Abstract
Rodent models of retinal angiogenesis play a pivotal role in angiogenesis research. These models are a window to developmental angiogenesis, to pathological retinopathy, and are also in vivo tools for anti-angiogenic drug screening in cancer and ophthalmic research. The mouse model of oxygen-induced retinopathy (OIR) has emerged as one of the leading in vivo models for these purposes. Many of the animal studies that laid the foundation for the recent breakthrough of anti-angiogenic treatments into clinical practice were performed in the OIR model. However, readouts from the OIR model have been time-consuming and can vary depending on user experience. Here, we present a computer-aided quantification method that is characterized by (i) significantly improved efficiency, (ii) high correlation with the established hand-measurement protocols, and (iii) high intra-and inter-individual reproducibility of results. This method greatly facilitates quantification of retinal angiogenesis while at the same time increasing lab-to-lab reproducibility of one of the most widely used in vivo models in angiogenesis research.
Keywords: Oxygen-induced retinopathy, OIR, Retina, Neovascularization, Quantification, SWIFT_NV
In 2006, Science Magazine ranked intraocular anti-VEGF therapy as one of the top 10 breakthroughs of the year [1] for increasing vision in patients with wet age-related macular degeneration—a blinding disease where slowing the rate of vision loss had previously been considered a treatment success [2]. Beyond ophthalmology, anti-angiogenic treatment strategies have also drawn increasing attention as adjuncts to established chemotherapy regimens in cancer patients [3].
Most of the basic work allowing the successful transition of these anti-angiogenic therapies from bench to bedside has been performed using rodent models of angiogenesis. From the plethora of useful angiogenic in vivo models, the mouse model of oxygen-induced retinopathy (OIR) [4] stands out as one of the earliest and most widely used models both for investigation of angiogenesis as well as in vivo testing of treatment strategies. The OIR model has been the first in vivo model to demonstrate the role of VEGF in the pathogenesis of retinal neovascularization [5, 6]. Subsequent studies using the OIR model have shown the beneficial effect of anti-VEGF treatment in hypoxia-driven ocular angiogenesis [7–11]. Results from the OIR model thus helped to lay the foundation for today’s clinical application of anti-VEGF therapies in human patients. There are more than 15,000 references to this model since it was first published in 1994 [12]. Standardizing and facilitating the readout of neovascularization from this model would be of great benefit to the research community.
In the mouse OIR model, pups with nursing mothers are placed into 75% oxygen from postnatal day 7 (P7) to P12. During this hyperoxic phase immature vessels in the central retina are lost and a zone of vaso-obliteration (VO) develops (Fig. 1a, b). Mice are returned to room air at P12. As metabolic demands of the developing retina increase, the avascular retina becomes hypoxic and triggers neovascularization (NV) at the interface between the avascular and vascularized retina. This NV can be quantified on postnatal day 17 (Fig. 1c, d).
Fig. 1.
Lectin-stained retinal whole mounts showing the zone of vaso-obliteration (VO) and neovascularization (NV). After oxygen incubation a central VO zone develops (a) that is quantified by manually outlining the edges of the VO area (b). After returning the mouse pups to room air the hypoxic retina in the VO zone triggers formation of retinal NV (c). In the traditional hand-measurement protocols all neovascular tufts (arrowheads) and clusters (arrow) are manually marked to quantify retinal NV (d)
Quantification of retinal NV, however, has been a variable that is difficult to standardize among different investigators. As a consequence, various approaches to NV quantification co-exist: (i) counting pre-retinal nuclei on retinal cross-sections [4–7, 13]; (ii) using retinal whole mounts to score retinal NV in a grading system [14, 15]; and (iii) using retinal whole mounts to manually outline individual neovascular tufts and clusters [16–19]. All these approaches can accurately quantify retinal NV but all are time-consuming and to some degree subjective due to manual counting, scoring, or outlining of individual NV tufts and clusters. Therefore, a more standardized and time-efficient protocol for NV quantification would be desirable. With SWIFT_NV, we present a semi-automated quantification method that allows accurate and time-efficient quantification of retinal NV.
