Abstract
Selenium supplementation in people with Hashimoto's thyroiditis might reduce antibody levels and result in a decreased dosage of levothyroxine (LT4) and may provide other beneficial effects (e.g. on mood and health-related quality of life). The aim of our systematic review was to assess the effects of selenium supplementation on Hashimoto's thyroiditis. We searched The Cochrane Library, MEDLINE, EMBASE and Web of Science for randomized controlled trials. Study selection, data extraction, assessment of risk of bias and analyses were carried out by two independent review authors. We assessed the quality of the evidence of included studies using GRADE. Four studies rated at unclear to high risk of bias comprising 463 participants were included. One study at high risk of bias showed statistically significant improvement in subjective well-being with sodium selenite 200 μg plus titrated LT4 compared with placebo plus titrated LT4 (RR 4.67, 95% CI 1.61-13.50). Selenomethionine 200 μg as a single treatment or combined with LT4 reduced the serum levels of anti-thyroid peroxidase antibodies compared with placebo (or placebo plus LT4) in three studies (p < 0.001). Although the changes from baseline were statistically significant in these three studies, their clinical relevance is unclear. In conclusion, the results of these four studies, assessed at unclear to high risk of bias, show that evidence to support or refute the efficacy of selenium supplementation in people with Hashimoto's thyroiditis is incomplete and not reliable to help inform clinical decision making.
Key Words : Selenium, Hashimoto's thyroiditis, Evidence-based medicine, Supplements
Introduction
Hashimoto's thyroiditis or chronic lymphocytic thyroiditis is a common autoimmune disorder which tends to run in families and affects both genders at any age, although it is most often seen in middle-aged women [1,2]. Its prevalence is influenced by ethnicity, environmental factors, such as iodine and selenium status, age and gender [1,2]. Clinical manifestations of the disease are caused primarily by low levels of thyroid hormones, for which the treatment is hormone replacement therapy, which usually consists of levothyroxine (LT4) [3].
Selenium is an essential trace element that is required for the correct functioning of the immune system with a recommended daily intake for adults of 55 μg [4]. Natural selenium-rich sources include Brazil nuts, organ meat, muscle meat, cereals, shellfish and fish [5]. Selenomethionine and sodium selenite are the two most common oral forms of selenium supplementation that are available in variable dosages (100 and 200 μg/day) [6,7]. It has recently been established that selenium plays a key role in thyroid cell physiology [8]. It is incorporated in the molecular structure of several enzymes in the thyroid gland [8,9]. One of these enzymes, glutathione peroxidase (GPx), is critically involved in protecting the gland against oxidative damage. Thyroid peroxidase uses hydrogen peroxide (H2O2), a free radical capable of inflicting oxidative damage, as a substrate in catalyzing the iodination and coupling of tyrosyl residues in thyroglobulin to produce thyroid hormone. The active form of thyroid hormone, tri-iodothyronine (T3), is produced by de-iodination of the prohormone T4 by type I and type II iodothyronine de-iodinasesin, a two-substrate ‘ping-pong’ mechanism of reaction, along with degradation of H2O2 to water by GPx. Iodothyronine de-iodinases are also selenoproteins, as is GPx.
If there is selenium deficiency, these two enzymes cannot function properly, which results in both ineffective production of T3 and inefficient protection against free radicals, the latter facilitating cell damage and autoimmune destruction of the gland [6,8,9]. In view of the pivotal role of selenium in thyroid physiology, it is conceivable that selenium supplementation may be of benefit to patients with Hashimoto's thyroiditis, particularly in clinical situations of selenium deficiency [6]. Several studies have suggested that selenium treatment reduces antibodies levels [10], allows lower dosage of LT4 supplementation and may provide other beneficial effects (e.g. on mood and health-related quality of life; HRQoL) in patients with Hashimoto's thyroiditis [11]. We performed a systematic review to assess the effects of selenium supplementation for Hashimoto's thyroiditis, and this report is a summary of the Cochrane systematic review [12].
