Abstract
Introduction: To date, a growing number of advanced anticancer nanomedicines (e.g., Doxil®, Lipoxal®, DepoCyte®) have entered into different phases of clinical trials. However, most of these medicaments fail to differentiate between diseased and normal cells. They also do not have capability of real time monitoring of disease status trough on-demand imaging/sensing of target molecule(s). Multifunctional nanomedicines and theranostics can resolve such limitations, while formulation of these advanced seamless systems appear to involve various sophisticated process, exploiting several bioconjugations.
Methods: Recent works upon multifunctional nanomedicines for simultaneous imaging and therapy of cancer have been systematically reviewed, focusing on surface modification and application of advanced nanobiomaterials.
Results: Ultimate therapy of malignancies, as complex systems, demands implementation of seamless nanosystems (NSs) that can specifically target the cancerous cells and smartly deliver the anticancer agent(s) into the desired target site. Engineering of such NSs requires in-situ coordination of various technologies (e.g., synthesis, surface modification and bioconjugation) in order to achieve improved pharmacokinetics and pharmacodynamics outcomes.
Conclusion: Seamless multimodal NSs have potential to simultaneously target and monitor the tumor cells through homing and imaging/sensing devices and deliver the therapeutic agents. However, to achieve superior pharmacokinetics with maximal efficacy and minimal side effects, these advanced NSs need to become much more intelligent to sense the disease condition and liberate therapeutics on demand.
Keywords: Cancer diagnosis, Cancer imaging, Cancer therapy, Nanomedicine, Theranostics
Introduction
Cancer is fundamentally characterized by the irregular wild proliferation of abnormal cells with aggressiveness to invade and metastasize. Since cancers are viewed as complex systems wherein a variety of cells are involved, its concurrent early detection and simultaneous therapy are of necessary steps for success of treatment modalities.1 The malignant cells, in comparison with normal cells, show some important genomic and/or epigenomic alterations (e.g., DNA mutations, DNA methylation status and overexpression of some genes and literally proteins) prior to macroscopic phenotypic changes. Such inadvertent alterations have led classification of various cancer marker molecules (CMMs) such as plasma membrane integrated proteins (the-so-called cell surface receptors) or intracellular biomolecules involved in cell signaling. The nature, specificity and level of expression of CMMs are largely dependent upon type of cancers, wherein their early detection is increasingly becoming important and useful steps in terms of diagnosis and prognosis of malignancies. For instance, the gastric cancer is one of the most devastating malignancies with high mortality rate worldwide, hence the only chance to reach better outcomes largely lays on an early-stage diagnosis and simultaneous therapy.2 In fact, the treatability of other types of solid tumors (e.g., pancreas, ovarian, bladder, colorectal, thyroid, and breast cancers) are almost similar. While monitoring of the level of the expressed CMMs can result in improvement of treatment strategies and detection of cancer recurrence, they possess intrinsic potential to be exploited as targets for early detection of tumor and simultaneous therapy. As a result, having exploited CMMs, several monoclonal antibodies (mAbs) and their fragments have been developed and translated into clinical applications.3 Further, there exist compelling evidences that most of the solid tumors are immunogenic tumors, therefore immunotherapy modalities can be pursued for effective therapy of these diseases.4 Nevertheless, similar to chemotherapy alone, immunotherapy appears not to be effective enough when used alone.5
Although cancer chemotherapy has been accepted as an effective treatment modality for various malignancies, this approach is often associated with inadvertent intrinsic side effects mainly because of cytotoxic nature of the most anticancer agents. To tackle this problem, , multifunctional nanomedicines and theranostics have been engineered to improve pharmacokinetic and pharmacodynamics impacts because they are able (a) to target cancer cells specifically through homing device, (b) to monitor the disease status through imaging device, and (c) to deliver the anticancer agent(s) actively to the target site. However, engineering of these long circulating smart “bioshuttles” demands several steps of synthesis, formulation and bioconjugation processes.6,7 In the current study, we will review the advanced materials used for engineering of surface modified multifunctional nanomedicines and theranostics as well as the commonly used conjugation materials and techniques.
Multifunctional nanomedicines and theranostics
From translational standpoint, it is the treatment strategy (e.g., cancer type, biological architecture at cellular/molecular dimension, and disease/patient conditions) that bestows the directionality and endpoint objectives of the seamless coordinated diagnostics and therapeutics of a single multifunctional nanomedicine (the-so-called “theranostics”). However, of enormous investigations towards development and advancement of multifunctional NSs, a very minor percentile studies have successfully been translated into clinical applications solely for diagnosis purposes using mostly advanced inorganic nanomaterials (e.g., functionalize gold and magnetic NPs) for imaging/detecting/sensing.6,8,9 Table 1 and Fig. 1 respectively represent the medical applications and schematic architectures of different types of multifunctional NSs.
Table 1. Selected examples of multifunctional nanomedicines and theranostics .
| Nanosystems | Size (nm) | Therapeutic/imaging agents | Application |
| Liposomal nanoparticles (NPs) | 30-300 | Maghemite nanocrystals | MR imaging and cancer therapy10 |
| Cisplatin | Cisplatin nanoliposomes11 | ||
| Herceptin | Antibody-labeled PEGylated liposomes12 | ||
| Micellar NPs | 20-200 | Herceptin | Targeted NIR QDs-loaded micelles13 |
| Solid lipid NPs | 50-500 | Doxorubicin | Overcoming multidrug resistance in cancer therapy14 |
| Dendrimeric NPs | <100 | Anticancer drugs, antibodies, genes | Dendrimeric theranostics nanocomposites15 |
| Gold NPs | <50 | - | Cancer cells imaging and PTT/PDT |
| Magnetic NPs (MNPs) | <50 | - | Cancer cells imaging and PTT/PDT |
| Silica NPs | 20-300 | Small anticancer drugs, antibodies, genes | Multifunctional porous silica NPs as DDS16-19 |
| Trastuzumab | Increased specificity of gold nanoshells for HER2+ breast cancer20 | ||
| Nanoshell | <100 | ||
| Anti-HER2 antibody | Immunotargeted nanoshells for NIR photothermal therapy using anti-HER2 antibody | ||
| Fullerenes | <50 | Small anticancer drugs, antibodies, genes | Non-invasive cancer imaging and therapy21 |
| Carbon nanotubes (CNTs) | <100 | Paclitaxel | PEG-graft-CNTs for potent cancer therapeutics22 |
| Small anticancer drugs and antibodies | Cancer cell targeting and photoacoustic therapy by CNTs as nanobombs23 | ||
| Nanorodes | <100 | Photosensitizer | ZnO nanorods for treatment of single cancer cells24 |
| Quantum dots (QDs) | <10 | - | Cancer cells imaging and PTT/PDT |
| Bioconjugated MNPs | 50-200 | PAION-Ab | HER2/neu antibody conjugated SPIONs for breast cancer MRI25 |
| Bioconjugated QDs | 20-100 | Cetuximab | Cetuximab-QDs bioconjugate targeting EGFR positive cancer cells26 |
| Bioconjugated aptamer | 50-200 | Small anticancer drugs, antibodies, genes | Aptamer-antibody sandwich ELISA for the early diagnosis of epithelial tumors27 |
| Bioconjugated antibody | 50-200 | Trastuzumab and Maytansinoid | Antibody-cytotoxic drug conjugate28 |
| BRCAA1 antbody | BRCAA1-MNPs for in vivo targeting of gastric cancer29 | ||
| Anti-EGFR antibodies | Anti-EGFR antibody conjugated gold NPs for cancer diagnostics30 | ||
| Bioconjugated CNTs | <200 | Small anticancer drugs, antibodies, genes | Targeted killing of cancer cells in vivo and in vitro with EGF-directed carbon nanotube-based drug delivery31 |
Fig. 1.

Schematic representation of various multifunctional nanosystems (NS) used as theranostics. For engineering multimodal nanosystems, various moieties (e.g., anticancer agent(s), antisense, siRNA, Aptamer, imaging agents, antibody fragments, targeting agents) are generally entrapped, encapsulated or conjugated with different delivery systems such as polymers/lipids. Therapeutics and homing devices can be conjugated to magnetic nanoparticles (MNPs) and quantum dots (QDs) for simultaneous detection and therapy. Note: not drawn to scale and not shown the actual mechanism of conjugation.
