Abstract
Thyroid hormone (TH) exerts its biological activity via the TH receptors TRα1 and TRβ1/2, which are encoded by the THRA and THRB genes. The first patients with mutations in THRB were identified decades ago. These patients had a clinical syndrome of resistance to TH associated with high serum TH and nonsuppressed thyroid-stimulating hormone levels. Until recently, no patients with mutations in THRA had been identified. In an attempt to predict the clinical phenotype of such patients, different TRα1 mutant mouse models have been generated. These mice have a variable phenotype depending on the location and severity of the mutation. Recently, the first humans with mutations in THRA were identified. Their phenotype consists of relatively low serum T4 and high serum T3 levels (and thus an elevated T3/T4 ratio), growth retardation, delayed mental and bone development, and constipation. While, in retrospect, certain features present in humans can also be found in mouse models, the first humans carrying a defect in TRα1 were not suspected of having a THRA gene mutation initially. The current review focuses on the clinical consequences of TRα1 mutations.
Key Words : Thyroid hormone receptor α, Resistance to thyroid hormone, LT4 therapy, Growth retardation, Delayed bone development
Introduction
Thyroid hormone (TH) is essential for normal development and metabolic activity of most tissues. This is illustrated by the severe consequences of congenital hypothyroidism, leading to growth failure and mental retardation when untreated [1,2]. The biological action of TH is mediated by the binding of the biologically active hormone T3 to its nuclear receptor (TR). TRs are bound to T3 response elements in the promoter region of target genes and function as ligand-dependent transcription factors [3,4]. Nongenomic effects of TH have also been reported [5], but those nongenomic actions are beyond the scope of this review.
Different TR isoforms are generated from THRA and THRB by usage of alternative splice sites and/or promoters, with TRα1, TRβ1, and TRβ2 as the T3-binding isoforms [4]. Both TRα1 and TRβ1 are widely expressed, with TRα1 as the predominant isoform in the brain, bone, heart, and intestine, and TRβ1 as the major isoform in the liver, kidney, and thyroid [4,6]. TRβ2 has a more restricted expression pattern (hypothalamus, pituitary, and retina) and is involved in the regulation of the hypothalamus-pituitary-thyroid axis as well as in the neurosensory development [7,8].
Heterozygous mutations in the ligand-binding domain of THRB are associated with resistance to TH (RTHβ). RTHβ is characterized by high serum TH levels in combination with nonsuppressed thyroid-stimulating hormone (TSH). The syndrome may comprise goiter, tachycardia, and raised energy expenditure [9,10,11], although many RTHβ patients are asymptomatic. Mice with specific TRβ defects exhibit a phenotype similar to that of RTHβ patients [12,13].
It has proven much more difficult to identify patients with mutations in THRA. In an attempt to predict the clinical consequences of TRα1 defects, different knockin and knockout mouse models have been generated. Interestingly, when mice devoid of all TRs are deprived of TH, they have fewer symptoms of hypothyroidism than wild-type hypothyroid mice due to the suppressive effect of unliganded TRs, in particular TRα1, on (positively regulated) gene transcription [14,15,16]. Mice with heterozygous TRα1 mutations are viable and have a heterogeneous phenotype, depending on the severity and the location of the mutation [17]. Most adult TRα1 mutant mice are euthyroid with a mildly elevated TSH. Other characteristics of the various mouse models include delayed endochondral ossification resulting in dwarfism, disturbed behavior, memory impairment, locomotor dysfunction, mild bradycardia, and insulin resistance [6,17,18,19,20,21,22,23]. For an excellent review of the different TRα1 mutant mouse models, please refer to Vennström et al. [17].
In the last 2 years, 4 patients have been identified with 3 different mutations in the TRα1 portion of the THRA gene [24,25,26,27]. Despite the existence of mouse models that, in retrospect, closely resemble certain features of the phenotype, the first identified patients were not suspected of having a THRA gene mutation initially. The first patient was identified via exome sequencing [24]. Around the same time, 2 additional patients were identified by a candidate gene approach after mutations in (at that time) more likely candidates such as TH transporters and deiodinases had been excluded [25]. This review describes the clinical consequences of the mutations in TRα1 in detail and discusses the relation with the different TRα1 mutant mouse models. One additional patient, with a mutation in THRA affecting both TRα1 and TRα2, was recently reported [28], but since a detailed description and analysis of this patient have not (yet) been published, the patient will not be discussed in this review.