Material and Methods
We conducted a systematic review of randomized controlled trials (RCTs) assessing the effects of selenium supplementation in adults with Hashimoto's thyroiditis. Selenium 100 μg or 200 μg (sodium selenite or selenomethionine) only or combined with titrated LT4 (to maintain basal TSH within normal range) was compared with: no selenium or no selenium plus titrated LT4, respectively; placebo tablets or placebo tablets plus titrated LT4, respectively (table 1).
Table 1.
Characteristics of included RCTs in the review
| Study | Methods | Participants | Interventions | Outcomes |
|---|---|---|---|---|
| Karanikas et al. [19] Vienna, Austria | RCT ‘blinded’ | 36 women with autoimmune thyroiditis mean age 47 years mean baseline selenium 75 µg/l | 3 months | (1) FT4, TSH, anti-TPOAb |
| A: LT4 + 200 µg sodium selenite once daily | (2) intracellular cytokine evaluation in CD4+ and CD8+ T cells of peripheral blood mononuclear cells | |||
| B: LT4 + placebo once daily | (3) plasma selenium | |||
| (4) subjective well-being of the patients (assessed with short form health survey, SF-12 [24]) | ||||
| Krysiak and Okopien [20] Katowice, Poland | RCT double-blind | 170 euthyroid women with recently diagnosed and previously untreated Hashimoto's thyroiditis mean age 38 years mean baseline selenium 57.5 µg/l, of women in this part of Poland (not measured in the participants) | 6 months | (1) monocyte and lymphocyte suppression |
| A: LT4 once daily | (2) systemic anti-inflammatory effects | |||
| B: 200 µg selenomethionine once daily | (3) anti-TPOAb | |||
| C: LT4 + 200 µg selenomethionine once daily | (4) adverse events | |||
| D: placebo once daily | ||||
| Negro et al. [21] Brindisi, Italy | RCT | 169 pregnant anti-TPOAb-positive women mean age 28 years mean baseline selenium 78.8 µg/l | From 12 weeks’ gestation to 12 months’ postpartum | (1) FT4, TSH, anti-TPOAb |
| A: 200 µg selenomethionine once daily | (2) selenium status | |||
| B: placebo once daily | (3) thyroid ultrasound | |||
| Turker et al. [7] Istanbul, Turkey | RCT ‘blinded’ | 88 women with autoimmune thyroiditis mean age 40 years no data on baseline selenium, but the study states that in Turkey there is mild selenium deficiency | 3 months | (1) TgAb, TSH, FT4, FT3 |
| A: LT4 + 200 µg selenomethionine | (2) anti-TPOAb | |||
| B: LT4 + placebo | ||||
LT4 = Levothyroxine; FT4 = free thyroxine; TSH = thyroid-stimulating hormone; TPOAb = thyroid peroxidase antibodies; TgAb = thyroglobulin antibodies; FT3 = free triiodothyronine.
Search Strategies
We searched for RCTs in The Cochrane Library, MEDLINE, EMBASE and Web of Science and in reference lists of articles (from inception until 2 October 2012). We also examined several online trial registries for on-going trials and attempted to contact trial investigators to provide missing data or to clarify study details. Two review authors (E.J.v.Z. and A.Y.A.) independently assessed the titles and abstracts for eligible RCTs.
Outcome Measures
Our three primary outcomes were: (1) change from baseline in HRQoL assessed using any validated quality-of-life instrument; (2) change from baseline in symptoms, such as mood, fatigue and muscle weakness, assessed using any validated instrument, and (3) proportion of participants reporting an adverse event throughout the study period. Secondary outcomes were: (a) change from baseline in serum levels of anti-thyroid peroxidase antibodies; (b) change from baseline in LT4 replacement dosage, and (c) economic costs.
