Abstract
Long-term macrolide therapy has been proven to improve survival in patients with diffuse panbronchiolitis. Although its mechanisms remain unknown, previous studies have suggested the effects of macrolide might be anti-inflammatory rather than antibacterial. To elucidate the molecular mechanisms of its action, we studied here the effects of erythromycin (EM) and its new derivative, EM703, which shows no antibacterial action, on the activation of the transcription factor nuclear factor-κB (NF-κB) in human bronchial epithelial cells. Western blotting analysis showed that EM did not inhibit the degradation of IκBα, suggesting the molecular target for EM was not the dissociation of NF-κB from IκB. An electrophoretic mobility shift assay showed that EM did not interrupt the NF-κB DNA-binding activity in the nucleus under the conditions tested. Moreover, not only EM but also EM703 suppressed the activation of NF-κB and the production of interleukin-8, demonstrating that the anti-inflammatory action of the macrolide is independent of its antibacterial activity. Taken together, these data suggest EM has an anti-inflammatory action, presumably via an interaction with the NF-κB signaling pathway in the downstream of the dissociation from IκB, resulting in the inhibition of NF-κB.
Erythromycin (EM), a macrolide antibiotic, has been reported to be effective for the treatment of chronic diseases, such as diffuse panbronchiolitis and chronic sinusitis, with its long-term and low-dose administration (8, 9). EM cannot be expected to act as an antibacterial agent at this concentration, suggesting that EM has some anti-inflammatory actions (14, 15). For instance, EM inhibits the production of proinflammatory cytokine such as interleukin-6 (IL-6) and IL-8, the expression of adhesion molecules on neutrophils, and mucus secretion from human airways (19, 20).
Expression of several genes, including IL-6, IL-8, and tumor necrosis factor alpha (TNF-α) genes, is controlled by several DNA-binding proteins that interact with specific sequence motifs in the promoter region of the gene. IL-8 expression is elicited by stimuli that cause translocation of nuclear factor-κB (NF-κB) and activator protein-1 (AP-1) to the nucleus. NF-κB is in an inactive form in the cytoplasm, complexed to an anchoring protein, IκB. Stimulation with a variety of agents, including phorbol myristate acetate (PMA) and TNF-α, leads to activation and translocation of NF-κB into the nucleus. During the activation, NF-κB is released from IκB through the phosphorylation and degradation of IκB. In the nucleus, reduction-oxidation (redox) regulation plays a pivotal role in the activation of NF-κB, since oxidation of NF-κB subunits in vitro abolishes their DNA-binding activity (7, 22). Previous studies in our laboratory have demonstrated that the release of IL-8, a neutrophil chemoattractant peptide, from bronchial epithelial cells was increased after stimulation with IL-1α, which was inhibited by macrolide antibiotics (19). Moreover, we have already reported that EM inhibited the activation of both NF-κB and AP-1 in human bronchial epithelial cells (5). However, it remains unknown which process is involved in the inhibition of transcription factors with EM. Therefore, the pathway for signaling cytokine production was investigated.
In the experiments described here, we examined how EM has an inhibitory effect on the activation of transcription factors such as NF-κB in the process of the signal transduction and DNA-binding activity. Moreover, to clarify whether anti-inflammatory action of EM is independent of antimicrobial action, we examined the suppressive effect of EM703, a new EM derivative with no antibacterial action, on the activation of NF-κB and AP-1. We showed that EM703 demonstrates an anti-inflammatory action, presumably through inhibiting the activation of NF-κB, suggesting that this new macrolide derivative has some immunomodulatory action independent of its antibacterial one.
MATERIALS AND METHODS
Culture of bronchial epithelial cells.
