Abstract
Introduction: Application of nanoparticles as radio sensitizer is a promising field to improve efficiency of radiotherapy.
Methods: This study was conducted to review nano radio sensitizers. PubMed, Ovid Medline, Science Direct, Scopus, ISI web of knowledge, and Springer databases were searched from 2000 to May 2013 to identify relevant studies. Search was restricted to English language.
Results: We included any study that evaluated nanoparticles, volunteer of radio enhancement at radiotherapy on animals or cell lines. Nanoparticles can increase radio sensitivity of tumor cells. This effect was shown in vivo and in vitro, at kilovltage or megavoltage energies, in 24 reviewed studies. Focus of studies was on gold nanoparticles. Radio sensitizing effects of nanoparticles depend on nanoparticles’ size, type, concentration, intracellular localization, used irradiation energy and tested cell line.
Conclusion: Literature suggests potency of nanoparticles for increasing cell radio sensitivity. Reviewed results are promising and warrant future clinical trials.
Keywords: Radio sensitizer, Nanoparticles, Radiation therapy, Gold nanoparticles
Introduction
Radiotherapy is one of the main modalities for the treatment of cancer. From its first reported medical use in 1896, radiotherapy is still the most common cancer treatment.1 Almost 52% of cancer patients undergo radiotherapy at least once during their treatment course.2 Comparing the radiotherapy with surgical treatment, it is not invasive and patients would have easier recovery.
Radiotherapy mechanism is irradiation with high-energy radiation. Radiation (X-rays, gamma rays and fast-moving charged particle like electron, proton) destroys intracellular components like DNA. Irradiation interacts with DNA and produces a series of free electrons, ions and radicals such as hydroxyl OH•, hydrogen H•, water H2O+, H3O+, superoxide O2 −.3 These products especially hydroxyl radicals damage DNA.4
One of the greatest challenges in radiotherapy is that ionizing radiation affects both healthy tissue and solid tumors. Since the tissue around tumor is affected by radiation, there is limitation in increasing radiation dose. Hence, healthy tissue undergoes less radiation dose. Radiotherapy needs some improvement in radiation delivery techniques in order to reduce injury to the surrounding tissues. To overcome this problem, radio sensitizers are appropriate solution. Radio sensitizers are adjunctive treatments which make tumor cells more susceptible to radiation. They are designed to improve tumor cell killing while having much less effect on normal tissues.5
Many substances and materials have been reported as radio sensitizers. Recent progresses have been made towards nanoparticles to propose them as novel radio sensitizers. Nanoparticles (NPs) are defined as particles between 1 and 100 nm.6 They have more cell penetration and less adverse effects than conventional radio sensitizers.7 Among nanomaterials which have this radiosensitizing nature, carbon nanotubes,8 gold nanoparticles (GNPs)9 and other metallic nanoparticles10 can be mentioned.
Application of NPs as radio sensitizers is a promising strategy to increase the efficiency of radiotherapy. The assessment of preclinical studies helps to shape further trials. Further, it prevents unnecessary study replication and opens way for successful clinical trials. Thus we intended to conduct this review with a focus on study methods and results. This is the first review of the literature which assessed the application of NPs in radiotherapy as radio sensitizer.
Methods
Search strategy
Our review was compatible with the PRISMA guidelines.11 The search was performed in the databases of PubMed, Ovid Medline, Science Direct, Scopus, ISI web of knowledge and Springer from 2000 to May 2013. Searches were restricted to English language. The last search was done on July 11, 2013. The following search terms were used: (nanoparticles or nanotubes or nanomaterials or “gold nano particles” /gnp or “carbon nano*” or “quantum dots”) and (radio sensitizer or radiosensitization or “radiation dose-enhancing” or “radiation sensitizing agents” or “enhanced X-ray therapy” or “enhancement of radiation sensitivity”) or (radiation therapy or radiotherapy). Synonyms and derivates of the terms were also used for finding more articles.
To have a comprehensive search and to find possible relevant articles, manual search was conducted on the reference list of articles. If any single study resulted in multiple publications, only the principal paper was reviewed.
Inclusion criteria
Well designed and executed studies provide the most reliable evidence for inclusion in any review. We included articles:
Studying the NPs as the volunteer of a radio sensitizer substance.
In which ionizing radiation was used.
The aim of study was cancer radiotherapy.
In which cell lines/animals were tested.
Exclusion criteria
Studies that have evaluated drug formulation based on NPs or NPs binded drug were excluded, as an example “Polymeric Nanoparticle Micelle Formulation of Paclitaxel”.
