Few brain chemicals have experienced as vibrant a renaissance in neuroendocrinology as the neurohypophyseal peptides oxytocin and vasopressin. Oxytocin (derived from Greek, meaning quick birth) is named for its role in the progression of labor. Oxytocin was the first peptide to have its structure defined and to be synthesized, resulting in a Nobel prize for Vincent du Vigneaud in 1955. The structure and synthesis of vasopressin (derived from Latin, meaning vessel pressure) soon followed, placing these peptides on center stage for chemists and endocrinologists alike. Both peptides have had tremendous success clinically for promoting labor and lactation and for treating diabetes insipidus.
Beginning in the mid 1960's researchers examined the central effects of oxytocin and vasopressin on learning and memory (De Wied, 1965; de Wied et al., 1993). Indeed the effects of intranasal vasopressin on learning and memory in humans were examined as early as 1981 (Weingartner et al., 1981). The specificity of many of these effects on learning and memory was later challenged and attributed to the modulation of arousal (Ettenberg et al., 1983), and that line of investigation dwindled. In the early 1980's the distribution of oxytocin and vasopressin neurons in the hypothalamus and extrahypothalamic brain regions, and their projections were mapped using immunohistochemical techniques (Swanson and Sawchenko, 1983). During this time the neurohypophyseal system became the model system for the study of neurosecretion, and the regulation of oxytocin and vasopressin release was studied extensively, particularly in relation to peripheral release in the regulation of labor, nursing and water homeostasis.
But today, oxytocin and vasopressin may be best known for their contribution to the regulation of social behaviors. The first evidence linking oxytocin and vasopressin to complex social behaviors came in the late 1970's and 1980's. Cort Pedersen found that centrally administered oxytocin promoted maternal nurturing in virgin, steroid-primed female rats, suggesting that this molecule coordinates not only the peripheral physiology of reproduction, but also transforms the mother's brain to ensure the survival of the offspring (Pedersen and Prange, 1979; Bosch and Neumann, 2012-this issue). This observation was not without controversy but there is now compelling evidence that oxytocin modulates maternal behavior in several species (Ross and Young, 2009). Keith Kendrick, Barry Keverne et al. went on to show that oxytocin plays a specific role in the formation of the mother-infant bond in sheep moments after birth (Kendrick et al., 1997). Oxytocin was also found to play an important role in regulating lordosis behavior in rats (Arletti and Bertolini, 1985; Borrow and Cameron, 2012-this issue). Craig Ferris and Elliott Albers then reported that vasopressin acting in the hypothalamus initiated scent marking behavior and territorial aggression in hamsters (Ferris et al., 1984; Albers, 2012-this issue).
The behavioral roles of oxytocin and vasopressin soon expanded in the mid 1990's as Sue Carter, Thomas Insel, Geert DeVries et al. reported that these peptides play important roles in regulating pair bonding, mate guarding and paternal care in monogamous prairie voles (Winslow et al., 1993; Wang et al., 1994; Williams et al., 1994). These studies and those that followed in prairie voles have sparked the imagination of the media and placed these molecules squarely in the public's attention. It is now not uncommon to hear references to oxytocin and vasopressin on prime time television dramas or talk shows like “The View”.
The prominence of these peptides in the public's attention has been further elevated in the past few years by a plethora of studies in humans suggesting that oxytocin and vasopressin modulate human social behavior and cognition. In 2005 a landmark paper published in Nature suggested that oxytocin enhanced interpersonal trust (Kosfeld et al., 2005). Dozens of intranasal oxytocin papers followed, suggesting that this peptide modulates many aspects of social cognition, ranging from gaze into the eyes of another, to “in group love” (De Dreu, in press; Graustella and MacLeod, 2012-this issue). Oxytocin has even been shown to enhance some aspects of social function in psychiatric disorders such as autism (Graustella and MacLeod, 2012-this issue; Higashida et al., 2012-this issue; Modi and Young, 2012-this issue). Polymorphisms in the oxytocin and vasopressin receptor genes have been linked to attachment, generosity, and even pair bonding behaviors in humans (Ebstein et al., 2012-this issue). Once known almost exclusively for their roles in birth, milk ejection and water balance, it is not uncommon for these peptides or receptors to be referred to (regrettably) as the “trust” hormone, the “monogamy gene”, or even the “cheating gene”. One BBC news website boldly proclaimed that “Scientists have for the first time shown how a “trust” hormone is released in the brains of breastfeeding mothers”.
