Abstract
Background
Guidelines for the follow-up of differentiated thyroid cancer (DTC) recommend the measurement of TSH-stimulated thyroglobulin (s-Tg) instead of basal Tg on T4 therapy (b-Tg). However, these guidelines were established using first-generation Tg assays with a functional sensitivity (FS) of 0.5-1.0 ng/ml. Current more sensitive second-generation Tg assays (Tg2G; FS 0.05-0.10 ng/ml) have shown that low-risk DTC patients with undetectable b-Tg rarely have recurrences.
Objectives
This study was undertaken to compare b-Tg using a chemiluminescent Tg2G assay (Tg2GICMA; FS 0.1 ng/ml) with s-Tg in DTC patients with an intermediate risk of recurrence.
Methods
We evaluated 168 DTC patients with a low (n = 101) and intermediate (n = 67) risk of recurrence treated by total thyroidectomy (147 also treated with radioiodine), with a mean follow-up of 5 years.
Results
b-Tg was undetectable with the Tg2GICMA in 142 of 168 patients. s-Tg was <2 ng/ml in 138 of these 142 patients, and only 3 of these 138 (2%) presented metastases on cervical ultrasound (US). Of the 4 of 142 patients with s-Tg >2 ng/ml, 1 had cervical metastases seen after radioiodine. Furthermore, 26 of 168 patients presented detectable b-Tg with the Tg2GICMA; 17 of these 26 patients also presented s-Tg >2 ng/ml. In 10 of these 17 patients, metastases were detected. Cervical US or b-Tg were positive in 14 of 15 patients with recurrent disease. Globally, the sensitivity and negative predictive value of the Tg2GICMA plus US were 93 and 99%, respectively.
Conclusion
b-Tg measured with a Tg2GICMA and cervical US, used together, are equivalent to s-Tg in identifying metastases in patients with DTC with a low or intermediate risk of recurrence.
Key Words : Differentiated thyroid cancer, Thyroglobulin, Highly sensitive thyroglobulin assay, TSH-stimulated thyroglobulin test
Introduction
Patients with differentiated thyroid carcinoma (DTC) generally have a very good prognosis, with 10-year overall mortality of less than 4% [1]. However, persistent or recurrent disease, which is mainly loco-regional, occurs in approximately 20% of patients [2,3,4,5]. Because DTC can recur at any time for years after initial treatment, long-term follow-up is necessary. Follow-up should be guided by an approach with a high negative predictive value (NPV) to exclude patients with a nonsignificant risk of recurrence from undergoing unnecessary investigations and identify those individuals with a higher risk who merit closer follow-up [6,7].
The measurement of serum thyroglobulin (Tg) levels is one of the most important modalities used to monitor patients for residual or recurrent disease. Tg has high sensitivity and specificity in detecting thyroid cancer, especially after total thyroidectomy and remnant ablation, with the highest sensitivity noted after thyroid hormone withdrawal (WD-Tg) or stimulation with recombinant human TSH (rhTSH-Tg) [2,3,4,5]. Basal Tg measurement during thyroid hormone suppression of TSH (b-Tg) using first-generation Tg (Tg1G) assays with functional sensitivity (FS) of approximately 1.0 ng/ml can fail to identify patients with relatively small amounts of residual tumor and cervical metastases [8,9,10].
However, over the last decade, assays with lower FS have been developed and evaluated, primarily to study patients with DTC with a low risk of recurrence [11,12,13]. These studies have shown that in low-risk DTC patients, when these second-generation Tg (Tg2G) assays are used (FS 0.05-0.1 ng/ml), b-Tg is correlated with WD-Tg and rhTSH-Tg [14,15,16,17,18,19,20,21]. The purpose of the present study was to prospectively evaluate a cohort of patients treated for DTC with a low or intermediate risk of recurrence using a Tg2G assay during a long-term follow-up.
Subjects and Methods
Patient Selection and Tg Stimulation Tests
We evaluated 168 DTC patients, 149 women and 19 men, with a mean age at diagnosis of 40 years (range 7-72 years), who were followed by the same team in the combined thyroid clinics of the Division of Endocrinology, Department of Medicine, Escola Paulista de Medicina, Federal University of São Paulo, and the Thyroid Diseases Center, Instituto Israelita de Ensino e Pesquisa Albert Einstein, both in Sao Paulo, Brazil. All of the diagnostic procedures were performed in accordance with the regulations of the local ethics committees. Written informed consent was obtained from each patient.
Seventeen patients presented with follicular thyroid carcinoma, and 151 patients presented with papillary thyroid carcinoma. All of the patients had been treated by total thyroidectomy, and 147 of 168 had also received 131I for thyroid remnant ablation (30 or 100 mCi depending on the hospital) or treatment of local disease or lymph node metastases (200 mCi). At the Federal University of São Paulo, we used to administer 100 mCi for ablation before 2003, and 30 mCi after this period. At Albert Einstein Hospital, we administered 100 mCi for ablation. A dose of 200 mCi was used when local disease was suspected according to surgeon and histopathology descriptions or according to cervical ultrasound (US) 3-4 months after surgery. Also, 27 of 147 patients had received more than one 131I treatment (total of 250-600 mCi) for the treatment of recurrent local disease or lymph node metastases that were not suitable for surgery, before the beginning of the study. After thyroidectomy, 131I postablative whole-body scan (WBS) and initial US, the DTC patients were classified according to the American Thyroid Association's assessment for risk of recurrence [5]; 101 patients were found to have a low risk of recurrence, and 67 patients had an intermediate risk of recurrence. The mean length of follow-up before the Tg stimulation test was 5 years (range 1-21 years).