Of these NSs, the macromolecules with globular structures (e.g., liposomes, micelles and dendrimers) can entrap/encapsulate the diagnostic and therapeutic agent(s) and improve both the solubility and the blood circulation period, while protecting them from quick elimination and/or biodegradations. As shown in Fig. 1, multimodal NSs may harbor the entrapped anticancer agents such as doxorubicin (DOX) and paclitaxel (PTX), which are also conjugated with homing devices such as antibody (Ab) or aptamer (Ap) and and imaging devices such as gold NPs (AuNPs) and quantum dots (QDs). Such bioshuttle can result in increased accumulation of drug in tumor tissue, the so called enhanced permeation and retention (EPR) effect, as a result of the leaky vasculature surrounding rapidly growing neoplasm. To be maximally effective, the surface of NSs need to be modified with hydrophilic materials such as poly(ethylene glycol) (PEG), a process the so-called PEGylation, and conjugated with homing and imaging devices. Fig. 2 represents a simple conjugation scheme for PEGylation and Ab bioconjugation of NPs functionalized with carboxylic groups such as acid terminated poly(lactic-co-glycolic acid) (PLGA) NPs. These NPs can be activated using N-(3-dimethylaminopropyl)-N′-ethylcarbodiimide hydrochloride (EDC) and N-hydroxysulfosuccinimide (NHS), which can be then PEGylated and conjugated with Ab through a one-/two-step processes.
Fig. 2 .

Schematic illustration of PEGylation and antibody (Ab) conjugation. A) Activation of poly (D,L-lactide-co-glycolide) (PLGA) nanoparticles (NPs) by N-(3-dimethylaminopropyl)-N′-ethylcarbodiimide hydrochloride (EDC), N-hydroxysulfosuccinimide (NHS). B) One-step PEGylation and Ab conjugation. C) Two-step PEGylation and Ab conjugation. SHK: shikonin. FSN: fluorescein. scFv: single-chain variable fragment. PEG: polyethylene glycol.
Impacts of advanced nanomaterials as imaging devices
A prerequisite for simultaneous imaging and therapy of cancerous cells using theranostics is implantation of photo-acoustic nanomaterials with desirable characteristics.6,32 So far, anticancer chemotherapies, Abs or Aps conjugated with AuNPs, magnetic nanoparticles (MNPs), QDs and carbon nanotubes (CNTs) have been used for the engineering of multifunctional NSs since simultaneous pinpointing of cancerous cells by such NSs can even impart existence of a single cancerous cell at the course period of treatment. It is clear now that the real time optical monitoring of diseased cells at molecular/cellular levels can significantly favor the targeted therapy – an approach that is largely dependent upon the exquisite sensitivity and versatility of optical technologies.
Quantum Dots
The quantum dot semiconductors are the most studied nanocrystals used as an imaging agent in formulation of cancer nanomedicine and theranostics because they display superior fluorescent properties as compared with the conventional chromophores and contrast agents.33 When excited with laser beam, the QDs can emit fluorescent light based on their size, in which the band gap energy determines the energy, and hence the color of a QD fluorescent light which is inversely proportional to the size of the QD. As a simple rule regarding the size and color of QDs, it can be stated that the smaller the bluer, the larger the redder. This means the smaller the size of the QD nanocrystal, the larger the band gap will be; thus, the energies of the emitted photon increases while the wavelength decreases. Fig. 3 represents optical spectra of various QDs (panel A), architecture of a CdSe/ZnS QD (panel B), and emission spectra of monodispersed CdSe/ZnS QDs with diameters from 3 to 6 nm (panel C). In fact, four samples of monodispersed CdSe/ZnS, QDs are excitable with one wavelength (e.g., 488-nm of an argon-ion laser), but emit fluorescence depending on their size, from the blue region to the red region of the optical spectrum. Of these inorganic fluorophores, semiconductor nanocrystals are typically composed of atoms from groups II-VI elements (e.g., CdSe‚ CdS‚ CdTe, ZnSe), III-V (InP and InAs) and IV-VI (PbSe).34,35 Among various QDs preparation methods, a common method to produce bulk quantities of QD semiconductor particles is the colloidal suspension synthesis in organic solvent with nucleation of semiconductor metals under high-temperature conditions.36,37 In a study, the routinely used breast cancer marker “Her2” was profoundly detected on the surface of both fixed and live cancer cells using bioconjugates of anti-Her2 IgG-QD, showing superiority of these inorganic dyes to commonly used organic fluorophores.38 These researchers showed profoundly longer photo-stability of QDs bioconjugates in comparison with Alexa 488 bioconjugates. In addition to such synchronous cancer diagnosis and treatment, as shown in Fig. 4 , the conjugated QDs to macromolecular medicaments (e.g., oligonucleotides, Abs, Aps, peptides) can be used in assays involving fluorescence resonance energy transfer (FRET), or bioluminescence resonance energy transfer (BRET).39-43 The most important pitfall of QDs for in vivo applications, similar to various advanced materials such as cationic lipids and polymers used as DDSs or gene delivery systems (GDSs),44-53 is their intrinsic toxicity which is yet to be fully understood. For example, in human umbilical vein ECs (HUVECs), QDs (10µg/mL of CdTe QDs) were shown to elicit significant oxidative stress, mitochondrial network fragmentation as well as disruption of mitochondrial membrane potential, leading to apoptosis through upregulation of Bax, downregulation of Bcl-2, release of mitochondrial cytochrome c and cleavage of caspase-9/caspase-3.54 However, surface modification is deemed to alter the toxicity of QDS because it has been shown that the QD-capping material, rather than the core metalloid complex, is responsible for the majority of their toxicity and biological activity. Therefore, unlike molecules covered with a toxic agent that display cytotoxicity, the surface-modified QDs conjugated with biomolecules seem to retain the biological effects of the conjugate.55
Fig. 3 .

Schematic illustration of quantum dots (QDs). A) Optical spectra of various QDs. The ranges of size dependent emission wavelengths are: CdSe = ~ 470–660 nm, CdTe = ~ 520–750 nm; InP = ~ 620–720 nm; PbS = ~ 900 nm; and PbSe = ~ 1,000 nm. B) Architecture of a CdSe/ZnS QD. C) Different fluorescence emission spectra of mondispersed CdSe/ZnS QDs (3 to 6 nm in diameter) by a single 488-nm beam of an argon-ion laser. Note: not drawn to scale.
Fig. 4 .

Schematic representation of fluorescence resonance energy transfer (FRET) by means of quantum dots (QDs). Image shows the conjugation mechanism of streptavidin-QD with the hybridized probe DNA strand and target DNA. Note: not drawn to scale.
AuNPs
In addition to QDs, other types of inorganic nanomaterials (e.g., AuNPs, gold nanoshells, AuroShell and ferrofluid, silica NPs) have successfully been exploited for sensing and/or therapy of cancers resistant to immunotherapy or chemotherapy.20,56 In 2011, Carpin et al. reported successful targeting and ablation of trastuzumab-resistant cells using anti-HER2-conjugated silica-gold nanoshells and a near-infrared laser. As the main concept for enhancing thermal ablation of cancer by AuNPs, bioconjugation of CMMs targeting mAbs/aptamers with AuNPs appears to provide a useful platform for AuNP-based photothermal therapy (PTT) and imaging of cancer.56 AuNPs can simply be conjugated through ionic interactions, hydrophobic interactions, or dative binding (e.g., thiolation) using an appropriate linker such as N-hydroxysuccinimidyl (NHS) ester that is mainly used for engineering of immunosensors and biochips. Technically, the electrolyte-mediated coagulation phenomenon is the basis of formation of gold-mAbs bioconjugates, in which if mAbs are present in the colloidal suspension, adsorption of mAbs can occur as the electrolyte concentration (NaCl or buffer salts) is raised to surpass the negative repulsion effects. It should be noted that spontaneous adsorption of protein on the surface of AuNPs happens because of electrostatic, hydrophobic, and Van der Waals interactions between AuNPs and mAbs.
