Abstract
Picornaviruses (PV) include human rhinovirus (HRV), the primary cause of the common cold, and the enteroviruses (EV), which cause serious diseases such as poliomyelitis, meningoencephalitis, and systemic neonatal disease. Although no compounds for PV infections have been approved in the United States, pirodavir was one of the most promising capsid-binding compounds to show efficacy in human clinical trials for chemoprophylaxis of the common cold. Susceptibility to hydrolysis precluded its use as an oral agent. We have developed orally bioavailable pyridazinyl oxime ethers that are as potent as pirodavir. Compounds BTA39 and BTA188 inhibited a total of 56 HRV laboratory strains and three clinical isolates as determined by neutral red uptake assay. At concentrations of <100 nM, BTA39 inhibited 69% of the HRV serotypes and isolates evaluated, BTA188 inhibited 75%, and pirodavir inhibited 59% of the serotypes and isolates. The 50% inhibitory concentrations (IC50s) for the two compounds ranged from 0.5 nM to 6,701 nM. The compounds also inhibited EV, including coxsackie A and B viruses (IC50 = 773 to 3,608 nM) and echoviruses (IC50 = 193 to 5,155 nM). BTA39 only inhibited poliovirus strain WM-1 at 204 nM, and BTA188 only inhibited poliovirus strain Chat at 82 nM. EV 71 was inhibited by BTA39 and BTA188, with IC50s of 1 and 82 nM, respectively. Both compounds were relatively nontoxic in actively growing cells (50% cytotoxic doses, ≥4,588 nM). These data suggest that these oxime ethers warrant further investigation as potential agents for treating selected PV infections.
The virus family Picornaviridae is a large group of plus-strand RNA viruses with different host ranges and disease symptoms but with similar genome organization and replication strategies. These viruses are small icosahedral particles composed of four capsid proteins coating the single-strand RNA. The viral groups in this family causing significant human disease are the Enterovirus, Rhinovirus, Hepatovirus, and Parechovirus genera. The diseases caused by the viruses in these four genera include a range of febrile colds and sore throats, acute hepatitis, poliomyelitis, neurological disease, ocular disease, cardiac and muscular disease, and severe neonatal disease (5). The coxsackieviruses and the echoviruses of the Enterovirus genus have also been implicated as the etiological agents of hemolytic-uremic syndrome (5). For decades, coxsackie B viruses have also been speculatively linked to juvenile-onset insulin-dependent diabetes mellitus and to chronic (postviral) fatigue syndrome because of their predilection for muscle tissue (5).
Because this virus family causes a variety of serious, acute infections and there are few effective vaccines against many of these viruses, there has been constant effort to find efficacious antiviral chemotherapeutic agents for their control. Most of the agents discovered thus far have either targeted key viral proteases (7) or viral RNA replication (10, 19) or have interacted with the viral capsid, some by preventing uncoating (2, 12, 17). AG7088 {ethyl-3-[(5′-methylisoxazole-3′-carbonyl)-ι-ValΨ-[COCH2]-l-Phe(4-F)-l-[(S)-Pyrrol-Ala]]-E-propeno-ate}, a protease inhibitor, was designed to irreversibly inhibit rhinovirus 3C protease, a key enzyme for viral polypeptide production (13, 16). Enviroxime {[2-amino-1-(isopropylsulfonyl)-6-benzimidazole phenyl ketone oxime]} is a rather broad-spectrum picornavirus inhibitor that is thought to inhibit RNA replication by targeting the 3A protein-coding region of the viral RNA (10). Among the substances interacting with capsids, zinc is thought to bind to the surface canyon sites of rhinovirus to prevent attachment (15), although there is disputed evidence whether zinc-containing lozenges actually are therapeutic (11). Ple-conaril {[3-[3,5-dimethyl-4-[(3-methyl-5-isoxazolyl)propyl]phenyl]-5-(trifluoromethyl)-1,2,4-oxadiazole]}, pirodavir {ethyl 4-[2-[1-(6-methyl-3-pyridazinyl)-4-piperidinyl]ethoxy]benzoate},and the Sterling-Winthrop set of compounds (e.g., disoxaril) inhibit viral attachment and/or virus uncoating (6). Some of these compounds are not very orally bioavailable (18), although pleconaril does have that capability. AG7088 and pleconaril have been or are in clinical trials in the United States (18), but neither has been approved for clinical use.
Most of the capsid-binding inhibitors reported on to date are represented by the compounds shown in Fig. 1 (8, 14). One of those agents, pirodavir, is a rather potent, broad-spectrum picornavirus inhibitor. It inhibited 80 of the 100 human rhinovirus (HRV) strains tested at a concentration of 64 ng/ml (2). In that same study, pirodavir was also effective in inhibiting 16 enteroviruses, with a mean 80% inhibitory concentration (IC80) of 1,300 ng/ml. In clinical trials, however, pirodavir was not therapeutically efficacious when given intranasally, although it was effective prophylactically (9). It appears that the lack of clinical therapeutic effect was due to the poor pharmacokinetics of pirodavir, probably because it undergoes facile hydrolysis of the ester functionality to an inactive acidic form in vivo (3).
FIG. 1.
Chemical structures of capsid-binding picornavirus inhibitors, including the ether oxime derivatives of pirodavir, BTA39 and BTA188.