Clinical Phenotype
The characteristics of the 4 patients with resistance to TH caused by mutations in TRα1 (RTHα) are summarized in table 1 [24,25,26,27,29]. The key features of RTHα are growth retardation, delayed mental and bone development, constipation, and elevated serum T3/T4 and T3/rT3 ratios [24,25,26,27]. All 4 patients identified so far have mutations leading to a truncated protein with a complete lack of T3 binding: the 6-year-old girl (P1) has a heterozygous nonsense mutation (E403X) [24], the 11-year-old girl (P2) and her 47-year-old father (P3) have a heterozygous single-nucleotide insertion (F397fs406X) [25,26], and the 45-year-old female patient (P4) has a heterozygous single-nucleotide deletion (A382fs388X) [27].
Table 1.
Genotype and phenotype comparison of patients with a mutation in the TRα1 portion of the THRA gene
| TRα1-E403X [24] | TRα1-F397fs406X [25, 26] | TRα1-A382PfsX7 [27] | |
|---|---|---|---|
| Subject | P1 | P2 and P3 | P4 |
| Genotype | |||
| Mutation | nonsense | frame shift | frame shift |
| Zygosity | heterozygous | heterozygous | heterozygous |
| Phenotype | |||
| Bone development | growth retardation | growth retardation | growth retardation |
| Mental development | mildly affected | mildly affected | cognitive impairment, seizures |
| Constipation | severe | mild | severe |
| TSH | normal | normal | high-normal |
| FT4 | low-normal | low-normal | low-normal |
| T3 | high-normal | high | normal |
| T3/T4 ratio | elevated | elevated | elevated |
| rT3 | low | low | low |
The 2 youngest patients (P1 and P2) initially presented with classical clinical features of hypothyroidism such as delayed motor development, delayed closure of the skull sutures, macroglossia, delayed tooth eruption, bone development abnormalities, macrocephaly, and growth delay [24,25]. The 2 adult patients (P3 and P4) also had symptoms of hypothyroidism at clinical evaluation, such as slow speech, drowsiness, slow reactions, dry skin and hair, and slow reflexes [24,25,26,27].
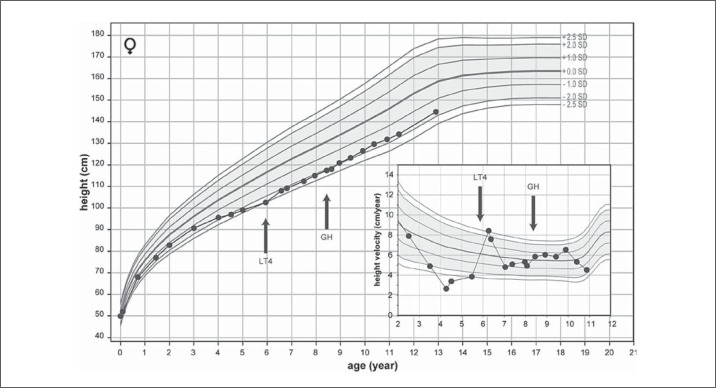
Growth retardation is a consistent component of the phenotype in all 4 patients (fig. 1) [24,25,27]. All patients have short stature, with leg length being more affected than sitting height [24,25,27], pers. commun. with Dionisios Chrysis]. Both young patients also have other bone development abnormalities. One (P2) had congenital hip dislocation around birth, with a delayed appearance of ossification centers on X-ray, whereas the other (P1) suffered from femoral epiphyseal dysgenesis [24,25]. In addition, both had delayed closure of the skull sutures, delayed tooth eruption, and delayed bone age relative to their age [24,25,26]. DXA scans revealed normal bone mineral densities in the juvenile and adult patients [26,27], but more detailed imaging data (such as qCT) are not yet available.
Fig. 1.
Growth chart of the 11-year-old girl (P2) with an inactivating mutation in the TRα1 portion of the THRA gene, with growth velocity in the inset. The growth curve is compared with the normal range for the Greek population. The initiation of LT4 and GH therapy is indicated by the arrows. Adapted from van Mullem et al. [25] with permission.
As mentioned above, knockin mouse models with various TRα1 defects have been generated (table 2): (1) TRα1-P394fs406X, also known as TRα1-PV, resulting in a frame shift and premature stop leading to a complete loss of T3 affinity [21] (this mutation is very similar to the TRα1-F397fs406X mutation that has been identified in patients P2 and P3); (2) TRα1-R384C, resulting in a 10-fold reduced T3 affinity [23]; (3) TRα1-L400R, resulting in a reduced T3 affinity as well as a reduced binding of coactivators [22], and (4) TRα1-P398H, resulting in a 3-fold reduced T3 affinity and interference with PPARα [19]. Similar to humans, all knockin mice except TRα1-P398H suffer from growth retardation [19,23,30,31]. Interestingly, TRα1-R384C mice with a reduced but not absent T3 affinity overcome the growth retardation in adulthood and reach a near-normal size, whereas the other mice remain dwarfed [17,21,22,23]. The growth retardation of TRα1-L400R mice is disproportional as also seen in the RTHα patients [22]. No data on possible disproportional growth retardation are available for the other mouse models.