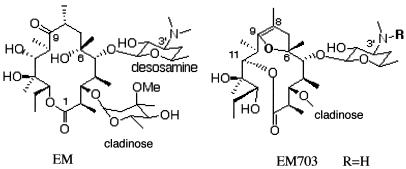
The human bronchial epithelial cell line BET-1A (kindly provided by J. F. Lechner and C. C. Harris, National Cancer Institute, Bethesda, Md.) was cultured by the method reported previously (18). Briefly, the cells were plated onto collagen-coated 24-well flat-bottom tissue culture plates (Koken, Tokyo, Japan) at the density of 5 × 104 cells/well in hormonally defined Ham F-12 medium (HD-F12) as reported. HD-F12 contained 1% penicillin-streptomycin, 5 μg of insulin (Life Technologies, Grand Island, N.Y.)/ml, 5 μg of transferrin (Life Technologies)/ml, 25 ng of epidermal growth factor (Collaborative Research, Lexington, Mass.)/ml, 15 μg of endothelial cell growth supplement (Collaborative Research)/ml, 2 × 10−10 M triiodothyronin (Life Technologies), and 10−7 M hydrocortisone(Life Technologies). The cells were incubated in a humidified atmosphere at 37°C and 5% CO2. The medium was changed at day 1 and subsequently every 2 days. EM703 was chemically semisynthesized in the laboratory of Kitasato Medical Institute, Tokyo, Japan (Fig. 1). For the evaluation of effects of EM and its derivative EM703, the cells were incubated with various concentrations of EM or EM703 before the stimulation by TNF-α or PMA (Sigma Chemical Co., St. Louis, Mo.). The number of the cells was counted by using a standard hemocytometer, and cell viability was assessed by the trypan blue dye exclusion method.
FIG. 1.
Structures of EM and EM703.
Western blot analysis for IκBα and phosphorylated IκBα.
BET-1A cells were washed once in phosphate-buffered saline and lysed in ice-cold lysis buffer (50 mM Tris [pH 8.0], 110 mM NaCl, 5 mM EDTA, and 1% Triton X-100). Protein concentrations were measured by using the Bradford assay (Bio-Rad, Richmond, Calif.). Cytoplasmic lysates containing 50 μg of protein were boiled with equal volumes of treatment buffer (0.125 M Tris [pH 6.8], 4% sodium dodecyl sulfate, 20% glycerol, and 10% 2-β-mercaptoethanol); separated by sodium dodecyl sulfate-10% polyacrylamide gel electrophoresis under reducing conditions; and electrotransferred to polyvinylidene difluoride membranes (Millipore, Bedford, Mass.) as described previously (12). Polyclonal antibodies against either IκBα or phosphorylated IκBα (New England Biolabs, Beverly, Mass.) were applied. Proteins were visualized by using the enhanced chemiluminescence kit from DuPont and Kodak X-Omat radiographic film (Eastman Kodak, Rochester, N.Y.).
Electrophoretic mobility shift assay (EMSA).
After the cells were washed with phosphate-buffered saline, the nuclear proteins were isolated by a previously reported method (21) with some modifications. In brief, 2 × 106 to 3 × 106 cells were harvested with the addition of trypsin-EDTA solution (Life Technologies), rinsed in Tris-buffered saline, resuspended in lysis buffer (10 mM HEPES, 10 mM KCl, 0.1 mM EGTA, 1 mM dithiothreitol [DTT], 0.1 mM EDTA, 0.5 mM phenylmethylsulfonyl fluoride), and incubated on ice for 15 min. Nonidet P-40 (10%) was added to lyse the cells, and then the cells were centrifuged for 6 min at 4°C at 600 × g. The nuclear pellet was resuspended in extraction buffer (20 mM HEPES, 50 mM KCl, 400 mM NaCl, 1 mM EDTA, 1 mM EGTA, 1 mM DTT, 1 mM phenylmethylsulfonyl fluoride) and vortexed for 15 min at 12,000 rpm at 4°C. The supernatant was collected, divided into aliquots, and stored at −70°C. The protein concentration was determined