Given the promise shown by pirodavir, a synthesis approach was developed leading to the discovery of novel pyridazinyl oxime ether compounds that are related to the structure of pirodavir and are orally bioavailable (20). We considered that a particularly important area for exploration was the preparation of analogues of pirodavir with stable bioisosteres of the ethyl ester group (Fig. 1). In previous work, we found that a chloropyridazine analogue (ethyl 4-{2-[1-(6-chloro-3-pyridazinyl)-4-piperidinyl]ethoxy}benzoate) of pirodavir had anti-HRV activity that was essentially equivalent to that of pirodavir; therefore, for ease of synthesis, we used chloropyridazineintermediates 1-(6-chloro-3-pyridazinyl)-4-piperdineethanoland 3-chloro-6-[4-(2-chloroethyl)-1-piperidinyl]pyridazine for reactions with a variety of phenols (20). We found, for example, that the aldehyde, ketone, and hydrazide analogues of the chloropyridazine analogue of pirodavir were all much less active than pirodavir. In addition, benzaldehyde 4-[2-[1-(6-chloro-3-pyridazinyl)-4-piperidinyl]ethoxy]-O-ethyloxime(BTA39), which was derived from 1-(6-chloro-3-pyridazinyl)-4-piperdineethanol, and benzaldehyde 4-[2-[1-(6-methyl-3-pyridazinyl)-4-piperidinyl]ethoxy]-O-ethyloxime (BTA188) were highly active against selected HRV strains, and both appeared to be orally bioavailable in animal models (18). We now present an extensive report on the antipicornavirus activity of these two pyridazinyl oxime ethers, comparing this activity to that of pirodavir, which was also evaluated in parallel experiments.
MATERIALS AND METHODS
Compounds.
The syntheses of the oxime ether pyridazinamine (BTA39), benzaldehyde 4-[2-[1-(6-chloro-3-pyridazinyl)-4-piperidinyl]ethoxy]-O-ethyloxime (Chemical Abstracts registry number 314062-79-8), and the ethyl oxime ether pyridazinamine (BTA188), benzaldehyde 4-[2-[1-(6-methyl-3-pyridazinyl)-4-piperidinyl]ethoxy]-O-ethyloxime (Chemical Abstracts registry number 314062-80-1), have been described by Watson et al. (20). Details of those syntheses can be downloaded at the website http://pubs.acs.org/ by selecting for the author's abstract (21). Pirodavir {ethyl 4-[2-[1-(6-methyl-3-pyridazinyl)-4-piperidinyl]ethoxy]benzoate}, BTA39, and BTA188 were provided by Biota Holdings Ltd. (Melbourne, Victoria, Australia).
Virus and cell lines.
All viruses were obtained from the American Type Culture Collection (Manassas, Va.), except for the HRV isolates (Biostar Cold Virus study), which were kindly provided by R. Turner (MUSC Children's Hospital, Charleston, S.C.). For HRV assays, 10 mM MgCl2 was added to the medium.
KB cells propagated from human epidermoid oral carcinoma cells, African green monkey kidney (CV-1 and Vero 76) cells, and human lung carcinoma (A549) cells were obtained from the American Type Culture Collection. African green monkey embryonic kidney (MA-104) cells were obtained from BioWhittaker (Walkersville, Md.). Human cervical epithelioid carcinoma (HeLa Ohio-I) cells were obtained from F. Hayden (University of Virginia). The cells were grown in minimal essential medium (Gibco-BRL, Gaithersburg, Md.) supplemented with 0.1% NaHCO3 and 5 to 9% fetal bovine serum (HyClone Laboratories, Logan, Utah), except for A549 cells, which were propagated in Dulbecco's minimal essential medium (Gibco-BRL) with high glucose and 10% fetal bovine serum. When performing antiviral assays, serum was reduced to 2% and 50 μg gentamicin (Sigma Chemical Company, St. Louis, Mo.) per ml was added to the medium.
CPE inhibition assay.
The protocol of Barnard et al. (4) was used. Test compounds were evaluated by using seven 1/2-log10 dilutions. Virus was used at a multiplicity of infection (MOI) of 0.001 to 0.01. Compound was added at the appropriate concentration to near-confluent cell monolayers in 96-well tissue culture plates, immediately followed by the addition of an equal volume of virus. Each concentration of drug was assayed in quadruplicate. The MOIs used were virus dependent and chosen for each strain such that 100% of the cells in the virus controls showed cytopathic effects (CPE) within 5 to 7 days. An appropriate positive control drug (pirodavir) for each virus was included for each set of compounds tested. The CPE results were then quantified spectrophotometrically by neutral red (NR) uptake assay (see below).
NR assay of CPE inhibition and compound cytotoxicity.
The NR assay was done on the same test plates described above to quantitate the antiviral activity and the cytotoxicity of the compounds. The NR assay was performed as described by Barnard et al. (4). Absorbances at 540 and 450 nm were read with a microplate reader (Bio-Tek EL 1309; Bio-Tek Instruments, Inc., Winooski, Vt.). Absorbance values were expressed as percentages of those for untreated controls. The IC50s were calculated by regression analysis of the means of the absorbances expressed as percentages of untreated, uninfected control values for each concentration. The 50% cytotoxic doses (CC50) were calculated by regression analysis, and a selectivity index (SI) was calculated using the formula SI = CC50/IC50.
Confirmatory virus yield reduction assay.
Infectious virus yields from each well used in a second CPE inhibition assay were determined as previously described by Barnard et al. (4). After the CPE was scored as described above, each plate was frozen at −80°C and thawed. Sample wells at each compound concentration tested were pooled, and titers of infectious virus were determined by CPE assay. The wells were scored for CPE and virus titers calculated as described by Barnard et al. (4). A 1 log10 reduction in virus yield (IC90) was then calculated by regression analysis when possible.
Virucidal assay.
A virucidal test was done as previously described (4) to exclude the possibility that the compounds inhibited the virus by physically inactivating or disrupting the virion. Equal volumes of undiluted virus stock and a single concentration of compound were incubated at 37°C for 1 h. Surviving virus was quantified by CPE assay, and titers were calculated as described previously (4). The concentrations tested bracketed the concentration determined to represent the IC50. Each concentration of test compound was assayed in quadruplicate.
Cell yield assay.
Cytotoxicity in rapidly dividing cells was evaluated by determining the total number of cells as reflected by a NR uptake assay after a 3-day exposure to several concentrations of compound (4). To quantitate cell growth after 72 h in the presence or absence of drug, the plates were treated as described above for the NR assay. Absorbance values were expressed as percentages of those for the untreated controls, and CC50s were calculated by regression analysis.
MOI assay.