Silica NPs
Mesoporous silica nanoparticles (MSNPs) have great potential to be used as multimodal drug delivery system (DDS). The mesoporous structures of these biodegradable ceramic based matrices appear to provide a shelter for incorporation of various agents (e.g., drugs, proteins, imaging agents, photosensitizers), while the outer surface can simply be modified and functionalized.16 For example, multimodal silica NPs (7 nm), which have recently been approved for clinical trial, were used as imaging agents.57 The MSNPs displayed high potential for dye-encapsulating, surface functionalization with cyclic arginine-glycine-aspartic acid peptide ligands and radioiodine as well as safe kidney clearance. In fact, the high binding affinity of these NSs makes them tumor-selective NPs as reported in serial in vivo positron emission tomography (PET) imaging of tumor-selective targeting and nodal mapping through multi-scale near infrared (NIR) optical fluorescence imaging.57 Further, to circumvent the P-gp mediated efflux, endosomal pH-sensitive MSNPs have successfully been used to control the release of DOX in vitro and in vivo, which resulted in profound induction of apoptosis through upregulation of caspase-3.58 Silica NPs (SNPs) show ability to entrap a large number of fluorescent dye molecules and the resultant fluorescence SNPs (FSNPs) with bright optical properties can be further modified for specific targeting of CMMs.59
Carbon nanotubes
As another advanced DDSs, CNTs have been shown to display high potential of photothermal (PT) and photoacoustic (PA) properties, which make them very suitable NSs for imaging and treating tumors.9,32,60,61 CNTs are able to absorb NIR radiation (700 and 1100 nm), in which body tissues are most transparent, and transform the adsorbed NIR energy into PT and/or PA signals. As a result, they can be used as an imaging agent more deeply within tissues than other optical modalities can offer, resulting in an efficient heating within the surrounding environment.32,62-65 In addition to being highly mechanically flexible, the small size and high surface area make CNTs very attractive nanomaterials for development of seamless multifunctional NSs for simultaneous diagnosis and therapy of cancer.60 CNTs can be functionalized with targeting device (e.g., Abs, Fabs, scFvs, Aps), magnetic nanoparticles (MNPs) and also cytotoxic agent (e.g., DOX, PTX) mainly via molecular adsorption or chemical conjugation methods (e.g., cleavable ester bond, amide bond).61,66 It has been reported that the growth head and neck squamous carcinoma cells, which overexpress the epidermal growth factor receptor (EGFR), can significantly be inhibited by CNTs loaded with cisplatin (CP) and armed with EGF (CNT-CP-EGF). These NSs were shown to be highly selectively taken up by cancerous cells and hence result in profound inhibition of malignancies.31 The distribution and clearance study of PEGylated CNTs carrying CP molecules (PEG-CNT-CP) in mice have revealed that the PEG-CNT-CP were highly dispersed in aqueous media, and upon conjugation with EGF, they were able to efficiently inhibit the growth of squamous cell tumors, in large part due to better cellular internalization.67 Besides, single wall CNTs (SWCNTs) were shown to be heated up under a radiofrequency (RF) field – a de novo safe method for selective elimination of malignant cells. Hence, application of 13.56-megahertz RF field had a heating impacts on injected functionalized SWCNTs in the hepatic VX2 tumors in rabbits, so that at 48 hours, all treated tumors displayed complete necrosis.68 Taken all, it seems that such promising PT and PA properties of CNTs can be used for selective destruction of cancer cells and may change the directionality of the cancer diagnosis and therapy in the near future.
Magnetic nanoparticles
The other important group of inorganic NSs are MNPs and superparamagnetic iron oxide NPs (SPIONs), which are deemed to provide a robust platform for cancer targeting and imaging. These NPs may be categorized as (a) ultra-small superparamagnetic iron oxide (IO) NPs (USPIONs) with 10-50 nm in diameter, (b) small superparamagnetic IO NPs (SPIONs) with 50-150 nm in diameter, and (c) monocrystalline IO NPs (MIONs) with 100-200 nm in diameter.69 They are superior to traditional gadolinium-based magnetic resonance (MR) contrast agents mainly because of lower toxicity and stronger enhancement of proton relaxation resultant in lower detection limit.70 MNPs have increasingly been used for clinical applications such as magnetic resonance imaging (MRI), drug delivery and magnetic fluid hyperthermia. The MNPs-based thermal therapy has been examined in prostate cancer, showing good tolerability.71,72 Further, SPIONs-enhanced MRI (Ferumoxtran-10) has successfully been used for diagnosis of nodal staging in patients with head and neck cancer. From a total of 63 nodes studied (36 nonmetastatic, 25 metastatic, and 2 inflammatory), SPIONs-enhanced MRI resulted in diagnosis of 24 metastatic and 30 nonmetastatic nodes, i.e. yielding a sensitivity of 96%, a specificity of 78.9%, a positive predictive value of 75%, and a negative predictive value of 96.8%, while the overall accuracy of the technique was about 85.7%.73 SPIONs with diameter around 30 nm are currently under clinical trials for prostate cancer imaging and thermal therapy.74,75 Functionalized MNPs have also shown great potential as theranostics.76 For example, using selected surface modification methods, we have recently engineered PEGylated MNPs functionalized with folic acid (FA) and loaded with either mitoxantrone (MTX) or tamoxifen (TMX) to target the folate receptor (FR) overexpressing cancer cells for specific delivery of the anticancer agents.77,78 Based on our findings, we proposed both MTX- and TMX-loaded FA-armed PEGylated MNPs as novel multifunctional theranostics for concurrent targeting, imaging and therapy of the FR-positive cancer cells, which can be translated into clinical applications with high efficacy and safety. In a study, MNPs were coated with oleic acid (OA) and PEG to form water-dispersible NSs which were then exploited to adsorb DOX onto the OA layer. Such coated MNPs conjugated to anti-HER2 mAb (~184 nm diameter with ~8 nm iron-oxide core) were successfully used for active targeting of the human MCF-7 breast cancer cells.79 Taken all, although the MNPs need further characterization and optimization prior to their applications in clinic to ensure upon their early/late biologic impacts, they provide promising platform for advancement of multimodal NSs.
Biocompatibility of polymers and lipids for development of multifunctional NSs
A large variety of natural, semisynthetic (modified natural polymers), synthetic polymers (linear, branched and dendritic architectures) and lipids have so far been examined for their safety and potential as DDSs or gene delivery systems (GDSs). However, unfortunately, very few polymers have successfully been translated into clinical applications. In fact, many of these materials (e.g., cationic polymers, dendrimers and lipids) were shown to elicit intrinsic cytotoxicity and toxicogenomics.44-53,80 There exist several important biodegradable and natural polymers that possess promising characteristics and suitability for further development towards clinical uses. Issues relating to the suitability of polymers and/or their conjugates for development towards clinical uses have previously been well reviewed.81,82 Pivotal parameters for an ideal polymer/lipid based DDsS/GDSs for clinical applications include (a) maximal drug delivery capacity, (b) minimal toxicity following acute or chronic uses by the NS or its metabolite(s), (c) reproducibly in manufacturing, (d) appropriateness for pharmaceutical formulation, (e) acceptable stability (both physicochemical and biological), (f) suitable in vitro (cellular) and in vivo (whole body) pharmacokinetics properties, and (g) the cost-effectiveness for the large scale production.83
Surface modification and bioconjugation paradigms
Technically, the development of NSs demands surface modification and conjugation steps to some extent. In fact, in the most cases, there exists a need for alteration of the native structure of a biomacromolecule to provide functional groups on their surface.84 For example, a simple polymeric NS may have a tripartite structure including (a) the backbone polymer, (b) the linker molecule and (c) the payload molecule(s) such as small drugs, peptides, or proteins. In the case of multimodal theranostics, some other moieties such as targeting and/or imaging agents are also linked to the NSs using cross-linking agents. In the following section, we will briefly provide an overview on different types of cross-linkers.
Cross-linking agents
The smallest available reagent systems (the-so-called zero-length cross-linkers) are routinely used for bioconjugation, in which they mediate the conjugation of two molecules through formation of a bond containing no additional atoms. The widely used zero-length cross-linkers are carbodiimides such as 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide hydrochloride (EDC), 1-cyclohexyl-3-(2-morpholinoethyl)carbodiimide (CMC), dicyclohexyl carbodiimide (DCC), diisopropyl carbodiimide (DIC)), Woodward’s reagent K (N-ethyl-3-phenylisoxazolium-3′-sulfonate), N,N′-carbonyldiimidazole (CDI). Further, both homofunctional and hetrofunctional agents have also been successfully utilized as cross-linkers for modification and conjugation of macromolecules.84 Fig. 5 represents molecular structures of some selected zero-length cross-linkers (panel A) and homobifunctional cross-linkers (panel B).
Fig. 5 .
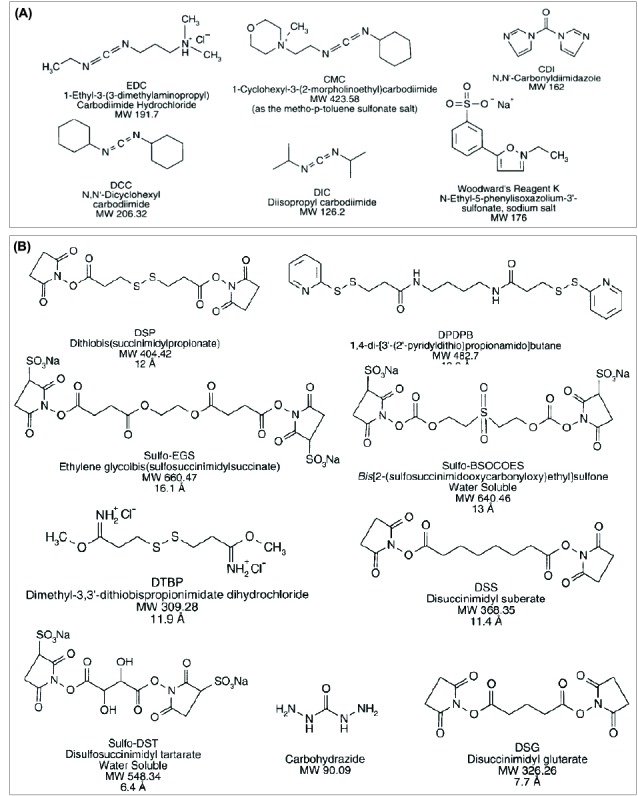
Molecular structures of important zero-length cross-linkers (A) and homobifunctional cross-linkers (B). For detailed information, reader is referred to the textbook of “Bioconjugate Techniques”.84
It should be pointed out that, the use of homobifunctional reagents may generate a broad range of undesired conjugates. For example, conjugation of two different scFv Ab fragments may result in formation of scaffolds with the same type of scFvs instead of two different scFvs. However, heterobifunctional systems provide greater control on bioconjugation process, wherein one scFv Ab fragment can be modified through the cross-linker’s most reactive or most labile end and purified from excess reagents (using gel filtration or rapid dialysis) and then conjugated with the second scFv Ab fragment. In fact, most heterobifunctional cross-linkers contain at least one reactive group with good stability in aqueous settings, and hence providing possibility towards extensive purification of the intermediate scaffold prior to conjugation of the second moiety. The NHS ester–maleimide heterobifunctional epitomizes such reactivity through its NHS ester end with the amine groups of a scFv Ab fragment, while its maleimide functional end can be used for the conjugation of the second scFv Ab fragment after purification step. Fig. 6 represents some selected heterobifunctional cross-linkers.