Thesis, meetings and other unpublished data were excluded.
First, titles and abstracts of the searched studies were read to determine their potential eligibility for the review. Then the full text of each possibly relevant study was retrieved and assessed independently by authors. Relevant articles were chosen for more investigation. Articles which met our inclusion criteria were included and some were excluded based on our exclusion criteria. After the assessment, the authors agreed on the reporting of 24 articles in a consensus meeting selection and any disagreement between the authors was resolved through discussion. For assessing the agreement between the authors, Cohen’s kappa statistic was used (Cohen’s kappa =0.85).
Independent extraction of articles was performed. Following data were extracted: paper citation, publication year, type of nanoparticle, radiation dose and type, and NPs’ size. We also noted outcomes of studies regardless of author, affiliation and journal. We gathered data from all studies identified irrespective of nanoparticle synthesis method.
Due to the heterogeneous nature of the studies identified, the data available did not allow us to use formal statistical techniques such as meta-analysis. Heterogeneity results from variations in the studies’ methods, outcome measures, sizes and types of NPs and cell line types.
Results
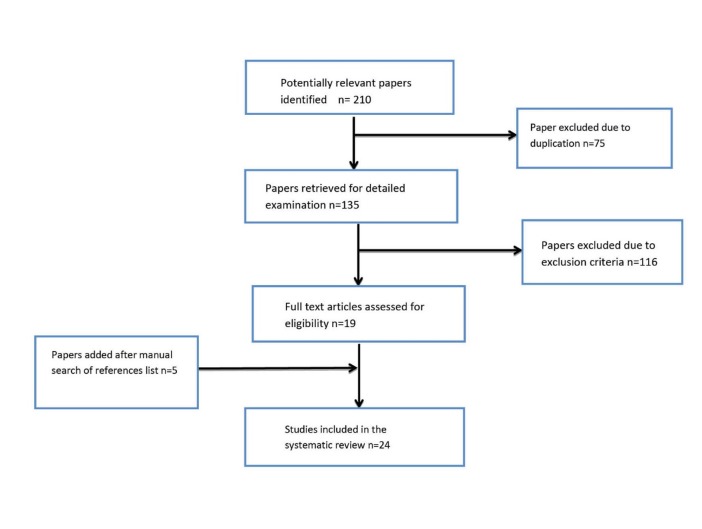
The search of databases yielded 210 publications. Duplicates were eliminated (n=75). Leaving 135 publications for initial evaluation, 116 of articles were excluded based on the exclusion criteria. Five articles were added after checking the references list of included articles. Finally, 24 articles were reviewed. Fig. 1 shows the algorithm of the study selection procedure.
Fig. 1 .
Study following diagram
Fifteen papers were carried out based on GNPs, one paper carried out using nanosilver and nanogold particles and eight papers carried out using other NPs. Two papers were in vivo, 18 papers were in vitro and four papers were both in vivo and in vitro.
Noteworthy studies have been done to show NPs’ radio sensitization effect. Supplementary 1 shows a summary of the studies’ conclusion. The majority of studies have evaluated GNPs. GNPs have received special attention during last decade.12 It has been shown that by using GNPs, less radiation dose is needed.13 Supplementary 2 shows GNPs’ sizes, cell types and radiation doses and types used for each study.
A pioneering study was conducted on mice bearing subcutaneous EMT-6 mammary carcinomas by Hainfeld.14 Mice were divided into two groups: treated with GNPs and radiation, treated with radiation alone. These two groups had 86% and 20% one-year survival, respectively. Another in vivo study was done by Hainfeld recently. He used the same size used in the previous study (1.9 nm GNPs). Mice bearing murine squamous cell carcinoma (SCCVII) were radiated with X-ray (68 keVp, 42 and 30 Gy). Significant tumor growth delay and long-term tumor control were seen with 42 Gy but not with 30 Gy.15 Moreover, mice were radiated with 157-keV photons; more tumor radiosenstivity was seen with GNPs accompanied by 50.6 Gy than 44 Gy.
Another animal study was conducted in 2008.16 They injected melanoma cells (B16F10) to mice. After GNP injection, mice were irradiated with electron (25 Gy). They showed that GNPs radiosensitized the melanoma cells. In comparison with control group, tumor growth rate was decreased; apoptic signals and survival rates were increased.