by the Bradford dye-binding procedure (Bio-Rad Protein Assay), standardized with bovine serum albumin. For the detection of NF-κB DNA binding, a gel shift assay system Promega (Madison, Wis.) was used. The sequences of the oligonucleotides containing a tandem repeat of the consensus sequence for NF-κB DNA-binding site were as follows: 5′-AGTTGAGGGGACTTTCCCAGGC-3′ and 3′-TCAACTCCCCTGAAAGGGTCCG-5′. The specific oligonucleotide sequences for double-stranded DNA for AP-1 were as follows: 5′-CGCTTGATGAGTCAGCCGGAA-3′ and 3′-GCGAACTACTCAGTCGGCCTT-5′. Synthetic double-stranded oligonucleotides were labeled with [γ-32P]ATP by using T4 polynucleotide kinase as recommended by the manufacturer. The DNA-binding reaction was conducted at room temperature for 20 min in a volume of 25 μl. The reaction mixture contained 10 μg of nuclear extract, 10 mM Tris (pH 7.5), 1 mM EDTA, 100 mM NaCl, 1 mM DTT, 1 mM EGTA, 4% (vol/vol) glycerol, 0.08 mg of sonicated salmon sperm DNA/ml, and 32P-labeled double-stranded oligonucleotides at 0.7 fmol/μg of nuclear extract. After incubation, the samples were loaded onto a 4% polyacrylamide gel (polyacrylamide-bispolyacrylamide, 30:0.8% [wt/vol]-2.5% glycerol in 0.5× Tris-borate-EDTA) and run at 120 V for 2 h. Each gel was then dried and subjected to autoradiography.
For supershift studies, 2 μl of anti-p65, anti-p50 (Santa Cruz Biotechnology, Inc., Santa Cruz, Calif.), or control antiserum was added to the reaction mixture containing the NF-κB oligonucleotide. Binding of the antibody to the appropriate transcription factor was indicated by a supershift in the EMSA. N-Acetylcysteine (NAC; Sigma) was added to nuclear extracts from BET-1A for determination of the DNA-binding activity of NF-κB, as well as ampicillin sodium salt (ABPC).
Cytokine assay.
The specific immunoreactivity for IL-8 in culture supernatants was measured by enzyme-linked immunosorbent assay (ELISA) kits (R&D Systems, Minneapolis, Minn.). Each sample was assayed in duplicates as recommended by the manufacturer.
Statistical analysis.
The results were analyzed by nonparametric equivalents of analysis of variance (ANOVA) for multiple comparison as reported previously (3, 4).
RESULTS
Effect of EM on TNF-α-mediated activation of NF-κB.
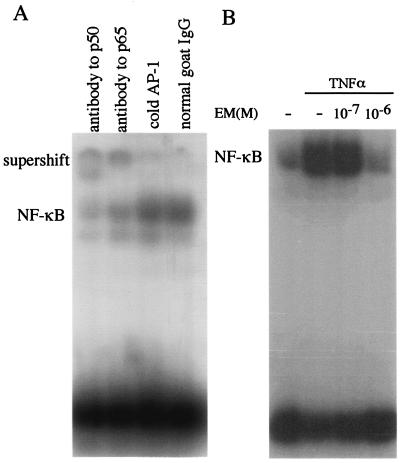
BET-1A cells were treated with EM for 24 h before the addition of 10 ng of TNF-α/ml, and the nuclear extracts were isolated 10 min after the addition of TNF-α for EMSA as described in Materials and Methods. The specificity of the NF-κB binding was ascertained by the supershift of the bands with the antibody to p50 and p65, as well as the increased intensity of the signals with excess amounts of cold DNA AP-1 probe (Fig. 2A). The activation of NF-κB induced by TNF-α was inhibited by the preincubation with EM at the concentration of 10−6 M (Fig. 2B).
FIG. 2.
EMSA for NF-κB binding in BET-1A cells in vitro. (A) The cells were stimulated by TNF-α, and the nuclear extracts were isolated for EMSA as described in Materials and Methods. NF-κB was activated by stimulation with TNF-α. The specificity of the binding was ascertained by the supershift of the band with the antibody to p50, as well as to p65, compared to control antisera (normal goat immunoglobulin G). The addition of excess cold AP-1 probe showed no effect on NF-κB binding. (B) The activation of NF-κB induced by TNF-α was inhibited by incubation with EM at 10−6 M.