None of the patients had structural evidence of disease at the beginning of the study, because they all presented with negative cervical US, negative WBS, b-Tg <1.0 ng/ml using a first-generation immunofluorometric assay (Tg1GIFMA) with an FS of 1.0 ng/ml and negative anti-Tg antibodies (TgAb) for at least 1 year (at least one evaluation every 6 months) after the initial treatment or after the last radioiodine treatment.
All of the patients were submitted to a Tg stimulation test either by withdrawal of L-T4 (WD-Tg) or by rhTSH-Tg. The hypothyroidism protocol (n = 98) consisted of obtaining a serum sample for the measurement of b-Tg; then, the Tg stimulation test was performed under L-T4 withdrawal for 4 weeks. At this time, while the patients were in hypothyroidism, a second serum sample was collected for measurements of TSH, Tg and TgAb, and a cervical US was performed, followed by the WBS. During the follow-up, measurements of serum TSH, Tg and TgAb were obtained at least once every 6 months.
The rhTSH protocol consisted of a Tg stimulation test during suppressive therapy. All of the patients (n = 70) received one injection of rhTSH (0.9 mg, i.m.; Thyrogen, Genzyme Corp., Cambridge, Mass., USA) for 2 consecutive days. Serum samples for TSH, Tg and TgAb measurements were collected before the first rhTSH injection and 72 h after the second injection, when WBS was also performed. Imaging was performed 48 h after the administration of a 4-mCi tracer dose of radioiodine.
All of the patients with negative stimulated Tg (s-Tg; hypothyroidism <2 ng/ml or rhTSH <1 ng/ml) were followed up for a mean of 55 months (range 28-92 months) to confirm the negative results.
Most of the patients with s-Tg >2 ng/ml and negative US were treated with therapeutic activities of 131I and then submitted to WBS. The patients with suspicious images on US that were confirmed as metastases by fine-needle aspiration cytology (FNAC) and Tg measurements of the needle washing fluid (FNA-Tg) underwent lymph node surgery. Additional imaging procedures, primarily cervical and chest computed tomography (CT) scanning and 18FDG positron emission tomography (PET)-CT, were performed with those patients with s-Tg greater than 2 ng/ml, negative US and negative posttherapeutic WBS to rule out the presence of metastases.
Serum Assays: Tg, TgAb and TSH
Serum Tg was assayed following international recommendations [22,23,24,25] in duplicate using 2 methods. The first, which was used previously to classify patients as not having evidence of disease and for the Tg stimulation tests, was a Tg fluoroimmunoassay (Delfia®, PerkinElmer Life and Analytical Sciences, Wallac Oy, Turku, Finland) which presented FS of 1.0 ng/ml at a coefficient of variation (CV) of 20%. Between-run precision assessed over a 12-month period was 4.7, 4.8 and 3.8% for serum Tg concentrations of 3.5, 31.2 and 751 ng/ml, respectively. Within-run precision was 3.0, 2.9 and 3.0% at concentrations of 3.4, 18.9 and 689 ng/ml, respectively. This assay is referred to throughout this study as ‘Tg1GIFMA’.
The second assay, which was used for the comparison with s-Tg, was a highly sensitive chemiluminescent assay (Tg Access immunoassay, Beckman Coulter, Brea, Calif., USA); its FS was 0.1 ng/ml at a CV of 20% according to a previous study [17]. Within-run precision was 1.4% for a pool with a mean value of 4.2 ng/ml, 1.4% for a pool with a mean value of 21.6 ng/ml, 4.4% for a pool with a mean value of 130.4 ng/ml and 2.0% for a pool with a mean value of 344.7 ng/ml. Between-run precision assessed over a period of 12 months was 1.7, 1.8, 4.9 and 4.0% for the same pools. The total CV was 4.9% for a pool with a mean value of 4.2 ng/ml, 5.2% for a pool with a mean value of 5.3 ng/ml, 6.4% for a pool with a mean value of 34.0 ng/ml, 3.8% for a control with a mean value of 42.5 ng/ml and 4.6% for a control with a mean value of 213.0 ng/ml [26]. This assay is referred to throughout this study as ‘Tg2GICMA’.
Serum samples from each patient were processed in the same analytical course, and all sera were screened for the presence of interfering TgAb using a specific in-house immunofluorometric assay test [27]. The assay was standardized against the International Reference Preparation 65/93 standard. Between-run precision was <5% along the standard curve. The sensitivity was 40 IU/ml; therefore, values less than or equal to 40 IU/ml were regarded as negative. The positivity observed for DTC patients was 15%.
Serum TSH levels were measured using a third-generation in-house immunofluorometric assay that provided an FS of 0.05 mIU/l [28]. Suppression of TSH was defined as TSH values <0.1 mIU/l during L-T4 therapy and hypothyroidism as TSH >30 mIU/l.