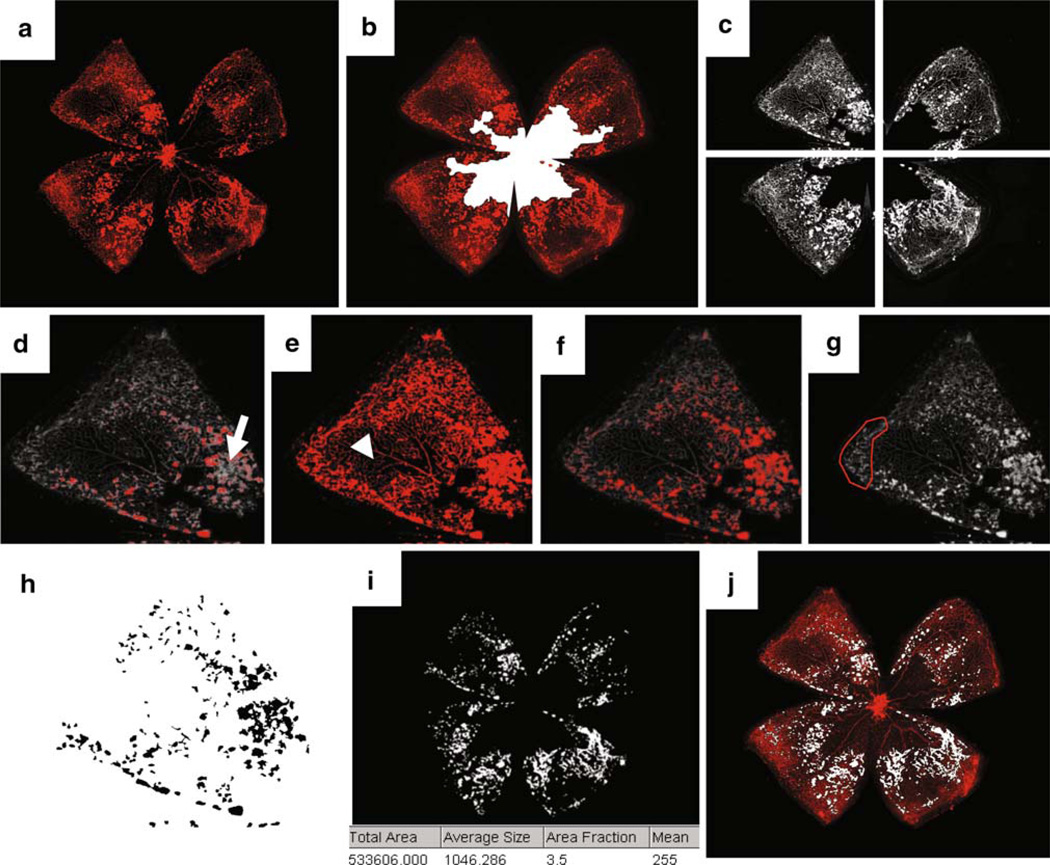
SWIFT_NV consists of a set of macros that was developed to run on NIHs free ImageJ platform. The macros together with a detailed manual can be obtained from the authors free of charge for all academic institutions. Similar to the established hand-measurement protocols, SWIFT_NV analyzes retinal NV from lectin-stained retinal whole mounts. It requires both the original retinal whole mount images as well as an image with a manually outlined VO zone (Fig. 2a, b). This approach was chosen because outlining the VO zone requires only a negligible amount of time compared to the amount of time that is needed for manual quantification of NV tufts and clusters. Manual VO measurement is also less susceptible to interuser variability. The SWIFT_NV method therefore focuses solely on NV quantification.
Fig. 2.