It is demonstrated that radio sensitization is cell line-dependent, as Jain et al. showed that GNPs’ radio sensitization occurred in MDA-MB-231 cell line but not in DU145 or L132 cell line despite GNP uptake.17
Bonded GNPs
Different functional groups can be attached to GNPs such as PEG, thiol, peptides and antibodies. Binding ligands and molecules bestows numerous characteristics to the particle. Daniel et al. after in vitro experiments showed that intravenously injected PEGylated-GNPs radiosensitized the human glioblastoma cells to radiotherapy and increased mice survival.18 Another study about PEGylated-GNPs showed that in the presence of this nanoparticle, EMT-6 and CT26 cell survival rates were decreased.19
A recent study has evaluated effects of targeted GNPs on tumor radiation sensitivity.17 This study had two parts: in vivo and in vitro. At the in vivo part, athymic mice bore the subcutaneous MDA-MB-361 xenografts. Mice were injected Human Epidermal Growth Factor Receptor-2 targeted GNPs or saline intratumorally. After 24 hours, mice received a single dose radiation of x-rays (100 kVp, 11Gy). These mice had slower growth rate than control mice (which were only radiated). Remarkably, in vivo results were in agreement with in vitro’. Survival curve of cells exposed to targeted GNPs and radiation was significantly lower than that of cells exposed to x-radiation alone. But, survival curves for cells exposed to GNPs and radiation versus radiation alone were not significantly different. Thus, targeted GNPs caused more radio sensitivity than neutral GNPs.
Glucose capped GNPs-enhanced (Glu-GNPs) radiation sensitivity in radiation-resistant human prostate cancer cells study is another bonded GNPs study.20 It has been shown that Glu-GNPs activate cell cycle acceleration in the G0/G1 phase and restrain cell in the G2/M phase. This activation occurs with sensitivity to ionizing radiation. Similar studies about Glu-GNPs showed that irradiation of HeLa cells with Glu-GNPs results in enhanced radiation sensitivity.21 Also similar effects is seen on lung cancer cells and ovarian cancer cells.22,23 Another study showed that Glu-GNPs have a greater reduction in cellular proliferation than neutral GNPs.24
Binding groups bring about changes in GNPs location. Kong et al. compared thioglucose and cysteamine-capped GNPs in breast-cancer cell line (MCF-7) versus a nonmalignant breast-cancer cell line (MCF-10A).25 This study showed that cysteamine-capped GNPs were mostly bound to the MCF-7 cell membrane, but thioglucose –capped GNPs entered the cells and scattered in the cytoplasm.
Other NPs as radio sensitizers
Apart from GNPs, other NPs are also radio sensitizers. It was shown that silver NPs radiosensitized HCC cell lines (HepG2) like GNPs.26 A recent research paper showed that titanate nanotubes radiosensitized two human glioblastoma cell lines.27 Also titanium dioxide NPs proved their effect in presence of 60Co gamma rays on human breast cancer (MCF-7) and gastric cancer (MKN-45) cell lines.28
Lin et al. demonstrated that germanium NPs could enhance the radiation sensitivity of cell.29 NH2-Silicone NPs increased radiation sensitivity of breast cancer and mouse fibroblast cells by oxygen free radicals’ formation.30 Hafnium oxide NPs have acted as radio sensitizers in xenograft mouse.31 The ammonium persulfate functionalized multi-walled carbon nanotubes have also been introduced as novel radio sensitizers due to biocompatibility and bio absorption.32 Also fullerene-C60 (nano-C60) is another volunteer for possible radiosensitazation effect.33
Since hydroxyapatite NPs (nano-HAPs) had retarded glioma growth, a study was conducted to show the radiation sensitization induced by hydroxyapatite NPs. They concluded that nano-HAPs could increase the radiation sensitivity of tumor cells in vitro and in vivo.8
Discussion
Reviewed studies have demonstrated radio enhancement effect of NPs. NPs particularly GNPs have been developed for cancer radiotherapy. They have unique properties like biocompatibility and modifiable surfaces that make them great volunteer for radio sensitization. The sensitizing characteristics of NPs have been tested on various cell lines and animals. Different sizes, concentrations, cell lines, radiation sources and doses have been used at the reviewed studies. Radiation sensitivity using NPs depends on nanoparticle type, cell line, irradiation energy, nanoparticle size, concentration and intracellular localization. Great studies were done on GNPs and 15 out of 24 reviewed articles had assessed the GNPs. Thus our focus was on GNPs.