Data Extraction and Synthesis
Three review authors (E.J.v.Z., A.Y.A. and Z.F.) extracted study details and data using a structured data extraction form and any disagreements were resolved by discussion. The risk of bias in each study was assessed independently by the same 3 authors using the Cochrane Collaboration's domain-based evaluation tool as described in Chapter 8, Section 8.5, in the Cochrane Handbook for Systematic Reviews of Interventions [13]. We presented continuous outcomes data on the original scale as reported in each individual study. Dichotomous outcomes data were presented as risk ratios (RRs) and if significant were converted to the number needed to treat (NNT) for an additional beneficial outcome. All outcomes data were reported with their associated 95% CIs and were analyzed using a random-effects model and the Mantel-Haenzel test for dichotomous outcomes data and invariance analysis for continuous outcomes data, unless stated otherwise. The degree of heterogeneity between the studies was assessed using the I2 statistic. We reported heterogeneity as important if it was at least moderate to substantial (I2 between 50 and 90%). If heterogeneity could be explained by clinical reasoning and a coherent argument could be made for combining the studies, we planned to enter these into a meta-analysis.
Results
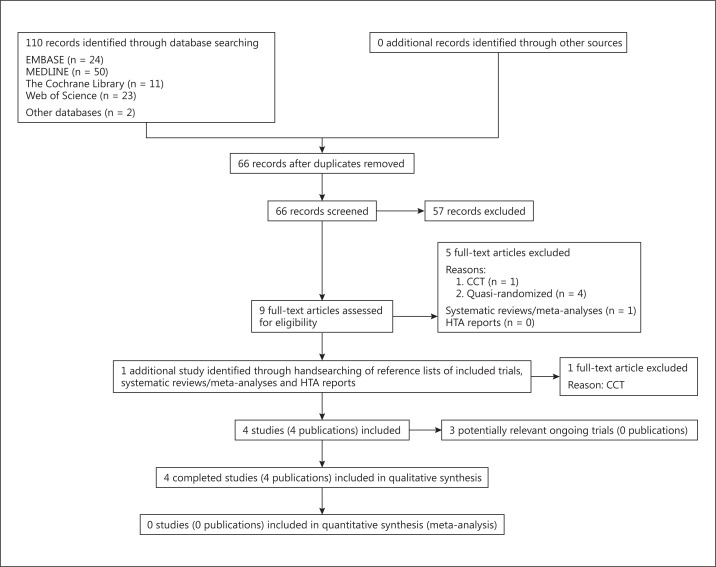
From the searches, we identified 110 studies for abstract review (fig. 1). Six studies were excluded after careful evaluation of the full text of the publication [10,14,15,16,17,18]. These appeared to be controlled (i.e. nonrandomized) clinical trials, of which four [10,16,17,18] were confirmed to be quasi-randomized after communication with the trialists (i.e. allocation was done on the basis of a pseudo-random sequence, e.g. odd/even hospital number or date of birth, alternation). Eventually, only four studies comprising 463 participants met our inclusion criteria and were included [7,19,20,21].
Fig. 1.
Study flow diagram.
Risk of Bias of the Included Studies
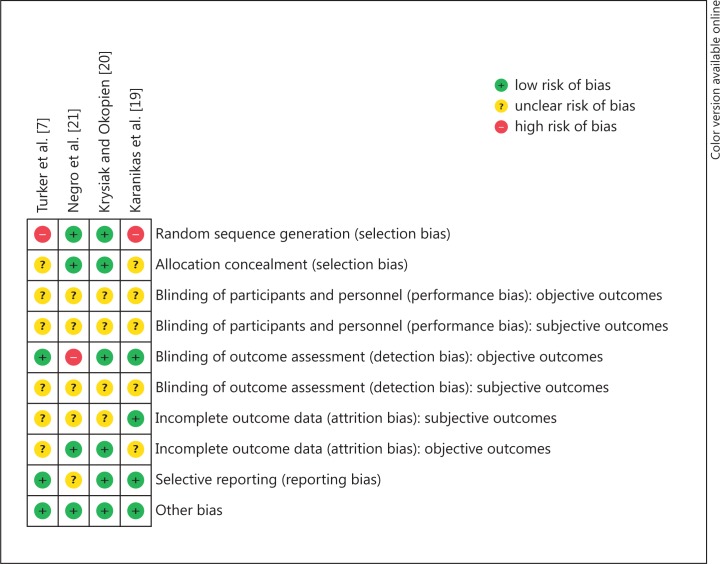
We assessed the studies for risk of bias and have reported the judgements for the individual domains in the ‘risk of bias’ table (fig. 2). The overall ‘risk of bias’ of the four studies was assessed as ‘unclear risk of bias’ to ‘high risk of bias’ (plausible bias that raises some to serious doubts about the results). In two studies [7,19], one of the key domains, ‘sequence generation’, was assessed as high risk of bias and, in all of the studies, 4-6 domains were assessed as ‘unclear risk of bias’.
