Abstract
Background
The significance of perturbations of thyroid-stimulating hormone (TSH) and thyroid hormones within the laboratory reference ranges after hemithyroidectomy is unknown. Our aim was to examine changes in TSH and thyroid hormones after hemithyroidectomy for benign euthyroid goiter, focusing on tissue response by examining the mitochondrial membrane potential (MMP) of peripheral blood mononuclear cells (PBMCs) and basal oxygen consumption (V˙O2).
Materials and Methods
In a prospective study on 28 patients and controls, we examined serum TSH and thyroid hormones before hemithyroidectomy and 1, 3, 6 and 12 months after hemithyroidectomy for benign euthyroid goiter. In the hemithyroidectomy group, flow cytometry was used to measure the MMP of tetramethylrhodamine methyl ester (TMRM)- and MitoTracker Green (MTG)-stained PBMCs, and V˙O2 was measured by an Oxycon Pro apparatus.
Results
One year after hemithyroidectomy, TSH had increased from a median of 0.97 mIU/l (interquartile range, IQR: 0.69-1.50 mIU/l) to 2.10 mIU/l (IQR: 1.90-3.00 mIU/l; p < 0.001); free thyroxine (fT4) had decreased from a median of 16.0 pmol/l (IQR: 14.9-17.0 pmol/l) to 14.8 pmol/l (IQR: 14.1-16.4 pmol/l; p = 0.009), whereas total triiodothyronine variations did not differ from those in controls. Concomitantly, the MMP of TMRM- and MTG-stained PBMCs was increased by 58% (p < 0.001) and 22% (p = 0.008), respectively. V˙O2 was increased by 14% (p = 0.01).
Conclusion
Hemithyroidectomy for benign euthyroid goiter induced persistently increased TSH and decreased fT4, sustained mitochondrial hyperpolarization and increased V˙O2. Our results demonstrate a decrease after hemithyroidectomy of the metabolic state to which the individual is adapted, with persistent cellular metabolic changes in a hemithyroidectomized patient group which is normally considered clinically and biochemically euthyroid.
Key Words : Thyroidectomy, Mitochondrial function, Thyrotropin, Thyroid hormone, Hypothyroidism
Introduction
Whereas hemithyroidectomy is associated with a recognized risk of overt hypothyroidism [1], the significance of perturbations of thyroid-stimulating hormone (TSH) and thyroid hormones within the laboratory reference ranges after hemithyroidectomy has not previously been addressed, and these patients are generally considered euthyroid. Thyroid hormones regulate a number of cellular functions, a major one of which is regulation of mitochondrial energy production [2] and biogenesis [3], and we have previously described impaired thyroid hormone-regulated mitochondrial function in patients with subclinical hypothyroidism [4]. Therefore, we aimed to examine individual changes in TSH and thyroid hormones after hemithyroidectomy, focusing on tissue response to hemithyroidectomy-induced thyroid functional changes by examining the mitochondrial function of peripheral blood mononuclear cells (PBMCs) before and during the first year after hemithyroidectomy for benign euthyroid goiter.
Materials and Methods
We performed a pilot study to evaluate the magnitude of thyroid functional changes in hemithyroidectomized patients compared with those in healthy persons over time, in which we compared TSH changes in 28 hemithyroidectomized patients after 2 years of follow-up with TSH changes in a healthy control group. In a following prospective study, we evaluated the tissue response to these hemithyroidectomy-induced thyroid functional changes by measuring mitochondrial function, TSH and thyroid hormone levels in 28 patients before and during the first year after hemithyroidectomy for benign euthyroid goiter.
