopic system. We noted details of laser treatment including postconceptional age at treatment, number of spots, number of laser sittings, time to regression, and outcome. The criteria for unfavorable outcome at 6 months were: (1) Retinal detachment (stage 4a/4b/5), (2) Falciform fold involving the macula, and (3) A retro-lental tissue/mass obscuring the view of the posterior pole.
Results
Sixteen infants ≥1500 g birth weight underwent laser photocoagulation for APROP during the study period. We excluded one infant due to hydrocephalus. Details of the remaining 15 infants were included [Table 1].
Table 1.
Profile and disease characteristics of infants developing APROP
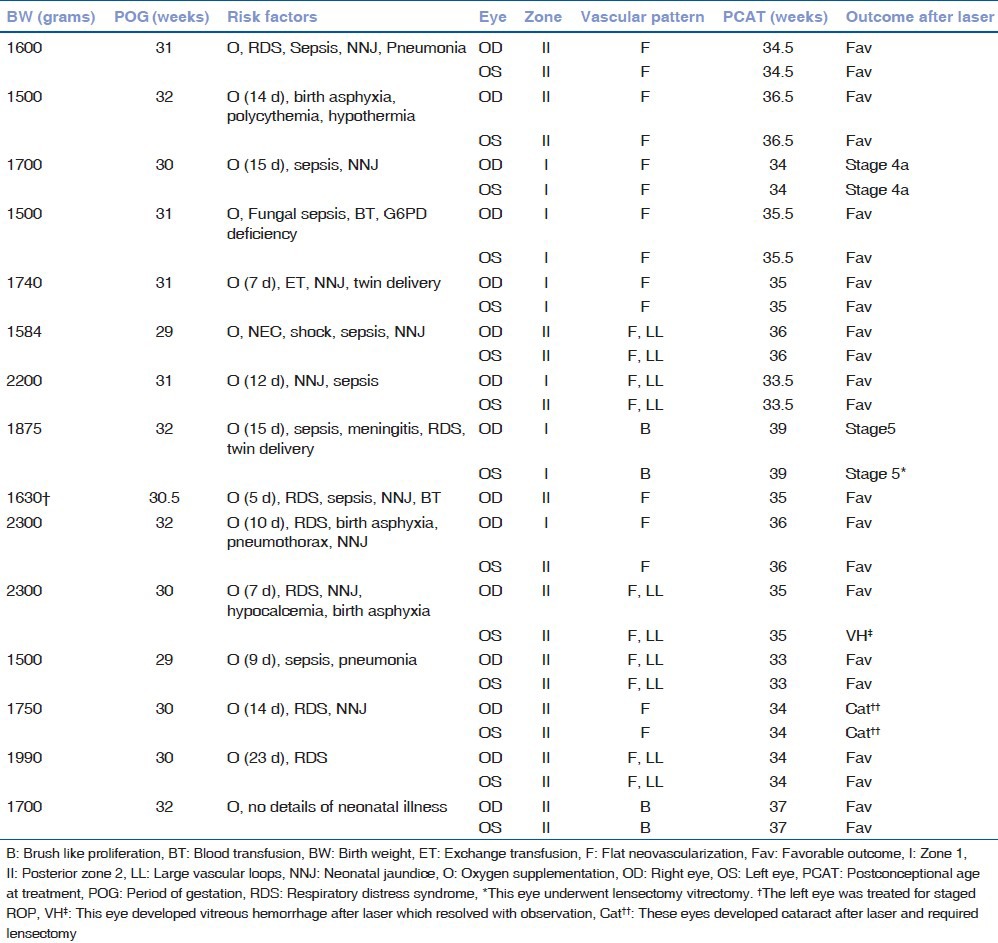
The mean birth weight and gestational age were 1791.27 ± 281.86 g (range, 1500-2300 g) and 30.7 ± 1.03 weeks (range, 29-32 weeks), respectively. All infants were outborn and referred from level I/II accredited nurseries, which had a limited level of neonatal care with paucity or nonavailability of saturation monitors and oxygen blenders. All infants received supplemental unmonitored oxygen. Duration of oxygen exposure was known for 11 infants and ranged from 7 to 23 days. Systemic co-morbidities were common [Table 2].
Table 2.