Rhinovirus 2 (strain HGP) (HRV-2) was added to cells at different concentrations to determine the efficacy of viral inhibition of the compounds at different viral MOIs (4). The drugs were added to infected cells at various concentrations in the presence of virus at a selected MOI and were evaluated for inhibition of viral replication by CPE inhibition assay and verified by NR assay. IC50s and CC50s were determined as described above.
Timing assay.
The virus yield assay used in this part of the study was performed as described above, although the time that the compound was added to the plates varied relative to the time of virus exposure (4). Virus was added to the cells at an MOI of 2 to ensure that most every cell was exposed to virus. At the appropriate time after virus exposure (0, 1, 2, 4, 6, and 8 h postexposure), 10 μM test compound in a small volume was added to the cells without removal of the virus. When a pretreatment was done (−1 h), compound was added to the wells and incubated for 1 h at 37°C, after which virus was added to each well in a small volume. At “0” time, the virus was added within 5 min of drug addition. In addition, drug was added at 24 h postinfection to determine the extent of drug carryover that might be present. At 25 h after virus exposure, all plates were frozen, and virus was quantified by virus yield reduction assay. Reduction in virus yield was calculated by subtracting the log10 viral titer at each time period from the log10 viral titer of the untreated control for each given time period. The experiment was done twice.
RESULTS
Rhinovirus inhibition.
Initially, the pyridazinyl oxime ethers were evaluated against HRV-2 (Table 1). By NR uptake assay, the IC50 for BTA39 was equal to 1.0 nM. The compound was still very inhibitory when IC90s were calculated from the same assays (IC90 = 1.5 nM). Since the CC50 was 12,887 nM in non-actively growing cells and 7,465 nM in actively growing cells, the compound was extremely virus specific. The SI using the CC50 value derived from actively growing cells was 7,465. The cytotoxicity profile of BTA39 was similar to that of pirodavir. To determine if this potent HRV inhibitory activity also reduced virus released during the infection, the compounds were also evaluated against HRV-2 in a virus yield reduction assay (Table 1). The concentration required to drop the viral titer at least one log10 was 0.4 nM for BTA39. Pirodavir was very potent in a virus yield reduction assay (IC90 = 2.3 nM). Compared to BTA39, the pyridazinyl oxime ether, BTA188, was similarly inhibitory to HRV-2 replication with an IC50 of 0.8 nM and an IC90 of 11 nM, but it was slightly less effective by virus yield reduction assay (IC90 = 2.1 nM). BTA188 was more toxic than BTA39, with IC50s of 5,420 nM in resting cells and 4,706 nM in actively growing cells. None of the three compounds physically disrupted the virus or apparently prevented binding of the virus to the cell at the concentrations tested (BTA39, ≤25,000 nM; BTA188, ≤27,000 nM; pirodavir, ≤27,000 nM), as determined by a virucidal assay.
TABLE 1.
Effects of pyridazinyl oxime capsid-binding agents on HRV-2 (strain HGP) replication in human oral epidermoid carcinoma (KB) cells
| Compound | NR uptake assaya
|
Resting cell SI (CC50/IC50) | Virus yield reduction assay IC90 (nM)b | Actively growing cell CC50 (nM)b | Actively growing cell SI (CC50/IC50) | ||
|---|---|---|---|---|---|---|---|
| IC50 (nM) | IC90 (nM) | CC50 (nM) | |||||
| BTA39 | 1.0 ± 0.9 | 1.5 ± 1 | 12,887 ± 3,534 | 12,387 | 0.4 ± 0.2 | 7,465 ± 13 | 7,465 |
| BTA188 | 0.8 ± 0.4 | 11 ± 2 | 5,420 ± 3,556 | 6,775 | 2.1 ± 0.1 | 4,706 ± 167 | 5,882 |
| Pirodavir | 0.8 ± 0.3 | 6 ± 0.8 | >13,350c | >16,688 | 2.3 ± 0.4 | >13,350 | >16,688 |
Assay was done in cells not actively growing. Values represent the means of six experiments ± the standard deviations for each value.
Values represent the means of two experiments ± the standard deviations for each value.
Concentrations greater than indicated were not evaluated.
Having established that both pyridazinyl oxime ether compounds were highly active against HRV-2, they were then evaluated against 57 other HRV laboratory strains representing the two major receptor-binding groups: those that bind to ICAM-1 and those that bind to low-density lipoprotein receptor (5). The compounds were also evaluated against serotype 87, the only serotype that binds a sialoprotein (5). For the other 57 laboratory serotypes tested, the IC50s for BTA39 ranged from 2 nM (HRV-11, -24, -59, -89, and -92) to >7,474 nM (HRV-64 and -70); for BTA188, the IC50s ranged from 0.5 nM (HRV-39 and -40) to >4,588 nM (RV-25 and -64); and for pirodavir, the IC50s ranged from 1 nM (HRV-39 and -40) to 8,130 nM (HRV-32) (Table 2). Serotypes 8, 32, 64, 70, and 87 were relatively resistant to inhibition by all three compounds.
TABLE 2.