Study Groups
In the pilot study, the hemithyroidectomy group was formed by review of the hospital charts of all patients (n = 74) who had undergone hemithyroidectomy in the time period from September 2008 to July 2009 at the Department of Otorhinolaryngology – Head and Neck Surgery, Slagelse Hospital, Hospital South, Region Zealand, Denmark. The criteria for inclusion in the study groups were: (1) no previous goiter surgery; (2) no previous radioiodine treatment for goiter; (3) no previous or present medical treatment of hyperthyroidism or thyroid hormone substitution therapy; (4) preoperative value of TSH in the laboratory reference range (0.3-4.0 mIU/l); (5) benign pathologic diagnosis of resected tissue; (6) preoperative BMI between 20 and 35; (7) no endocrine diseases; and (8) age between 18 and 75 years. Values for preoperative TSH were obtained from nonfasting venous blood samples drawn at the routine examination prior to hemithyroidectomy. Forty-six patients (11 men and 35 women) aged 32-74 years fulfilled the inclusion criteria and were invited to take part in the study; 28 patients responded to the invitation (6 men and 22 women), resulting in a 61% participation rate.
Control persons were recruited from participants in the Danish General Suburban Population Study (GESUS) [5]. The 80 control persons invited (18 men and 62 women) had reported absence of thyroid or other endocrine disease in the GESUS questionnaire, and their values for TSH (from the GESUS database) were within the laboratory reference ranges; 53 persons responded to the invitation, resulting in a 66% participation rate. Six of these were excluded due to other ethnicity than Caucasian (n = 2) or subsequent endocrine disease (n = 4). In total, 47 control persons (9 men and 38 women) were included. At the follow-up visit, the hemithyroidectomized patients and controls reported their medical history and current medication, and nonfasting venous blood samples were collected and analyzed for serum concentrations of TSH, thyroid hormones and antibodies against thyroid peroxidase (TPOAb). Due to the retrospective study design, and for practical reasons, measurement of mitochondrial function in controls was not possible.
Eligible for enrollment in the prospective study were a total of 106 patients, scheduled to undergo hemithyroidectomy between June 1, 2011, and July 1, 2012, at the Department of Otorhinolaryngology – Head and Neck Surgery at Slagelse Hospital. Of the 106 patients, 55 fulfilled the inclusion criteria and were consecutively invited to the study. Ultimately, 33 patients were included (60% participation rate). Postoperatively and during the year of follow-up, 5 patients were excluded from the study due to either malignant pathologic diagnosis of resected tissue (n = 3) or subsequent severe disease (n = 2), resulting in a study group of 28 patients (6 men and 22 women). In this study group, 25 of the 28 patients had been diagnosed with 2 or more thyroid nodules at the preoperative ultrasound examination.
Anthropometric Measurements
The patients in the prospective study were examined by an experienced endocrinologist before hemithyroidectomy and at 4 time points (1, 3, 6 and 12 months) after hemithyroidectomy. At each time point, the patients underwent a medical examination including medical history and current medication, physical examination and measurement of basal oxygen consumption (V˙O2), and a nonfasting venous blood sample was drawn (between 10 and 14 a.m.) and submitted to analysis. TPOAb was measured at the examination before hemithyroidectomy.
Analysis of PBMCs by Flow Cytometry
Venous blood samples were drawn in EDTA Vacutainer tubes and immediately cooled. Red blood cells were lysed with a 1-ml lysing solution (VersaLyse; Beckman Coulter, Brea, Calif., USA), and the PBMCs were washed once in PBS. For each patient, 4 pellets were resuspended in a total volume of 1,000 μl of PBS, including an unstained control and 3 samples with the following final concentrations of 1 µg/ml propidium iodide (Sigma-Aldrich, Copenhagen, Denmark), 0.01 μM MitoTracker Green FM (MTG; Invitrogen A/S, Taastrup, Denmark) and 0.1 μM tetramethylrhodamine methyl ester (TMRM; Invitrogen A/S), respectively. Two different fluorescent dyes were used because they are considered to reflect different aspects of the mitochondrial membrane potential (MMP) [6] and as our own laboratory research has demonstrated that addition of an uncoupler of mitochondrial oxidative phosphorylation, FCCP (carbonyl cyanide-p-trifluoromethoxyphenylhydrazone), markedly decreases TMRM as well as MTG fluorescence. The resuspended cells were incubated for 30 min at room temperature in the dark. After incubation, the test tubes were chilled on ice until the fluorescence intensity of the stained PBMCs was measured by flow cytometry (BD Accuri™ C6 Flow Cytometer) according to a previously described method [7]. MTG fluorescence was recorded in the FL1 (FITC) channel and TMRM fluorescence in the FL2 (PE) channel. Lymphocytes were identified by forward and side scatter, and gated for further analysis. A total of 20,000 cells per sample were acquired. Median values for fluorescence intensity of lymphocytes were measured in relative fluorescence units (hereafter denoted as arbitrary units, AU).