Table 2.
Genotype and phenotype comparison of TRα1 mutant mice
| TRα1-PV [21] | TRα1-R384C [23] | TRα1-L400R [22] | TRα1-P398H [19, 22] | |
|---|---|---|---|---|
| Model | l | 2 | 3 | 4 |
| Phenotype | ||||
| T3 affinity | lost | reduced | reduced | reduced |
| Bone development | dwarfism | growth retardation | dwarfism | normal length |
| TSH | elevated | normal in adulthood | normal | elevated |
| FT4 | normal | normal in adulthood | normal | slightly elevated |
| T3 | elevated | normal | normal | slightly elevated |
The phenotype of the various models includes delayed tooth eruption and delayed closure of the skull sutures as well [22,23,30,31,32,33]. In addition, delayed endochondral and intramembranous ossification has been documented. Again, only the TRα1-R384C mice overcome most of these defects in adulthood [23].
Both young TRα1 patients (P1 and P2) had a delayed motor and mental development [24,25]. All 4 patients have cognitive deficits (IQ range 52-90), although patient P2 attends normal school with average grades [24,25,27]. The patient with the most severe cognitive impairment (P4) has seizures, which have also been described in TRα1 mutant mice [17,27].
The psychomotor phenotype has been documented in greatest detail in TRα1-R384C mice, which suffer from reduced grip strength, poor limb coordination, and an abnormal gait [34]. The locomotor dysfunctions correlate with an aberrant development of GABAergic interneurons and can be ameliorated by TH treatment during early fetal and postnatal development. In addition, TRα1-R384C mice exhibit extreme anxiety and reduced cognition [35]. This behavior can be normalized by treating the adult animals with T3. It is therefore very tempting to speculate about possible benefits for human patients with TRα1 defects leading to a reduced, but not fully abolished, affinity of the receptor for T3. However, no such patients have yet been identified. TRα1-L400R mice have an ataxic walk, and TRα1-PV mice have reduced glucose utilization in the brain [22,36].
All patients suffer from mild to severe constipation [24,25,26,27]. The most severely affected patient (P1) also has bowel dilatation and delayed intestinal transit [24]. This is very similar to the intestinal phenotype of TRα-/-mice, which suffer from a delayed development, intestinal hypoplasia, hypotrophy, and reduced function [32,37].
The 2 adult patients with RTHα (P3 and P4) have an increased BMI, but this is less pronounced in the younger patients (P1 and P2) [24,26,27]. The metabolic phenotype is also very variable between the different mutant mouse models. Although TRα1-PV and TRα1-R384C mice have a reduced body weight, TRα1-P398H mice have an increased amount of body fat in combination with insulin resistance [17,19,21,23]. This difference may very well depend on the exact position of the mutation, as well as on differences in the genetic background of the various mouse strains.
RTHα patients have a normal body temperature [26], but data on cold intolerance are not yet available. TRα1-P398H mice have a lower core body temperature, and both TRα1-P398H and TRα1-L400R mice have reduced cold-induced thermogenesis [17,19,22]. The basal metabolic rate (BMR) was low to low-normal in the 2 human RTHα patients studied (P1 and P4). This is similar to TRα1-P398H mice but in contrast to TRα1-R384C mice which have an increased BMR [17]. TRα1-R384C mice are hyperphagic and resistant to obesity, due to a centrally induced hypermetabolism caused by apo-TRα1 [38].
Two patients (P1 and P4) had a low resting heart rate and a low blood pressure, while these parameters were normal in the other 2 patients (P2 and P3) [24,26,27]. TRα1-R384C, TRα1-L400R, and TRα1-P398H mice all have a lower heart rate as well [19,20,22].
Laboratory Findings
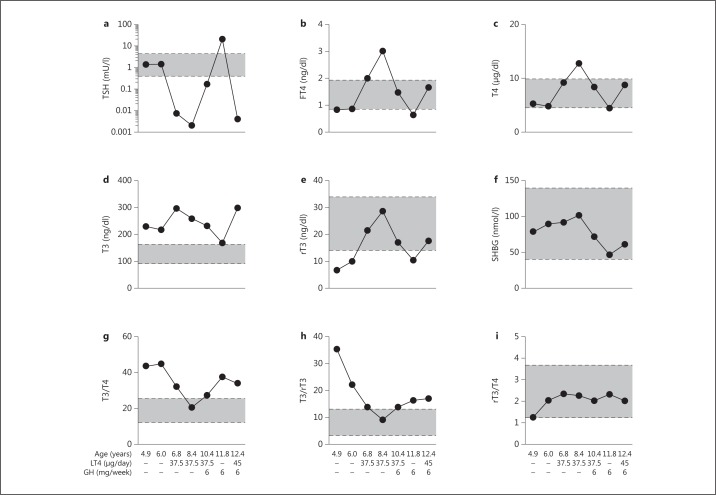
All 4 RTHα patients had low to low-normal serum (F)T4, high-normal to high T3, and low rT3, in combination with normal TSH levels (fig. 2, 3) [24,25,26,27]. As a consequence, their T3/T4 and T3/rT3 ratios were clearly elevated, suggesting an altered peripheral TH metabolism (fig. 2) [26,27]. These altered thyroid function tests did not fully agree with what was seen in the different mouse models, since TRα1-R384C and TRα1-L400R mice had normal TH levels, whereas thyroid function tests were only slightly altered in TRα1-PV and TRα1-P398H mice [17,19,21,22,23].