The parallels between the findings in animals and humans are indeed remarkable. We imagine that few of the veterans in the oxytocin and vasopressin field (including us) would have guessed that these peptides would have such profound effects on human social cognition, let alone have clinical applications for autism, social anxiety, or schizophrenia as seems to be the case today. Indeed, we sometimes ask ourselves “is this for real?” Do these peptides truly influence our trust, social bonding, social cohesion, attunement to others, or is the field experiencing an irrational exuberance that could burst after a few more years of careful scrutiny. We are reaching a point where the evidence is overwhelmingly convincing, but we must keep on our watch. There are important questions that have yet to be addressed. Does intranasal administration of oxytocin or vasopressin modulate behavior by acting centrally or peripherally? Are plasma oxytocin concentrations a proxy of central oxytocin activity? Do the effects of exogenous (intranasal) oxytocin tell us something about normal physiology? There are issues related to how plasma samples are prepared (e.g. extraction versus no extraction) which raise concern among veteran endocrinologists. Finally, are the effects of oxytocin and vasopressin on social cognition specific for those behaviors or could they be explained by a more general effect on anxiety or arousal as proposed for the early learning and memory studies. Churchland and Winkielman provide a thoughtful perspective on some of these questions in this issue (Churchland and Winkielman, 2012-this issue).
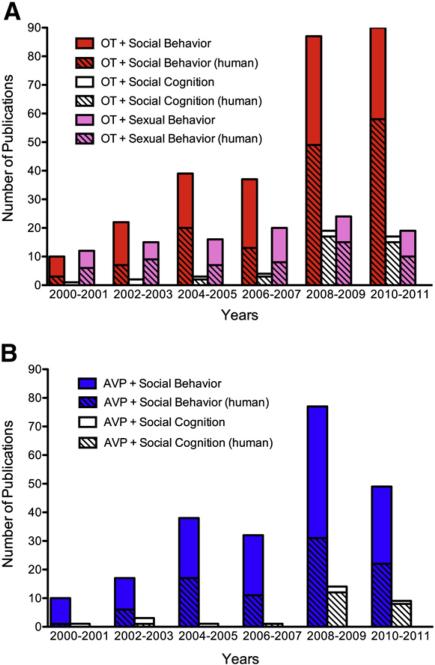
The growth in research focusing on the roles of oxytocin and vasopressin in regulating social behavior has been extraordinary over the last decade. A Medline search reveals more than a four-fold increase in the number of papers published in the past 10 years on the topic of (oxytocin or vasopressin) and (social cognition or social behavior) compared with the previous decade (368 versus 87). Fig. 1 illustrates the steady rise in papers during the past 10 years, including the jump in human research on this topic. As of January 2012 there were 37 studies classified as clinical trials related to social cognition or psychiatric disorders as reported on www.clinicaltrials.com. Therefore, we believe that this special issue focusing on oxytocin, vasopressin and social behavior is particularly timely.
Fig. 1.
Bar graphs illustrate the number of publications per two-year time bin that included the key words oxytocin (panel A) or vasopressin (panel B) and social behavior, social cognition, or sexual behavior. Note the steady climb in the subset of papers that uses human participants. In contrast to the sharp rise in papers on these peptides in social behavior and cognition, note that number of publications on oxytocin and sexual behavior has remained flat. The searches were conducted on the Medline database using the “basic search” option, and limited by years, English language, human (when applicable) and a five star ranking (“all search concepts are present and complete”).
This special issue collects twenty reviews on the many facets of neurohypophyseal hormones and social behavior, and we believe that this represents the most comprehensive and up to date coverage of the topic available today. The issue begins with two comparative reviews that examine the role of this family of peptides in fish and birds, which highlights the adaptive value of these peptides’ functions across speciation (Godwin and Thompson, 2012-this issue; Goodson et al., 2012-this issue). Then a series of reviews examine the role of oxytocin and vasopressin in regulating social recognition, sexual behavior and pregnancy (Albers, this issue; Borrow and Cameron, 2012-this issue; Ferri and Flanagan-Cato, 2012-this issue; Stevenson and Caldwell, 2012-this issue), with an eye toward revealing the underlying brain circuits that enable central oxytocin and vasopressin effects. Bosch and Neumann review the roles of oxytocin and vasopressin in regulating maternal care and aggression, challenging the notion that vasopressin primarily regulates male social behaviors (Bosch and Neumann, 2012-this issue). Two reviews then explore the developmental influences of oxytocin and vasopressin on later life behavior (Bales and Perkeybile, 2012-this issue; Veenema, 2012-this issue). Smith and Wang explore the possibility that oxytocin mediates the social buffering effects on the stress system (Smith and Wang, 2012-this issue).