Paired serum specimens for Tg testing from the 168 patients were collected between 2005 and 2008. The serum Tg concentrations from each patient were initially measured using the Tg1GIFMA. Our laboratory routinely stores (at −20°C) all specimens remaining after Tg measurement, so it was possible to remeasure all storage specimens using the Tg2GICMA method. Previous studies had demonstrated that Tg and TgAb measurements performed on frozen specimens stored for over 10 years were stable [29,30,31].
Neck US
Cervical US was performed with a linear, multi-frequency, 7.5- to 10-MHz transducer and integrated with color Doppler examination by a very well-trained radiologist. US was conducted in all of the patients 1-3 months before the hypothyroidism or rhTSH-Tg test, directly following the stimulation and every 6-12 months during the subsequent follow-up.
All suspicious findings were submitted to US-guided FNAC using a 23-gauge needle and 10-ml syringe. The cytology examinations were performed by a cytologist experienced in thyroid cancer. FNA-Tg was also obtained as previously described [32].
Radioiodine Imaging
WBS was performed 48-96 h after oral administration of a standard dose of 3-5 mCi of 131I. Two-head gamma cameras equipped with high-energy collimators were used to perform WBS (speed: 6 cm/min; matrix: 1,024 × 1,024 × 16) and collect planar images of the neck and mediastinum (matrix: 128 × 128; counts: 12345).
Results
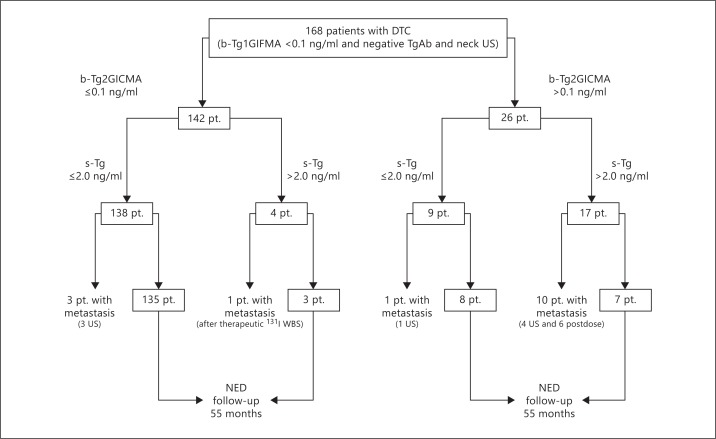
The cohort of 168 patients with DTC presented with b-Tg1GIFMA <1.0 ng/ml and negative TgAb and neck US for at least 1 year after initial treatment or after the last radioiodine treatment. When we used b-Tg2GICMA, 142 patients (84%) still demonstrated Tg <0.1 ng/ml; however, 26 patients presented with detectable b-Tg2GICMA (values varied from 0.3 to 1.5 ng/ml; fig. 1).
Fig. 1.
Follow-up and outcome of all 168 patients (pt.). NED = No evidence of disease.
Among the 142 patients with b-Tg1GIFMA <1.0 ng/ml and b-Tg2GICMA <0.1 ng/ml, 138 showed s-Tg <2 ng/ml, but 4 showed s-Tg >2 ng/ml. However, 3 of those 138 showed positive neck US findings, which presented positive cytology for cancer or elevated FNA-Tg during the follow-up, despite presenting with b-Tg2GICMA <0.1 ng/ml and s-Tg <2 ng/ml (fig. 1; table 1; patients 1, 2 and 3). One of the patients showed a positive neck US finding, positive cytology on FNAC and an elevated FNA-Tg level in a 0.5-cm cervical lymph node, which was detected immediately after the Tg stimulation test. The other 2 patients had positive US findings and presented positive cytology on FNAC and elevated FNA-Tg levels during the follow-up (30 and 48 months after the Tg stimulation test, respectively). Therefore, our study found that few patients with negative responses to s-Tg present with metastases in long-term follow-up. The remaining 135 of these 138 patients showed s-Tg <2 ng/ml and negative US and WBS and had at least 28 months of follow-up without evidence of disease (mean 55 months; fig. 1).
Table 1.