Analysis of Basal Oxygen Consumption
Respiratory gas analysis and volume measurements were performed with a canopy connected by a triple V (flow/gas sensor) to an Oxycon Pro apparatus (Jaeger Oxycon Champion, Breda, The Netherlands), as previously described [4]. The relation between V˙O2 and MMP was examined, and we observed a significant correlation between measurements of V˙O2 and the MMP of MTG-stained PBMCs (r = 0.46, p = 0.03), indicating that V˙O2 reflects inner mitochondrial membrane O2 consumption.
Biochemical Variables
Measurements of serum TSH, free thyroxine (fT4) and total triiodothyronine (tT3) were performed using an electrochemical luminescent immunoassay (Cobas 6000; Roche, Basel, Switzerland). The reference range for TSH was 0.3-4.0 mIU/l (coefficient of variation, CV: <7%), the fT4 reference range was 10.0-26.0 pmol/l (CV: <5%), and the one for tT3 was 1.20-2.80 nmol/l (CV: <4%). TPOAb was measured by KRYPTOR antiTPOn (BRAHMS, Henningsdorf, Germany), with a detection limit of 10 kU/l. TPOAb >60 kU/l was the cutoff value for TPOAb positivity.
Ethical Considerations
The study was approved by the regional ethics committee of Zealand, Denmark (SJ-10 and SJ-245) and by the Danish Data Protection Agency, and was registered in ClinicalTrials.gov (NCT01358136). The study conformed to the principles of the Declaration of Helsinki. Informed consent was obtained from the hemithyroidectomized patients as well as from the control persons prior to inclusion.
Hemithyroidectomy
Hemithyroidectomy was performed in an inpatient setting by experienced thyroid surgeons who all used an identical technique, which involved total left or right thyroid lobectomy and isthmusectomy and, if present, resection of the pyramidal lobe with preservation of the contralateral thyroid lobe. One of the 61 hemithyroidectomized patients suffered from postoperative permanent recurrent laryngeal nerve paresis. Otherwise, no surgical complications were observed.
Statistical Analysis
Statistical comparison between categorical variables was performed by the χ2 test. When the Shapiro-Wilk test demonstrated absence of normality, the Mann-Whitney U test was used for continuous variables. Statistical comparisons of outcome variables at baseline and at the different time points of follow-up were performed by the Wilcoxon signed-rank test. Significant difference was defined as p < 0.05. The STATA statistical package software program (version 12.0; StataCorp, College Station, Tex., USA) was used for statistical analyses.
Results
Characteristics of Study Groups
The baseline and clinical data on the study groups are provided in table 1. The median time interval between preoperative examination and hemithyroidectomy was 8 days (interquartile range, IQR: 4-15 days), and between hemithyroidectomy and the 4 visits 1, 3, 6 and 12 months postoperatively, it was 35 (IQR: 31-43), 102 (IQR: 97-112), 192 (IQR: 182-216) and 379 days (IQR: 363-390 days), respectively.
Table 1.