Fig. 6 .

Molecular structures of heterobifunctional cross-linkers (A) and nanoparticles bioconjugations process by SATA (B). For detailed information, reader is referred to the textbook of “Bioconjugate Techniques”.84 Note: not drawn to scale.
It should be also pointed out that there exist some other cross-linkers such as trifunctional cross-linkers as well as dendrimers, dendrons, photoreactive and cleavable cross-linking systems.
Of various methods, the sulfhydryl group is considered as a popular target in many modification strategies. Technically, the crosslinking agents that possess more than one reactive group often employ a sulfhydryl-reactive function (the-so-called thiolation) at one end to direct the conjugation reaction to a particular part of a target macromolecule. Thiolation can also be performed using water-soluble Traut’s reagent (2-iminothiolane), while a versatile reagent for introducing sulfhydryl groups onto target particle (e.g., proteins such as mAbs and NPs) appears to be the heterobifunctional cross-linkers such as N-succinimidyl S-acetylthioacetate (SATA)/sulfo-SATA (Fig. 6A ). The active NHS ester end of SATA reacts with amino-groups of proteins and other molecules to form a stable amide linkage. Accordingly, the modified protein/NP with SATA contain a protected sulfhydryl end that can be stored without degradation and subsequently deprotected (Fig. 6B ). SATA has successfully been used for conjugation of oligomers of a peptidomimetic integrin alphavbeta3 antagonist (i.e., 4-[2-(3,4,5,6-tetrahydropyrimidine-2-ylamino)ethyloxy]benzoyl-2-(S)-aminoe thylsulfonylamino-β-alanine) (IA) to mAbs to increase the binding avidity of integrin αvβ3 receptor.85 To generate sulfhydryl groups, these researchers conjugated SATA to both mAb and IA by undertaking stepwise reactions using S-acetylthioacetato (ATA)-mAb or ATA-IA for generation of IA-(A-SH) to mAb-(A-SH)n; and homobifunctional cross-linker, 1,8-bis(maleimido)diethylene glycol (BM[PEO]2). They showed that monomeric mAb-(A-S-S-[PEO]2-S-S-A-IA)10 (mAb-IA10) radiolabeled with 111In by 2-(p-isothiocyanatobenzyl)cyclohexyl-DTPA and with 125I by iodogen method showed over 70% bindability to the integrin αvβ3 receptor (0.4 µM). Upon intravenous injection to nude mice with the receptor-positive M21 tumor, the mAb-IA10 radiolabeled with both 111 In and 125I accumulated rapidly and retained in the tumor for a period of 44 h, while the radioactivity cleared quickly from the blood, thereby resulted in increased tumor-to-blood ratios over the time. As a proof of concept, the fluorescence microscopic revealed a rapid blood clearance, a short peak tumor uptake time, and a low peak tumor uptake value with prolonged tumor retention for mAb-IA10. It was shown that mAb-IA10 can primarily bind to the integrin αvβ3 receptors on angiogenic vessels, but not on the tumor.85 SATA has also been used for preparation of multimodal proteins, or proteins labeled with both fluorescent and magnetic reporter groups, which can be used in a wide range of in vitro and in vivo imaging such as FACS flow cytometry, fluorescence microscopy, MRI and/or NIR optical imaging as well as fractionation of cells by magnetic cell sorting.86 To avoid problems such as loss of bioactive sites due to modification points during preparation of multimodal proteins, Schellenberger et al. (2004) reported the synthesis of a magneto/optical form of annexin V, which was performed by reacting the amino-CLIO NPs with Cy5.5 and N-succinimidyl 3-(2-pyridyldithio)propionate (SPDP) to produce a fluorescent, sulfhydryl reactive NPs. To pursue such aim, these researchers added a single reactive sulfhydryl group to annexin V using SATA cross-linking, by which they were enabled to preserve the protein’s ability to bind apoptotic Jurkat T cells. Then, reacting SATAylated annexin V with an SPDP activated NP yielded Anx-CLIO-Cy5.5 (i.e., a magneto/optical form of annexin V). Having showed high specific binding of Anx-CLIO-Cy5.5 to apoptotic Jurkat T, they proposed such conjugate to preserve the strength of the interaction between annexin V and apoptotic cells, with capability to develop NPs including colloidal QDs and AuNPs.87
Further, similar to SATA (Fig. 7A), SPDP can react with amine-containing molecules through its NHS ester end to form amide bonds. The pyridyl disulfide group then can be then coupled to a sulfhydryl-containing molecule to create a cleavable disulfide bond (Fig. 7B). This cross-linker agent is technically extensively used to conjugate proteins such as Ab scaffolds (e.g., mAb, Fab, scFv) to form multispecific systems and also immunotoxin) that can be used for in vivo applications.26,88,89 Succinimidyl-4-( N-maleimidomethyl)cyclohexane-1-carboxylate (SMCC) is a heterobifunctional reagent with significant utility in crosslinking proteins, particularly in the preparation of Ab – enzyme.26,28,84,90 For example, for the tumor-specific imaging through targeting EGFR using QD-cetuximab conjugates, Lee et al. (2010) reported three different conjugation strategies. Successful conjugation of cetuximab to QDs was reported upon exploitation of PEG conjugated polymer-coated QDs and two long-chain heterobifunctional linkers (i.e., sulfo-LC-SPDP and sulfo-SMCC) with dissociation constant of the QD-cetuximab conjugates to EGFR of 0.61 +/- 0.28 nM and efficient internalization. Since the cellular imaging experiments using the QD-cetuximab conjugates resulted in a clear endocytosis and colocalization of the QD-cetuximab conjugates with dye-labeled transferrin, the QD-cetuximab conjugates were suggested to be used as an imaging modality for EGFR overexpressing cancer cells.26 In another study, for the characterization of QDs and their conjugates to biological molecules by capillary electrophoresis coupled with laser-induced fluorescence, non-selective and selective methods were used for preparation of QDs conjugated to some biomolecules.91 For the non-selective approach, 1-ethyl-3- [3-dimethylaminopropyl]carbodiimide hydrochloride (EDCHCl)/sulfo-NHS was used for the conjugation of BSA and myoglobin to carboxylic acid-functionalized QDs. For the selective approach, heterobifunctional cross-linker sulfo-SMCC was utilized for the conjugation of partially reduced IgG to amine-functionalized QDs and the conjugation of periodate-oxidized IgGs to hydrazide-functionalized QDs.In general, there are different approaches for surface modification and bioconjugation of NPs, including: (a) use of a bifunctional ligand such as mercaptoacetic acid, (b) trioctylphosphine/trioctylphosphine oxide (TOP/TOPO)-capped NPs bound to a modified acrylic acid polymer through hydrophobic forces, (c) NPs solubilization and bioconjugation using a mercaptosilane compound, (d) positively charged biomolecules linked to negatively charged NPs by electrostatic attraction, and (e) incorporation of NPs into microbeads and nanobeads.92-94 For example, immunoQDs (i.e., Ab-QD bioconjugates) can be produced through different methods, including (a) QDs conjugation to Ab fragments via disulphide reduction and sulfhydryl-amine coupling, (b) covalent coupling between carboxylic acid (-COOH) coated QDs and primary amines (-NH2) on intact Abs using EDC or EDC/NHS chemistry, (c) site-directed conjugation via oxidized carbohydrate groups on the Ab Fc portion and covalent reactions with hydrazide-modified NPs, (d) conjugation of histidine-tagged peptides or Abs to Ni-NTA modified QDs, and (e) noncovalent conjugation of streptavidin-coated QDs to biotinylated Abs.95 Fig. 8 exemplifies two different bioconjugations processes including thiolation of an amine-containing scFv Ab fragment with methyl 3-mercaptopropionimidate (panel A) and conjugation of two scFv Ab fragments with carboxylic acid group and amine group through 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide hydrochloride (EDC) (panel B).