Characteristics of patients with metastasis
| Patient No. | Sex | Age at diagnosis, years | Histology | TNM | b-Tg2GICMA, ng/ml | s-Tg, ng/ml | Diagnosis of metastasis |
|---|---|---|---|---|---|---|---|
| 1 | F | 29 | DSPTC | T1N0M0 | <0.1 | <1.0 | positive US + FNAC |
| 2 | F | 35 | CPTC | T1N0M0 | <0.1 | 1.1 | positive US + FNAC 2 years after s-Tg |
| 3 | F | 26 | CPTC | T1N0M0 | <0.1 | 1.7 | positive US + FNAC 4 years after s-Tg |
| 4 | M | 36 | TCPTC | T3N1bM0 | <0.1 | 5.6 | positive WBS after therapeutic 131I activity (outside the TB) |
| 5 | F | 48 | CPTC | T3N1aM0 | 0.3 | 1.4 | positive US + FNAC 2 years after s-Tg |
| 6 | F | 27 | FVPTC | T1N1bM0 | 0.3 | 4.2 | positive WBS after therapeutic 131I activity (mediastinum) |
| 7 | F | 24 | CPTC | T3N1aM0 | 0.5 | 5.6 | positive WBS after therapeutic 131I activity (outside the TB) |
| 8 | F | 46 | FVPTC | T3N1aM0 | 0.3 | 7.5 | positive WBS after therapeutic 131I activity (outside the TB) |
| 9 | F | 32 | FVPTC | T2N0M0 | 0.8 | 7.6 | positive US + FNAC |
| 10 | F | 49 | CPTC | T2N1aM0 | 0.4 | 7.6 | positive WBS after therapeutic 131I activity (outside the TB) |
| 11 | F | 19 | CPTC | T2N1bM0 | 0.5 | 11.4 | positive US + FNAC |
| 12 | F | 48 | CPTC | T1N0M0 | 1.5 | 11.4 | positive US + FNAC |
| 13 | F | 35 | DSPTC | T3N1aM0 | 0.6 | 16.0 | positive WBS after therapeutic 131I activity (mediastinum) |
| 14 | F | 45 | CPTC | T2N1bM0 | 1.3 | 33.1 | positive WBS after therapeutic 131I activity (outside the TB) |
| 15 | F | 56 | CPTC | T1N0M0 | 1.5 | 48.3 | positive US + FNAC |
F = Female; M = male; CPTC = classical papillary thyroid carcinoma; FVPTC = follicular variant of PTC; TCPTC = tall cell PTC; DSPTC = diffuse sclerosing PTC; TB = thyroid bed; out TB = outside thyroid bed; FNAC = fine needle aspiration cytology.
The remaining 4 of the 142 patients with b-Tg2GICMA <0.1 ng/ml presented with s-Tg >2 ng/ml, but only 1 patient showed evidence of recurrence by the end of the study (fig. 1; table 1, patient 4). This patient was classified as having an intermediate risk of recurrence according to the American Thyroid Association guidelines [5], and cervical metastases were detected after a therapeutic 131I WBS. Therefore, our study demonstrated that 1 of the 142 patients with b-Tg2GICMA <0.1 ng/ml and negative US presented with metastasis during follow-up. The remaining 3 patients with b-Tg2GICMA<0.1 ng/ml and s-Tg >2 ng/ml were monitored, and no evidence of disease was found by the end of the study, even after therapeutic 131I WBS and cervical and chest CT. Additionally, 2 of the patients presented with negative 18FDG PET-CT.
Therefore, b-Tg2GICMA<0.1 ng/ml showed an NPV of 97%, which increased to 99% when neck US was also used (table 2). In contrast, Tg stimulation testing had to be performed in 142 patients with b-Tg2GICMA<0.1 ng/ml to detect only 1 case of local recurrence, which was not seen on US but was suspected by s-Tg >2.0 ng/ml and seen in the posttherapeutic WBS.
Table 2.
Sensitivity, specificity, positive predictive value and NPV of b-Tg2GICMA and b-Tg2GICMA + US-guided FNAC
| b-Tg2GICMA | b-Tg2GICMA + US-guided FNAC | |
|---|---|---|
| Sensitivity, % | 73 | 93 |
| Specificity, % | 93 | 93 |
| PPV, % | 52 | 58 |
| NPV, % | 97 | 99 |
| Accuracy, % | 91 | 93 |
The b-Tg2GICMA has an FS of 0.1 ng/ml. PPV = Positive predictive value.
As shown in figure 1, 26 of the 168 patients in the cohort (15%) showed detectable Tg levels without stimulation when b-Tg2GICMA was used (values varied from 0.3 to 1.5 ng/ml). Nine of the patients presented with s-Tg <2 ng/ml and 17 with s-Tg >2 ng/ml. One patient in the nonstimulated group presented with an increased Tg trend with b-Tg2GICMA, and metastatic disease was found on neck US and PET-CT 28 months after the Tg stimulation test (table 1, patient 5). Therefore, it is possible to have a patient with metastasis and a previously negative s-Tg. The remaining 8 patients from this group of 9 did not show any evidence of disease after at least 28 months of follow-up. In contrast, in the group of 17 of 26 patients with s-Tg >2 ng/ml, 10 of the 17 showed evidence of recurrence (fig. 1; table 1, patients 6-15). Of the remaining 7 of the 17 patients with s-Tg >2 ng/ml, 5 had negative neck US with positive thyroid bed uptake on posttherapeutic WBS, which was considered to be thyroid tissue remnant. Three of the patients did not receive 131I for thyroid ablation, and 1 received 30 mCi of 131I after thyroidectomy. The other 2 patients were followed up for 39 and 66 months, respectively, and they did not show any evidence of disease, even after therapeutic 131I WBS, cervical and chest CT and PET-CT.
In this group of 168 patients with a low or intermediate risk of recurrence who presented with b-Tg1GIFMA <1.0 ng/ml, we identified 149 patients without evidence of disease after a mean of 55 months of follow-up (s-Tg <2 ng/ml, negative US, negative WBS), 15 patients with demonstrated recurrent disease and 4 patients with s-Tg >2 ng/ml but without evidence of disease, either on neck US or on diagnostic and therapeutic 131I WBS, cervical and chest CT or 18FDG PET-CT.