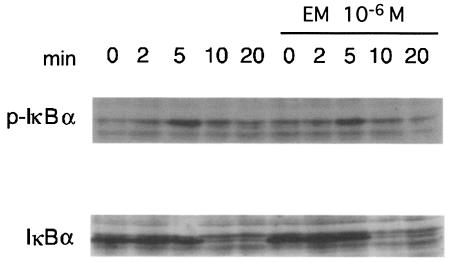
Effect of EM on TNF-α-mediated degradation of IκBα.
IκBα is a potent inhibitor of NF-κB, and the degradation of IκBα via the ubiquitin pathway is necessary for NF-κB nuclear translocation. Therefore, we examined the effect of EM on the degradation of IκBα. The addition of 10 ng of TNF-α/ml resulted in the degradation of IκBα in 5 min (Fig. 3). Incubation with 10−6 M EM for 24 h had no effect on this degradation of IκBα. These results suggest that EM acts in the signaling pathway besides the interaction with IκBα.
FIG. 3.
Effect of EM on TNF-α-mediated degradation of IκBα: BET-1A cells were stimulated with TNF-α. Cytoplasmic lysates were separated by polyacrylamide gel electrophoresis and then analyzed by blotting them with anti-IκBα and anti-phosphorylated IκBα antibodies. The addition of TNF-α resulted in the degradation of IκBα within 5 min. Incubation with EM had no effect on the degradation of IκBα.
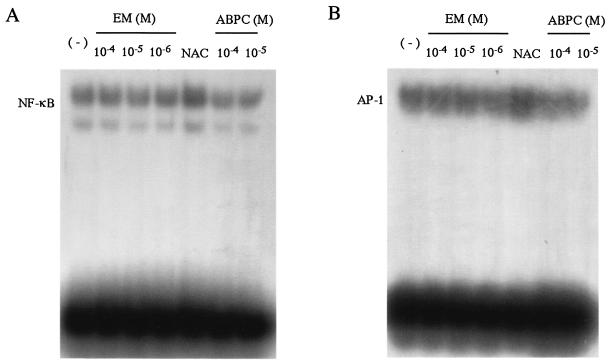
Effect of EM on the DNA-binding activity of NF-κB.
Activation of BET-1A cells results in dissociation of NF-κB from IκB and its translocation to nucleus. The DNA-binding activity of NF-κB in the nucleus is regulated by an oxidoreductive mechanism (22). Oxidation of NF-κB in the nucleus abolishes the DNA-binding activity, and the subsequent reduction restores the activity (7). Therefore, we used NAC as a positive control for the reduction of NF-κB to examine the DNA-binding activity. Nuclear extracts were isolated from BET-1A cells for EMSA as described in Materials and Methods and were preincubated with various concentrations of EM and ABPC, as well as 1 mM NAC, prior to the addition of radiolabeled NF-κB probe. NAC increased the protein-DNA binding, whereas EM or ABPC did not show any effects under the conditions tested (Fig. 4A). We previously showed that EM suppressed AP-1 activation, as well as NF-κB in BET-1A cells (5); we examined here the DNA-binding activity of AP-1 in the nucleus. EMSA showed that NAC increased the protein-DNA binding, whereas EM or ABPC did not show any effects (Fig. 4B).
FIG. 4.
EMSA for the DNA-binding activity of NF-κB in BET-1A cells in vitro. Nuclear extracts from the cells were preincubated with EM, NAC, and ABPC before being incubated with radiolabeled NF-κB probe (A) or AP-1 probe (B). Samples were analyzed by EMSA at the final concentrations of EM and ABPC indicated. Neither EM nor ABPC showed any effects on the binding of NF-κB and AP-1 to each target motif, whereas NAC increased the DNA binding with NF-κB and AP-1.
Effect of EM703 on the activation of NF-κB and AP-1.