Fig. 7 .

Schematic representation of functionalization of monoclonal antibody (mAb) and conjugation of single chain fragment variable (scFv) antibody fragments. A) SATA-based conjugation of a model mAb. B) SPDP-based conjugation of amine group with sulfhydryl group in two different scFvs. The modified protein (mAb) with a protected sulfhydryl end using SATA can be stored without degradation and subsequently deprotected with an excess of hydroxylamine. SATA: N-succinimidyl S-acetylthioacetate; SPDP: N-succinimidyl 3-(2-pyridyldith io)propionate; NHS: N-hydroxysuccinimide (NHS). Note: not drawn to scale.
Fig. 8 .
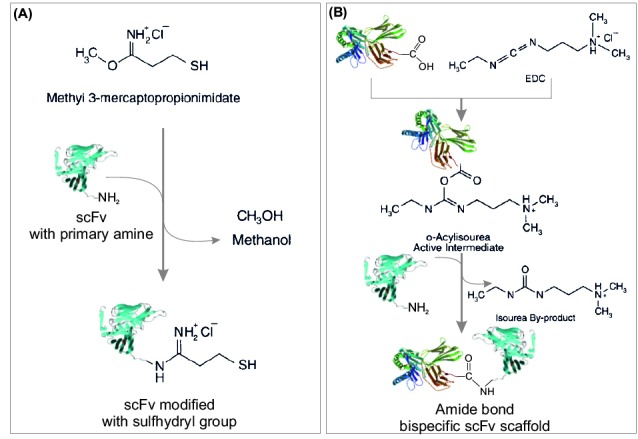
Schematic representation of conjugations of scFv antibody fragments. A) Thiolation of an amine-containing compound with methyl 3-mercaptopropionimidate. B) Application of 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide hydrochloride (EDC). For detailed information, reader is referred to the textbook of “Bioconjugate Techniques”.84
It should be also stated that different cross-linkers have been exploited for surface modification of QDs, including (a) bifunctional linkage (via homo/hetrobifuntional cross-linkers), (b) hydrophobic attraction (TOPO-capped QDs bound to a modified acrylic acid polymer), (c) silanization, and (d) electrostatic attraction. Technically, most QDs’ surfaces for biological applications contain negatively charged carboxylates for conjugation with amine-containing molecules via a carbodiimide reaction with EDC and sulfo-NHS.84,93,96 The QDs have been also exploited for live cell imaging, nonetheless these NSs showed cytotoxic effects to some extent. Accordingly, recent developments in silicon QDs, non-blinking QDs, and QDs with reduced-size and controlled-valence further make these QDs bioanalytically attractive because of their low toxicity, biocompatibility, high quantum yields, and diverse surface modification flexibility. The potential of multiplexed sensing using QDs with different wavelengths of emission is promising for simultaneous detection of multiple biomarkers of disease.97,98 It should be stated that various types of NPs (e.g., polymeric/lipidic NPs, QDs, MNPs and AuNPs) can simply be PEGylated and conjugated using NHS-PEG-maleimide (Fig. 9), which has been widely used for production of multifunctional nanomedicines and theranostics.99-102
Fig. 9 .

Schematic representation for PEGylation and conjugation of nanoparticles using NHS-PEG-Maleimide. NHS: N-hydroxysuccinimide. PEG: poly(ethylene glycol). Note: not drawn to scale.
For example, in 2013, Chan et al. have exploited NHS-PEG-maleimide for development of PEGylated fluorescent polystyrene NPs conjugated with anti-EGFR M225 Abs, which were successfully used for optical molecular imaging in human epidermoid carcinoma A431 cells and lung squamous cell carcinoma NCI-H520 cells as well as human esophageal tissue.
Biological implications of surface modified nanomedicines
The foremost biological barrier against injected NSs is reticuloendothelial system (RES) that can predominantly limit the clinical efficacy of nanomedicines and theranostics. In general, the anatomy and size of NPs play a key role in terms of RES function, that is, NPs >250 nm can be physically trapped by the fenestrations in the spleen while NPs <70 nm can be accumulated in liver. Thus, NPs in a range of 70–200 nm are able to stay in blood stream for a longer periode of time.103 However, these NPs are also subjected to opsonization, which is a process that can lead the foreign particulate invaders to be covered by opsonins and subsequently seen by phagocytic cells that are responsible for sequestration and immune clearance of the invading NPs.104,105 Hence, blocking the electrostatic and hydrophobic interactive surface of NPs by means of surface adsorbed or grafted shielding groups (e.g., long hydrophilic polymer chains and non-ionic surfactants) can help to circumvent the opsonization. Hydrophilic materials (e.g., polysaccharides, polyacrylamide, poly(vinyl alcohol), poly(Nvinyl- 2-pyrrolidone), PEG, and PEG-containing copolymers poloxamers, poloxamines, polysorbates and PEG copolymers) have successfully been exploited.104 Of these, PEGylation is the most widely used method for making stealth NPs. However, regardless of being PEGylated, a 250 nm PEGylated NP can be cleared from the blood stream much quicker than a 70 nm PEGylated NP.
Further, consequences for activation of the complements by NPs may be the reactogenicity functions such as hypersensitivity reactions as reported for liposomal drugs (e.g., Doxil®). To understand the mechanism of such adverse immune reaction, the-so-called C activation-related pseudoallergy (CARPA), Szebeni et al.(2011) analyzed the relationship among liposomes’ features, C activation in human serum in vitro, and liposome-induced cardiovascular distress in a pig model for human CARPA. These researchers found that among the structural variables (e.g., surface charge, presence of saturated/ unsaturated moieties, PEGylation, and use of CP/DOX in liposomal formulations), high negative surface charge and the presence of DOX were the significant contributors in terms of the reactogenicity both in vitro and in vivo, where the effect of DOX appeared to be indirect perhaps through distorting the morphology of liposomes.106 Doxil® mediated complement opsonic fragments was shown to elicit C3b deposition and degradation (65 and 40/43 kDa fragments) that can reach the plateau within 5 min, followed by generation of high molecular weight C3b- and iC3b-containing complexes (C3-X).107 Complement activation by Doxil® has also been reported in cancer patients through significant elevation of SC5b-9 (the terminal complex activation marker of complement system) levels in plasma within 10–30 min of infusion.108 In addition to reactogenicity, multifunctional NPs may act as immunomodulators, activating immune responses where needed. Recently, polysaccharide-based pH-sensitive NSs have been engineered to target mannose-ligands based cell-surface receptors which was able to enhance internalization and activation of antigen presenting cells (APCs).109 This may lead us towards tunable modulation of immune responses. Cui et al. showed that the mannosylated NPs exhibited enhanced antigen presentation in the context of major histocompatibility complex (MHC) class I molecules in dendritic cells (DCs). Such functionalized pH-sensitive NSs seem to open new avenue for vaccine development, in which the conjugation of cell-surface receptor ligands can deliver antigens to specific intracellular pathways and accordingly provide a tool for better controlling the antigen presentation to T cells, or even produce specific signals to manipulate the cytokine production and activation of APCs.
Regarding clinical impacts, specific/nonspecific effects of multifunctional nanomedicines have yet to be fully understood. In general, it seems that the clinical impacts of multifunctional nanomedicines and theranostics, in most of the cases, are largely dependent upon their ability to cross biological membranes and barriers efficiently, to target the desired cells specifically and to interact with/to internalize into the target cells. Upon interaction of nanomedicines with the target CMMs, they are mostly prone to endocytosis through fluid-phase or receptor-mediated endocytosis.51 Various cell surface receptors have so far been reported to be involved in endocytosis phenomenon, including: clathrin coated pits, caveolin proteins, transferrin, EGFR. For example, in ovarian cancer cells, cisplatin (CP) nanocapsules endocytosis and toxicity was showen to be cell-dependent and high cytotoxicity of CP nanocapsules appeared to be largely dependent on expression of caveolin-1 endocytosis followed by release of the drug from a late endosomal/lysosomal compartment and CP-DNA-adduct formation. Thus, cells with higher expression of caveloin-1 (e.g., Igrov-1 cells) shows higher responsiveness to CP nanocapsules compared to those with lower/no expression of caveloin-1 (e.g., Ovcar-3 cells).110 This concept should be taken into account for development of anticancer nanomedicines.