Baseline data on study groups
| Control group | Hemithyroidectomy group I (pilot study) | Hemithyroidectomy group II (prospective study) | p |
||
|---|---|---|---|---|---|
| C vs. H I | C vs. H II | ||||
| n | 47 | 28 | 28 | ||
| Males, % | 19 | 21 | 21 | 0.811 | 0.811 |
| Age, years | 53 (43–62) | 51 (43–60) | 49 (43–59) | 0.591 | 0.591 |
| Weight, kg | 69.3 (62.2–81.4) | 75.0 (63.5–85.0) | 75.7 (67.3–90.4) | 0.403 | 0.121 |
| BMI, kg/m2 | 25.0 (23.0–29.0) | 26.0 (23.0–28.0) | 27.5 (23.0–31.5) | 0.601 | 0.244 |
| Smokers, % | 9 | 14 | 14 | 0.433 | 0.433 |
| TPOAb positivity, % | 13 | 14 | 7 | 0.851 | 0.445 |
| TSH, mUI/l | 1.70 (1.10–2.20) | 1.23 (0.91–1.59) | 0.97 (0.69–1.50) | 0.005 | <0.001 |
| fT4, pmol/l | 15.0 (14.0–17.0) | – | 16.0 (14.9–17.0) | – | 0.167 |
| tT3, nmol/l | 1.70 (1.53–2.02) | – | 1.89 (1.67–2.18) | – | 0.071 |
Data are presented as medians with interquartile ranges in parentheses unless specified otherwise. p values by Mann-Whitney U test and χ2 test when appropriate. C = Controls; H I = hemithyroidectomy group I; H II = hemithyroidectomy group II.
Postoperative Thyroid Function
In the pilot study, 8 of 28 patients (28.6%) received levothyroxine replacement therapy 2 years after hemithyroidectomy following the current guidelines of the department. The biochemically euthyroid patients without replacement therapy demonstrated a TSH increase of 89% from a median of 1.0 mIU/l (IQR: 0.89-1.56 mIU/l) to 1.89 mIU/l (IQR: 1.38-2.33 mIU/l; p = 0.001). In contrast, the TSH and fT4 levels of the controls were unchanged – from a median of 1.70 mIU/l (IQR: 1.10-2.20 mIU/l) to 1.60 mIU/l (IQR: 1.10-1.90 mIU/l) and from 15.0 pmol/l (IQR: 14.0-17.0 pmol/l) to 15.6 pmol/l (IQR: 13.6-16.5 pmol/l), respectively – after 2 years of follow-up. Unexpectedly, serum tT3 of the controls had decreased from 1.7 nmol/l (IQR: 1.53-2.02 nmol/l) to 1.6 nmol/l (IQR: 1.45-1.87 nmol/l; p < 0.001) after 2 years of follow-up.
In the prospective study, 8 of the 28 patients (29%) were diagnosed with posthemithyroidectomy hypothyroidism during 1 year of follow-up, and levothyroxine therapy was started. Levothyroxine-treated patients were no longer included in the analysis of follow-up results, reflected in the decreasing number of patients in the analysis of follow-up results. The median time interval from hemithyroidectomy to start of levothyroxine replacement therapy was 120 days (IQR: 102-198 days).