Inhibitory effects of pyridazinyl oxime ether capsid-binding agents on a spectrum of rhinoviruses in KB cellsa
| Group and virus | Strain | BTA39
|
BTA188
|
Pirodavir
|
|||
|---|---|---|---|---|---|---|---|
| IC50 (nM) | IC90 (nM) | IC50 (nM) | IC90 (nM) | IC50 (nM) | IC90 (nM) | ||
| Sialoprotein receptor group | |||||||
| 87 | FO2-3607 | 2,320 ± 231 | 4,897 ± 2,827 | 3,342 ± 81 | >4,588b | 2,439 ± 542 | >13,550 |
| Low-density lipoprotein receptor group | |||||||
| 1A | 2020 | 4 ± 25 | 44 ± 51 | 11 ± 4 | 54 ± 10 | 54 ± 5 | 81 ± 33 |
| 1B | B632 | 9 ± 7 | 67 ± 14 | 16 ± 40 | 54 ± 40 | 3 ± 7 | 8 ± 7 |
| 29 | 5582 | 8 ± 2 | 13 ± 7 | 5 ± 5 | 8 ± 2 | 8 ± 2 | 11 ± 4 |
| 30 | 106F | 52 ± 8 | 232 ± 10 | 272 ± 18 | 1,087 ± 65 | 271 ± 46 | 2,168 ± 1,699 |
| 47 | Baylor 3 | 173 ± 38 | 387 ± 34 | 117 ± 46 | 299 ± 165 | 298 ± 98 | 678 ± 179 |
| 49 | 8213 | 3 ± 1 | 57 ± 2 | 3 ± 1 | 38 ± 16 | 33 ± 5 | 434 ± 29 |
| 62 | 1963M-CV40 | 696 ± 188 | 3,840 ± 704 | 92 ± 20 | 326 ± 20 | 2,846 ± 103 | >13,550 |
| ICAM-1 receptor group | |||||||
| 3 | FEB | 19 ± 50 | 70 ± 25 | 26 ± 80 | 299 ± 26 | 49 ± 189 | 217 ± 54 |
| 4 | 16/60 | 23 ± 8 | 82 ± 149 | 22 ± 10 | 435 ± 450 | 108 ± 61 | 2,710 ± 556 |
| 5 | Norman | 8 ± 2 | 77 ± 20 | 5 ± 7 | 27 ± 12 | 16 ± 3 | 108 ± 41 |
| 6 | Thompson | 3 ± 4 | 10 ± 48 | 7 ± 2 | 19 ± 8 | 5 ± 3 | 10 ± 12 |
| 8 | MRH-CV12 | 773 ± 853 | >7,474 | 543 ± 761 | >4,588 | 5,420 ± 1,563 | >13,550 |
| 9 | 211-CV13 | 15 ± 4 | 119 ± 35 | 8 ± 7 | 245 ± 130 | 22 ± 15 | 27 ± 63 |
| 10 | 204-CV14 | 773 ± 1,102 | >7,474 | 82 ± 20 | 54 ± 68 | 596 ± 147 | >13,550 |
| 11 | 1-CV15 | 2 ± 3 | 25 ± 5 | 3 ± 3 | 8 ± 8 | 271 ± 27 | 271 ± 190 |
| 12 | 181-CV16 | 14 ± 3 | 258 ± 50 | 27 ± 20 | 299 ± 190 | 54 ± 20 | 201 ± 25 |
| 13 | 353 | 26 ± 11 | 258 ± 110 | 109 ± 57 | 543 ± 171 | 108 ± 30 | 542 ± 85 |
| 14 | 1059 | 52 ± 12 | 108 ± 25 | 68 ± 33 | 163 ± 81 | 27 ± 43 | 163 ± 64 |
| 16 | 11757 | 8 ± 3 | 258 ± 68 | 8 ± 2 | 215 ± 37 | 16 ± 34 | 214 ± 49 |
| 17 | 33342 | 19 ± 2 | 204 ± 50 | 8 ± 8 | 141 ± 270 | 27 ± 14 | 144 ± 270 |
| 18 | 5986 | 155 ± 28 | 799 ± 180 | 652 ± 132 | 3,152 ± 220 | 298 ± 279 | 1,003 ± 534 |
| 21 | 47-CV21 | 258 ± 50 | >7,474 | 5 ± 3 | 8 ± 6 | 271 ± 40 | >13,550 |
| 24 | 5146 | 2 ± 2 | 16 ± 2 | 16 ± 4 | 33 ± 12 | 6 ± 5 | 30 ± 36 |
| 25 | 5426-CV26 | 6,701 ± 157 | >7,474 | >4,588 | >4,588 | 108 ± 27 | 2,710 ± 270 |
| 32 | 363 | 1,031 ± 556 | 2,577 ± 154 | 1,359 ± 339 | >4,588 | 8,130 ± 883 | 10,840 ± 900 |
| 33 | 1200 | 3 ± 5 | 23 ± 31 | 1.0 ± 0.5 | 11 ± 5 | 54 ± 98 | 81 ± 285 |
| 38 | CH79 | 23 ± 2 | 26 ± 3 | 24 ± 4 | 82 ± 26 | 27 ± 39 | 190 ± 114 |
| 39 | 209 | 3 ± 6 | 8 ± 10 | 0.5 ± 0.5 | 4 ± 1.5 | 1.0 ± 0.6 | 1 ± 1 |
| 40 | 1794 | 3 ± 2 | 93 ± 10 | 0.5 ± 0.1 | 46 ± 2 | 1.0 ± 0.5 | 11 ± 3 |
| 42 | 56822 | 103 ± 50 | 258 ± 77 | 136 ± 50 | 598 ± 50 | 41 ± 5 | 60 ± 10 |
| 43 | 58750 | 77 ± 21 | 515 ± 250 | 41 ± 8 | 217 ± 25 | 89 ± 21 | 2,710 ± 500 |
| 45 | Baylor 1 | 258 ± 360 | 2,577 ± 692 | 174 ± 134 | 1,902 ± 4,114 | 271 ± 531 | 1,626 ± 1,081 |
| 48 | 1505 | 103 ± 15 | 2,577 ± 250 | 158 ± 103 | 272 ± 180 | 136 ± 103 | 813 ± 180 |