Systemic risk factors in infants ≥ 1500 g developing APROP (n=14)*
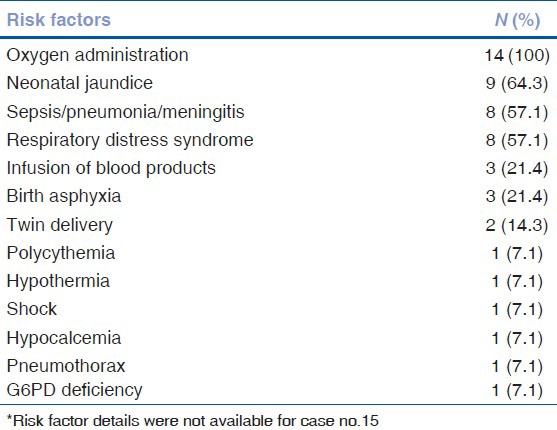
Twenty-nine eyes of 15 infants developed APROP. One eye was excluded as it developed stage 2 ROP in zone 2. Of the 29 eyes, 10 (34.5%) had zone 1 and 19 (65.5%) had posterior zone 2 disease. Twenty-five (86.2%) eyes had flat neovascularization [Fig. 1]. Four (13.8%) eyes had brush like proliferation [Fig. 2]. We noticed large vascular loops in 10 (34.5%) eyes [Fig. 3].
Figure 1.
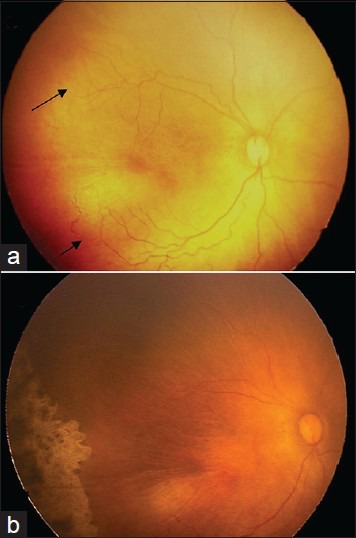
Fundus photographs from a male infant weighing 1500 g at birth and 29 weeks of gestation demonstrate APROP in posterior zone 2 and regression following confluent laser photocoagulation. (a) Fundus photograph prior to laser photocoagulation demonstrates plus disease and flat new vessels in posterior zone 2. The posterior arcade vessels look mature and well developed. Confluent laser photocoagulation was started at 33 weeks postmenstrual age. (b) Fundus photograph at 37 weeks shows complete regression
Figure 2.
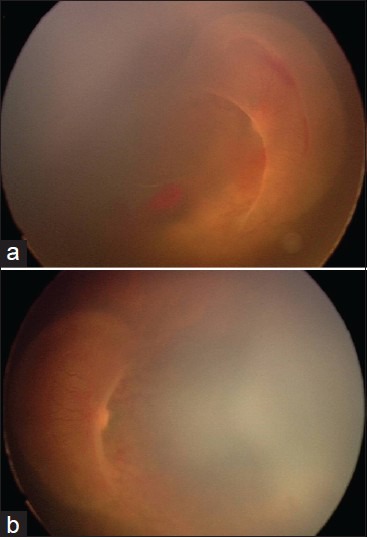
Fundus photographs from a male infant weighing 1875 g at birth and 32 weeks of gestation demonstrate APROP in zone 1 with extensive brush like proliferation projecting into the vitreous and covering the optic disc and posterior pole in right eye (a) and left eye (b), respectively. Both eyes had an unfavorable outcome after laser
Figure 3.