There is growing evidence that oxytocin may have important clinical applications in disorders such as autism and addiction, and a series of reviews explores translational research in this area (Higashida et al., 2012-this issue; McGregor and Bowen, 2012-this issue; Modi and Young, 2012-this issue). Several reviews explore the evidence that the oxytocin and vasopressin system regulate human social behaviors based on genetic studies, circulating hormonal correlations, brain imaging studies and pharmacological manipulations (De Dreu, 2012-this issue; Ebstein et al., 2012-this issue; Feldman, 2012-this issue; Graustella and MacLeod, 2012-this issue; Zink and Meyer-Lindenberg, 2012-this issue).
Also included in this Special Issue are four original articles within the theme of the special issue, which also span the continuum from basic to translational to clinical research. These articles utilize freely behaving prairie voles as a model for studying the role of oxytocin receptors in social bonds (Ophir et al., 2012), murine models to investigate the role of different oxytocin receptor populations in the brain on maternal behavior (Curley et al., 2012), a transgenic mouse model of autistic behavior (Pobbe et al., 2012), and natural variation in human maternal behaviors to investigate the role of endogenous oxytocin (Strathearn et al., 2012).
One of the most interesting aspects of oxytocin and vasopressin biology pertains to the evolution of their function. What was the evolutionary journey that led to these peptide's roles in empathy, cooperation and pair bonding? Ancestral homologues of oxytocin and vasopressin have been reported in several invertebrate taxa. Indeed these homologues regulate egg laying behavior in earthworms and sexual behavior in leeches (Goodson et al., 2012-this issue). In mammals, reproduction requires not only delivery and lactation, but also the motivation to nurture the offspring, and the timing is right for oxytocin to take on a role in motivating the mother to adopt motherly behaviors. Perhaps maternal nurturance is the evolutionary antecedent to many more recently evolved behaviors such as empathy, pair bonding, and group cohesion. Likewise, vasopressin's role in social communication may be evolutionarily linked to it role in regulating water balance and concentrating urine (Albers, 2012-this issue). This led to scent marking and then other territorial behaviors. Indeed vasopressin's role in pair bonding in male prairie voles may be related to this ancestral function where the female partner may be an extension of the male's territory.
There are other issues that the field as a whole should consider. For example, there is significant cross-talk between oxytocin and vasopressin and their receptors. Oxytocin can activate the vasopressin receptor and vasopressin can activate the oxytocin receptor. Thus, some of the effects of exogenously administered oxytocin could be due to their activation of vasopressin receptors. Indeed, perhaps we should consider referring to oxytocin and vasopressin receptors as Type I, Type II, etc., neurohypophyseal peptide receptors.
We do not know much about distribution of oxytocin and vasopressin receptors in the human brain. A single paper published twenty years ago reported the localization of oxytocin and vasopressin receptors in the human brain, but this issue needs to be re-addressed with more selective tools (Loup et al., 1991). It turns out that the radioligands that are selective for oxytocin and vasopressin receptors in rodents are not selective for primate or human receptors. New receptor autoradiographic techniques involving human selective compounds are needed. Even more valuable would be the development of PET ligands for these receptors that would allow for in vivo imaging in the living human brain.
The translational implications for oxytocin are remarkable. While intranasal oxytocin has been found to improve some aspects of social cognition, we do not know whether these are due to central actions or peripheral effects. We do not know whether intranasal oxytocin even penetrates to subcortical brain regions. The development of novel pharmacological approaches to stimulate the oxytocin system could profoundly improve treatment strategies for disorders characterized by impairments in social cognition (Modi and Young, 2012-this issue). But research also is needed to determine the possible long-term health consequences of exogenous oxytocin treatment, the potential for abuse, and the ethical considerations of neuro-enhancement with oxytocin treatment.
We are grateful for the opportunity to assemble this exciting Special Issue and we hope that it will become a valuable resource for students and researchers interested in the oxytocin and vasopressin system and their role in the regulation of social cognition and behavior. We trust that this area of research will continue to connect with other domains of neurobiology, to deliver exciting discoveries and fresh insights into proximate and ultimate mechanisms of behavior.