| 50 | A2#58 | 23 ± 8 | 155 ± 52 | 1 ± 2 | 9 ± 6 | 27 ± 6 | 271 ± 69 |
| 52 | FO 1-3772 | 52 ± 6 | 59 ± 10 | 11 ± 3 | 54 ± 5 | 54 ± 11 | 190 ± 17 |
| 54 | FO 1-3774 | 3.0 ± 0.7 | 23 ± 18 | 190 ± 5 | 272 ± 2 | 108 ± 82 | 2,168 ± 130 |
| 56 | Ch82 | 4 ± 6 | 36 ± 10 | 2.0 ± 0.4 | 7 ± 8 | 22 ± 3 | 108 ± 9 |
| 57 | Ch47 | 103 ± 13 | 258 ± 25 | 109 ± 18 | 272 ± 27 | 79 ± 16 | 195 ± 24 |
| 59 | 611-CV35 | 2 ± 2 | 59 ± 21 | 8 ± 4 | 68 ± 45 | 16 ± 1 | 54 ± 31 |
| 60 | 2268-CV37 | 258 ± 82 | 1,546 ± 643 | 82 ± 18 | 1,087 ± 463 | 38 ± 12 | 190 ± 18 |
| 61 | 6669-CV39 | 121 ± 40 | 387 ± 25 | 73 ± 7 | 353 ± 25 | 2,846 ± 1,463 | >13,550 |
| 63 | 6360-CV41 | 13 ± 21 | 111 ± 192 | 2 ± 4 | 7 ± 8 | 27 ± 8 | 222 ± 87 |
| 64 | 6258-CV44 | >7,474 | >7,474 | >4,588 | >4,588 | 813 ± 539 | 1,084 ± 780 |
| 66 | 1983-CV48 | 20 ± 8 | 80 ± 7 | 1 ± 7 | 20 ± 32 | 2 ± 3 | 21 ± 5 |
| 67 | 1857-CV51 | 13 ± 3 | 41 ± 5 | 16 ± 4 | 33 ± 17 | 54 ± 24 | 271 ± 25 |
| 68 | FO2-2317 | 59 ± 77 | 214 ± 250 | 38 ± 22 | 114 ± 62 | 19 ± 35 | 114 ± 512 |
| 69 | FO2-2513 | 52 ± 30 | 155 ± 53 | 54 ± 50 | 272 ± 24 | 136 ± 42 | 813 ± 61 |
| 72 | K2207 | 77 ± 10 | 129 ± 21 | 109 ± 50 | 272 ± 50 | 136 ± 15 | 271 ± 25 |
| 70 | FO2-2547 | >7,474 | >7,474 | 2,174 ± 2,528 | >4,588 | 1,897 ± 2,081 | 2,737 ± 2,710 |
| 73 | 107E | 3 ± 2 | 90 ± 80 | 27 ± 3 | 84 ± 9 | 18 ± 2 | 41 ± 13 |
| 85 | 50-525-CV54 | 10 ± 5 | 26 ± 7 | 8 ± 13 | 33 ± 54 | 54 ± 21 | 54 ± 77 |
| 86 | 121564 | 21 ± 3 | 77 ± 19 | 41 ± 3 | 84 ± 7 | 81 ± 30 | 114 ± 151 |
| 89 | 41467 | 2 ± 4 | 8 ± 22 | 2 ± 9 | 14 ± 36 | 22 ± 26 | 35 ± 46 |
| 91 | JM1 | 258 ± 15 | 1,031 ± 16 | 60 ± 15 | 815 ± 21 | 271 ± 269 | 1,084 ± 520 |
| 92 | SF-1662 | 2.0 ± 0.2 | 121 ± 20 | 1 ± 2 | 8 ± 3 | 3 ± 1 | 43 ± 4 |
| 100 | K6579 | 26 ± 5 | 258 ± 50 | 14 ± 2 | 125 ± 54 | 27 ± 24 | 81 ± 44 |
| Biostar Cold Virus study isolatesc | |||||||
| 1 | Clinical isolate | 82 ± 33 | 272 ± 82 | 76 ± 14 | 271 ± 130 | 271 ± 62 | 1,626 ± 1,162 |
| 36 | Clinical isolate | 27 ± 5 | 82 ± 10 | 27 ± 11 | 84 ± 21 | 108 ± 12 | 190 ± 22 |
| 43 | Clinical isolate | 299 ± 141 | 763 ± 333 | 68 ± 58 | 136 ± 110 | 81 ± 33 | 260 ± 50 |
Values represent the means ± the standard deviations of one assay done in quadruplicate. Values for each compound determined by NR assay.
Concentrations greater than indicated were not evaluated or could not be calculated due to cytotoxicity.
Provided by R. Turner (MUSC Children's Hospital).
BTA39 and BTA188 also inhibited three rhinovirus clinical isolates (Table 2). All the clinical isolates were inhibited at concentrations of ≤299 nM by BTA39 and BTA188. Both BTA39 (mean IC50 for three isolates, 136 nM) and BTA188 (mean IC50 for three isolates, 57 nM) inhibited the isolates at lower concentrations than did pirodavir (mean IC50 for three isolates, 153 nM).
Enterovirus inhibition.
BTA39 significantly inhibited enterovirus 71 replication with an IC50 value of 1 nM and an IC90 value of 2 nM. BTA188 inhibited the virus with an IC50 and IC90 of 82 and 109 nM, respectively (Table 3). Pirodavir was a much less effective inhibitor of the virus, with an IC50 of 5,420 nM and an IC90 of >13,350 nM.
TABLE 3.