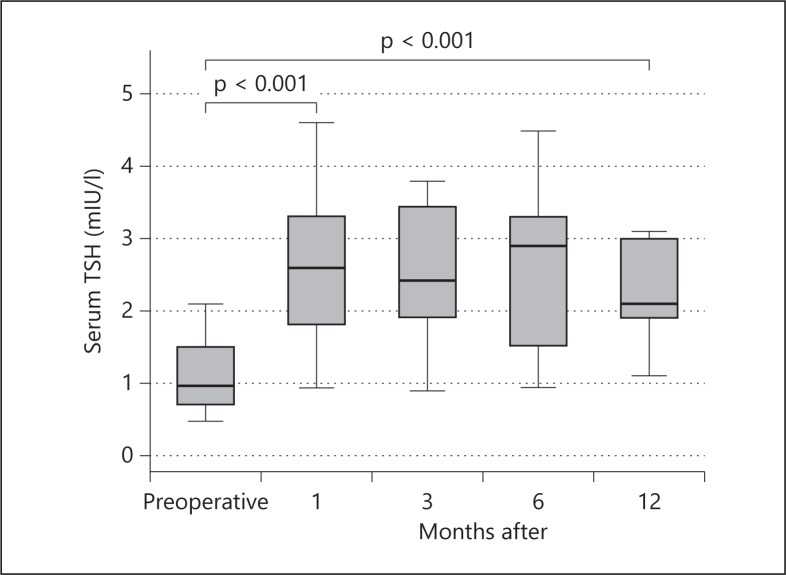
Figure 1 demonstrates a persistently increased TSH level within the reference range 1 year after hemithyroidectomy, unchanged with regard to values 1 month after hemithyroidectomy. The patients did not receive postoperative levothyroxine therapy. Table 2 presents serum fT4 and tT3 levels, the T4/T3 ratio and V˙O2 before hemithyroidectomy and 1, 3, 6 and 12 months after hemithyroidectomy for the patients who did not receive postoperative levothyroxine therapy. The table demonstrates a significant increase in V˙O2 1 year after hemithyroidectomy, unchanged relative to values 1 month after hemithyroidectomy. FT4 had persistently decreased within the reference range 1 year after hemithyroidectomy, unchanged with regard to values 1 month after hemithyroidectomy. The T4/T3 ratio was unchanged relative to the preoperative value during 1 year of follow-up after hemithyroidectomy.
Fig. 1.
The figure presents serum TSH levels before hemithyroidectomy for benign euthyroid goiter and during a 1-year follow-up. Patients did not receive levothyroxine replacement therapy. Data are presented as medians and IQR. The Wilcoxon signed-rank test was used.
Table 2.
Serum fT4 and tT3 levels, T4/T3 ratio and VO2 for patients without postoperative levothyroxine therapy
| Preoperative | 1 month after operation | 3 months after operation | 6 months after operation | 12 months after operation | p (baseline vs. 1 month after operation) | p (baseline vs. 12 months after operation) | |
|---|---|---|---|---|---|---|---|
| n | 28 | 27 | 24 | 23 | 19 | ||
| fT4, pmol/l | 16.0 (14.9–17.0) | 14.1 (13.3–16.5) | 14.5 (13.8–15.2) | 14.9 (13.8–16.0) | 14.8 (14.1–16.4) | <0.001 | 0.02 |
| tT3, nmol/l | 1.89 (1.67–2.18) | 1.75 (1.54–1.91) | 1.73 (1.51–2.04) | 1.75 (1.51–1.92) | 1.69 (1.44–1.86) | <0.001 | <0.001 |
| T4/T3 ratio | 8.2 (7.1–9.8) | 8.6 (6.7–9.6) | 8.2 (7.2–9.4) | 8.5 (7.9–9.7) | 8.8 (7.3–9.8) | NS | NS |
| Vo2, ml O2/min·m2 | 108.2 (90.3–119.6) | 119.6 (109.6–127.6) | 111.4 (107.6–125.2) | 118.2 (102.7–124.7) | 121.5 (117.6–125.2) | 0.03 | 0.01 |
Data are presented as medians with IQR in parentheses unless specified otherwise. NS = Not significant.
Postoperative Mitochondrial Function
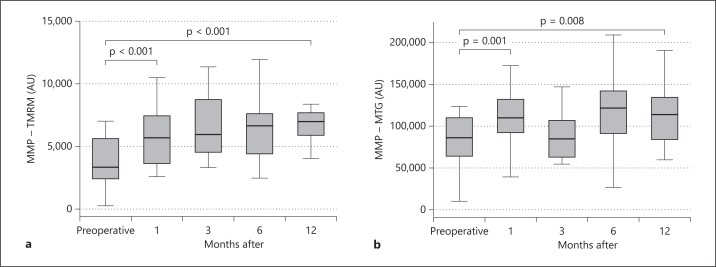
Figure 2 presents the MMP of TMRM- and MTG-stained PBMCs before hemithyroidectomy and 1, 3, 6 and 12 months after hemithyroidectomy in patients who did not receive postoperative levothyroxine therapy. One year after hemithyroidectomy, the MMP of the TMRM- and MTG-stained PBMCs was persistently increased, unchanged with regard to values 1 month postoperatively.