Fig. 2.
Risk of bias summary: review of the authors' judgments about each risk of bias item for each included study.
Effects of Interventions
See table 2 for a summary of the key results.
Table 2.
Summary of results of included studies
| Study | Interventions | Summary outcomes | Comments |
|---|---|---|---|
| Karanikas et al. [19] |
|
Subjective well-being was improved in 14/18 women receiving sodium selenite compared with 3/18 in the placebo group (RR 4.67, 95% CI 1.61 – 13.50; p = 0.004; number needed to treat = 2; 95% CI 2 – 3) Anti-TPOAb changed from 524 ± 452 IU/ml at baseline to 505 ± 464 IU/ml for the sodium selenite group and from 521 ± 349 to 527 ± 354 IU/ml for the placebo group The mean difference was estimated to be −25 (95% CI −181 to 131; p = 0.75; 36 participants) | This study was assessed as high risk of bias as randomization seemed to be based on prognostic factors, with no mention of stratified randomization Although it was stated to be a blinded study, the report did not provide sufficient detail about the specific measures used to blind study participants and personnel from knowledge of which intervention a participant received |
| Krysiak and Okopien [20] |
|
B versus D: the mean difference in anti-TPOAb was estimated as −917 IU/ml (95% CI −1,029.16 to −804.84; p < 0.001; 85 participants) No adverse events were reported in either group C versus D: the mean difference was estimated to be −1,508 U/ml (95% CI −1,672 to −1,345; p < 0.001; 86 participants) In group C, 1/43 reported an adverse event versus 0/42 in group D (RR 2.93, 95% CI 0.12–70.00) | |
| Negro et al. [21] |
|
The mean difference in anti-TPOAb was estimated as −345.0 IU/ml (95% CI −358.79 to −331.21; p < 0.001; 169 participants) | No report on any blinding might have influenced assessment of thyroid ultrasound |
| Turker et al. [7] |
|
The mean difference in anti-TPOAb was estimated to be −235 IU/ml (95% CI −374 to −95; p = 0.001; 88 participants) 1/48 reported an adverse event (gastric discomfort) in group A versus 0/40 in group B (RR 2.63, 95% CI 0.11–62.95) | This study was assessed as high risk of bias as randomization seemed to be based on prognostic factors, with no mention of stratified randomization Although it stated to be a blinded study the report did not provide sufficient detail about the specific measures used to blind study participants and personnel from knowledge of which intervention a participant received |
TPOAb= Thyroid peroxidase antibodies.
The primary outcome of HRQoL was not addressed in any study. Two of our secondary outcomes (‘change from baseline in LT4 replacement dosage at the end of the study’ and ‘economic costs’) were not assessed either. One study at high risk of bias showed a statistically significant improvement in subjective well-being with sodium selenite 200 μg plus titrated LT4 compared with placebo plus titrated LT4[19]. In three studies [7,20,21], selenomethionine 200 μg supplementation was associated with a decrease in serum levels of anti-thyroid peroxidase antibodies, and although the changes from baseline were statistically significant, their clinical significance is unclear. Pooling of the studies by Krysiak and Okopien [20] and Negro et al. [20] was not feasible due to extreme heterogeneity (I2 = 99%).