Effects of pyridazinyl oxime ether capsid-binding agents on enterovirus replication in human and monkey cell culturesa
| Virus | Strain | Cell line | BTA39
|
BTA188
|
Pirodavir
|
|||
|---|---|---|---|---|---|---|---|---|
| IC50 (nM) | IC90 (nM) | IC50 (nM) | IC90 (nM) | IC50 (nM) | IC90 (nM) | |||
| Coxsackievirus | ||||||||
| A7 | 275/58 | CV-1 | >7,474b | >7,474 | 2,717 ± 2,384 | >4,588 | 10,840 ± 5,992 | >13,350 |
| A21 | Kuykendall | A549 | 825 ± 303 | 1,546 ± 650 | 1,359 ± 1,157 | >4,588 | 8,130 ± 2,282 | >13,350 |
| B2 | Ohio-1 | Vero 76 | >7,474 | >7,474 | >4,588 | >4,588 | 2,710 ± 771 | 8,130 ± 1,440 |
| B3 | Nancy | A549 | >7,474 | >7,474 | >4,588 | >4,588 | >13,350 | >13,350 |
| B4 | J.V.B. | KB | 773 ± 20 | >7,474 | 1,603 ± 195 | >4,588 | 3,794 ± 110 | >13,550 |
| B5 | Faulkner | Vero 76 | 3,608 ± 129 | >7,474 | 2,717 ± 77 | >4,588 | 10,840 ± 103 | >13,350 |
| B6 | Schmitt | LLC-MK2 | 2,062 ± 213 | >7,474 | 2,174 ± 117 | >4,588 | 8,130 ± 598 | >13,350 |
| Echovirus | ||||||||
| 2 | Cornelius | A549 | 5,155 ± 620 | >7,474 | 543 ± 68 | 3,804 ± 518 | 5,420 ± 1,850 | >13,350 |
| 3 | Morrisey | A549 | 1,031 ± 67 | 1,804 ± 121 | 815 ± 157 | 2,446 ± 179 | 1,897 ± 135 | 5,420 ± 266 |
| 4 | Pesascek | MA104 | 464 ± 203 | 2,320 ± 121 | 193 ± 114 | 1,087 ± 398 | 542 ± 336 | 5,420 ± 650 |
| 9 | Hill | A549 | 67 ± 26 | 216 ± 50 | 50 ± 50 | 250 ± 77 | 163 ± 81 | 623 ± 813 |
| 11 | Gregory | KB | 5,155 ± 248 | >7,474 | 543 ± 12 | 3,804 ± 136 | 5,420 ± 205 | >13,350 |
| 22 | Harris | A549 | 1,082 ± 1,361 | 3,093 ± 2,098 | 543 ± 449 | >4,588 | 8,672 ± 11,173 | 12,195 ± 5,892 |
| 30 | Bastianni | A549 | 1,031 ± 377 | 1,804 ± 500 | 815 ± 252 | 2,446 ± 217 | 1,897 ± 86 | 5,420 ± 407 |
| 31 | Caldwell | MA104 | >7,474 | >7,474 | >4,588 | >4,588 | >13,350 | >13,350 |
| Enterovirus | ||||||||
| 71 | BrCr | BS-C-1 | 1.0 ± 0.2 | 2.0 ± 0.2 | 82 ± 139 | 109 ± 103 | 5,420 ± 491 | >13,350 |
| Poliovirus | ||||||||
| 1 | Chat | Vero 76 | >7,474 | >7,474 | 82 ± 25 | 109 ± 50 | >13,350 | >13,350 |
| 2 | Fox | Vero 76 | >7,474 | >7,474 | >4,588 | >4,588 | 10,840 ± 250 | >13,350 |
| 3 | WM-1 | Vero 76 | 204 ± 250 | 2,191 ± 500 | >4,588 | >4,588 | 2,331 ± 129 | 5,420 ± 179 |
Means with the standard deviations represent one assay done in quadruplicate.
Concentrations greater than indicated were not evaluated or could not be calculated due to cytotoxicity.
Most coxsackieviruses, echoviruses, and polioviruses were only weakly inhibited by the pyridazinyl oxime ethers (Table 3). Nevertheless, the two compounds still inhibited some of these viruses at higher nanomolar levels of compound. When evaluating BTA39 for inhibition of coxsackieviruses, IC50s ranged from 773 to >7,474 nM, for BTA188 they were from 1,359 to >4,588 nM, and for pirodavir they ranged from 2,710 to >13,350 nM. IC50s for echoviruses were from 67 to >7,474 nM for BTA39, for BTA188 they were from 50 to >4,588 nM, and for pirodavir the range was from 163 to >13,350 nM. Poliovirus type 1 (strain Chat) and poliovirus 2 (strain Fox) were not inhibited by BTA39, although BTA188 inhibited strain Chat at 82 nM. However, poliovirus type 3 (strain WM-1) was sensitive to inhibition by BTA39 (IC50 = 204 nM) but not to inhibition by BTA188 (IC50, >4,588 nM). Pirodavir only weakly inhibited the three poliovirus strains evaluated, the IC50s ranging from 2,331 to >13,350 nM.
Effect of viral MOI on the activity of pyridazinyl oxime ethers.
The effects of increasing viral load on compound efficacy were also evaluated using BTA188 and pirodavir. For this purpose, various concentrations of virus were added to a constant amount of cells, and then each compound was evaluated for anti-HRV activity in an NR assay. The compounds inhibited HRV-2 replication at MOIs up to 2 (Table 4).
TABLE 4.
Effects of MOI on the sensitivity of HRV-2 (strain HGP) replication in KB cells to inhibition by BTA39, BTA188, and pirodavir
| Compound | IC50 (nM) at MOI ofa:
|
|||||||
|---|---|---|---|---|---|---|---|---|
| 15 | 7.5 | 2.0 | 1.0 | 0.1 | 0.01 | 0.001b | 0.0001 | |
| BTA39 | >7,474 | 2,320 ± 335 | 67 ± 44 | 14 ± 9 | 9.0 ± 5.4 | 2.0 ± 1.0 | 0.6 ± 0.2 | <0.6 |
| BTA188 | >4,588 | >4,588 | 41 ± 19 | 27 ± 21 | 21 ± 8 | 5.1 ± 0.3 | 2.0 ± 0.9 | 0.6 ± 0.2 |
| Pirodavir | >13,350 | >13,350 | 201 ± 22 | 3.8 ± 1.6 | 7.2 ± 1.4 | 1.0 ± 0.5 | 2.3 ± 0.2 | 3.5 ± 2.4 |
IC50s represent the averages of two separate experiments ± the standard deviations.
MOI used in normal antiviral test.
Efficacy of BTA188 throughout the HRV replication cycle.
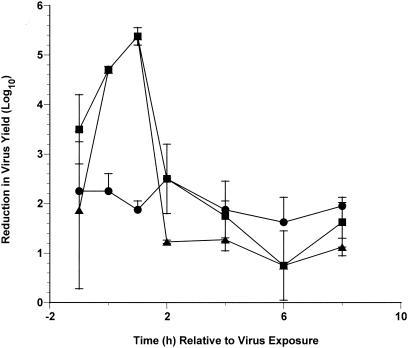
A time-of-addition assay was done to determine the efficacy of BTA39 and BTA188 for inhibiting HRV-2 replication when added at various times before or during virus infection (Fig. 2). The results indicated that virus replication, at an MOI at which almost every cell was simultaneously infected, was inhibited most effectively during the early parts of the virus replication cycle by the oxime ether pyridazinamines. After 1 h post-virus exposure, viral replication was greatly inhibited by BTA188 and BTA39. All drugs demonstrated inhibition of virus production at all time periods sampled. This inhibition at later time periods was due to residual drug present in those samples set aside for titration, since drug added to the culture at the expected end of the viral replication was as inhibitory as when drug was added at 2 to 8 h after virus exposure (data not shown).