Among NPs, GNP is the most studied nanoparticle in cancer therapeutics. In vitro radio sensitization and in vivo tumor growth retardation accompanied by longer survival give researchers the proof of using GNPs. All reviewed studies showed consistency of their result and confirmed the enhancement of radiotherapy by using GNPs. Such enhancement takes place as long as GNPs are accompanied by radiation. GNPs without radiation result is similar to no treatment.14
Probable mechanism involved in GNP radio enhancement is cell cycle changes and elevated reactive oxygen species production.20,23 In the presence of GNPs, more radicals and electrons are produced.34 It is suggested that radio sensitivity of GNPs can be attributed to enhanced localized absorption of X-rays, release of low-energy electrons from GNPs and efficient deposition of energy in the form of radicals and electrons.35 Most of studies have compared using the GNPs with none-use of them.
Six factors affecting GNPs as radio sensitizer
1-Intracellular localization
Accumulation of GNPs inside the cells and intracellular localization enhance the radiation effects as probable photon and electron interaction increase. Study of Kong et al. and Chattopadhyay et al. suggested that localization of GNPs within the cells is an important factor in increasing the radiation cytotoxicity.17,25
2-Size
GNPs can be synthesized over a wide range of sizes (0.4-5000 nm). Some of GNP properties are attributed to size. Size is a strong factor in presence time in blood. Smaller GNPs are filtrated through kidneys quickly, while larger ones avoid clearing. Toxicity can be controlled by size. Although Gold is inert, it can be toxic at high levels as any other substance.
GNPs’ size affects cellular uptake. Since only GNPs of size 1-100 nm can enter cells, optimal size design can increase the cell internalization.35 The majority of studies point out that size is an influencing radiation sensitivity parameter. Large-sized GNPs have the most efficient dose enhancement factor (DEF).36,37 It is demonstrated that GNPs with 50-nm diameter could have the highest radio enhancement factor (REF) (1.43 at 220 kVp) compared with GNPs of 14 and 74 nm (1.20 and 1.26, respectively).35 This diameter has also the highest cellular uptake.38 Although a recent report has shown that 18 nm GNPs have more cell internalization than larger particles of 35 and 65 nm.39
3-Concentration
The effect of GNPs’ concentrations on dose enhancement is much greater than GNPs’ size.39 Increasing the GNPs’ concentration decreases cells growth rate.40 This reduction seems logical as increasing the concentration of GNPs causes an increase in the number of GNPs, and in turn, the number of gold atoms. Therefore, more photoelectric interactions between photons and gold atoms occur.39 Higher GNP concentrations seem to carry higher risk of toxicity. Therefore, the balance between dose enhancement effect and toxicity should be set.
4-Radiation dose
Several reports have shown GNPs’ radio sensitization with kV (proton and X-ray) and KeV. Also such radio enhancement is shown at MV X-rays and MeV energies.9,13,16,21,35,41 Dose enhancement factor (DEF) depends on radiation energy and amount of GNPs.42 Such following DEF for different radiations have been reported: DEFs of 2.9 and 3.7 using 0.5 mM, a concentration of 1.9 nm GNPs at 6 MeV and 12 MeV were reported.13 DEFs of 1.66, 1.43 and 1.17 were observed with 105kVp, 220kVp and 6MV X-rays, respectively.35 DEFs of 1.44, 1.1 and 1.32 were achieved with 8keV, 160kVp and 6MV X-rays, respectively.43 DEF=2-3.7 and DEF=1.8-3 were noted while using 90 keV and 50 keV, respectively (for different sizes and concentrations).39 DEF of 2 was reported using 7 mg AU/g with 140 kVp.44
5-Cell type
Cytotoxicity of GNPs alters in different cell types.25,45 GNPs could enhance the sensitivity of some cells to irradiation but not all cells, as glucose capped GNPs did not radiosensitize human diploid fibroblast cells but did enhance human prostate carcinoma cells.20 As another proof, despite cellular uptake in human prostate cancer cells and lung epithelial cells, radio sensitization was not observed in neither of them.9
GNPs’ cellular uptake levels and cell cycle phases might justify it. Metallic materials block cells at the G2/M phase, the most radiosensitive phase of the cell cycle, and therefore augment cell radio sensitivity.46
6-Modifing GNP’s Surface
A 0.8-nm GNP has seven ligand sites, a 2-nm has ~100, and a 15-nm has approximately 4000.PEG, carboxyl or amino groups, thiol, derivative drugs, DNA, lipids, carbohydrates, antibodies, peptides or organic moiety can be attached to GNPs. Any of these bindings confer beneficial properties to GNP. As an example, PEG binding helps GNPs to avoid reticuloendothelial system uptake.42 Glucose binding GNPs enter the cells and spread in the cytoplasm more than neutral GNPs,25 as it was shown in 6 out of 15 review studies.20-25 Cancer cells have more metabolisms than normal cells, which render a greater need to glucose. Therefore, when glucose is coated on the surface of GNPs, cancer cells take up the glucose with GNPs attached to it. Glucose increases cell internalization and afterwards increases radio sensitivity.