Fig. 2.
The figure presents the MMP of PBMCs before hemithyroidectomy for benign euthyroid goiter and during a 1-year follow-up. Patients did not receive levothyroxine replacement therapy. Data are presented as medians and IQR. The Wilcoxon signed-rank test was used.
Discussion
In concordance with previous studies of thyroid function after hemithyroidectomy [1], we observed persistently increased serum TSH levels within the laboratory reference range. Moreover, we confirmed 2 previous reports of concomitantly decreased serum fT4 [8,9]. The individual normal ranges for serum TSH, T4 and T3 are much narrower than the population-based reference ranges [10], and a thyroid functional change induced by hemithyroidectomy may not be noticed in the wide population-based reference ranges, although it represents a thyroid function that is abnormal for the individual. The main finding of the study is persistently increased TSH and decreased serum fT4 within the reference range as well as concomitantly sustained mitochondrial hyperpolarization and increased V˙O2 in patients who develop neither overt nor subclinical hypothyroidism after hemithyroidectomy.
Thyroid hormones are major regulators of mitochondrial function and biogenesis [3]. In the mitochondrion, ATP production by the electron transport chain is driven by the electrochemical gradient across the inner mitochondrial membrane. The inner MMP reflects the functional status of a mitochondrion, and an increased MMP might be anticipated to reflect increased energy production, but mitochondrial hyperpolarization might also represent increased mitochondrial production of reactive oxygen species (ROS) [11,12]. It may be argued that the preoperative measurements of MMP represent decreased values due to slightly increased metabolism in patients with euthyroid goiter, as TSH was lower in the hemithyroidectomy group than in the controls. However, preoperative values of fT4 and tT3 did not differ significantly compared with controls, and V˙O2 was not significantly different from the reference range we have established in our laboratory. There is no definite way to determine whether we observe a decrease in a slightly increased metabolism toward normal metabolism, or a decrease from a normal to slightly decreased metabolism. In either situation, hemithyroidectomy induces a decrease in the metabolic state to which the individual was adapted preoperatively. We have previously demonstrated increased mitochondrial processes, and thereby increased MMP, in PBMCs of patients with subclinical hypothyroidism compared with controls [4], and the present study has reproduced these results in a hemithyroidectomized patient group, using two different measures of MMP. Subclinical hypothyroidism is associated with an increased risk of coronary heart disease [13,14], and recent studies have demonstrated an association between low thyroid function within the euthyroid range and various cardiovascular risk markers [15,16]. It may be speculated whether this risk is also be present in hemithyroidectomized patients with individual TSH increase, fT4 decrease and mitochondrial hyperpolarization.
Increased MMP and V˙O2 in patients with decreased serum T4 after hemithyroidectomy may seem a paradox. In euthyroid humans, serum T3 is predominantly generated by outer-ring deiodination of T4 to T3 by type 2 deiodinase (D2) [17]. We have previously demonstrated how in vitro T3 and T2 stimulation is able to increase the MMP [18] and mitochondrial ROS production in PBMCs [7], while others reported thyroid hormone-induced increases in ROS production in PBMCs in vivo [19]. Although the T4/T3 ratio did not change after hemithyroidectomy, mitochondrial hyperpolarization might be a result of an increased conversion of T4 to T3 by D2 and subsequently to T2, known to stimulate mitochondria directly [18], as decreased levels of serum T4/T3 in rodent models of hypothyroidism increase D2 activity in white adipose tissue [20] and skeletal muscle [21]. Since there were no clinical signs of a raised metabolic rate after hemithyroidectomy, an increased MMP and increased V˙O2 do not seem to promote ATP production or to increase heat production. We propose the hypothesis that mitochondrial hyperpolarization after hemithyroidectomy might lead to increased mitochondrial ROS formation and subsequently to unfavorable cellular effects [22].