The computer-aided quantification method SWIFT_NV requires both the original retinal whole mount image (a) as well as an image with manually marked area of vaso-obliteration (VO; b). SWIFT_NVs algorithm automatically divides the retinal image into four quadrants and subtracts background fluorescence (c). During the next step the user is asked to set an intensity threshold for each quadrant individually. Setting the threshold too high misses NV tufts and clusters (arrow in d). Setting the threshold too low falsely includes normal vessels (arrowhead in e). Correct thresholding marks tufts and clusters, but not normal vessels (f). During this step the user can also manually mark areas that should be excluded from the quantification; i.e., hyperfluorescent retinal edges, remaining hyaloid vessels, or fluorescent debris (g). SWIFT_NV will then automatically quantify all pixels in the image that lie above the set threshold and are part of an object that has a minimum size of 100 pixels. By setting a cut-off in object size, small hyperfluorescent artifacts like vessel branchpoints get removed (h). After quantifying all four quadrants SWIFT_NV re-assembles the retinal image and displays the total number of NV pixels (i). Finally, an overlay of original image and NV image is created and automatically saved (j). The supplementary file in this article contains a movie showing SWIFT_NV quantification in realtime
To run SWIFT_NV, the user copies the five SWIFT_NV macros into the plugin folder of NIHs ImageJ software and assigns the appropriate shortcut for each macro. After this initial setup, the user opens a retinal whole mount image and starts SWIFT_NV. The first macro will retrieve information on the directory and name of the original image. SWIFT_NV then opens the appropriate VO image, isolates the red channel and divides the image into four quadrants (Fig. 2c). Dividing the image is important as the four quadrants often have different color intensities and need to be processed separately. In the same step SWIFT_NV also removes background fluorescence to allow for the NV structures to stand out clearly against the background fluorescence of normal vessels.
The background subtraction in this step allows for the user to define a fluorescence threshold that marks NV tufts and clusters, but not normal vessels. SWIFT_NVs image pre-processing renders this normally user-dependent step to become more user-independent. Setting a fluorescence threshold in SWIIFT_NV is comparatively intuitive even for users with no prior experience in retinal NV quantification, especially when comparing it with the traditional quantification methods where all individual NV tufts and clusters must be correctly identified and marked by the investigator. By using a slide bar to either increase or decrease a particular quadrant’s intensity threshold the SWIFT_NV user designates a threshold that marks NV structures, but not normal vessels (Fig. 2d–f). After setting the appropriate threshold, ImageJ’s freehand tool can be used to mark any artifacts that should be excluded from the quantification. These artifacts include remaining hyaloid vessels, cellular debris, or hyperfluorescent retinal edges that can occur during the process of retinal whole mount preparation (Fig. 2g).
Once all artifacts are removed and a correct threshold is chosen, SWIFT_NV analyzes all pixels in the image that lie above the set threshold and are part of an object that has a minimum size of 100 pixels. By setting this cut-off in object size, small artifacts like vessel branch points are removed. ImageJ’s “Despeckle”-algorithm is used additionally for the same purpose. Subsequently, holes within tufts or clusters are filled to obtain solid staining of the marked NV structures (Fig. 2h).
The above-mentioned steps are repeated for the remaining three quadrants. SWIFT_NV then creates a composite from all four NV images and displays the quantification result for the whole retina as “total area” (Fig. 2i). Additional to displaying the quantification result, SWIFT_NV re-opens the original retinal whole mount and creates an overlay of NV image and whole mount image (Fig. 2j). The merged image is automatically saved for documentation. The supplementary file in this article contains a movie showing SWIFT_NV quantification in realtime.