Fig. 2.
Results of serum thyroid function tests and SHBG levels in samples collected from female patient P2 with an inactivating mutation in the TRα1 portion of the THRA gene under different treatment modalities (on and off LT4 and/or GH therapy) at different time points. The horizontal lines represent the different reference ranges. Adapted from van Mullem et al. [26] with permission.
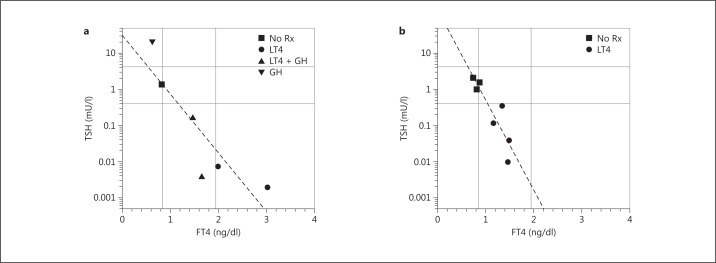
Fig. 3.
Serum TSH plotted as a function of serum FT4 levels in a female patient (P2; a) and her father (P3; b) with an inactivating mutation in the TRα1 portion of the THRA gene. Different symbols represent different treatment modalities (on and off LT4 and/or GH therapy). The regression line is based on all data points. Reference ranges are indicated by the horizontal and vertical lines. Rx = Medication. Adapted from van Mullem et al. [26] with permission.
The elevated T3/T4 and T3/rT3 ratios in all affected humans are likely explained by an altered expression of deiodinases, which are the most important enzymes in the peripheral TH metabolism [39]. Type 1 deiodinase (D1) is present in the liver, kidney, and thyroid and plays a key role in the production of the active hormone T3 from T4 and in clearance of the metabolite rT3. Type 2 deiodinase (D2) is expressed in the brain, pituitary, brown adipose tissue, thyroid, skeletal muscle, and osteoblasts. In tissues such as the brain, D2 is important for the local production of T3, whereas D2 in skeletal muscle may also contribute to plasma T3 production. Type 3 deiodinase (D3) is present in the brain, skin, placenta, pregnant uterus, and various fetal tissues during development. D3 is the major T3 and T4 inactivating enzyme and contributes to TH homeostasis by protecting tissues from excess active TH or its precursor. T3/T4 and T3/rT3 ratios are considered to be sensitive indicators of the peripheral metabolism of TH, being positively influenced by D1 and D2 and negatively influenced by D3. These ratios are relatively independent of thyroidal T4 production and of variations in serum binding proteins. The elevated ratios could be due to increased D1 or D2 activity, decreased D3 activity, or a combination of both [26]. It has been shown that TRα1-PV mice have increased levels of hepatic D1, while D1 is normal in the liver of TRα1-L400R mice [21,22]. TRα0/0 mice have normal liver D1 expression but impaired regulation of brain D3 expression, which leads to a reduced production of rT3 and degradation of T3 [40]. Further research is necessary to elucidate these alterations in deiodinase activities in humans with RTHα [26].
Other laboratory findings were low-normal IGF1, with insufficient growth hormone (GH) responses to both clonidine and L-DOPA stimulation tests in P2 and P3 [24,25,26,27]. Alterations in the GH-IGF1 axis have been described in different mouse models as well. TRα1-R384C mice have lower pituitary GH mRNA levels before adolescence, although their IGF1 levels are in the normal range [23]. Also TRα1-L400R mice have a reduced GH expression, whereas TRα1-PV mice have normal pituitary GH levels [22,30].
The patients with the same frame shift mutation (P2 and P3) had clearly elevated levels of cholesterol and elevated LDL/HDL ratios, whereas cholesterol was normal in the other 2 patients (P1 and P4) [25,26,27]. TRα1-P398H mice had slightly elevated serum lipids as well, whereas all other mutant mice had reduced serum lipid levels [17].