This reduction in serum anti-thyroid peroxidase antibodies was not confirmed in the study which assessed sodium selenite 200 μg plus titrated LT4[19]. The two studies that reported on adverse events demonstrated that selenium supplementation did not lead to a statistically significant increase in the number of adverse events when compared with placebo. The effectiveness and safety of selenium (+ LT4) versus placebo (+ LT4) are presented in a GRADE summary of findings table (table 3) [22].
Table 3.
Summary of findings: selenium (+LT4) compared to placebo (+LT4) for participants with Hashimoto's thyroiditis
| Outcomes | Illustrative comparative risks* (95% CI) |
Relative effect (95% CI) | Participants, n (studies) | Quality of the evidence (GRADE) | Comments | |
|---|---|---|---|---|---|---|
| assumed risk placebo (+LT4) | corresponding risk selenium (+LT4) | |||||
| Change from baseline in HRQoL – not reported | see comment | see comment | not estimable | – | see comment | not reported in any study |
|
167 per 1,000 | 778 per 1,000 (268 – 1,000) | RR 4.67 (1.61 – 13.5) | 36 (1 study) | ⊕⊕⊖⊖ low123 | |
|
RR 2.71 (0.29 – 25.66) | 258 (3 studies4) | ⊕⊕⊖⊖ low1 | participants in placebo group counted twice (same participants in both comparisons) | ||
|
see comment | see comment | not estimable | 252 (4 studies4) | ⊕⊕⊖⊖ low1 | data could not be pooled because of substantial clinical heterogeneity of participants, interventions and controls |
| Change from baseline in LT4 replacement dosage at the end of the study – not reported | see comment | see comment | not estimable | – | see comment | not reported in any study |
| Economic costs – not reported | see comment | see comment | not estimable | – | see comment | not reported in any study |
Patient or population: participants with Hashimoto's thyroiditis. Settings: hospital-outpatient department. Intervention: selenium (+LT4). Karanikas et al. [19] and Turker et al. [7] included LT4 in both treatment arms. Krysiak and Okopien [20] had LT4 included in one arm combined with selenium. Comparison: placebo (+LT4). GRADE Working Group grades of evidence: (a) High quality: further research is very unlikely to change our confidence in the estimate of effect. (b) Moderate quality: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. (c) Low quality: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. (d) Very low quality: we are very uncertain about the estimate.
The basis for the assumed risk (e.g. the median control group risk across studies) is provided in the footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI).
Randomization was probably based on prognostic factors, and no mentioning of stratified randomization [19].
Wide confidence interval.
RR 4.67.
Krysiak and Okopien [20] provided 2 comparisons.
Discussion
Based on the four studies assessed at unclear to high risk of bias that provided limited data, no clinically relevant conclusions can be drawn. As Hashimoto's thyroiditis is associated with many debilitating symptoms, outcomes, such as change in HRQoL, and improvement in symptoms, such as mood, fatigue and muscle weakness, are important and clinically meaningful markers. The results of these studies provide incomplete evidence to support or refute the efficacy of selenium in people with Hashimoto's thyroiditis. We identified three on-going studies that may eventually help to fill in some of the gaps in evidence for the efficacy of selenium as a supplement in people with Hashimoto's thyroiditis.
Another systematic review, which attempted ‘to summarize available data and provide an evidence-based recommendation regarding selenium supplementation in the treatment of Hashimoto's thyroiditis’ included a meta-analysis of data extracted exclusively from studies that were ‘blinded, randomized, placebo-controlled in design’ [6]. Although the review could be regarded as fairly transparent, as it relied on a consensus process negotiated between the reviewers, we dispute the robustness of its methodological approach. Incomplete reporting of some of the critical steps taken in study assessment and dealing with missing trial details and data illustrated a lack of clarity in the process and limitation in its reproducibility. There was no indication if contact had been made with the principal investigators of the included studies for clarification of the methods used to generate the sequence, allocation concealment or blinding or to retrieve missing data. Risk of bias assessment was not undertaken and two of the studies included in the meta-analysis by Toulis et al. [6] were excluded in our review as after E-mail contact with the trial investigators these were confirmed to have been quasi-randomized [10,16]. Furthermore, it was unclear from the review how the quality of evidence was rated, or how the strength of the subsequent recommendations was graded.