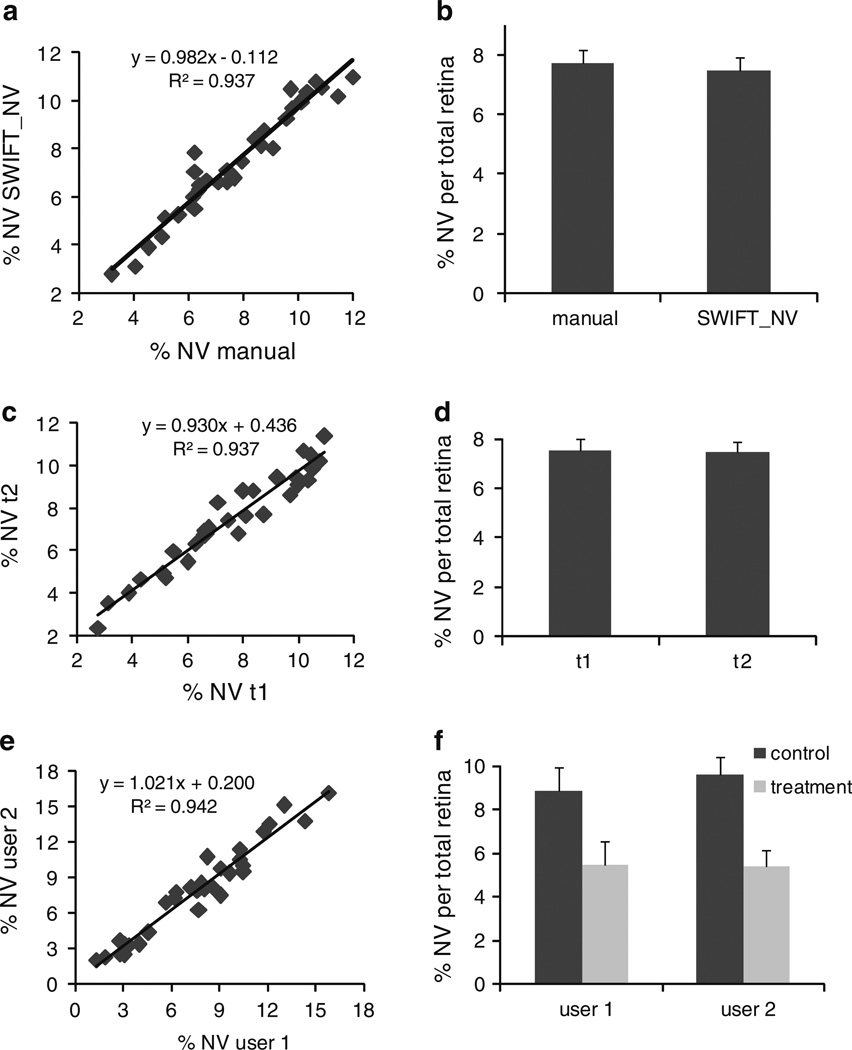
In order to analyze the accuracy of the SWIFT_NV method, we compared traditional hand measurements of an experienced researcher to quantifications performed with SWIFT_NV. Plotting SWIFT_NV quantifications against hand measurements revealed an R2-value of 0.9372 (Fig. 3a). Comparison of mean values and standard errors of SWIFT_NV versus hand quantification demonstrated almost exact congruence (7.76 ± 0.40 vs. 7.51 ± 0.41% NV per total retina; Fig. 3b). In order to assess the intra-individual reproducibility of SWIFT_NV quantifications, we had one user reassess his SWIFT_NV quantifications after a 3-month time interval. The repeated measurement correlated well with the initial data set (R2 = 0.9376; Fig. 3c) and both mean value and standard error were almost exactly reproduced (7.51 ± 0.41 vs. 7.46 ± 0.42% NV per total retina; Fig. 3d). In order to additionally assess the inter-individual reproducibility of SWIFT_NV measurements, two users analyzed the same images independently. The data set used for this inter-individual comparison contained retinal images from both a control and a treatment group. The two independent measurements correlated well (R2 = 0.9424; Fig. 3e) and both users obtained almost identical values for the control and treatment groups (Fig. 3f).
Fig. 3.