Of those 15 of 168 patients with recurrence (9%), 1 showed thyroid bed recurrence and 14 showed cervical lymph node metastasis, including 2 with positive scans of the mediastinum. None of the patients showed evidence of distant metastasis. Serum b-Tg2GICMAwas detectable (0.2-1.5 ng/ml) in 11 of 15 patients. Therapeutic 131I WBS identified 7 of 15 patients with recurrent disease, and neck US-guided FNAC identified 8 of these 15 patients. Globally, by combining b-Tg2GICMAand US examination, 14 of 15 patients with recurrent disease were correctly identified (table 1).
Sensitivity, specificity, accuracy, positive predictive value and NPV of b-Tg and b-Tg plus US, in monitoring DTC patients considering s-Tg measurement (by using 2 positivity cutoff values of 1 and 2 ng/ml, for rhTSH-Tg and WD-Tg, respectively) and cervical US as reference standards, were obtained from all 168 patients.
Discussion
Guidelines for patients with DTC have indicated that after total thyroidectomy and 131I ablation, these patients must undergo s-Tg testing during follow-up, in association with neck US, to distinguish those patients free of disease from the small minority of patients with persistent disease [2,3,4,5,8,9,10]. Consequently, although these few cases do need to be identified, the investigation should be as minimally invasive and inexpensive as possible [5,33]. Furthermore, because the large majority of these patients have a good prognosis, not all of them will warrant the same strict follow-up investigations, instead favoring risk-adapted management during follow-up [6,7].
In addition, those guidelines were based on studies using Tg1G assays with an FS of 0.5-1.0 ng/ml [10,14]. However, over the last decade, Tg2G assays that present an FS tenfold greater, in the order of 0.05-0.1 ng/ml, have been developed and validated, and these assays are now routinely used in laboratories worldwide [12,13,14,15,16,17,18,19,20,21]. These studies have shown that when a second-generation assay is employed for Tg measurement, undetectable serum b-Tg has the same predictive value as s-Tg. The authors of these studies analyzed a total of 953 patients, mainly belonging to the group of patients with a low risk of recurrence, using Tg2G assays (0.1 or 0.2 ng/ml), and only 19 of the 953 patients showed evidence of recurrent disease (16 of them seen on neck US). The authors concluded that these patients with undetectable b-Tg rarely have s-Tg greater than 1.0 or 2.0 ng/ml (1.7%) and that they rarely present with metastasis (1.3%) [12,13,14,15,16,17,18,19,20,21].
Our study, which included many patients with an intermediate risk of recurrence, is in agreement with these previous data. We confirm that it is possible, in most cases, to predict a negative Tg response after stimulation in the presence of b-Tg2GICMA<0.1 ng/ml, based on the institutional clinical cutoff of 2.0 ng/ml for WD-Tg or of 1.0 ng/ml for rhTSH-Tg [5,34]. Our data showed that of the 142 patients with b-Tg2GICMA<0.1 ng/ml, 138 (97%) presented with Tg <2.0 ng/ml after s-Tg testing. The remaining 4 patients showed s-Tg greater than 2 ng/ml, and only 1 presented with metastasis after therapeutic 131I WBS. The NPV for b-Tg2GICMA<0.1 ng/ml was 97%, and it reached 99% when combined with neck US. s-Tg testing identified only 1 local recurrence missed by both US and b-Tg2GICMA.
Our data also suggest that patients with b-Tg2GICMA>0.1 ng/ml must be carefully evaluated, because 17 of 26 patients (65%) showed s-Tg >2.0 ng/ml, and in 11 of the 26 patients (42%), metastases were found. More recently, Spencer et al. [33] found that second-generation b-Tg measurements were correlated with the degree of rhTSH-Tg stimulation and thus with the likelihood of having rhTSH-Tg >2.0 ng/ml, whereas b-Tg, which was measured by first-generation assay, did not show this correlation.
Additionally, in the present study, 11 of 15 patients who presented with identifiable metastases were correctly diagnosed by a detectable (0.2-1.5 ng/ml) Tg2G assay, and 14 of 15 were detected when a Tg assay while patients were on T4 therapy was associated with neck US.
Finally, there were 9 patients with evidence of thyroid tissue remnants. They did not receive radioiodine ablation, and in 6 of these 9 patients, b-Tg was detectable, showing that these second-generation assays are also very sensitive to the presence of normal thyroid tissue. During follow-up, b-Tg remained stable or decreased in those patients, and none of them have shown evidence of disease. According to the new guidelines, the number of patients not receiving radioiodine ablation will increase worldwide, as radioiodine treatment becomes more targeted to high-risk patients. Therefore, in the near future, there will be many DTC patients with low b-Tg concentrations. In the follow-up of those patients, b-Tg trend is more important than isolated b-Tg measurements and s-Tg. The b-Tg trend will naturally reflect stability or changes in thyroid tissue mass, whereas the fixed rhTSH-Tg consensus cutoff of 1.0 or 2.0 ng/ml is patient-specific, blunted with poorly differentiated tumors and compromised by between-method differences [33].
While this study was in progress, Trimboli et al. [35] also demonstrated that a second-generation b-Tg assay can avoid s-Tg, and Giovanella et al. [36] confirmed these data in a meta-analysis study.