The recently updated summary in DynaMed, a clinical reference derived from systematic literature surveillance, was largely in concordance with our assessments of the overall quality of the evidence and conclusions on the efficacy of selenium supplementation for Hashimoto's thyroiditis [23].
The results of our systematic review demonstrate that at present, there is insufficient objective evidence to support clinical decision making regarding the use of selenium supplementation for the treatment of patients with Hashimoto's thyroiditis. Well-designed randomized placebo-controlled trials to evaluate the effects of selenium in people with Hashimoto's thyroiditis are still needed and can ultimately provide reliable evidence to support clinical decision making.
Disclosure Statement
The authors have no conflict of interest to declare.
Acknowledgements
The authors would like to thank Dr. Brian Alper, who provided free access to the DynaMed summary on this topic.
References
- 1.Fink H, Hintze G. Autoimmune thyroiditis (Hashimoto's thyroiditis): current diagnostics and therapy. Med Klin. 2010;105:485–493. doi: 10.1007/s00063-010-1082-y. [DOI] [PubMed] [Google Scholar]
- 2.Stathatos N, Daniels GH. Autoimmune thyroid disease. Curr Opin Rheumatol. 2012;24:70–75. doi: 10.1097/BOR.0b013e32834ddb27. [DOI] [PubMed] [Google Scholar]
- 3.Özen S, Berk Ö, Şimşek DG, Darcan S. Clinical course of Hashimoto's thyroiditis and effects of levothyroxine therapy on the clinical course of the disease in children and adolescents. J Clin Res Pediatr Endocrinol. 2011;3:192–197. doi: 10.4274/jcrpe.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hu Y, McIntosh GH, Young GP. Selenium-rich foods: a promising approach to colorectal cancer prevention. Curr Pharm Biotechnol. 2012;13:165–172. doi: 10.2174/138920112798868809. [DOI] [PubMed] [Google Scholar]
- 5.Rayman MP. Food-chain selenium and human health: emphasis on intake. Br J Nutr. 2008;100:254–268. doi: 10.1017/S0007114508939830. [DOI] [PubMed] [Google Scholar]
- 6.Toulis KA, Anastasilakis AD, Tzellos TG, Goulis DG, Kouvelas D. Selenium supplementation in the treatment of Hashimoto's thyroiditis: a systematic review and a meta-analysis. Thyroid. 2010;20:1163–1173. doi: 10.1089/thy.2009.0351. [DOI] [PubMed] [Google Scholar]
- 7.Turker O, Kumanlioglu K, Karapolat I, Dogan I. Selenium treatment in autoimmune thyroiditis: 9-month follow-up with variable doses. J Endocrinol. 2006;190:151–156. doi: 10.1677/joe.1.06661. [DOI] [PubMed] [Google Scholar]
- 8.Köhrle J, Jakob F, Contempré B, Dumont JE. Selenium, the thyroid, and the endocrine system. Endocr Rev. 2005;26:944–984. doi: 10.1210/er.2001-0034. [DOI] [PubMed] [Google Scholar]
- 9.Brown KM, Arthur JR. Selenium, selenoproteins and human health: a review. Public Health Nutr. 2001;4:593–539. doi: 10.1079/phn2001143. [DOI] [PubMed] [Google Scholar]
- 10.Gärtner R, Gasnier BC, Dietrich JW, Krebs B, Angstwurm MW. Selenium supplementation in patients with autoimmune thyroiditis decreases thyroid peroxidase antibodies concentrations. J Clin Endocrinol Metabol. 2002;87:1687–1691. doi: 10.1210/jcem.87.4.8421. [DOI] [PubMed] [Google Scholar]