The present study is limited by its small number of patient as the laboratory technique restricted the number of patients possible to include; therefore it should be considered hypothesis generating. Moreover, due to the laboratory technique, it was not possible to examine MMP and V˙O2 in the control group. However, consecutive inclusion and examination of the patients during a year has minimized the effect of the unstable laboratory technique. We examined the longitudinal variations in TSH and T4 in the control group (which were not detected) in order to clearly demonstrate a shift of hemithyroidectomized patients toward subclinical hypothyroidism, reflected by the persistently increased TSH and decreased T4 levels after hemithyroidectomy for benign euthyroid goiter.
In conclusion, hemithyroidectomy for benign euthyroid goiter induced persistently increased TSH and decreased fT4 levels with sustained mitochondrial hyperpolarization and increased V˙O2. In a hemithyroidectomized patient group that is normally considered clinically and biochemically euthyroid, we have demonstrated a decrease in the metabolic state to which the individual was adapted preoperatively, with persistent cellular metabolic changes.
Disclosure Statement
The authors declare no conflicts of interest.
Acknowledgements
The authors thank technicians Jette Ellehauge and Carina Foldager at the Department of Clinical Biochemistry, Næstved Hospital, Denmark, for their skilled efforts. This study was supported by research grants from the Zealand (Medical) Research Foundation, from the Medical Research Foundation of Hospital South, Region Zealand, the Agnes and Knut Mørk Foundation and the Hans Skouby and Wife Emma Skouby Foundation, Denmark.
References
- 1.Verloop H, Louwerens M, Schoones JW, et al. Risk of hypothyroidism following hemithyroidectomy: systematic review and meta-analysis of prognostic studies. J Clin Endocrinol Metab. 2012;97:2243–2255. doi: 10.1210/jc.2012-1063. [DOI] [PubMed] [Google Scholar]
- 2.Kim B. Thyroid hormone as a determinant of energy expenditure and the basal metabolic rate. Thyroid. 2008;18:141–144. doi: 10.1089/thy.2007.0266. [DOI] [PubMed] [Google Scholar]
- 3.Weitzel JM, Iwen AK. Coordination of mitochondrial biogenesis by thyroid hormone. Mol Cell Endocrinol. 2011;342:1–7. doi: 10.1016/j.mce.2011.05.009. [DOI] [PubMed] [Google Scholar]
- 4.Kvetny J, Wilms L, Pedersen PL, Larsen J. Subclinical hypothyroidism affects mitochondrial function. Horm Metab Res. 2010;42:324–327. doi: 10.1055/s-0030-1248261. [DOI] [PubMed] [Google Scholar]
- 5.Bergholdt H, Bathum L, Kvetny J, et al. Study design, participation, and characteristics of The Danish General Suburban Population Study. Dan Med J. 2013;60:A4693. [PubMed] [Google Scholar]
- 6.Plásek J, Vojtísková A, Houstek J. Flow-cytometric monitoring of mitochondrial depolarisation: from fluorescence intensities to millivolts. J Photochem Photobiol B. 2005;78:99–108. doi: 10.1016/j.jphotobiol.2004.09.011. [DOI] [PubMed] [Google Scholar]
- 7.Anthonsen S, Larsen J, Pedersen PL, Dalgaard LT, Kvetny J. Basal and T3-induced ROS production in lymphocyte mitochondria is increased in type 2 diabetic patients. Horm Metab Res. 2013;45:261–266. doi: 10.1055/s-0032-1327590. [DOI] [PubMed] [Google Scholar]
- 8.Cheung P, Boey J, Wong J. Thyroid function after hemithyroidectomy for benign nodules. World J Surg. 1986;10:718–723. doi: 10.1007/BF01655566. [DOI] [PubMed] [Google Scholar]