Contributor Information
Larry J. Young, Center for Translational Social Neuroscience, Department of Psychiatry and Behavioral Sciences Yerkes National Primate Research Center, Emory University, USA lyoun03@emory.edu
Loretta M. Flanagan-Cato, Department of Psychology, University of Pennsylvania, USA flanagan@psych.upenn.edu
References
- Albers HE. The regulation of social recognition, social communication and aggression: Vasopressin in the social behavior neural network. Horm. Behav. 2012;61:283–292. doi: 10.1016/j.yhbeh.2011.10.007. (this issue) [DOI] [PubMed] [Google Scholar]
- Arletti R, Bertolini A. Oxytocin stimulates lordosis behavior in female rats. Neuropeptides. 1985;6:247–253. doi: 10.1016/0143-4179(85)90095-2. [DOI] [PubMed] [Google Scholar]
- Bales KL, Perkeybile AM. Developmental experiences and the oxytocin receptor system. Horm Behav. 2012;61:313–319. doi: 10.1016/j.yhbeh.2011.12.013. (this issue) [DOI] [PubMed] [Google Scholar]
- Borrow AP, Cameron NM. The role of oxytocin in mating and pregnancy. Horm. Behav. 2012;61:266–276. doi: 10.1016/j.yhbeh.2011.11.001. (this issue) [DOI] [PubMed] [Google Scholar]
- Bosch OJ, Neumann ID. Both oxytocin and vasopressin are mediators of maternal care and aggression in rodents: From central release to sites of action. Horm. Behav. 2012;61:293–303. doi: 10.1016/j.yhbeh.2011.11.002. (this issue) [DOI] [PubMed] [Google Scholar]
- Churchland PS, Winkielman P. Modulating social behavior with oxytocin: How does it work? What does it mean? Horm. Behav. 2012;61:392–399. doi: 10.1016/j.yhbeh.2011.12.003. (this issue) [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Dreu CKW. Oxytocin modulates cooperation within and competition between groups: An integrative review and research agenda. Horm. Behav. 2012;61:419–428. doi: 10.1016/j.yhbeh.2011.12.009. (this issue) [DOI] [PubMed] [Google Scholar]
- De Wied D. The influence of the posterior and intermediate lobe of the pituitary and pituitary peptides on the maintenance of a conditioned avoidance response in rats. Int. J. Neuropharmacol. 1965;4:157–167. doi: 10.1016/0028-3908(65)90005-5. [DOI] [PubMed] [Google Scholar]
- de Wied D, Diamant M, Fodor M. Central nervous system effects of the neurohypophyseal hormones and related peptides. Front. Neuroendocrinol. 1993;14:251–302. doi: 10.1006/frne.1993.1009. [DOI] [PubMed] [Google Scholar]
- Ebstein RP, Knafo A, Mankuta D, Chew SH, Lai PS. The contributions of oxytocin and vasopressin pathway genes to human behavior. Horm. Behav. 2012;61:359–379. doi: 10.1016/j.yhbeh.2011.12.014. (this issue) [DOI] [PubMed] [Google Scholar]
- Ettenberg A, van der Kooy D, Le Moal M, Koob GF, Bloom FE. Can aversive properties of (peripherally-injected) vasopressin account for its putative role in memory? Behav. Brain Res. 1983;7:331–350. doi: 10.1016/0166-4328(83)90024-4. [DOI] [PubMed] [Google Scholar]
- Feldman R. Oxytocin and social affiliation in humans. Horm. Behav. 2012;61:380–391. doi: 10.1016/j.yhbeh.2012.01.008. (this issue) [DOI] [PubMed] [Google Scholar]
- Ferri SL, Flanagan-Cato LM. Oxytocin and dendrite remodeling in the hypothalamus. Horm. Behav. 2012;61:251–258. doi: 10.1016/j.yhbeh.2012.01.012. (this issue) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferris CF, Albers HE, Wesolowski SM, Goldman BD, Luman SE. Vasopressin injected into the hypothalamus triggers a stereotypic behavior in golden hamsters. Science. 1984;224:521–523. doi: 10.1126/science.6538700. [DOI] [PubMed] [Google Scholar]
- Godwin J, Thompson R. Nonapeptides and Social Behavior in Fishes. Horm. Behav. 2012;61:230–238. doi: 10.1016/j.yhbeh.2011.12.016. (this issue) [DOI] [PubMed] [Google Scholar]
- Goodson J, Kelly A, Ma K. Evolving Nonapeptide Mechanisms of Gregariousness and Social Diversity in Birds. Horm Behav. 2012;61:239–250. doi: 10.1016/j.yhbeh.2012.01.005. (this issue) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graustella AJ, MacLeod C. A critical review of the influence of oxytocin nasal spray on social cognition in humans: Evidence and future directions. Horm. Behav. 2012;61:410–418. doi: 10.1016/j.yhbeh.2012.01.002. (this issue) [DOI] [PubMed] [Google Scholar]