FIG. 2.
Effects of varying the time of addition of compound on the inhibition of HRV-2 (strain HGP) replication in KB cells by pyridazinyl oxime capsid-binding agents. ▪, BTA39; ▴, BTA188; •, pirodavir. Virus was added to the cells at an MOI of 2. The values represent mean values from two virus yield reduction assays ± the standard deviations for each value.
DISCUSSION
The family Picornaviridae contains many human virus pathogens of clinical importance. Some can cause very severe, even life-threatening, diseases in neonates (5). Although a number of compounds have entered clinical trials for the treatment of picornavirus infections, none have been approved for use. Most of the compounds target the virus capsid or the viral 3C protease, and in only a few instances have they shown beneficial effects (18).
One of the more potent types of inhibitors of HRV is the capsid binders, such as pleconaril (6) and pirodavir (2). Pirodavir also inhibits some of the enteroviruses of the Picornaviridae as well. Unfortunately, the compound was not efficacious in clinical trials for treating rhinovirus infections, due to lack of oral bioavailability (9) and probably lack of stability due to hydrolysis (3). Because of the potential of pirodavir to be a broad-spectrum picornavirus inhibitor, a new class of oxime ether derivatives of pirodavir has been developed, and they have been shown to have potent antirhinoviral activity; they also appear to be orally bioavailable in animal models (20).
We found that two of these compounds, BTA39 and BTA188, inhibit 56 rhinovirus laboratory strains and three of the clinical isolates tested. BTA39 inhibited 69% and BTA188 inhibited 75% of the rhinovirus serotypes and isolates evaluated with IC50s of <100 nM. This was comparable to pirodavir, which inhibited 59% of the serotypes and isolates with IC50s of <100 nM. BTA39 and BTA188 inhibited many of the virus serotypes in antiviral groups A and B, described by Andries et al. (1), and many of the serotypes in the two major receptor groups at concentrations equal to or better than those for pirodavir. In addition, there appeared to be no selective inhibition of any of the HRV serotype classification groups mentioned above by the three compounds. Serotypes 8, 32, 64, 70, and 87 were relatively resistant to inhibition by all three compounds. Of these five resistant viruses, four belonged to the ICAM-1 receptor-binding group, and the other virus, serotype 87, is the only rhinovirus that uses a sialoprotein as a receptor (5). Serotypes 8, 32, 70, and 87 belong to antiviral group A, and serotype 64 belongs to antiviral group B; these are the two groups of rhinoviruses defined by their sensitivities (or lack of) to capsid-binding drugs as described by Andries et al. (1). Thus, these oxime ethers appear to be potent inhibitors of most rhinoviruses, regardless of the virus receptor group to which a particular serotype might belong. This suggests that the compounds bind virus and not a receptor to achieve an antiviral effect. It is also consistent with BTA188 and BTA39 acting as capsid binders, a mechanism of action shared with structurally related inhibitors such as pleconaril and pirodavir which bind to the capsid and prevent uncoating or attachment to the cellular receptor. The data from the timing assays also suggest that BTA39 and BTA188 inhibited virus production most effectively at early time periods associated with virus binding.
In addition, the two compounds were tested for inhibition of coxsackieviruses. Four of the seven coxsackie group A and B viruses tested were inhibited by both BTA39 and BTA188, albeit at much higher concentrations than were the rhinoviruses. Coxsackie B4, implicated in some of the more serious diseases caused by this group, was one of the viruses more sensitive to inhibition.
A number of echoviruses were also evaluated for sensitivity to BTA39 and BTA188. This group of enteroviruses was slightly more sensitive to inhibition by the oxime ethers than were the coxsackieviruses. A number of the strains known to be etiological agents for aseptic meningitis (i.e., 4, 11, 30, and 31) were inhibited at concentrations as low as 193 nM by BTA188 and 464 nM by BTA39, although type 31 was resistant to both drugs.
Of all the enteroviruses tested (poliovirus, coxsackievirus, echovirus, and enterovirus), enterovirus type 71 was the most sensitive to inhibition by BTA39 and BTA188. The IC50s were 1 and 82 nM, respectively. This particular virus is involved in such serious diseases as meningoencephalitis and other severe central nervous system diseases (5).
Polioviruses were relatively insensitive to inhibition by all compounds, although BTA39 did inhibit the WM strain of poliovirus type 1 at 204 nM.
A second generation of compounds of the pyridazinamine series, BTA39 and BTA188, were potent inhibitors of rhinoviruses, and of enterovirus 71 in vitro in the case of BTA39, with IC50s comparable to those of pirodavir. Other enteroviruses were sensitive to inhibition by the oxime ethers and pirodavir as well, but at somewhat higher concentrations. However, pirodavir is not very orally bioavailable (19), while BTA39 and BTA188 are (20). Thus, these data and the oral bioavailability of the compounds suggest that BTA39 and BTA188 may be potential clinically useful chemotherapeutic agents for treating infections caused by the viruses in the Picornaviridae.
Acknowledgments
This research was supported by Biota Holdings Ltd., Melbourne, Victoria, Australia.
REFERENCES
- 1.Andries, K., B. Dewindt, J. Snoeks, L. Wouters, H. Moereels, P. J. Lewi, and P. A. Janssen. 1990. Two groups of rhinoviruses revealed by a panel of antiviral compounds present sequence divergence and differential pathogenicity. J. Virol. 64:1117-1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andries, K., B. Dewindt, J. Snoeks, R. Willebrords, K. van Eemeren, R. Stokbroekx, and P. A. J. Janssen. 1992. In vitro activity of pirodavir (R 77975), a substituted phenoxy-pyridazinamine with broad-spectrum antipicornaviral activity. Antimicrob. Agents Chemother. 36:100-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Andries, K. 1993. Discovery of pirodavir, a broad-spectrum inhibitor of rhinoviruses, p. 179-209. In J. Adams and V. J. Merluzzi (ed.), The search for antiviral drugs. Birkhauser, Boston, Mass.