In conclusion, b-Tg2GICMA<0.1 ng/ml combined with negative US presents high NPV in DTC patients after thyroidectomy and primary thyroid ablation and should be used instead of s-Tg. Serum Tg assay must always be performed using a highly sensitive method, and detectable b-Tg with second-generation assays in patients with thyroid tissue remnants is expected and should be followed by monitoring the b-Tg trend but usually is not of concern.
Disclosure Statement
The authors have nothing to declare.
Acknowledgements
This work was supported by the São Paulo State Research Foundation Grants 09/50573-1 (to R.M.B.M.) and 09/50574-8 (Research Fellowship Grant to R.P.P.) and by a grant from the Brazilian Ministry of Health (25000.168513/2008-11). J.G.H.V., R.P.M.B. and R.M.B.M. are investigators of the Fleury Group.
References
- 1.Davies L, Welch HG. Thyroid cancer survival in the United States: observational data from 1973 to 2005. Arch Otolaryngol Head Neck Surg. 2010;136:440–444. doi: 10.1001/archoto.2010.55. [DOI] [PubMed] [Google Scholar]
- 2.Schlumberger M, Berg G, Cohen O, Duntas L, Jamar F, Jarzab B, Limbert E, Lind P, Pacini F, Reiners C, Franco FS, Toft A, Wiersinga WM. Follow-up of low-risk patients with differentiated thyroid carcinoma: a European perspective. Eur J Endocrinol. 2004;150:105–112. doi: 10.1530/eje.0.1500105. [DOI] [PubMed] [Google Scholar]
- 3.Pacini F, Schlumberger M, Dralle H, Elisei R, Smit JW, Wiersinga W, European Thyroid Cancer Taskforce European consensus for the management of patients with differentiated thyroid carcinoma of the follicular epithelium. Eur J Endocrinol. 2006;154:787–803. doi: 10.1530/eje.1.02158. [DOI] [PubMed] [Google Scholar]
- 4.Rosário PW, Ward LS, Carvalho GA, Graf H, Maciel RMB, Maciel LMZ, Maia AL, Vaisman M. Thyroid nodules and differentiated thyroid cancer: update on the Brazilian consensus. Arq Bras Endocrinol Metab. 2013;57:240–264. doi: 10.1590/s0004-27302013000400002. [DOI] [PubMed] [Google Scholar]
- 5.Cooper DS, Doherty GM, Haugen BR, Kloss RT, Lee SL, Mandel SJ, Mazzaferri EL, McIver B, Pacini F, Schlumberger M, Sherman SI, Steward D, Tuttle RM. Revised American Thyroid Association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid. 2009;19:1–48. doi: 10.1089/thy.2009.0110. [DOI] [PubMed] [Google Scholar]
- 6.Tuttle RM. Risk-adapted management of thyroid cancer. Endocr Pract. 2008;14:764–774. doi: 10.4158/EP.14.6.764. [DOI] [PubMed] [Google Scholar]
- 7.Tuttle RM, Tala H, Shah J, Leboeuf R, Ghossein R, Gonen M, Brokhin M, Omry G, Fagin JA, Shaha A. Estimating risk of recurrence in differentiated thyroid cancer after total thyroidectomy and radioactive iodine remnant ablation: using response to therapy variables to modify the initial risk estimates predicted by the new American Thyroid Association staging system. Thyroid. 2010;20:1341–1349. doi: 10.1089/thy.2010.0178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pacini F, Molinaro E, Lippi F, Castagna MG, Agate L, Taddei CCD, Elisei R, Capezzone M, Pinchera A. Prediction of disease status by recombinant human TSH-stimulated serum Tg in the postsurgical follow-up of differentiated thyroid carcinoma. J Clin Endocrinol Metab. 2001;86:5686–5690. doi: 10.1210/jcem.86.12.8065. [DOI] [PubMed] [Google Scholar]
- 9.Mazzaferri EL, Kloss RT. Is diagnostic iodine-131 scanning with recombinant human TSH useful in the follow-up of differentiated thyroid cancer after thyroid ablation? J Clin Endocrinol Metab. 2002;87:1490–1498. doi: 10.1210/jcem.87.4.8338. [DOI] [PubMed] [Google Scholar]
- 10.Mazzaferri EL, Robbins RJ, Spencer CA, Braverman LE, Pacini F, Wartofsky L, Haugen BR, Sherman SI, Cooper DS, Braunstein GD, Lee S, Davies TF, Arafah BM, Ladenson PW, Pinchera A. A consensus report of the role of serum thyroglobulin as a monitoring method for low-risk patients with papillary thyroid carcinoma. J Clin Endocrinol Metab. 2003;88:1433–1441. doi: 10.1210/jc.2002-021702. [DOI] [PubMed] [Google Scholar]
- 11.Wunderlich G, Zophel K, Crook L, Smith S, Smith BR, Franke WG. A high-sensitivity enzyme-linked immunosorbent assay for serum thyroglobulin. Thyroid. 2001;11:819–824. doi: 10.1089/105072501316973064. [DOI] [PubMed] [Google Scholar]