Furthermore, all RTHα patients had anemia, which is frequently seen in hypothyroidism and is in line with the impaired erythropoiesis seen in TRα-/-mice [26,27,41,42]. SHBG, which is a marker of thyroid status in the liver, varied between different patients from normal to high (fig. 2) [24,26,27]. Creatine kinase, which is elevated in hypothyroidism, was slightly raised in all patients [26,27,43].
Mechanisms of Disease
A functional analysis of the TRα1 mutants identified in humans showed that none of them were capable of T3 binding and/or of stimulating the expression of T3-responsive genes [24,25,26,27]. For 2 of the mutants, it has been shown that the dissociation from corepressors under the influence of T3 is affected as well [24,27]. All mutations identified were in the ligand-binding domain of TRα1 and thus did not appear to affect DNA binding. All patients are heterozygous, suggesting dominant negative effects of these mutant receptors in vivo. This was confirmed by in vitro studies, demonstrating dominant negative effects of the mutant receptors on wild-type TRα, and by an ex vivo analysis of patient-derived cells, showing a marked reduction in T3-mediated induction of the known TR target gene KLF9 [24,25,26,27]. Furthermore, a dominant negative effect of TRα1-F397fs406X on wild-type TRβ has been demonstrated as well. The clinical relevance of this effect on TRβ remains to be determined in future studies. The dominant negative activity of these TRα1 mutants is similar to that of heterozygous TRβ mutants identified in patients with RTHβ [9].
As mentioned above, all THRA mutations identified so far in patients with RTHα result in a complete loss of T3 binding affinity. It is expected that variants in THRA with a less detrimental effect on the function of TRα1 will have a more subtle effect on the clinical phenotype. The identification of these patients in future years will be very relevant, since studies in mice with these milder mutations (TRα1-R384C) have shown beneficial effects of T3 treatment on developmental and behavioral clinical features [35].
Consequences of Therapy
LT4 therapy has beneficial effects on certain components of the phenotype, but cognitive and fine motor skill defects remain. During LT4 treatment, serum (F)T4 and rT3 normalize while serum T3 remains elevated, resulting in suppressed TSH (fig. 2)[24,25,26,27]. Certain peripheral markers of TH action have been reported to respond to therapy as well. LT4 therapy induced a rise in serum SHBG or it remained elevated, while serum creatine kinase levels decreased (fig. 2) [24,26,27]. Although total and LDL cholesterol levels were only elevated in 2 patients (P2 and P3), it decreased with LT4 therapy in all 4 patients [24,25,26,27]. Serum copper levels have been reported to rise with LT4 treatment as well, whereas selenium levels remain unchanged [27]. Moreover, IGF1 levels normalized in both young patients, whereas no clear effect was induced on IGF1 levels in the affected adults [24,26,27]. The anemia observed in all patients was normalized in only 1 adult (P3) during LT4 therapy [26,27]. In 1 affected adult (P4), bone turnover markers rose progressively upon LT4 treatment, with certain markers (procollagen type 1 N-propeptide, C-telopeptide cross-linked collagen type I) becoming frankly elevated [27].
One patient reported being more energetic (P2), while another patient (P4) showed greater alertness after the initiation of LT4 therapy [26,27]. However, the cognitive deficits did not clearly improve with LT4 treatment [26]. The anxiety and memory problems of TRα1-R384C mice can be remedied with T3 treatment during adulthood [35], but it is important to realize that this mutation results in a reduced T3 affinity, a defect that can be overcome with high levels of T3. In contrast, all RTHα patients identified so far have a mutation that is completely devoid of T3 binding.
LT4 therapy further induced an initial catch-up growth in 1 patient (P2), but the effect was less clear in the other young patient (P1) (fig. 1) [24,25]. Additional GH therapy had little effect on growth [25]. In all patients, LT4 treatment had a beneficial effect on constipation [24,25,26,27] without improving intestinal motility [24]. While BMR also normalized with LT4 therapy, there was no difference in body temperature during treatment [24,26,27]. Blood pressure and heart rate partially normalized in 1 of the affected adults (P4) [27], whereas the other showed no clear response, independently of basal blood pressure and heart rate [24,26]. In all patients, the cardiac response was blunted, particularly considering the elevated T3 levels during treatment [24,26,27]. This is in line with the dampened cardiac response in TRα1-/-mice [44].
To what extent TRβ1 is involved in the beneficial effects of TH treatment by taking over the role of the impaired TRα1 is not yet clear.