GNPs’ surface can be modified for targeting of cancer cells by antibodies or hormones.44 If GNPs can be localized in cancerous cells, cancerous tissue receives higher dose compared with normal tissue during a radiotherapy treatment. Also, less radiation dose is needed.
Rationale behind other NPs
High-Z NPs absorb more radiation, therefore more beam energy deposits in tumor loaded with NPs. Final outcome is local dose enhancement. In addition, free radicals are produced as a result of radiation and metallic NPs interaction. They damage DNA, thus cell apoptosis occurs. Cell damage by free radicals could be the principal mechanism involved in the sensitivity of metallic and also non-metallic NPs. Raised production of cellular ROS causes radio enhancement activity of nano-C60.8 TiO2 nanoparticle and amino silanized oxidized silicon NPs provoke oxidative stress by embedding in mitochondrial membranes and thus cause generation of ROS.28,30 Multi-walled carbon nanotubes modified by ammonium persulfate possess negatively charged carbonyl and hydroxyl groups. While radiation, these groups produce free radicals. Free radicals destroy cancer cells directly. Besides, multi-walled carbon nanotubes modified by ammonium persulfate are soluble and can enter cancer cells.32
Hafnium oxides NPs (NBTXR3) have been established for enhancing the radiation sensitivity of nine folds and tumor growth delay.31 The rationale for choosing NBTXR3 was its capacity to deposit high energy within tumors and their chemically inert style in vivo, thus decreasing potential damages.
Another novel radio sensitizer is TiO2 nanoparticle. It has a lot of water, oxygen and hydroxides in its structure. While TiO2 NPs interact with radiation, free radicals such as OH•, H• and HO•2 (which are well known radio sensitizers) are generated.47 Also,TiO2 NPs increase number of cells in the G2/M cell cycle phase.27
There are some other NPs such as CaF,48 LaF,49 ZnS,50 ZnO,51 quantum dots and carbon dots,52 volunteer of having possible effects of radio sensitizer but further efficacy and toxicity studies are needed.
In the dearth of nano clinical trials, novel nanomedicines are being introduced for cancer therapy and there is a need to bring these products to clinical trials, after considering ethical issues.
Conclusion
Up-to-date literature supports using NPs as radio sensitizer for neoplasms irradiation. By altering factors affecting radio sensitivity, desirable result in clinical applications would be achieved. These findings show signs of future success of the NPs in cancer treatment.
Acknowledgements
This paper has no financial support.
Ethical issues
Ethical issues are not applicable.
Competing interests
The authors declare no conflict of interests.
Supplementary materials
Supplementary file contains the supplementaries 1, 2
References
- 1. Hall EJ, Giaccia AJ. Radiobiology for the Radiologist. Philadelphia: Lippincott Williams & Wilkins; 2006.
- 2.Delaney G, Jacob J, Featherstone C, Barton M. The role of radiotherapy in cancer treatment: estimating optimal utilization from a review of evidence-based clinical guidelines. Cancer. 2005;104:1129–37. doi: 10.1002/cncr.21324. [DOI] [PubMed] [Google Scholar]
- 3.Neuman D, Ostrowski AD, Mikhailovsky AA, Absalonson RO, Strouse GF, Ford PC. Quantum dot fluorescence quenching pathways with Cr(III) complexesPhotosensitized NO production from trans-Cr(cyclam)(ONO)2+ J Am Chem Soc. 2008;130:168–75. doi: 10.1021/ja074164s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Salomeh J, Chithrani DB. Gold Nanostructures as a Platform for Combinational Therapy in Future. Cancer Therapeutics. 2011;3:1081–110. doi: 10.3390/cancers3011081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Khan FM. The physics of radiation therapy. fourth edition. Philadelphia: Lippincott Williams & Wilkins; 2003.