- 12.Mongenthaler NG, Froelich J, Rendl J, Willnich M, Alonso C, Bergmann A, Reiners C. Technical evaluation of a new immunoradiometric and an immunoluminometric assay for thyroglobulin. Clin Chem. 2002;48:1077–1083. [PubMed] [Google Scholar]
- 13.Zophel K, Wunderlich G, Smith BR. Serum thyroglobulin measurements with a high-sensitivity enzyme-linked immunosorbent assay: is there a clinical benefit in patients with differentiated thyroid carcinoma? Thyroid. 2003;13:861–865. doi: 10.1089/105072503322401050. [DOI] [PubMed] [Google Scholar]
- 14.Giovanella L, Ceriani L, Ghelfo A, Keller F, Sacchi A, Maffioli M, Spriano G. Thyroglobulin assay during thyroxine treatment in low-risk differentiated thyroid cancer management: comparison with recombinant assay and imaging procedures. Clin Chem Lab Med. 2006;44:648–652. doi: 10.1515/CCLM.2006.107. [DOI] [PubMed] [Google Scholar]
- 15.Smallridge RC, Meek SE, Morgan MA, Gates GS, Fox TP, Grebe S, Fatourechi V. Monitoring thyroglobulin in a sensitive immunoassay has comparable sensitivity to recombinant human TSH-stimulated thyroglobulin in follow-up of thyroid cancer patients. J Clin Endocrinol Metab. 2007;92:82–87. doi: 10.1210/jc.2006-0993. [DOI] [PubMed] [Google Scholar]
- 16.Schlumberger M, Hitzel A, Toubert ME, Corone C, Troalen F, Schlageter MH, Claustrat F, Koscielny S, Taieb D, Toubeau M, Bonichon F, Borson-Chazot F, Leenhardt L, Schvartz C, Dejax C, Brenot-Rossi I, Torlontano M, Tenenbaum F, Bardet S, Bussière F, Girard JJ, Morel O, Schneegans O, Schlienger JL, Prost A, So D, Archambeaud F, Ricard M, Benhamou E. Comparison of seven serum thyroglobulin assays in the follow-up of papillary and follicular thyroid cancer patients. J Clin Endocrinol Metab. 2007;92:2487–2495. doi: 10.1210/jc.2006-0723. [DOI] [PubMed] [Google Scholar]
- 17.Iervasi A, Iervasi G, Ferdeghini M, Solimeo C, Bottoni A, Rossi L, Colato C, Zucchelli GC. Clinical relevance of highly sensitive Tg assay in monitoring patients treated for differentiated thyroid cancer. Clin Endocrinol. 2007;67:434–441. doi: 10.1111/j.1365-2265.2007.02907.x. [DOI] [PubMed] [Google Scholar]
- 18.Rosario PW, Purisch S. Does a highly sensitive thyroglobulin (Tg) assay change the clinical management of low-risk patients with thyroid cancer with Tg on T4 < 1 ng/ml determined by traditional assays? Clin Endocrinol (Oxf) 2008;68:338–342. doi: 10.1111/j.1365-2265.2007.03043.x. [DOI] [PubMed] [Google Scholar]
- 19.Giovanella L, Maffioli M, Ceriani L, De Palma D, Spriano G. Unstimulated high sensitive thyroglobulin measurement predicts outcome of differentiated thyroid carcinoma. Clin Chem Lab Med. 2009;47:1001–1004. doi: 10.1515/CCLM.2009.216. [DOI] [PubMed] [Google Scholar]
- 20.Castagna MG, Tala Jury HP, Cipri C, Belardini V, Fioravanti C, Pasqui L, Sestini F, Theodoropoulou A, Pacini F. The use of ultrasensitive thyroglobulin assays reduces but does not abolish the need for TSH stimulation in patients with differentiated thyroid carcinoma. J Endocrinol Invest. 2011;34:219–223. doi: 10.3275/7571. [DOI] [PubMed] [Google Scholar]
- 21.Chindris AM, Diehl NN, Crook JE, Fatourechi V, Smallridge RC. Undetectable sensitive serum thyroglobulin (<0.1 ng/ml) in 163 patients with follicular cell-derived thyroid cancer: results of rhTSH stimulation and neck ultrasonography and long-term biochemical and clinical follow-up. J Clin Endocrinol Metab. 2012;97:2714–2723. doi: 10.1210/jc.2011-3017. [DOI] [PubMed] [Google Scholar]
- 22.Spencer CA, Takeuchi M, Kazarosyan M. Current status and performance goals for serum thyroglobulin assays. Clin Chem. 1996;42:164–173. [PubMed] [Google Scholar]
- 23.Baloch Z, Carayon P, Conte-Devolx B, Demers LM, Feldt-Rasmussen U, Henry JF, LiVosli VA, Niccoli-Sire P, John R, Ruf J, Smyth PP, Spencer CA, Stockigt JR. Laboratory medicine practice guidelines: laboratory support for the diagnosis and monitoring of thyroid disease. Thyroid. 2003;13:57–67. doi: 10.1089/105072503321086962. [DOI] [PubMed] [Google Scholar]
- 24.Grebe SKG. Diagnosis and management of thyroid carcinoma: a focus on serum thyroglobulin. Expert Rev Endocrinol Metab. 2009;4:25–43. [Google Scholar]