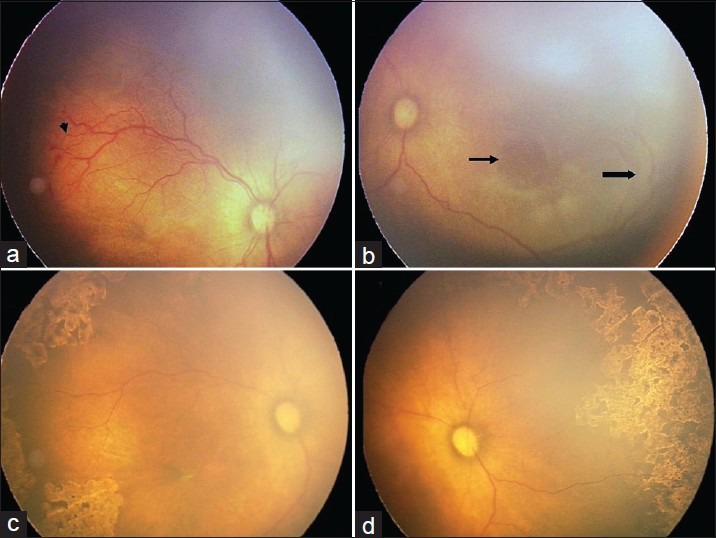
Fundus photographs from a male infant weighing 1990 g at birth and 30 weeks of gestation demonstrate APROP in posterior zone 2 and large vascular loops. (a) Fundus photograph prior to laser demonstrates plus disease, flat new vessels and hemorrhages in posterior zone 2. (b) The nasal aspect of same eye demonstrates a large vascular loop (block arrow) enclosing a grayish avascular retina (arrow). Confluent laser photocoagulation was started at 34 weeks postmenstrual age. (c) Complete regression was observed at 37 weeks. (d) The nasal aspect of same eye shows laser scars inside the vascular loop
The mean post conceptional age at laser treatment was 35.2 ± 1.54 weeks (range, 33-39 weeks). The mean number of spots were 2323.83 ± 1211.20 (range, 840-5210). Five (17.2%) eyes required retreatment. Of the 29 eyes, 22 (75.9%) eyes had complete regression after laser. The mean time to regression was 6.14 ± 2.05 weeks (range, 3-10 weeks).
Seven (24.1%) eyes had an unfavorable outcome (Stage 5: Two eyes, Stage 4a: Two eyes, Cataract: Two eyes, vitreous hemorrhage: One eye). One eye with stage 5 underwent lensectomy vitrectomy with an unfavorable outcome. The other eye with stage 5 did not undergo intervention. Both eyes of an infant had localized (2 clock hours) stage 4a detachments which remained stable until a follow-up of 34 months. Two eyes developed cataract after laser and required lensectomy. Complete regression of disease was noted at the time of lensectomy. One eye had vitreous hemorrhage that resolved after 12 weeks of observation. Overall, a complete regression was achieved in 25 (86.2%) of the 29 eyes.
Discussion
Various studies from the west and Japan describe APROP in infants <30 weeks of gestational age and <1000 g birth weight.[1,2,9] However, recent studies from India report APROP in older and heavier infants.[3,4,6,7] In a recent study from north India, 15.91% infants developing APROP had a birth weight above 1500 g.[3]
The reported risk factors for zone 1 APROP include extreme prematurity, disruption of vasculogenesis, and a low platelet count.[1,7,10] These factors do not explain APROP in older and heavier infants. A recent study has observed the use of supplemental unblended oxygen in heavier infants developing APROP.[6] Most of the infants in the present series had multiple co-morbidities and received supplemental oxygen in centers with poor neonatal care. It is plausible that early and excessive exposure to unmonitored oxygen therapy may lead to APROP-like morphology in these infants. However, the present study is too small to prove causal association of any risk factor with APROP in heavier infants.
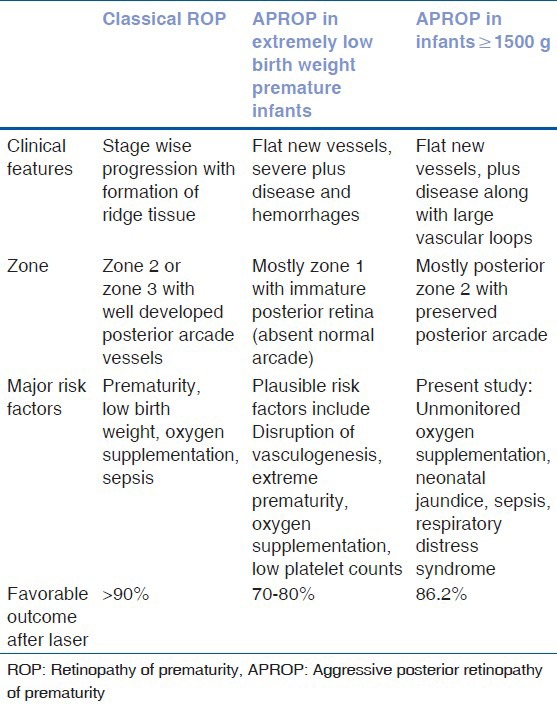
APROP in heavier infants differs in certain aspects from disease seen in extremely low birth weight premature infants [Table 3]. First, there is a preponderance of posterior zone 2 APROP with more mature central vasculature as compared with poorly developed vasculature in zone 1 APROP. Second, vessels extend for a considerable distance into the nasal retina forming large loops and enclosing an underlying avascular retina. Finally, we did not observe relentless fibrovascular proliferation after laser treatment, which might limit outcomes in typical APROP.[9] These differences suggest that APROP in heavier infants may have a different pathogenesis.