- 9.Lindblom P, Valdemarsson S, Lindergård B, Westerdahl J, Bergenfelz A. Decreased levels of ionized calcium one year after hemithyroidectomy: importance of reduced thyroid hormones. Horm Res. 2001;55:81–87. doi: 10.1159/000049975. [DOI] [PubMed] [Google Scholar]
- 10.Andersen S, Pedersen KM, Bruun NH, Laurberg P. Narrow individual variations in serum T4 and T3 in normal subjects: a clue to the understanding of subclinical thyroid disease. J Clin Endocrinol Metab. 2002;87:1068–1072. doi: 10.1210/jcem.87.3.8165. [DOI] [PubMed] [Google Scholar]
- 11.Gergely P, Jr, Grossman C, Niland B, et al. Mitochondrial hyperpolarization and ATP depletion in patients with systemic lupus erythematosus. Arthritis Rheum. 2002;46:175–190. doi: 10.1002/1529-0131(200201)46:1<175::AID-ART10015>3.0.CO;2-H. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Widlansky ME, Wang J, Shenouda SM, et al. Altered mitochondrial membrane potential, mass, and morphology in the mononuclear cells of humans with type 2 diabetes. Transl Res. 2010;156:15–25. doi: 10.1016/j.trsl.2010.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rodondi N, den Elzen WP, Bauer DC, et al. Subclinical hypothyroidism and the risk of coronary heart disease and mortality. JAMA. 2010;304:1365–1374. doi: 10.1001/jama.2010.1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kvetny J, Heldgaard PE, Bladbjerg EM, Gram J. Subclinical hypothyroidism is associated with a low-grade inflammation, increased triglyceride levels and predicts cardiovascular disease in males below 50 years. Clin Endocrinol (Oxf) 2004;61:232–238. doi: 10.1111/j.1365-2265.2004.02088.x. [DOI] [PubMed] [Google Scholar]
- 15.Roos A, Bakker SJ, Links TP, Gans RO, Wolffenbuttel BH. Thyroid function is associated with components of the metabolic syndrome in euthyroid subjects. J Clin Endocrinol Metab. 2007;92:491–496. doi: 10.1210/jc.2006-1718. [DOI] [PubMed] [Google Scholar]
- 16.Ruhla S, Weickert MO, Arafat AM, et al. A high normal TSH is associated with the metabolic syndrome. Clin Endocrinol (Oxf) 2010;72:696–701. doi: 10.1111/j.1365-2265.2009.03698.x. [DOI] [PubMed] [Google Scholar]
- 17.Gereben B, Zavacki AM, Ribich S, et al. Cellular and molecular basis of deiodinase-regulated thyroid hormone signaling. Endocr Rev. 2008;29:898–938. doi: 10.1210/er.2008-0019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kvetny J, Bomholt T, Pedersen P, et al. Thyroid hormone effect on human mitochondria measured by flow cytometry. Scand J Clin Lab Invest. 2009;69:772–776. doi: 10.3109/00365510903154752. [DOI] [PubMed] [Google Scholar]
- 19.Magsino CH, Jr, Hamouda W, Ghanim H, et al. Effect of triiodothyronine on reactive oxygen species generation by leukocytes, indices of oxidative damage, and antioxidant reserve. Metabolism. 2000;49:799–803. doi: 10.1053/meta.2000.6263. [DOI] [PubMed] [Google Scholar]
- 20.Calvo RM, Obregon MJ. Presence and regulation of D1 and D2 deiodinases in rat white adipose tissue. Metabolism. 2011;60:1207–1210. doi: 10.1016/j.metabol.2011.01.014. [DOI] [PubMed] [Google Scholar]
- 21.Marsili A, Ramadan W, Harney JW, et al. Type 2 iodothyronine deiodinase levels are higher in slow-twitch than fast-twitch mouse skeletal muscle and are increased in hypothyroidism. Endocrinology. 2010;151:5952–5960. doi: 10.1210/en.2010-0631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sivitz WI, Yorek MA. Mitochondrial dysfunction in diabetes: from molecular mechanisms to functional significance and therapeutic opportunities. Antioxid Redox Signal. 2010;12:537–577. doi: 10.1089/ars.2009.2531. [DOI] [PMC free article] [PubMed] [Google Scholar]