- 25.Verburg FA, Luster M, Cupini C, Chiovato L, Duntas L, Elisei R, Feldt-Rasmussen U, Rimmele H, Seregni E, Smit JWA, Thimer C, Giovanella L. Implications of thyroglobulin antibody positivity in patients with differentiated thyroid cancer: a clinical position paper. Thyroid. 2013;23:1211–1225. doi: 10.1089/thy.2012.0606. [DOI] [PubMed] [Google Scholar]
- 26.Nakabashi CC, Biscolla RP, Kasamatsu TS, Tachibana TT, Barcelos RN, Malouf EZ, Andreoni DM, Maciel RMB, Vieira JGH. Development, characterization and clinical validation of new sensitive immunofluorometric assay for the measurement of serum thyroglobulin. Arq Bras Endocrinol Metabol. 2012;56:658–665. doi: 10.1590/s0004-27302012000900010. [DOI] [PubMed] [Google Scholar]
- 27.Vieira JGH, Tachibana TT, Fonseca RMG, Nishida SK, Maciel RMB. Development of an immunofluorimetric assay for the measurement of anti-thyroglobulin antibodies. Arq Bras Endocrinol Metabol. 1996;40:232–237. [Google Scholar]
- 28.Vieira JGH, Kunii IS, Nishida SK, Matsumura LK, Russo EMK, Maciel RMB. Development of an immunofluorimetric assay for the measurement of human thyrotropin (TSH) in serum and in total blood collected in filter paper. Arq Bras Endocrinol Metabol. 1992;36:7–12. [Google Scholar]
- 29.Spencer CA, Bergoglio LM, Kazarosyan M, Fatemi S, LoPresti JS. Clinical impact of Thyroglobulin (Tg) and Tg autoantibody method differences on the management of patients with differentiated thyroid carcinomas. J Clin Endocrinol Metab. 2005;90:5566–5575. doi: 10.1210/jc.2005-0671. [DOI] [PubMed] [Google Scholar]
- 30.Männistö T, Surcel HM, Bloigu A, Ruokonen A, Hartikainen AL, Järvelin MR, Pouta A, Vääräsmäki M, Suvanto-Luukkonen E. The effect of freezing, thawing, and short- and long-term storage on serum thyrotropin, thyroid hormones, and thyroid autoantibodies: implications for analyzing samples stored in serum banks. Clin Chem. 2007;53:1986–1987. doi: 10.1373/clinchem.2007.091371. [DOI] [PubMed] [Google Scholar]
- 31.Spencer C, Fatemi S. Thyroglobulin antibody (TgAb) methods – strengths, pitfalls and clinical utility for monitoring TgAb-positive patients with differentiated thyroid cancer. Best Pract Res Clin Endocrinol Metab. 2013;27:701–712. doi: 10.1016/j.beem.2013.07.003. [DOI] [PubMed] [Google Scholar]
- 32.Biscolla RP, Ikejiri ES, Mamone MC, Nakabashi CC, Andrade VP, Kasamatsu TS, Crispim F, Chiamolera MI, Andreoni DM, Camacho CP, Hojaij FC, Vieira JG, Furlanetto RP, Maciel RMB. Diagnosis of metastases in patients with papillary thyroid cancer by the measurement of thyroglobulin in fine needle aspirate. Arq Bras Endocrinol Metabol. 2007;51:419–425. doi: 10.1590/s0004-27302007000300009. [DOI] [PubMed] [Google Scholar]
- 33.Spencer C, Fatemi S, Singer P, Nicoloff J, LoPresti J. Serum basal thyroglobulin measured by a second-generation assay correlates with the recombinant human thyrotropin-stimulated thyroglobulin response in patients treated for differentiated thyroid cancer. Thyroid. 2010;20:587–595. doi: 10.1089/thy.2009.0338. [DOI] [PubMed] [Google Scholar]
- 34.Haugen BR, Pacini F, Reiners C, Schlumberger M, Ladenson PW, Sherman SI, Cooper DS, Graham KE, Braverman LE, Skarulis MC, Davies TF, DeGroot LJ, Mazzaferri EL, Daniels GH, Ross DS, Luster M, Samuels MH, Becker DV, Maxon HR, 3rd, Cavalieri RR, Spencer CA, McEllin K, Weintraub BD, Ridgway EC. A comparison of recombinant human thyrotropin and thyroid hormone withdrawal for the detection of thyroid remnant or cancer. J Clin Endocrinol Metab. 1999;84:3877–3885. doi: 10.1210/jcem.84.11.6094. [DOI] [PubMed] [Google Scholar]
- 35.Trimboli P, La Torre D, Ceriani C, Condorelli E, Laurenti O, Romanelli F, Ventura C, Signore A, Valabrega S, Giovanella L. High sensitive thyroglobulin assay on thyroxine therapy: can it avoid stimulation test in low and high risk differentiated thyroid carcinoma patients? Horm Metab Res. 2013;45:664–668. doi: 10.1055/s-0033-1345185. [DOI] [PubMed] [Google Scholar]
- 36.Giovanella L, Treglia G, Sadeghi R, Trimboli P, Ceriani L, Verburg F. Unstimulated high-sensitive thyroglobulin in follow-up of differentiated thyroid cancer patients: a meta-analysis. J Clin Endocrinol Metab. 2014;99:440–447. doi: 10.1210/jc.2013-3156. [DOI] [PubMed] [Google Scholar]