Future Perspectives
In order to fully understand the clinical phenotype associated with RTHα, it is important to identify additional subjects with THRA mutations. A detailed characterization of additional individuals will not only improve our understanding of the physiologic role of TRα1, but it will also provide a detailed insight into the molecular mechanisms underlying the specific phenotype associated with TRα1 defects. Expectedly, variants in THRA with a less detrimental effect on the function of TRα1 will have a more subtle effect on the clinical phenotype. Parallel to the different mouse models, the phenotype of new patients may also vary depending on the exact position of the mutation. This is supported by the recent report of a RTHα patient (P4) with more severe cognitive impairment than the other 3 patients [27].
The identification of additional subjects with less severe mutations in THRA is even more important because studies in TRα1 mutant mice predict that such individuals will benefit from treatment with TH [17,35]. Although some beneficial effects of LT4 treatment have already been observed in patients with mutations resulting in a complete lack of T3 binding, such as an increase in growth velocity and a response of peripheral markers of TH action, more beneficial effects can be expected in patients with a TRα1 mutant having residual T3 binding affinity.
Conclusion
The first 4 patients identified with inactivating mutations in THRA have a phenotype consisting of growth retardation, delayed bone development, mildly delayed motor and mental development, slightly abnormal thyroid function tests, low GH and IGF1 levels, anemia, and constipation. Seizures, changes in cardiac function, and dyslipidemia may also occur. The identification of additional patients is necessary to fully understand the clinical phenotype.
Although these patients may present with hypothyroid symptoms and growth retardation, the diagnosis can easily be missed when only TSH and (F)T4 are analyzed, since these may be normal. When THRA mutations are suspected, serum T3 and rT3 should be measured as well, and especially elevated T3/T4 and T3/rT3 ratios could indicate RTHα.
Disclosure Statement
The authors have nothing to disclose.
Acknowledgment
This work was supported by a ZonMW-TOP grant (921.12.044), as well as an Erasmus MC MRACE grant to Robin P. Peeters.
References
- 1.Clause M. Newborn screening for congenital hypothyroidism. J Pediatr Nurs. 2013;28:603–608. doi: 10.1016/j.pedn.2013.03.009. [DOI] [PubMed] [Google Scholar]
- 2.Rastogi MV, LaFranchi SH. Congenital hypothyroidism. Orphanet J Rare Dis. 2010;5:17. doi: 10.1186/1750-1172-5-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhang J, Lazar MA. The mechanism of action of thyroid hormones. Annu Rev Physiol. 2000;62:439–466. doi: 10.1146/annurev.physiol.62.1.439. [DOI] [PubMed] [Google Scholar]
- 4.Cheng SY, Leonard JL, Davis PJ. Molecular aspects of thyroid hormone actions. Endocr Rev. 2010;31:139–170. doi: 10.1210/er.2009-0007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Moeller LC, Cao X, Dumitresco AM, et al. Thyroid hormone mediated changes in gene expression can be initiated by cytosolic action of the thyroid hormone receptor beta through the phosphatidylinositol 3-kinase pathway. Nucl Recept Signal. 2006;4:e020. doi: 10.1621/nrs.04020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cheng SY. Thyroid hormone receptor mutations and disease: beyond thyroid hormone resistance. Trends Endocrinol Metab. 2005;16:176–182. doi: 10.1016/j.tem.2005.03.008. [DOI] [PubMed] [Google Scholar]
- 7.Abel ED, Boers ME, Pazos-Moura C, et al. Divergent roles for thyroid hormone receptor beta isoforms in the endocrine axis and auditory system. J Clin Invest. 1999;104:291–300. doi: 10.1172/JCI6397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nunez J, Celi FS, Ng L, et al. Multigenic control of thyroid hormone functions in the nervous system. Mol Cell Endocrinol. 2008;287:1–12. doi: 10.1016/j.mce.2008.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Refetoff S, Dumitrescu AM. Syndromes of reduced sensitivity to thyroid hormone: genetic defects in hormone receptors, cell transporters and deiodination. Best Pract Res Clin Endocrinol Metab. 2007;21:277–305. doi: 10.1016/j.beem.2007.03.005. [DOI] [PubMed] [Google Scholar]
- 10.Mitchell CS, Savage DV, Dufour S, et al. Resistance to thyroid hormone is associated with raised energy expenditure, muscle mitochondrial uncoupling, and hyperphagia. J Clin Invest. 2010;120:1345–1354. doi: 10.1172/JCI38793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Visser WE, van Mullem AA, Visser TJ, et al. Different causes of reduced sensitivity to thyroid hormone: diagnosis and clinical management. Clin Endocrinol (Oxf) 2013;79:595–605. doi: 10.1111/cen.12281. [DOI] [PubMed] [Google Scholar]