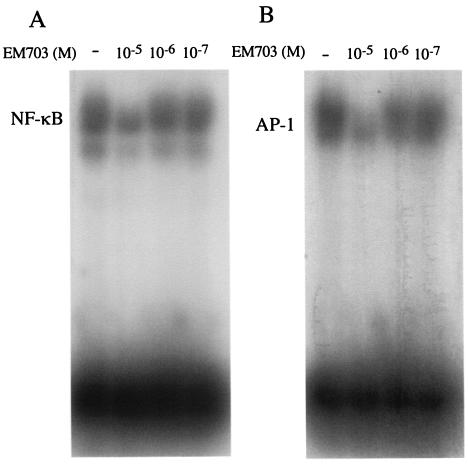
EM703 (Fig. 1) is a new EM derivative that has no antimicrobial or gastrointestinal motor-stimulating (GMS) activity. We examined the inhibitory effect of EM703 on the activation of NF-κB and AP-1 to clarify whether the anti-inflammatory action of EM is independent of its antibacterial action. BET-1A cells were treated with EM703 for 24 h at several concentrations before the addition of 10−7 M PMA, and the nuclear extracts were isolated 1 h after the addition of PMA for EMSA as described in Materials and Methods. The activation of NF-κB, as well as AP-1, induced by PMA was inhibited by the preincubation with EM703 at the concentration of 10−5 M (Fig. 5).
FIG. 5.
Effect of EM703 on the activation of NF-κB and AP-1. The cells were treated with EM703 before the stimulation of PMA, and the nuclear extracts were isolated for EMSA assays. The activations of both NF-κB (A) and AP-1 (B) induced by PMA were inhibited by the incubation of EM703 at a concentration of 10−5 M.
Effect of EM703 on production of IL-8.
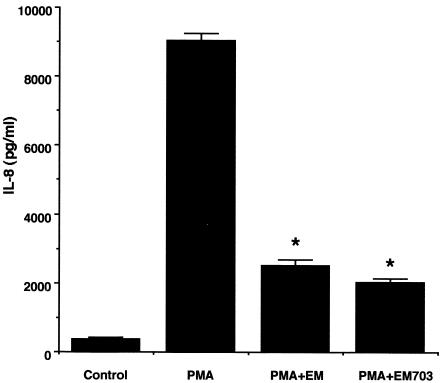
BET-1A cells spontaneously released IL-8 protein into the extracellular medium, as assessed by ELISA. The addition of exogenous PMA (at 1.0 × 10−7 M) to these cells for 6 h induced a significant release of IL-8 (Fig. 6, P < 0.01 [ANOVA]). PMA was administered for 6 h after the cells were incubated with EM703 or EM at a concentration of 10−5 M for 24 h, and IL-8 proteins in the supernatants were measured by ELISA. Both EM703 and EM inhibited the release of IL-8 protein (Fig. 6, percent inhibition = 77.5% ± 2.3% with EM703 and 72.4% ± 3.8% with EM, P < 0.01 compared to PMA alone [ANOVA]). EM703 and EM at this concentration had no significant cytotoxicity to BET-1A cells, as assessed by trypan blue dye exclusion.
FIG. 6.
Effects of EM and EM703 on the production of IL-8. BET-1A cells stimulated by PMA were preincubated with EM or EM703 (10−5 M), and the supernatants were harvested after 12 h for IL-8 measurements. Both EM703 and EM inhibited the production of IL-8. ✽, P < 0.01 (ANOVA). The data are shown as the means ± the standard error of the mean.
DISCUSSION
In the present study, we examined the inhibitory effect of EM on the signaling pathway through the activation of NF-κB. Cell activation by inflammatory mediators, such as TNF-α, causes rapid degradation of the regulatory protein IκBα, allowing nuclear translocation of the NF-κB complex, which results in transcriptional activation of target genes (10). We showed that EM inhibits the TNF-α-mediated activation of NF-κB. In the cytosol, NF-κB is in an inactive form bound to an inhibitory molecule, IκBα. Degradation of IκBα by the ubiquitin-dependent proteosome is necessary for NF-κB activation and nuclear translocation. We demonstrated that EM does not have any effect on the degradation of IκBα in human bronchial epithelial cells, suggesting that EM acts in the signaling pathway other than the interaction with IκBα.