Table 3.
Clinical features, risk factors and outcomes for various types of ROP
The present study is retrospective and lacks a control group. A prospective study is difficult, given the fact that infants come from different centers. In conclusion, APROP may be seen in infants ≥ 1500 g birth weight. Further studies should evaluate the risk factors, pathogenesis, and genetic mechanisms for APROP in these infants.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.International committee for the classification of retinopathy of prematurity. The international classification of retinopathy of prematurity revisited. Arch Ophthalmol. 2005;123:991–9. doi: 10.1001/archopht.123.7.991. [DOI] [PubMed] [Google Scholar]
- 2.Drenser KA, Trese MT, Capone A., Jr Aggressive posterior retinopathy of prematurity. Retina. 2010;30:S37–40. doi: 10.1097/IAE.0b013e3181cb6151. [DOI] [PubMed] [Google Scholar]
- 3.Sanghi G, Dogra MR, Das P, Vinekar A, Gupta A, Dutta S. Aggressive posterior retinopathy of prematurity in Asian Indian babies: Spectrum of disease and outcome after laser treatment. Retina. 2009;29:1335–9. doi: 10.1097/IAE.0b013e3181a68f3a. [DOI] [PubMed] [Google Scholar]
- 4.Jalali S, Kesarwani S, Hussain A. Outcomes of a protocol-based management for zone 1retinopathy of prematurity: The Indian Twin Citities ROP screening program report number. Am J Ophthalmol. 2011;151:719–24. doi: 10.1016/j.ajo.2010.10.007. [DOI] [PubMed] [Google Scholar]
- 5.Early Treatment for Retinopathy of Prematurity Cooperative Group. Revised indications for the treatment of retinopathy of prematurity: Results of the early treatment for retinopathy of prematurity randomized trial. Arch Ophthalmol. 2003;121:1684–96. doi: 10.1001/archopht.121.12.1684. [DOI] [PubMed] [Google Scholar]
- 6.Shah PK, Narendran V, Kalpana N. Aggressive posterior retinopathy of prematurity in large preterm babies in South India. Arch Dis Child Fetal Neonatal Ed. 2012;97:F371–5. doi: 10.1136/fetalneonatal-2011-301121. [DOI] [PubMed] [Google Scholar]
- 7.Vinekar A, Hegde K, Gilbert C, Braganza S, Pradeep M, Shetty R, et al. Do platelets have a role in the pathogenesis of aggressive posterior retinopathy of prematurity? Retina. 2010;30:S20–3. doi: 10.1097/IAE.0b013e3181cafc30. [DOI] [PubMed] [Google Scholar]
- 8.Stark AR. American Academy of Pediatrics Committee on Fetus and Newborn. Levels of neonatal care. Pediatrics. 2004;114:1341–7. doi: 10.1542/peds.2004-1697. [DOI] [PubMed] [Google Scholar]
- 9.Azuma N, Ishikawa K, Hama Y, Hiraoka M, Suzuki Y, Nishina S. Early vitreous surgery for aggressive posterior retinopathy of prematurity. Am J Ophthalmol. 2006;142:636–43. doi: 10.1016/j.ajo.2006.05.048. [DOI] [PubMed] [Google Scholar]
- 10.Flynn JT, Chan-Ling T. Retinopathy of prematurity: Two distinct mechanisms that underlie zone1 and zone 2 disease. Am J Ophthalmol. 2006;142:46–59. doi: 10.1016/j.ajo.2006.02.018. [DOI] [PubMed] [Google Scholar]