- Higashida H, Yokoyama S, Kikuchi M, Munesue T. CD38 and its role in oxytocin secretion and social behavior. Horm. Behav. 2012;61:351–358. doi: 10.1016/j.yhbeh.2011.12.011. (this issue) [DOI] [PubMed] [Google Scholar]
- Kendrick KM, Costa APCD, Broad KD, Ohkura S, Guevara R, Levy F, Keverne EB. Neural control of maternal behavior and olfactory recognition of off-spring. Brain Res. Bull. 1997;44:383–395. doi: 10.1016/s0361-9230(97)00218-9. [DOI] [PubMed] [Google Scholar]
- Kosfeld M, Heinrichs M, Zak PJ, Fischbacher U, Fehr E. Oxytocin increases trust in humans. Nature. 2005;435:673–676. doi: 10.1038/nature03701. [DOI] [PubMed] [Google Scholar]
- Loup F, Tribollet E, Dubois-Dauphin M, Dreifuss JJ. Localization of high-affinity binding sites for oxytocin and vasopressin in the human brain. An autoradiographic study. Brain Res. 1991;555:220–232. doi: 10.1016/0006-8993(91)90345-v. [DOI] [PubMed] [Google Scholar]
- McGregor IS, Bowen MT. Breaking the loop: Oxytocin as a potential treatment for drug addiction. Horm Behav. 2012;61:331–339. doi: 10.1016/j.yhbeh.2011.12.001. (this issue) [DOI] [PubMed] [Google Scholar]
- Modi ME, Young LJ. The oxytocin system in drug discovery for autism: Animal models and novel therapeutic strategies. Horm. Behav. 2012;61:340–350. doi: 10.1016/j.yhbeh.2011.12.010. (this issue) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen CA, Prange AJ., Jr. Induction of maternal behavior in virgin rats after intracerebroventricular administration of oxytocin. Proc. Natl. Acad. Sci. U. S. A. 1979;76:6661–6665. doi: 10.1073/pnas.76.12.6661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross HE, Young LJ. Oxytocin and the neural mechanisms regulating social cognition and affiliative behavior. Front. Neuroendocrinol. 2009;30:534–547. doi: 10.1016/j.yfrne.2009.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith AS, Wang Z. Salubrious effects of oxytocin on social stress-induced deficits. Horm. Behav. 2012;61:320–330. doi: 10.1016/j.yhbeh.2011.11.010. (this issue) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevenson EL, Caldwell HK. The vasopressin 1b receptor and the neural regulation of social behavior. Horm. Behav. 2012;61:277–282. doi: 10.1016/j.yhbeh.2011.11.009. (this issue) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson LW, Sawchenko PE. Hypothalamic integration: organization of the paraventricular and supraoptic nuclei. Annu. Rev. Neurosci. 1983;6:269–324. doi: 10.1146/annurev.ne.06.030183.001413. [DOI] [PubMed] [Google Scholar]
- Veenema AH. Toward understanding how early-life social experiences alter oxytocin- and vasopressin-regulated social behaviors. Horm. Behav. 2012;61:304–312. doi: 10.1016/j.yhbeh.2011.12.002. (this issue) [DOI] [PubMed] [Google Scholar]
- Wang Z, Ferris CF, De Vries GJ. Role of septal vasopressin innervation in paternal behavior in prairie voles (Microtus ochrogaster). Proc. Natl. Acad. Sci. U. S. A. 1994;91:400–404. doi: 10.1073/pnas.91.1.400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weingartner H, Gold P, Ballenger JC, Smallberg SA, Summers R, Rubinow DR, Post RM, Goodwin FK. Effects of vasopressin on human memory functions. Science. 1981;211:601–603. doi: 10.1126/science.7455701. [DOI] [PubMed] [Google Scholar]
- Williams JR, Insel TR, Harbaugh CR, Carter CS. Oxytocin administered centrally facilitates formation of a partner preference in prairie voles (Microtus ochrogaster). J. Neuroendocrinol. 1994;6:247–250. doi: 10.1111/j.1365-2826.1994.tb00579.x. [DOI] [PubMed] [Google Scholar]
- Winslow J, Hastings N, Carter CS, Harbaugh C, Insel T. A role for central vasopressin in pair bonding in monogamous prairie voles. Nature. 1993;365:545–548. doi: 10.1038/365545a0. [DOI] [PubMed] [Google Scholar]
- Zink CF, Meyer-Lindenberg A. Human Neuroimaging of Oxytocin and Vasopressin in Social Cognition. Horm. Behav. 2012;61:400–409. doi: 10.1016/j.yhbeh.2012.01.016. (this issue) [DOI] [PMC free article] [PubMed] [Google Scholar]