Comparison between manual NV quantification and the SWIFT_NV method shows close correlation between the two methods over a wide range of NV (a). Mean values and standard error do not differ between manual measurement and SWIFT_NV quantification (b). Repeated measurements of the same data set by the same user after a 3-month time interval show high intra-individual reproducibility (c, d). The second quantification (t2) correlates well with the original measurements (t1; c). Mean value and standard error are almost exactly reproduced (d). Comparison of two users independently quantifying the same images shows high inter-individual correlation (e). Both users obtained almost identical values for the control and treatment group contained in the data set (f). All measurements were done on n = 30 retinas
In summary, this study presents a computer-aided quantification method for retinal NV with reduced user-dependency and favorable time-effectiveness. The robust intra-and inter-individual reproducibility of SWIFT_NV measurements is achieved by combining computer-aided image processing with limited user-interference. Time requirements for SWIFT_NV lie between 3 and 5 min per retina. This compares favorably to the time required for accurate hand measurements which in our experience lies between 10 and 40 min per retina depending on the amount of NV.
Although the traditional hand measurements still represent the gold standard for NV quantification in the hands of an experienced investigator, the SWIFT_NV method has the potential to complement the existing protocols by offering a robust quantification method with reduced user-dependency and favorable time-effectiveness. We feel that the SWIFT_NV method will help to standardize the quantification of retinal NV between different investigators and different labs and therefore will add further robustness to one of the most widely used models of in vivo angiogenesis research.
Supplementary Material
Footnotes
Electronic supplementary material The online version of this article (doi:10.1007/s10456-009-9155-3) contains supplementary material, which is available to authorized users.
Contributor Information
A. Stahl, Department of Ophthalmology, Harvard Medical School, Children’s Hospital Boston, Fegan 4, 300 Longwood Avenue, Boston, MA 02115, USA University Eye Hospital Freiburg, Killianstr. 5, 79106 Freiburg, Germany.
K. M. Connor, Department of Ophthalmology, Harvard Medical School, Children’s Hospital Boston, Fegan 4, 300 Longwood Avenue, Boston, MA 02115, USA
P. Sapieha, Department of Ophthalmology, Harvard Medical School, Children’s Hospital Boston, Fegan 4, 300 Longwood Avenue, Boston, MA 02115, USA
K. L. Willett, Department of Ophthalmology, Harvard Medical School, Children’s Hospital Boston, Fegan 4, 300 Longwood Avenue, Boston, MA 02115, USA
N. M. Krah, Department of Ophthalmology, Harvard Medical School, Children’s Hospital Boston, Fegan 4, 300 Longwood Avenue, Boston, MA 02115, USA
R. J. Dennison, Department of Ophthalmology, Harvard Medical School, Children’s Hospital Boston, Fegan 4, 300 Longwood Avenue, Boston, MA 02115, USA
J. Chen, Department of Ophthalmology, Harvard Medical School, Children’s Hospital Boston, Fegan 4, 300 Longwood Avenue, Boston, MA 02115, USA
K. I. Guerin, Department of Ophthalmology, Harvard Medical School, Children’s Hospital Boston, Fegan 4, 300 Longwood Avenue, Boston, MA 02115, USA
L. E. H. Smith, Email: lois.smith@childrens.harvard.edu, Department of Ophthalmology, Harvard Medical School, Children’s Hospital Boston, Fegan 4, 300 Longwood Avenue, Boston, MA 02115, USA.