- 12.Hashimoto K, Curt FH, Borges PP, et al. An unliganded thyroid hormone receptor causes severe neurological dysfunction. Proc Natl Acad Sci USA. 2001;98:3998–4003. doi: 10.1073/pnas.051454698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kaneshige M, Kaneshige K, Zhu X, et al. Mice with a targeted mutation in the thyroid hormone beta receptor gene exhibit impaired growth and resistance to thyroid hormone. Proc Natl Acad Sci USA. 2000;97:13209–13214. doi: 10.1073/pnas.230285997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Flamant F, Poguet Al, Plateroti M, et al. Congenital hypothyroid Pax8(-/-) mutant mice can be rescued by inactivating the TRalpha gene. Mol Endocrinol. 2002;16:24–32. doi: 10.1210/mend.16.1.0766. [DOI] [PubMed] [Google Scholar]
- 15.Göthe S, Wang Z, Ng L, et al. Mice devoid of all known thyroid hormone receptors are viable but exhibit disorders of the pituitary-thyroid axis, growth, and bone maturation. Genes Dev. 1999;13:1329–1341. doi: 10.1101/gad.13.10.1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Morte B, Manzano J, Scanlan T, et al. Deletion of the thyroid hormone receptor alpha 1 prevents the structural alterations of the cerebellum induced by hypothyroidism. Proc Natl Acad Sci USA. 2002;99:3985–3989. doi: 10.1073/pnas.062413299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vennström B, Mittag J, Wallis K. Severe psychomotor and metabolic damages caused by a mutant thyroid hormone receptor alpha 1 in mice: can patients with a similar mutation be found and treated? Acta Paediatr. 2008;97:1605–1610. doi: 10.1111/j.1651-2227.2008.01031.x. [DOI] [PubMed] [Google Scholar]
- 18.Bassett JH, Williams GR. The skeletal phenotypes of TRalpha and TRbeta mutant mice. J Mol Endocrinol. 2009;42:269–282. doi: 10.1677/JME-08-0142. [DOI] [PubMed] [Google Scholar]
- 19.Liu YY, Schultz JJ, Brent GA. A thyroid hormone receptor alpha gene mutation (P398H) is associated with visceral adiposity and impaired catecholamine-stimulated lipolysis in mice. J Biol Chem. 2003;278:38913–38920. doi: 10.1074/jbc.M306120200. [DOI] [PubMed] [Google Scholar]
- 20.Mittag J, Wallis K, Vennström B. Physiological consequences of the TRalpha1 aporeceptor state. Heart Fail Rev. 2010;15:111–115. doi: 10.1007/s10741-008-9119-5. [DOI] [PubMed] [Google Scholar]
- 21.Kaneshige M, Suzuki H, Kaneshige K, et al. A targeted dominant negative mutation of the thyroid hormone alpha 1 receptor causes increased mortality, infertility, and dwarfism in mice. Proc Natl Acad Sci USA. 2001;98:15095–15100. doi: 10.1073/pnas.261565798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Quignodon L, Vincent S, Winter H, et al. A point mutation in the activation function 2 domain of thyroid hormone receptor alpha1 expressed after CRE-mediated recombination partially recapitulates hypothyroidism. Mol Endocrinol. 2007;21:2350–2360. doi: 10.1210/me.2007-0176. [DOI] [PubMed] [Google Scholar]
- 23.Tinnikov A, Nordström K, Thoren P, et al. Retardation of post-natal development caused by a negatively acting thyroid hormone receptor alpha1. EMBO J. 2002;21:5079–5087. doi: 10.1093/emboj/cdf523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bochukova E, Schoenmakers N, Agostini M, et al. A mutation in the thyroid hormone receptor alpha gene. N Engl J Med. 2012;366:243–249. doi: 10.1056/NEJMoa1110296. [DOI] [PubMed] [Google Scholar]
- 25.van Mullem A, van Heerebeek R, Chrysis D, et al. Clinical phenotype and mutant TRalpha1. N Engl J Med. 2012;366:1451–1453. doi: 10.1056/NEJMc1113940. [DOI] [PubMed] [Google Scholar]
- 26.van Mullem AA, Chrysis D, Eythimiadou A, et al. Clinical phenotype of a new type of thyroid hormone resistance caused by a mutation of the TRalpha1 receptor: consequences of LT4 treatment. J Clin Endocrinol Metab. 2013;98:3029–3038. doi: 10.1210/jc.2013-1050. [DOI] [PubMed] [Google Scholar]
- 27.Moran C, Schoenmakers N, Agostini M, et al. An adult female with resistance to thyroid hormone mediated by defective thyroid hormone receptor alpha. J Clin Endocrinol Metab. 2013;98:4254–4261. doi: 10.1210/jc.2013-2215. [DOI] [PubMed] [Google Scholar]
- 28.Espiard S, Savagner F, D'herbomez M, et al. Polymalformation, dyserythropoietic anemia, primary hyperparathyroidism and diarrhoea in a patient with mutation of the thyroid hormone receptor alpha gene (THRα). Abstracts – Orals, Featured Poster Presentations, and Posters. 95th Annu Meet Endocr Soc, ENDO, San Francisco, 2013.