NF-κB is a redox-sensitive transcription factor known to be activated by oxidants, as well as reductants (2). The redox response of transcription factors appears to be complex, with slight differences in redox state resulting in either activation or repression. Redox-sensitive transcription factors, including NF-κB, share the common requirement of basic regions containing highly conserved cysteine residues in the DNA-binding domains to facilitate transcriptional activation. Several studies have shown that reducing agents increase NF-κB binding to DNA and that a reduced cysteine in the p50 subunit is required for DNA binding (2, 7, 17, 22). An oxidative environment is associated with NF-κB activation, since NF-κB activation through IκBα degradation occurs under oxidized conditions in the cytosol. However, once inside the nucleus, a reducing environment favors the binding of NF-κB to its consensus sequence. Thus, the biphasic property of the NF-κB signaling pathway can be regulated by oxidants, as well as antioxidants. In the reduced state, the redox balance prevents the IκBα degradation in the cytosol, while accelerating the DNA binding processes in the nucleus. EM was proved to inhibit the superoxide generation of neutrophils exposed to PMA (23). Sato et al. reported that EM treatment improved the survival ratio of influenza virus-infected mice by decreasing free radicals such as nitric oxide (16). To investigate the effects of EM on the DNA binding of NF-κB in the nucleus, we added EM to the EMSA reaction mixture in vitro. NAC was used as a positive control to activate the DNA binding of NF-κB in the nucleus, since it is a glutathione precursor and an important antioxidant for regulating the redox balance. We demonstrated that EM had no effect on the DNA-binding activity of NF-κB, whereas NAC induced its activation in the nucleus.
The Fos and Jun proteins are components of another family of nuclear transcription factors that have been implicated in the regulation of inflammatory responses. Fos and Jun form a heterodimeric complex known as the activator protein-1 (AP-1) that interacts with a DNA sequence (6). The transcription of IL-8 gene requires the coordinated activation of several transcription factors, including NF-κB and AP-1. NF-κB is the most crucial factor for IL-8 gene transcription, although AP-1 is required for maximal expression (11). The DNA-binding region of Fos and Jun also contains a characteristic sequence with cysteine residues. Therefore, we also investigated the effects of EM on the DNA binding of AP-1 in the nucleus. We demonstrated that EM had no effect on the DNA-binding activity of AP-1, whereas NAC induced its activation. These results suggest that the activation of AP-1 is inhibited by the oxidative environment in the nucleus and is susceptible to redox regulation as well as NF-κB. However, EM had no effects on the redox environment that regulates the activation of transcription factors, including NF-κB and AP-1.
EM has a GMS activity in vivo, and this GMS activity is specific for EM and closely related 14-membered macrolides (13). The structure of macrolides gives diverse biological properties, such as antibacterial, GMS, and immunomodulating activities. EM does not always have the most desirable properties for anti-inflammatory action in the treatment of patients with diffuse panbronchiolitis, since the long-term use of EM may produces drug-resistant bacteria. Moreover, strong GMS activity of some EM derivatives may induce nausea and diarrhea, preventing the clinical use of these derivatives. Therefore, many efforts have been made to resolve problems such as spectrum of activities, drug resistance, GMS activity, and toxicities. Chemical modification and microbial transformation of the lactone ring or sugar moiety result in the increase of one action and the decrease of another. The antimicrobial activity of EM is extremely sensitive to small changes in the configuration of the lactone ring. Cleavage of the lactone ring by esterase destroys the antimicrobial activity (1). EM703 is a contracted 12-membered derivative of EM with neither antimicrobial nor GMS activity. However, our data showed that this derivative has an inhibitory action on the activation of NF-κB and AP-1, as well as on the production of proinflammatory chemokine such as IL-8, demonstrating that the anti-inflammatory action of macrolides is independent of antibacterial action. Although the structural factors of EM and its derivatives have not yet been clarified in relation to their anti-inflammatory activities, this new derivative of EM exhibited anti-inflammatory action without bactericidal activity. Since anti-inflammatory actions include antineutrophilic activities by the inhibition of IL-8, we expect EM703 to be useful for the treatment of systemic inflammatory diseases, including chronic airway diseases, chronic bowel disease, and skin diseases.