- 6.Praetorius NP, Mandal TK. Engineered nanoparticles in cancer therapy. Recent Pat Drug Deliv Formul. 2007;1:37–51. doi: 10.2174/187221107779814104. [DOI] [PubMed] [Google Scholar]
- 7.porcel E, Liehn S, Remita H, Usami N, Kobayashi K, Furusawa Y. et al. Platinum nanoparticles: a promising material for future cancer therapy? Nanotechnology. 2010;21:85103. doi: 10.1088/0957-4484/21/8/085103. [DOI] [PubMed] [Google Scholar]
- 8.Ni J, Wu Q, Li Y, Guo Z, Tang G, Sun D. et al. Cytotoxic and radiosensitizing effects of nano-C60 on tumor cells in vitro. J Nanopart Res. 2008;10:643–651. [Google Scholar]
- 9.Jain S, Coulter JA, Hounsell AR, Butterworth KT, McMahon SJ, Hyland WB. et al. Cell-specific radiosensitization by gold nanoparticles at megavoltage radiation energies. Int J Radiat Oncol Biol Phys. 2011;79:531–9. doi: 10.1016/j.ijrobp.2010.08.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Le Sech C, Kobayashi K, Usami N, Furusawa Y, Porcel E, Lacombe S. Comment on ‘Therapeutic application of metallic nanoparticles combined with particle-induced x-ray emission effect’. Nanotechnology. 2012;23:078001. doi: 10.1088/0957-4484/23/7/078001. [DOI] [PubMed] [Google Scholar]
- 11.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Medicine. 2009;6:e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lechtman E, Mashouf S, Chattopadhyay N, Keller BM, Lai P, Cai Z. et al. A Monte Carlo-based model of gold nanoparticle radiosensitization accounting for increased radiobiological effectiveness. Phys Med Biol. 2013;58:3075–87. doi: 10.1088/0031-9155/58/10/3075. [DOI] [PubMed] [Google Scholar]
- 13.Rahman WN, Bishara N, Ackerly T, He CF, Jackson P, Wong C. et al. Enhancement of radiation effects by gold nanoparticles for superficial radiation therapy. Nanomedicine. 2009;5:136–42. doi: 10.1016/j.nano.2009.01.014. [DOI] [PubMed] [Google Scholar]
- 14.Hainfeld JF, Slatkin DN, Smilowitz HM. The use of gold nanoparticles to enhance radiotherapy in mice. Phys Med Biol. 2004;49:309–15. doi: 10.1088/0031-9155/49/18/n03. [DOI] [PubMed] [Google Scholar]
- 15.Hainfeld JF, Dilmanian FA, Zhong Z, Slatkin DN, Kalef-Ezra JA, Smilowitz HM. Gold nanoparticles enhance theradiation therapy of a murine squamous cell carcinoma. Phys Med Biol. 2010;55:3045–59. doi: 10.1088/0031-9155/55/11/004. [DOI] [PubMed] [Google Scholar]
- 16.Chang MY, Shiau AL, Chen YH, Chang CJ, Chen HH, Wu CL. Increased apoptotic potential and dose-enhancing effect of gold nanoparticles in combination with single-dose clinical electron beams on tumor-bearing mice. Cancer Sci. 2008;99:1479–84. doi: 10.1111/j.1349-7006.2008.00827.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jain S, Coulter JA, Hounsell AR, Butterworth KT, McMahon SJ, Hyland WB. et al. Cell-specific radiosensitization by gold nanoparticles at megavoltage radiation energies. Int J Radiat Oncol Biol Phys. 2011;79:531–9. doi: 10.1016/j.ijrobp.2010.08.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Joh DY, Sun L, Stangl M, Al Zaki A, Murty S, Santoiemma PP. et al. Selective Targeting of Brain Tumors with Gold Nanoparticle-Induced Radiosensitization. PLoS One. 2013;8:e62425. doi: 10.1371/journal.pone.0062425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu CJ, Wang CH, Chen ST, Chen HH, Leng WH, Chien CC. et al. Enhancement of cell radiation sensitivity by pegylated gold nanoparticles. Phys Med Biol. 2010;55:931–45. doi: 10.1088/0031-9155/55/4/002. [DOI] [PubMed] [Google Scholar]
- 20.Roa W, Zhang X, Guo L, Shaw A, Hu X, Xiong Y. et al. Gold nanoparticle sensitize radiotherapy of prostate cancer cells by regulation of the cell cycle. Nanotechnology. 2009;20:375101. doi: 10.1088/0957-4484/20/37/375101. [DOI] [PubMed] [Google Scholar]