Final remarks
Of various advancements for improved targeted therapy of cancer, seamless multifunctional nanomedicines and theranostics appear to hold great promises. These NSs can be used for simultaneous imagining (optical/non-optical) and therapy of cancerous cells. Ideally, they should represent some important physicochemical and biological features such as (a) long blood circulation time, (b) high tumor-accumulation through passive targeting (EPR effect), (c) specific interaction with cancer cells through active targeting by homing devices, (d) high drug-loading capacity, (e) no/low toxicity, (f) low polydispersity index, and finally (g) simple method of formulation. Formulation of these NSs demand several steps of surface modifications such as PEGylation and conjugation with targeting and imaging devices, which demands integration of several domains for successful engieering of smart and safe seamless NSs. Further, such smart multifunctional NSs must be equipped with suitable stimuli to be able to trigger the liberation of drugs on demand during monitoring of the status of patients with malignancies. Taken all, smart multifunctional NSs provide new promising premises for simultaneous diagnosis and therapy of cancer, and to be much more efficient, they need to be designed based on disease condition leading to personalized targeted therapy of cancer.
Ethical issues
The authors declare no ethical issues.
Competing interests
The authors declare no conflict of interests.
References
- 1.Barar J, Omidi Y. Dysregulated pH in Tumor Microenvironment Checkmates Cancer Therapy. Bioimpacts. 2013;3:149–62. doi: 10.5681/bi.2013.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cappellani A, Zanghi A, Di Vita M, Zanet E, Veroux P, Cacopardo B. et al. Clinical and biological markers in gastric cancer: update and perspectives. Front Biosci (Schol Ed) 2010;2:403–12. doi: 10.2741/s73. [DOI] [PubMed] [Google Scholar]
- 3.Majidi J, Barar J, Baradaran B, Abdolalizadeh J, Omidi Y. Target therapy of cancer: implementation of monoclonal antibodies and nanobodies. Hum Antibodies. 2009;18:81–100. doi: 10.3233/HAB-2009-0204. [DOI] [PubMed] [Google Scholar]
- 4.Kandalaft LE, Powell DJ,Jr, Singh N, Coukos G. Immunotherapy for ovarian cancer: what’s next? J Clin Oncol. 2010;29:925–33. doi: 10.1200/JCO.2009.27.2369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vafadar-Isfahani B, Laversin SA, Ahmad M, Ball G, Coveney C, Lemetre C. et al. Serum biomarkers which correlate with failure to respond to immunotherapy and tumor progression in a murine colorectal cancer model . Proteomics Clin Appl. 2010;4:682–96. doi: 10.1002/prca.200900218. [DOI] [PubMed] [Google Scholar]
- 6.Omidi Y. Smart Multifunctional Theranostics: Simultaneous Diagnosis and Therapy of Cancer. BioiImpacts. 2011;1:145–7. doi: 10.5681/bi.2011.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Grimm J, Scheinberg DA. Will nanotechnology influence targeted cancer therapy? Semin Radiat Oncol. 2011;21:80–7. doi: 10.1016/j.semradonc.2010.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Omidi Y, Dolatabadi JEN, Mashinchian O, Ayoubi B, Jamali AA, Mobed A. et al. Optical and electrochemical DNA nanobiosensors. Trac-Trends in Analytical Chemistry. 2011;30:459–72. [Google Scholar]
- 9.Dolatabadi JEN, Omidi Y, Losic D. Carbon Nanotubes as an Advanced Drug and Gene Delivery Nanosystem. Curr Nanosci. 2011;7:297–314. [Google Scholar]
- 10.Plassat V, Martina MS, Barratt G, Menager C, Lesieur S. Sterically stabilized superparamagnetic liposomes for MR imaging and cancer therapy: pharmacokinetics and biodistribution . Int J Pharm. 2007;344:118–27. doi: 10.1016/j.ijpharm.2007.05.018. [DOI] [PubMed] [Google Scholar]
- 11.Ramachandran S, Quist AP, Kumar S, Lal R. Cisplatin nanoliposomes for cancer therapy: AFM and fluorescence imaging of cisplatin encapsulation, stability, cellular uptake, and toxicity . Langmuir. 2006;22:8156–62. doi: 10.1021/la0607499. [DOI] [PubMed] [Google Scholar]
- 12.Sofou S, Enmon R, Palm S, Kappel B, Zanzonico P, McDevitt MR. et al. Large anti-HER2/neu liposomes for potential targeted intraperitoneal therapy of micrometastatic cancer . J Liposome Res. 2010;20:330–40. doi: 10.3109/08982100903544185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nurunnabi M, Cho KJ, Choi JS, Huh KM, Lee YK. Targeted near-IR QDs-loaded micelles for cancer therapy and imaging. Biomaterials. 2010;31:5436–44. doi: 10.1016/j.biomaterials.2010.03.057. [DOI] [PubMed] [Google Scholar]
- 14.Kang KW, Chun MK, Kim O, Subedi RK, Ahn SG, Yoon JH. et al. Doxorubicin-loaded solid lipid nanoparticles to overcome multidrug resistance in cancer therapy . Nanomedicine. 2010;6:210–3. doi: 10.1016/j.nano.2009.12.006. [DOI] [PubMed] [Google Scholar]
- 15.Khan MK, Nigavekar SS, Minc LD, Kariapper MS, Nair BM, Lesniak WG. et al. In vivo biodistribution of dendrimers and dendrimer nanocomposites -- implications for cancer imaging and therapy . Technol Cancer Res Treat. 2005;4:603–13. doi: 10.1177/153303460500400604. [DOI] [PubMed] [Google Scholar]
- 16.Rosenholm JM, Sahlgren C, Linden M. Multifunctional mesoporous silica nanoparticles for combined therapeutic, diagnostic and targeted action in cancer treatment . Curr Drug Targets. 2011;12:1166–86. doi: 10.2174/138945011795906624. [DOI] [PubMed] [Google Scholar]
- 17.Mamaeva V, Rosenholm JM, Bate-Eya LT, Bergman L, Peuhu E, Duchanoy A. et al. Mesoporous silica nanoparticles as drug delivery systems for targeted inhibition of Notch signaling in cancer. Mol Ther. 2011;19:1538–46. doi: 10.1038/mt.2011.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Meng H, Liong M, Xia T, Li Z, Ji Z, Zink JI. et al. Engineered design of mesoporous silica nanoparticles to deliver doxorubicin and P-glycoprotein siRNA to overcome drug resistance in a cancer cell line . ACS Nano. 2010;4:4539–50. doi: 10.1021/nn100690m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cho YS, Yoon TJ, Jang ES, Soo Hong K, Young Lee S, Ran Kim O. et al. Cetuximab-conjugated magneto-fluorescent silica nanoparticles for in vivo colon cancer targeting and imaging . Cancer Lett. 2010;299:63–71. doi: 10.1016/j.canlet.2010.08.004. [DOI] [PubMed] [Google Scholar]
- 20.Carpin LB, Bickford LR, Agollah G, Yu TK, Schiff R, Li Y. et al. Immunoconjugated gold nanoshell-mediated photothermal ablation of trastuzumab-resistant breast cancer cells . Breast Cancer Res Treat. 2011;125:27–34. doi: 10.1007/s10549-010-0811-5. [DOI] [PubMed] [Google Scholar]
- 21.Krishna V, Singh A, Sharma P, Iwakuma N, Wang Q, Zhang Q. et al. Polyhydroxy fullerenes for non-invasive cancer imaging and therapy. Small. 2010;6:2236–41. doi: 10.1002/smll.201000847. [DOI] [PubMed] [Google Scholar]
- 22.Lay CL, Liu HQ, Tan HR, Liu Y. Delivery of paclitaxel by physically loading onto poly(ethylene glycol) (PEG)-graft-carbon nanotubes for potent cancer therapeutics. Nanotechnology. 2010;21:065101. doi: 10.1088/0957-4484/21/6/065101. [DOI] [PubMed] [Google Scholar]
- 23.Kang B, Yu D, Dai Y, Chang S, Chen D, Ding Y. Cancer-cell targeting and photoacoustic therapy using carbon nanotubes as “bomb” agents. Small. 2009;5:1292–301. doi: 10.1002/smll.200801820. [DOI] [PubMed] [Google Scholar]