- 29.Zavacki AM, Larsen PR. RTHalpha, a newly recognized phenotype of the resistance to thyroid hormone (RTH) syndrome in patients with THRA gene mutations. J Clin Endocrinol Metab. 2013;98:2684–2686. doi: 10.1210/jc.2013-2475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.O'Shea PJ, Bassett JH, Sriskantharajah S, et al. Contrasting skeletal phenotypes in mice with an identical mutation targeted to thyroid hormone receptor alpha1 or beta. Mol Endocrinol. 2005;19:3045–3059. doi: 10.1210/me.2005-0224. [DOI] [PubMed] [Google Scholar]
- 31.Bassett JH, Nordström K, Boyde A, et al. Thyroid status during skeletal development determines adult bone structure and mineralization. Mol Endocrinol. 2007;21:1893–1904. doi: 10.1210/me.2007-0157. [DOI] [PubMed] [Google Scholar]
- 32.Fraichard A, Chassande O, Plateroti M, et al. The T3R alpha gene encoding a thyroid hormone receptor is essential for post-natal development and thyroid hormone production. EMBO J. 1997;16:4412–4420. doi: 10.1093/emboj/16.14.4412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wojcicka A, Bassett JH, Williams GR. Mechanisms of action of thyroid hormones in the skeleton. Biochim Biophys Acta. 2013;1830:3979–3986. doi: 10.1016/j.bbagen.2012.05.005. [DOI] [PubMed] [Google Scholar]
- 34.Wallis K, Sjögren M, van Hogerlinden M, et al. Locomotor deficiencies and aberrant development of subtype-specific GABAergic interneurons caused by an unliganded thyroid hormone receptor alpha1. J Neurosci. 2008;28:1904–1915. doi: 10.1523/JNEUROSCI.5163-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Venero C, Guadano-Ferraz A, Herrero AI, et al. Anxiety, memory impairment, and locomotor dysfunction caused by a mutant thyroid hormone receptor alpha1 can be ameliorated by T3 treatment. Genes Dev. 2005;19:2152–2163. doi: 10.1101/gad.346105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Itoh Y, Esaki T, Kaneshige M, et al. Brain glucose utilization in mice with a targeted mutation in the thyroid hormone alpha or beta receptor gene. Proc Natl Acad Sci USA. 2001;98:9913–9918. doi: 10.1073/pnas.171319498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Plateroti M, Chassande O, Fraichard A, et al. Involvement of T3Ralpha- and beta-receptor subtypes in mediation of T3 functions during postnatal murine intestinal development. Gastroenterology. 1999;116:1367–1378. doi: 10.1016/s0016-5085(99)70501-9. [DOI] [PubMed] [Google Scholar]
- 38.Sjögren M, Alkemade A, Mittag J, et al. Hypermetabolism in mice caused by the central action of an unliganded thyroid hormone receptor alpha1. EMBO J. 2007;26:4535–4545. doi: 10.1038/sj.emboj.7601882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gereben B, Zavacki AM, Ribich S, et al. Cellular and molecular basis of deiodinase-regulated thyroid hormone signaling. Endocr Rev. 2008;29:898–938. doi: 10.1210/er.2008-0019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Barca-Mayo O, Liao XH, Alonso M, et al. Thyroid hormone receptor alpha and regulation of type 3 deiodinase. Mol Endocrinol. 2011;25:575–583. doi: 10.1210/me.2010-0213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Erdogan M, Kosenli A, Ganidagli S, et al. Characteristics of anemia in subclinical and overt hypothyroid patients. Endocr J. 2012;59:213–220. doi: 10.1507/endocrj.ej11-0096. [DOI] [PubMed] [Google Scholar]
- 42.Kendrick TS, Payne CJ, Epis MR, et al. Erythroid defects in TRalpha-/-mice. Blood. 2008;111:3245–3248. doi: 10.1182/blood-2007-07-101105. [DOI] [PubMed] [Google Scholar]
- 43.Graig FA, Smith JC. Serum creatine phosphokinase activity in altered thyroid states. J Clin Endocrinol Metab. 1965;25:723–731. doi: 10.1210/jcem-25-6-723. [DOI] [PubMed] [Google Scholar]
- 44.Wikström L, Johansson C, Salto C, et al. Abnormal heart rate and body temperature in mice lacking thyroid hormone receptor alpha 1. EMBO J. 1998;17:455–461. doi: 10.1093/emboj/17.2.455. [DOI] [PMC free article] [PubMed] [Google Scholar]