- 21.Kaura H, Pujaria G, Semwalb MK, Sarmaa A, Kumar Avasthi D. In vitro studies on radiosensitization effect of glucose capped gold nanoparticles in photon and ion irradiation of HeLa cell Nuclear Instruments and Methods in Physics Research Section B: Beam Interactions with Materials and Atoms. 2013;301:7–11. [Google Scholar]
- 22.Wang C, Li X, Wang Y, Liu Zh, Fu L, Hu L. Enhancement of radiation effect and increase of apoptosis in lung cancer cells by thio-glucose-bound goldnanoparticles at megavoltage radiation energies. J Nanopart Res. 2013;15:1642. [Google Scholar]
- 23.Geng F, Song K, Xing JZ, Yuan C, Yan S, Yang Q. et al. Thio-glucose bound gold nanoparticles enhance radiocytotoxic targeting of ovarian cancer. Nanotechnology. 2011;22:285101. doi: 10.1088/0957-4484/22/28/285101. [DOI] [PubMed] [Google Scholar]
- 24.Zhang X, Xing JZ, Chen J, Ko L, Amanie J, Gulavita S. et al. Enhanced radiation sensitivity in prostate cancer by goldnanoparticles. Clin Invest Med. 2008;31:E160. doi: 10.25011/cim.v31i3.3473. [DOI] [PubMed] [Google Scholar]
- 25.Kong T, Zeng J, Wang X, Yang X, Yang J, McQuarrie S. et al. Enhancement of radiation cytotoxicity in breast-cancer cells by localized attachment of gold nanoparticles. Small. 2008;4:1537–43. doi: 10.1002/smll.200700794. [DOI] [PubMed] [Google Scholar]
- 26.Zhenga Q, Yanga H, Weia J, Tonga J, Shub Y. The role and mechanisms of nanoparticles to enhance radiosensitivity in hepatocellular cell. Biomed Pharmacother. 2013;0753-3322:00057–7. doi: 10.1016/j.biopha.2013.04.003. [DOI] [PubMed] [Google Scholar]
- 27.Mirjolet C, Papa AL, Créhange G, Raguin O, Seignez C, Paul C. et al. The radiosensitization effect of titanate nanotubes as a new tool in radiation therapy for glioblastoma: A proof-of-concept. Radiother Oncol. 2013;108:136–142. doi: 10.1016/j.radonc.2013.04.004. [DOI] [PubMed] [Google Scholar]
- 28.Rezaei-Tavirani M, Dolat E, Hasanzadeh H, Seyyedi S, Semnani V, Sobhi S. TiO2 Nanoparticle as a Sensitizer Drug in Radiotherapy: in Vitro Study. Iran J Cancer Prev. 2013;6:37–44. [Google Scholar]
- 29.Lin MH, Hsu TS, Yang PM, Tsai MY, Perng TP, Lin LY. Comparison of organic and inorganic germanium compounds in cellular radiosensitivity and preparation of germanium nanoparticles as a radio sensitizer. Int J Radiat Biol. 2009;85:214–26. doi: 10.1080/09553000902748583. [DOI] [PubMed] [Google Scholar]
- 30.Klein S, Dell’Arciprete ML, Wegmann M, Distel LV, Neuhuber W, Gonzalez MC. et al. Oxidized silicon nanoparticles for radiosensitization of cancer and tissue cells. Biochem Biophys Res Commun. 2013;434:217–22. doi: 10.1016/j.bbrc.2013.03.042. [DOI] [PubMed] [Google Scholar]
- 31.Maggiorella L, Barouch G, Devaux C, Pottier A, Deutsch E, Bourhis J. et al. Nanoscale radiotherapy with hafnium oxide nanoparticles. Future Oncol. 2012;8:1167–1181. doi: 10.2217/fon.12.96. [DOI] [PubMed] [Google Scholar]
- 32.Yang Sh, Jing X, Li W, Hu X, Wei W, Wang Z. A new potential radio sensitizer- multi-walled carbon nanotubes modified by ammonium persulfate. Gene Ther Mol Biol. 2008;12:247–252. [Google Scholar]
- 33.Chu SH, Karri S, Ma YB, Feng DF, Li ZQ. In vitro and in vivo radiosensitization induced by hydroxyapatite nanoparticles. Neuro Oncol. 2013;15:880–90. doi: 10.1093/neuonc/not030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Carter JD, Cheng NN, Qu Y, Suarez GD, Guo T. Nanoscale energy deposition by x-ray absorbing nanostructures. J Phys Chem B. 2007;111:11622–5. doi: 10.1021/jp075253u. [DOI] [PubMed] [Google Scholar]
- 35.Chithrani DB, Jelveh S, Jalali F, van Prooijen M, Allen C, Bristow RG. et al. Gold nanoparticles as a radiation sensitizer in cancer therapy. Radiat Res. 2010;173:719–28. doi: 10.1667/RR1984.1. [DOI] [PubMed] [Google Scholar]