References
- 1.Kennedy D. Breakthrough of the Year. Science. 2006:1841. doi: 10.1126/science.1138510. [DOI] [PubMed] [Google Scholar]
- 2.Rosenfeld PJ, et al. Ranibizumab for neovascular age-related macular degeneration. N Engl J Med. 2006;355(14):1419–1431. doi: 10.1056/NEJMoa054481. [DOI] [PubMed] [Google Scholar]
- 3.Ranieri G, et al. Vascular endothelial growth factor (VEGF) as a target of bevacizumab in cancer: from the biology to the clinic. Curr Med Chem. 2006;13(16):1845–1857. doi: 10.2174/092986706777585059. [DOI] [PubMed] [Google Scholar]
- 4.Smith LE, et al. Oxygen-induced retinopathy in the mouse. Invest Ophthalmol Vis Sci. 1994;35(1):101–111. [PubMed] [Google Scholar]
- 5.Pierce EA, et al. Vascular endothelial growth factor/vascular permeability factor expression in a mouse model of retinal neovascularization. Proc Natl Acad Sci USA. 1995;92(3):905–909. doi: 10.1073/pnas.92.3.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Smith LE, et al. Regulation of vascular endothelial growth factor-dependent retinal neovascularization by insulin-like growth factor-1 receptor. Nat Med. 1999;5(12):1390–1395. doi: 10.1038/70963. [DOI] [PubMed] [Google Scholar]
- 7.Aiello LP, et al. Suppression of retinal neovascularization in vivo by inhibition of vascular endothelial growth factor (VEGF) using soluble VEGF-receptor chimeric proteins. Proc Natl Acad Sci USA. 1995;92(23):10457–10461. doi: 10.1073/pnas.92.23.10457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bainbridge JW, et al. Inhibition of retinal neovascularisation by gene transfer of soluble VEGF receptor sFlt-1. Gene Ther. 2002;9(5):320–326. doi: 10.1038/sj.gt.3301680. [DOI] [PubMed] [Google Scholar]
- 9.Ozaki H, et al. Blockade of vascular endothelial cell growth factor receptor signaling is sufficient to completely prevent retinal neovascularization. Am J Pathol. 2000;156(2):697–707. doi: 10.1016/S0002-9440(10)64773-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rota R, et al. Marked inhibition of retinal neovascularization in rats following soluble-flt-1 gene transfer. J Gene Med. 2004;6(9):992–1002. doi: 10.1002/jgm.586. [DOI] [PubMed] [Google Scholar]
- 11.Sone H, et al. Effects of intraocular or systemic administration of neutralizing antibody against vascular endothelial growth factor on the murine experimental model of retinopathy. Life Sci. 1999;65(24):2573–2580. doi: 10.1016/s0024-3205(99)00526-3. [DOI] [PubMed] [Google Scholar]
- 12.Google scholar search 6/15/09 for “oxygen induced retinopathy mice” [Google Scholar]
- 13.Vanhaesebrouck S, et al. Oxygen-induced retinopathy in mice: amplification by neonatal IGF-I deficit and attenuation by IGF-I administration. Pediatr Res. 2009;65(3):307–310. doi: 10.1203/PDR.0b013e3181973dc8. [DOI] [PubMed] [Google Scholar]
- 14.Higgins RD, et al. Diltiazem reduces retinal neovascularization in a mouse model of oxygen induced retinopathy. Curr Eye Res. 1999;18(1):20–27. doi: 10.1076/ceyr.18.1.20.5390. [DOI] [PubMed] [Google Scholar]
- 15.Spierer A, et al. An increase in superoxide dismutase ameliorates oxygen-induced retinopathy in transgenic mice. Eye. 2005;19(1):86–91. doi: 10.1038/sj.eye.6701424. [DOI] [PubMed] [Google Scholar]
- 16.Banin E, et al. T2-TrpRS inhibits preretinal neovascularization and enhances physiological vascular regrowth in OIR as assessed by a new method of quantification. Invest Ophthalmol Vis Sci. 2006;47(5):2125–2134. doi: 10.1167/iovs.05-1096. [DOI] [PubMed] [Google Scholar]
- 17.Chen J, et al. Erythropoietin deficiency decreases vascular stability in mice. J Clin Invest. 2008;118(2):526–533. doi: 10.1172/JCI33813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Connor KM, et al. Increased dietary intake of omega-3-polyunsaturated fatty acids reduces pathological retinal angiogenesis. Nat Med. 2007;13(7):868–873. doi: 10.1038/nm1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guma M, et al. Genetic and pharmacological inhibition of JNK ameliorates hypoxia-induced retinopathy through interference with VEGF expression. Proc Natl Acad Sci USA. 2009;106(21):8760–8765. doi: 10.1073/pnas.0902659106. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.