- 24.Kishwar S, Asif MH, Nur O, Willander M, Larsson PO. Intracellular ZnO Nanorods Conjugated with Protoporphyrin for Local Mediated Photochemistry and Efficient Treatment of Single Cancer Cell . Nanoscale Res Lett. 2010;5:1669–74. doi: 10.1007/s11671-010-9693-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yang HM, Park CW, Woo MA, Kim MI, Jo YM, Park HG. et al. HER2/neu Antibody Conjugated Poly(amino acid)-Coated Iron Oxide Nanoparticles for Breast Cancer MR Imaging. Biomacromolecules. 2010;11:2866–72. doi: 10.1021/bm100560m. [DOI] [PubMed] [Google Scholar]
- 26.Lee J, Choi Y, Kim K, Hong S, Park HY, Lee T. et al. Characterization and cancer cell specific binding properties of anti-EGFR antibody conjugated quantum dots. Bioconjug Chem. 2010;21:940–6. doi: 10.1021/bc9004975. [DOI] [PubMed] [Google Scholar]
- 27.Ferreira CS, Papamichael K, Guilbault G, Schwarzacher T, Gariepy J, Missailidis S. DNA aptamers against the MUC1 tumour marker: design of aptamer-antibody sandwich ELISA for the early diagnosis of epithelial tumours. Anal Bioanal Chem. 2008;390:1039–50. doi: 10.1007/s00216-007-1470-1. [DOI] [PubMed] [Google Scholar]
- 28.Lewis Phillips GD, Li G, Dugger DL, Crocker LM, Parsons KL, Mai E. et al. Targeting HER2-positive breast cancer with trastuzumab-DM1, an antibody-cytotoxic drug conjugate. Cancer Res. 2008;68:9280–90. doi: 10.1158/0008-5472.CAN-08-1776. [DOI] [PubMed] [Google Scholar]
- 29.Wang K, Ruan J, Qian Q, Song H, Bao C, Zhang X. et al. BRCAA1 monoclonal antibody conjugated fluorescent magnetic nanoparticles for in vivo targeted magnetofluorescent imaging of gastric cancer. J Nanobiotechnology. 2011;9:23. doi: 10.1186/1477-3155-9-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.El-Sayed IH, Huang X, El-Sayed MA. Surface plasmon resonance scattering and absorption of anti-EGFR antibody conjugated gold nanoparticles in cancer diagnostics: applications in oral cancer. Nano Lett. 2005;5:829–34. doi: 10.1021/nl050074e. [DOI] [PubMed] [Google Scholar]
- 31.Bhirde AA, Patel V, Gavard J, Zhang G, Sousa AA, Masedunskas A. et al. Targeted killing of cancer cells in vivo and in vitro with EGF-directed carbon nanotube-based drug delivery. ACS Nano. 2009;3:307–16. doi: 10.1021/nn800551s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Omidi Y. CNT Nanobombs for Specific Eradication of Cancer Cells: A New Concept in Cancer Theranostics. Bioimpacts. 2011;1:199–201. doi: 10.5681/bi.2011.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tan A, Yildirimer L, Rajadas J, De La Pena H, Pastorin G, Seifalian A. Quantum dots and carbon nanotubes in oncology: a review on emerging theranostic applications in nanomedicine. Nanomedicine (Lond) 2011;6:1101–14. doi: 10.2217/nnm.11.64. [DOI] [PubMed] [Google Scholar]
- 34.Michalet X, Pinaud FF, Bentolila LA, Tsay JM, Doose S, Li JJ. et al. Quantum dots for live cells, in vivo imaging, and diagnostics. Science. 2005;307:538–44. doi: 10.1126/science.1104274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sukhanova A, Nabiev I. Fluorescent nanocrystal quantum dots as medical diagnostic tools. Expert Opin Med Diagn. 2008;2:429–47. doi: 10.1517/17530059.2.4.429. [DOI] [PubMed] [Google Scholar]
- 36.Murray CB, Norris DJ, Bawendi MG. Synthesis and Characterization of Nearly Monodisperse CdE (E = S, Se, Te) Semiconductor Nanocrystallites. J Am Chem Soc. 1993;115:8706–15. [Google Scholar]
- 37.Dabbousi BO, Rodriguez-Viejo J, Mikulec FV, Heine JR, Mattouss IH, Ober R. et al. (CdSe)ZnS core-shell quantum dots: Synthesis and characterization of a size series of highly luminescent nanocrystallites. J Phys Chem. 1997;B101:9463–75. [Google Scholar]
- 38.Wu X, Liu H, Liu J, Haley KN, Treadway JA, Larson JP. et al. Immunofluorescent labeling of cancer marker Her2 and other cellular targets with semiconductor quantum dots. Nat Biotechnol. 2003;21:41–6. doi: 10.1038/nbt764. [DOI] [PubMed] [Google Scholar]
- 39.Jamieson T, Bakhshi R, Petrova D, Pocock R, Imani M, Seifalian AM. Biological applications of quantum dots. Biomaterials. 2007;28:4717–32. doi: 10.1016/j.biomaterials.2007.07.014. [DOI] [PubMed] [Google Scholar]
- 40.Biju V, Itoh T, Anas A, Sujith A, Ishikawa M. Semiconductor quantum dots and metal nanoparticles: syntheses, optical properties, and biological applications. Anal Bioanal Chem. 2008;391:2469–95. doi: 10.1007/s00216-008-2185-7. [DOI] [PubMed] [Google Scholar]
- 41.Obonyo O, Fisher E, Edwards M, Douroumis D. Quantum dots synthesis and biological applications as imaging and drug delivery systems. Crit Rev Biotechnol. 2010;30:283–301. doi: 10.3109/07388551.2010.487184. [DOI] [PubMed] [Google Scholar]
- 42.Rosenthal SJ, Chang JC, Kovtun O, McBride JR, Tomlinson ID. Biocompatible quantum dots for biological applications. Chem Biol. 2011;18:10–24. doi: 10.1016/j.chembiol.2010.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dolatabadi JEN, Mashinchian O, Ayoubi B, Jamali AA, Mobed A, Losic D. et al. Optical and electrochemical DNA nanobiosensors. TrAC Trends in Analytical Chemistry. 2011;30:459–72. [Google Scholar]
- 44.Omidi Y, Hollins AJ, Benboubetra M, Drayton R, Benter IF, Akhtar S. Toxicogenomics of non-viral vectors for gene therapy: a microarray study of lipofectin- and oligofectamine-induced gene expression changes in human epithelial cells. J Drug Target. 2003;11:311–23. doi: 10.1080/10611860310001636908. [DOI] [PubMed] [Google Scholar]
- 45.Omidi Y, Barar J, Akhtar S. Toxicogenomics of cationic lipid-based vectors for gene therapy: impact of microarray technology. Curr Drug Deliv. 2005;2:429–41. doi: 10.2174/156720105774370249. [DOI] [PubMed] [Google Scholar]
- 46.Omidi Y, Hollins AJ, Drayton RM, Akhtar S. Polypropylenimine dendrimer-induced gene expression changes: the effect of complexation with DNA, dendrimer generation and cell type. J Drug Target. 2005;13:431–43. doi: 10.1080/10611860500418881. [DOI] [PubMed] [Google Scholar]
- 47.Hollins AJ, Omidi Y, Benter IF, Akhtar S. Toxicogenomics of drug delivery systems: Exploiting delivery system-induced changes in target gene expression to enhance siRNA activity. J Drug Target. 2007;15:83–8. doi: 10.1080/10611860601151860. [DOI] [PubMed] [Google Scholar]
- 48.Omidi Y, Barar J, Heidari HR, Ahmadian S, Yazdi HA, Akhtar S. Microarray analysis of the toxicogenomics and the genotoxic potential of a cationic lipid-based gene delivery nanosystem in human alveolar epithelial a549 cells. Toxicol Mech Methods. 2008;18:369–78. doi: 10.1080/15376510801891286. [DOI] [PubMed] [Google Scholar]
- 49.Barar J, Hamzeiy H, Mortazavi Tabatabaei SA, Hashemi-Aghdam SE, Omidi Y. Genomic signature and toxicogenomics comparison of polycationic gene delivery nanosystems in human alveolar epithelial A549 cells . Daru. 2009;17:139–47. [Google Scholar]
- 50.Omidi Y, Barar J. Induction of human alveolar epithelial cell growth factor receptors by dendrimeric nanostructures . Int J Toxicol. 2009;28:113–22. doi: 10.1177/1091581809335177. [DOI] [PubMed] [Google Scholar]
- 51.Barar J, Omidi Y. Cellular Trafficking and Subcellular Interactions of Cationic Gene Delivery Nanomaterials. J Pharm Nut Sci. 2011;1:68–81. [Google Scholar]
- 52.Kafil V, Omidi Y. Cytotoxic Impacts of Linear and Branched Polyethylenimine Nanostructures in A431 Cells. BioiImpacts. 2011;1:23–30. doi: 10.5681/bi.2011.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Barar J, Omidi Y. Intrinsic bio-signature of gene delivery nanocarriers may impair gene therapy goals. Bioimpacts. 2013;3:105–9. doi: 10.5681/bi.2013.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yan M, Zhang Y, Xu K, Fu T, Qin H, Zheng X. An in vitro study of vascular endothelial toxicity of CdTe quantum dots. Toxicology. 2011;282:94–103. doi: 10.1016/j.tox.2011.01.015. [DOI] [PubMed] [Google Scholar]