- 36.Brun E, Sanche L, Sicard-Roselli C. Parameters governing gold nanoparticle X-ray radiosensitization of DNA in solution Colloids Surf B: Biointerfaces. 2009;72:128–34. doi: 10.1016/j.colsurfb.2009.03.025. [DOI] [PubMed] [Google Scholar]
- 37.Leung MK, Chow JC, Chithrani BD, Lee MJ, Oms B, Jaffray DA. Irradiation of gold nanoparticles by x-rays: Monte Carlo simulation of dose enhancements and the spatial properties of the secondary electrons production. Med Phys. 2011;38:624–31. doi: 10.1118/1.3539623. [DOI] [PubMed] [Google Scholar]
- 38.Chithrani BD, Ghazani AA, Chan WC. Determining the size and shape dependence of gold nanoparticle uptake into mammalian cells. Nano Lett. 2006;6:662–8. doi: 10.1021/nl052396o. [DOI] [PubMed] [Google Scholar]
- 39.Mesbahi A, Jamali F, Garehaghaji N. Effect of Photon Beam Energy, Gold Nanoparticle Size and Concentration on the Dose Enhancement in Radiation Therapy. Bioimpacts. 2013;3:29–35. doi: 10.5681/bi.2013.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jain S, Coulter JA, Hounsell AR, Butterworth KT, McMahon SJ, Hyland WB. et al. Cell-specific radiosensitization by gold nanoparticles at megavoltage radiation energies. Int J Radiat Oncol Biol Phys. 2011;79:531–9. doi: 10.1016/j.ijrobp.2010.08.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jeremic B, Aguerri AR, Filipovic N. Radiosensitization by gold nanoparticles. Clin Transl Oncol. 2013;15:593–601. doi: 10.1007/s12094-013-1003-7. [DOI] [PubMed] [Google Scholar]
- 42.Hainfeld JF, Dilmanian FA, Slatkin DN, Smilowitz HM. Radiotherapy enhancement with gold nanoparticles. J Pharm Pharmacol. 2008;60:977–85. doi: 10.1211/jpp.60.8.0005. [DOI] [PubMed] [Google Scholar]
- 43.Liu Y, Chen W, Wang Y, Joly AG. Investigation of water-soluble X-ray luminescence nanoparticles for photodynamic activation. Appl Phys Lett. 2008;92:043901–3. [Google Scholar]
- 44.Choi CHJ, Alabi CA, Webster P, Davis ME. Mechanism of active targeting in solid tumors with transferrin-containing gold nanoparticles. Proc Natl Acad Sci. 2010;107:1235–1240. doi: 10.1073/pnas.0914140107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Patra HK, Banerjee S, Chaudhuri U, Lahiri P, Dasgupta AK. Cell selective response to gold nanoparticles. Nanomedicine. 2007;3:111–9. doi: 10.1016/j.nano.2007.03.005. [DOI] [PubMed] [Google Scholar]
- 46.Turner J, Koumenis C, Kute TE, Planalp RP, Brechbiel MW, Beardsley D. Tachpyridine, a metal chelator, induces G2 cell-cycle arrest, activates checkpoint kinases, and sensitizes cells to ionizing radiation. Blood. 2005;106:3191–9. doi: 10.1182/blood-2005-03-1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Park EJ, Yi J, Chung KH, Ryu DY, Choi J, Park K. Oxidative stress and apoptosis induced by titanium dioxide nanoparticles in cultured BEAS-2B cells. Toxicol Lett. 2008;180:222–9. doi: 10.1016/j.toxlet.2008.06.869. [DOI] [PubMed] [Google Scholar]
- 48.Chen W, Westcott SL, Zhang J. Dose dependence of X-ray luminescence from CaF [sub 2]:Eu[sup 2+], Mn[sup 2+] phosphors. Appl Phys Lett. 2007;91:211103. [Google Scholar]
- 49.Liu Y, Chen W, Wang Y, Joly AG. Investigation of water-soluble X-ray luminescence nanoparticles for photodynamic activation. Appl Phys Lett. 2008;92:043901–3. [Google Scholar]
- 50.Wang YH, Chen Z, Zhou XQ. Synthesis and photoluminescence of ZnS quantum dots. J Nanosci Nanotechnol. 2008;8:1312–5. [PubMed] [Google Scholar]
- 51.Liu Y, Zhang Y, Wang S, Pope C, Chen W. Optical behaviors of ZnO-porphyrin conjugates and their potential applications for cancer treatment. Appl Phys Lett. 2008;92:143901–3. [Google Scholar]
- 52.Sun YP, Zhou B, Lin Y, Wang W, Fernando KA, Pathak P. et al. Quantum-sized carbon dots for bright and colorful photoluminescence. J Am Chem Soc. 2006;128:7756–7. doi: 10.1021/ja062677d. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary file contains the supplementaries 1, 2