The inhibitory effect on the activation of transcription factors in human bronchial epithelial cells reported here can play a pivotal role in the treatment of chronic airway inflammations. Although the molecular mechanism of anti-inflammatory action of EM remains unclear, characterization of the chemical structure responsible for its potential would be important to pursue, and further investigation for the molecular mechanism is necessary for a possible new type of anti-inflammatory agent.
Acknowledgments
We thank Makiko Baba for excellent technical support.
This study was supported in part by the Japan Antibiotics Research Association, The Diffuse Lung Disease Research Committee, and the Japan Ministry of Health and Welfare.
REFERENCES
- 1.Barthelemy, P., D. Autissier, G. Gerbaud, and P. Courvalin. 1984. Enzymic hydrolysis of erythromycin by a strain of Escherichia coli: a new mechanism of resistance. J. Antibiot. 37:1692-1696. [DOI] [PubMed] [Google Scholar]
- 2.Das, K. C. 2001. c-Jun NH2-terminal kinase-mediated redox-dependent degradation of IκB: role of thioredoxin in NF-κB activation. J. Biol. Chem. 276:4662-4670. [DOI] [PubMed] [Google Scholar]
- 3.Desaki, M., I. Sugawara, Y. Iwakura, K. Yamamoto, and H. Takizawa. 2002. Role of interferon-γ in the development of murine bronchus-associated lymphoid tissues induced by silica in vivo. Toxicol. Appl. Pharmacol. 185:1-7. [DOI] [PubMed] [Google Scholar]
- 4.Desaki, M., H. Takizawa, T. Kasama, K. Kobayashi, Y. Morita, and K. Yamamoto. 2000. Nuclear factor-κB activation in silica-induced interleukin 8 production by human bronchial epithelial cells. Cytokine 12:1257-1260. [DOI] [PubMed] [Google Scholar]
- 5.Desaki, M., H. Takizawa, T. Ohtoshi, T. Kasama, K. Kobayashi, T. Sunazuka, S. Omura, K. Yamamoto, and K. Ito. 2000. Erythromycin suppresses nuclear factor-κB and activator protein-1 activation in human bronchial epithelial cells. Biochem. Biophys. Res. Commun. 267:124-128. [DOI] [PubMed] [Google Scholar]
- 6.Galter, D., S. MIHM, and W. Droge. 1994. Distinct effects of glutathione disulphide on the nuclear transcription factors κB and the activator protein-1. Eur. J. Biochem. 221:639-648. [DOI] [PubMed] [Google Scholar]
- 7.Hayashi, T., Y. Ueno, and T. Okamoto. 1993. Oxidoreductive regulation of nuclear factor κB. J. Biol. Chem. 268:11380-11388. [PubMed] [Google Scholar]
- 8.Homma, H., A. Yamanaka, S. Tanimoto, M. Tamura, Y. Chijimatsu, S. Kira, and T. Izumi. 1983. Diffuse panbronchiolitis. A disease of the transitional zone of the lung. Chest 83:63-69. [DOI] [PubMed] [Google Scholar]
- 9.Kadota, J., O. Sakito, S. Kohno, H. Sawa, H. Mukae, H. Oda, K. Kawakami, K. Fukushima, K. Hiratani, and K. Hara. 1993. A mechanism of erythromycin treatment in patients with diffuse panbronchiolitis. Am. Rev. Respir. Dis. 147:153-159. [DOI] [PubMed] [Google Scholar]
- 10.Lentsch, A. B., B. J. Czermak, N. M. Bless, and P. A. Ward. 1998. NF-κB activation during IgG immune complex-induced lung injury: requirements for TNF-α and IL-1β but not complement. Am. J. Pathol. 152:1327-1336. [PMC free article] [PubMed] [Google Scholar]