- 4.Barnard, D. L., V. D. Stowell, K. L. Seley, V. R. Hegde, S. R. Das, V. P. Rajappan, S. W. Schneller, D. F. Smee, and R. W. Sidwell. 2001. Inhibition of measles virus replication by 5′-nor carbocyclic adenosine analogues. Antivir. Chem. Chemother. 12:241-250. [DOI] [PubMed] [Google Scholar]
- 5.Couch, R. B. 2001. Rhinoviruses, p. 777-797. In D. M. Knipe and P. M. Howley (ed.), Fields virology, 4th ed., vol. 1. Lippincott Williams and Wilkins, Philadelphia, Pa.
- 6.Diana, G. D., and D. C. Pevear. 1997. Antipicornavirus drugs: current status. Antivir. Chem. Chemother. 8:401-408. [Google Scholar]
- 7.Dragovich, P. S., T. J. Prins, R. Zhou, S. E. Webber, J. T. Marakovits, S. A. Fuhrman, A. K. Patick, D. A. Matthews, C. A. Lee, C. E. Ford, B. J. Burke, P. A. Rejto, T. F. Hendrickson, T. Tuntland, E. L. Brown, J. W. Meador, R. A. Ferre, J. E. V. Harr, and M. B. Kosa. 1999. Structure-based design, synthesis, and biological evaluation of irreversible human rhinovirus 3C protease inhibitors. 4. Incorporation of P-1 lactam moieties as L-glutamine replacements. J. Med. Chem. 42:1213-1224. [DOI] [PubMed] [Google Scholar]
- 8.Giranda, V. L., and G. D. Diana. 1987. Rhinoviral capsid-binding inhibitors: structural basis for understanding rhinoviral biology and for drug design, p. 487-524. In P. Veerapandian (ed.), Structure-based design. Marcel Dekker, Inc., New York, N.Y.
- 9.Hayden, F. G., G. J. Hipskind, D. H. Woerner, G. F. Eisen, M. Janssens, P. A. J. Janssen, and K. Andries. 1995. Intranasal pirodavir (R77,975) treatment of rhinovirus colds. Antimicrob. Agents Chemother. 39:290-294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Heinz, B. A., and L. M. Vance. 1995. The antiviral compound enviroxime targets the 3A-coding region of rhinovirus and poliovirus. J. Virol. 69:4189-4197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jackson, J. L., E. Lesho, and C. Peterson. 2000. Zinc and the common cold: a meta-analysis revisited. J. Nutr. 130:1512S-1515S. [DOI] [PubMed] [Google Scholar]
- 12.Kaiser, L., C. E. Crump, and F. G. Hayden. 2000. In vitro activity of pleconaril and AG7088 against selected serotypes and clinical isolates of human rhinoviruses. Antivir. Res. 47:215-220. [DOI] [PubMed] [Google Scholar]
- 13.Matthews, D. A., P. S. Dragovich, S. E. Webber, S. A. Fuhrman, A. K. Patick, L. S. Zalman, T. F. Hendrickson, R. A. Love, T. J. Prins, J. T. Marakovits, R. Zhou, C. E. Ford, J. W. Meador, R. A. Ferre, E. L. Brown, S. L. Binford, M. A. Brothers, and D. M. DeLisle. 1999. Structure-assisted design of mechanism-based irreversible inhibitors of human rhinovirus 3C protease with potent antiviral activity against multiple rhinovirus serotypes. Proc. Natl. Acad. Sci. USA 96:11000-11007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McKinlay, M. A., D. C. Pevear, and M. G. Rossmann. 1992. Treatment of the picornavirus common cold by inhibitors of viral uncoating and attachment. Annu. Rev. Microbiol. 46:635-654. [DOI] [PubMed] [Google Scholar]
- 15.Novick, S. G., J. C. Godfrey, N. J. Godfrey, and H. R. Wilder. 1996. How does zinc modify the common cold? Clinical observations and implications regarding mechanisms of action. Med. Hypotheses 46:295-302. [DOI] [PubMed] [Google Scholar]
- 16.Patick, A. K., S. L. Binford, M. A. Brothers, R. L. Jackson, C. E. Ford, M. D. Diem, F. Maldonado, P. S. Dragovich, R. Zhou, T. J. Prins, S. A. Fuhrman, J. W. Meador, L. S. Zalman, and D. A. Matthews. 1999. In vitro antiviral activity of AG7088, a potent inhibitor of human rhinovirus 3C protease. Antimicrob. Agents Chemother. 43:2444-2450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pevear, D. C., T. M. Tull, M. E. Seipel, and J. M. Groarke. 1999. Activity of pleconaril against enteroviruses. Antimicrob. Agents Chemother. 43:2109-2115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rotbart, H. A. 2002. Treatment of picornavirus infections. Antivir. Res. 53:83-98. [DOI] [PubMed] [Google Scholar]
- 19.Tebbe, M. J., W. A. Spitzer, F. Victor, S. C. Miller, C. C. Lee, T. R. Sattelberg, Sr., E. McKinney, and J. C. Tang. 1997. Antirhino/enteroviral vinylacetylene benzimidazoles: a study of their activity and oral plasma levels in mice. J. Med. Chem. 40:3937-3946. [DOI] [PubMed] [Google Scholar]
- 20.Watson, K. G., R. N. Brown, R. Cameron, D. K. Chalmers, S. Hamilton, B. Jin, G. Y. Krippner, A. Luttick, D. B. McConnell, P. A. Reece, J. Ryan, P. C. Stanislawski, S. P. Tucker, W. Y. Wu, D. L. Barnard, and R. W. Sidwell. 2003. An orally bioavailable oxime ether capsid binder with potent activity against human rhinovirus. J. Med. Chem. 46:3181-3184. [DOI] [PubMed] [Google Scholar]