- 11.Ott J, Promberger R, Kober F, Neuhold N, Tea M, Huber JC, Hermann M. Hashimoto's thyroiditis affects symptom load and quality of life unrelated to hypothyroidism: a prospective case-control study in women undergoing thyroidectomy for benign goiter. Thyroid. 2011;21:161–167. doi: 10.1089/thy.2010.0191. [DOI] [PubMed] [Google Scholar]
- 12.van Zuuren EJ, Albusta AY, Fedorowicz Z, Carter B, Pijl H. Selenium supplementation for Hashimoto's thyroiditis. Cochrane Database Syst Rev. 2013;6:CD010223. doi: 10.1002/14651858.CD010223.pub2. DOI: 10.1002/14651858.CD010223.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Higgins JPT, Green S. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane-handbook.org
- 14.Balázs C. The effect of selenium therapy on autoimmune thyroiditis. Orv Hetil. 2008;149:1227–1232. doi: 10.1556/OH.2008.28408. [DOI] [PubMed] [Google Scholar]
- 15.Contempré B, Duale NL, Dumont JE, Ngo B, Diplock AT, Vanderpas J. Effect of selenium supplementation on thyroid hormone metabolism in an iodine and selenium deficient population. Clin Endocrinol. 1992;36:579–583. doi: 10.1111/j.1365-2265.1992.tb02268.x. [DOI] [PubMed] [Google Scholar]
- 16.Duntas LH, Mantzou E, Koutras DA. Effects of a six month treatment with selenomethionine in patients with autoimmune thyroiditis. Eur J Endocrinol. 2003;148:389–393. doi: 10.1530/eje.0.1480389. [DOI] [PubMed] [Google Scholar]
- 17.Gärtner R, Gasnier BC. Selenium in the treatment of autoimmune thyroiditis. Biofactors. 2003;19:165–170. doi: 10.1002/biof.5520190309. [DOI] [PubMed] [Google Scholar]
- 18.Nacamulli D, Mian C, Petricca D, Lazzarotto F, Barollo S, Pozza D, Masiero S, Faggian D, Plebani M, Girelli ME, Mantero F, Betterle C. Influence of physiological dietary selenium supplementation on the natural course of autoimmune thyroiditis. Clin Endocrinol (Oxf) 2010;73:535–539. doi: 10.1111/j.1365-2265.2009.03758.x. [DOI] [PubMed] [Google Scholar]
- 19.Karanikas G, Schuetz M, Kontur S, Duan H, Kommata S, Schoen R, Antoni A, Kletter K, Dudczak R, Willheim M. No immunological benefit of selenium in consecutive patients with autoimmune thyroiditis. Thyroid. 2008;18:7–12. doi: 10.1089/thy.2007.0127. [DOI] [PubMed] [Google Scholar]
- 20.Krysiak R, Okopien B. The effect of levothyroxine and selenomethionine on lymphocyte and monocyte cytokine release in women with Hashimoto's thyroiditis. J Clin Endocrinol Metabol. 2011;96:2206–2215. doi: 10.1210/jc.2010-2986. [DOI] [PubMed] [Google Scholar]
- 21.Negro R, Greco G, Mangieri T, Pezzarossa A, Dazzi D, Hassan H. The influence of selenium supplementation on postpartum thyroid status in pregnant women with thyroid peroxidase autoantibodies. J Clin Endocrinol Metabol. 2007;92:1263–1268. doi: 10.1210/jc.2006-1821. [DOI] [PubMed] [Google Scholar]
- 22.Schünemann H, Brozek J, Oxman A.The GRADE Working Group: GRADE Handbook for Grading Quality of Evidence and Strength of Recommendation Version 3.2 [updated March 2009]. Available at: http://www.cc-ims.net/gradepro Accessed 17 July 2013.
- 23.Hashimoto thyroiditis. DynaMed [database online] EBSCO Publishing 2013. http://search.ebscohost.com/login.aspx?direct=true&site=DynaMed&id=113943 Updated August 14, 2012. Accessed July 17, 2013.
- 24.Jenkinson C, Layte R. Development and testing of the UK SF-12 (short form health survey) J Health Serv Res Policy. 1997;2:14–18. doi: 10.1177/135581969700200105. [DOI] [PubMed] [Google Scholar]