- 11.Mukaida, N., S. Okamoto, Y. Ishikawa, and K. Matsushima. 1994. Molecular mechanism of interleukin-8 gene expression. J. Leukoc. Biol. 56:554-558. [PubMed] [Google Scholar]
- 12.Okazaki, H., J. Zhang, M. M. Hamawy, and R. P. Siraganian. 1997. Activation of protein-tyrosine kinase Pyk2 is downstream of Syk in FcɛRI signaling. J. Biol. Chem. 272:32443-32447. [DOI] [PubMed] [Google Scholar]
- 13.Omura, S., K. Tsuzuki, T. Sunazuka, S. Marui, H. Toyoda, N. Inatomi, and Z. Itoh. 1987. Macrolides with gastrointestinal motor stimulating activity. J. Med. Chem. 30:1941-1943. [DOI] [PubMed] [Google Scholar]
- 14.Rubin, B. K., H. Druce, O. E. Ramirez, and R. Palmer. 1997. Effect of clarithromycin on nasal mucus properties in healthy subjects and in patients with purulent rhinitis. Am. J. Respir. Crit. Care Med. 155:2018-2023. [DOI] [PubMed] [Google Scholar]
- 15.Rubin, B. K., and J. Tamaoki. 2000. Macrolide antibiotics as biological response modifiers. Curr. Opin. Investig. Drugs 1:169-172. [PubMed] [Google Scholar]
- 16.Sato, K., M. Suga, T. Akaike, S. Fujii, H. Muranaka, T. Doi, H. Maeda, and M. Ando. 1998. Therapeutic effect of erythromycin on influenza virus-induced lung injury in mice. Am. J. Respir. Crit. Care Med. 157:853-857. [DOI] [PubMed] [Google Scholar]
- 17.Sun, Y., and L. W. Oberley. 1996. Redox regulation of transcriptional activators. Free Radic. Biol. Med. 21:335-348. [DOI] [PubMed] [Google Scholar]
- 18.Takizawa, H., M. Desaki, T. Ohtoshi, S. Kawasaki, T. Kohyama, M. Sato, J. Nakajima, M. Yanagisawa, and K. Ito. 1998. Erythromycin and clarithromycin attenuate cytokine-induced endothelin-1 expression in human bronchial epithelial cells. Eur. Respir. J. 12:57-63. [DOI] [PubMed] [Google Scholar]
- 19.Takizawa, H., M. Desaki, T. Ohtoshi, S. Kawasaki, T. Kohyama, M. Sato, M. Tanaka, T. Kasama, K. Kobayashi, J. Nakajima, and K. Ito. 1997. Erythromycin modulates IL-8 expression in normal and inflamed human bronchial epithelial cells. Am. J. Respir. Crit. Care Med. 156:266-271. [DOI] [PubMed] [Google Scholar]
- 20.Takizawa, H., M. Desaki, T. Ohtoshi, T. Kikutani, H. Okazaki, M. Sato, N. Akiyama, S. Shoji, K. Hiramatsu, and K. Ito. 1995. Erythromycin suppresses interleukin 6 expression by human bronchial epithelial cells. Biochem. Biophys. Res. Commun. 210:781-786. [DOI] [PubMed] [Google Scholar]
- 21.Takizawa, H., T. Ohtoshi, S. Kawasaki, T. Kohyama, M. Desaki, T. Kasama, K. Kobayashi, K. Nakahara, K. Yamamoto, K. Matsushima, and S. Kudoh. 1999. Diesel exhaust particles induce NF-κB activation in human bronchial epithelial cells in vitro: importance in cytokine transcription. J. Immunol. 162:4705-4711. [PubMed] [Google Scholar]
- 22.Toledano, M. B., and W. J. Leonard. 1991. Modulation of transcription factor NF-κB binding activity by oxidation-reduction in vitro. Proc. Natl. Acad. Sci. USA 88:4328-4332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Umeki, S. 1993. Anti-inflammatory action of erythromycin. Its inhibitory effect on neutrophil NADPH oxidase activity. Chest 104:1191-1193. [DOI] [PubMed] [Google Scholar]