- 55.Hoshino A, Hanada S, Yamamoto K. Toxicity of nanocrystal quantum dots: the relevance of surface modifications. Arch Toxicol. 2011;85:707–20. doi: 10.1007/s00204-011-0695-0. [DOI] [PubMed] [Google Scholar]
- 56.Kennedy LC, Bickford LR, Lewinski NA, Coughlin AJ, Hu Y, Day ES. et al. A new era for cancer treatment: gold-nanoparticle-mediated thermal therapies. Small. 2011;7:169–83. doi: 10.1002/smll.201000134. [DOI] [PubMed] [Google Scholar]
- 57.Benezra M, Penate-Medina O, Zanzonico PB, Schaer D, Ow H, Burns A. et al. Multimodal silica nanoparticles are effective cancer-targeted probes in a model of human melanoma . J Clin Invest. 2011;121:2768–80. doi: 10.1172/JCI45600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Huang IP, Sun SP, Cheng SH, Lee CH, Wu CY, Yang CS. et al. Enhanced chemotherapy of cancer using pH-sensitive mesoporous silica nanoparticles to antagonize P-glycoprotein-mediated drug resistance . Mol Cancer Ther. 2011;10:761–9. doi: 10.1158/1535-7163.MCT-10-0884. [DOI] [PubMed] [Google Scholar]
- 59.Santra S. Fluorescent silica nanoparticles for cancer imaging. Methods Mol Biol. 2010;624:151–62. doi: 10.1007/978-1-60761-609-2_10. [DOI] [PubMed] [Google Scholar]
- 60.Thakare VS, Das M, Jain AK, Patil S, Jain S. Carbon nanotubes in cancer theragnosis. Nanomedicine (Lond) 2010;5:1277–301. doi: 10.2217/nnm.10.95. [DOI] [PubMed] [Google Scholar]
- 61.Ilbasmis-Tamer S, Yilmaz S, Banoglu E, Degim IT. Carbon nanotubes to deliver drug molecules. J Biomed Nanotechnol. 2010;6:20–7. doi: 10.1166/jbn.2010.1099. [DOI] [PubMed] [Google Scholar]
- 62. Tao H, Yang K, Ma Z, Wan J, Zhang Y, Kang Z, et al. In Vivo NIR Fluorescence Imaging, Biodistribution, and Toxicology of Photoluminescent Carbon Dots Produced from Carbon Nanotubes and Graphite. Small 2011. [DOI] [PubMed]
- 63.Vilela D, Anson-Casaos A, Martinez MT, Gonzalez MC, Escarpa A. High NIR-purity index single-walled carbon nanotubes for electrochemical sensing in microfluidic chips . Lab Chip. 2012;12:2006–14. doi: 10.1039/c2lc40099e. [DOI] [PubMed] [Google Scholar]
- 64.Levi-Polyachenko NH, Merkel EJ, Jones BT, Carroll DL, Stewart JHt. Rapid photothermal intracellular drug delivery using multiwalled carbon nanotubes. Mol Pharm. 2009;6:1092–9. doi: 10.1021/mp800250e. [DOI] [PubMed] [Google Scholar]
- 65.Zhou F, Xing D, Ou Z, Wu B, Resasco DE, Chen WR. Cancer photothermal therapy in the near-infrared region by using single-walled carbon nanotubes . J Biomed Opt. 2009;14:021009. doi: 10.1117/1.3078803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Liu Z, Chen K, Davis C, Sherlock S, Cao Q, Chen X. et al. Drug delivery with carbon nanotubes for in vivo cancer treatment. Cancer Res. 2008;68:6652–60. doi: 10.1158/0008-5472.CAN-08-1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bhirde AA, Patel S, Sousa AA, Patel V, Molinolo AA, Ji Y. et al. Distribution and clearance of PEG-single-walled carbon nanotube cancer drug delivery vehicles in mice. Nanomedicine (Lond) 2010;5:1535–46. doi: 10.2217/nnm.10.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gannon CJ, Cherukuri P, Yakobson BI, Cognet L, Kanzius JS, Kittrell C. et al. Carbon nanotube-enhanced thermal destruction of cancer cells in a noninvasive radiofrequency field. Cancer. 2007;110:2654–65. doi: 10.1002/cncr.23155. [DOI] [PubMed] [Google Scholar]
- 69.Sajja HK, East MP, Mao H, Wang YA, Nie S, Yang L. Development of multifunctional nanoparticles for targeted drug delivery and noninvasive imaging of therapeutic effect. Curr Drug Discov Technol. 2009;6:43–51. doi: 10.2174/157016309787581066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Petri-Fink A, Hofmann H. Superparamagnetic iron oxide nanoparticles (SPIONs): from synthesis to in vivo studies--a summary of the synthesis, characterization, in vitro, and in vivo investigations of SPIONs with particular focus on surface and colloidal properties. IEEE Trans Nanobioscience. 2007;6:289–97. doi: 10.1109/tnb.2007.908987. [DOI] [PubMed] [Google Scholar]
- 71.Johannsen M, Thiesen B, Wust P, Jordan A. Magnetic nanoparticle hyperthermia for prostate cancer. Int J Hyperthermia. 2010;26:790–5. doi: 10.3109/02656731003745740. [DOI] [PubMed] [Google Scholar]
- 72.Johannsen M, Gneveckow U, Taymoorian K, Thiesen B, Waldofner N, Scholz R. et al. Morbidity and quality of life during thermotherapy using magnetic nanoparticles in locally recurrent prostate cancer: results of a prospective phase I trial. Int J Hyperthermia. 2007;23:315–23. doi: 10.1080/02656730601175479. [DOI] [PubMed] [Google Scholar]
- 73.Curvo-Semedo L, Diniz M, Migueis J, Juliao MJ, Martins P, Pinto A. et al. USPIO-enhanced magnetic resonance imaging for nodal staging in patients with head and neck cancer. J Magn Reson Imaging. 2006;24:123–31. doi: 10.1002/jmri.20602. [DOI] [PubMed] [Google Scholar]
- 74.Ross RW, Zietman AL, Xie W, Coen JJ, Dahl DM, Shipley WU. et al. Lymphotropic nanoparticle-enhanced magnetic resonance imaging (LNMRI) identifies occult lymph node metastases in prostate cancer patients prior to salvage radiation therapy. Clin Imaging. 2009;33:301–5. doi: 10.1016/j.clinimag.2009.01.013. [DOI] [PubMed] [Google Scholar]
- 75.Heesakkers RA, Hovels AM, Jager GJ, van den Bosch HC, Witjes JA, Raat HP. et al. MRI with a lymph-node-specific contrast agent as an alternative to CT scan and lymph-node dissection in patients with prostate cancer: a prospective multicohort study. Lancet Oncol. 2008;9:850–6. doi: 10.1016/S1470-2045(08)70203-1. [DOI] [PubMed] [Google Scholar]
- 76.Shubayev VI, Pisanic TR, 2nd, Jin S. Magnetic nanoparticles for theragnostics. Adv Drug Deliv Rev. 2009;61:467–77. doi: 10.1016/j.addr.2009.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Heidari Majd M, Asgari D, Barar J, Valizadeh H, Kafil V, Coukos G. et al. Specific targeting of cancer cells by multifunctional mitoxantrone-conjugated magnetic nanoparticles. J Drug Target. 2013;21:328–40. doi: 10.3109/1061186X.2012.750325. [DOI] [PubMed] [Google Scholar]
- 78.Heidari Majd M, Asgari D, Barar J, Valizadeh H, Kafil V, Abadpour A. et al. Tamoxifen loaded folic acid armed PEGylated magnetic nanoparticles for targeted imaging and therapy of cancer. Colloids Surf B Biointerfaces. 2013;106:117–25. doi: 10.1016/j.colsurfb.2013.01.051. [DOI] [PubMed] [Google Scholar]
- 79.Yallapu MM, Foy SP, Jain TK, Labhasetwar V. PEG-functionalized magnetic nanoparticles for drug delivery and magnetic resonance imaging applications. Pharm Res. 2010;27:2283–95. doi: 10.1007/s11095-010-0260-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Nakhlband A, Barar J, Bidmeshkipour A, Heidari HR, Omidi Y. Bioimpacts of anti epidermal growth factor receptor antisense complexed with polyamidoamine dendrimers in human lung epithelial adenocarcinoma cells. J Biomed Nanotechnol. 2010;6:360–9. doi: 10.1166/jbn.2010.1131. [DOI] [PubMed] [Google Scholar]
- 81.Vicent MJ, Ringsdorf H, Duncan R. Polymer therapeutics: clinical applications and challenges for development. Adv Drug Deliv Rev. 2009;61:1117–20. doi: 10.1016/j.addr.2009.08.001. [DOI] [PubMed] [Google Scholar]
- 82.Haag R, Kratz F. Polymer therapeutics: concepts and applications. Angew Chem Int Ed Engl. 2006;45:1198–215. doi: 10.1002/anie.200502113. [DOI] [PubMed] [Google Scholar]
- 83.Duncan R. Polymer therapeutics as nanomedicines: new perspectives. Curr Opin Biotechnol. 2011;22:492–501. doi: 10.1016/j.copbio.2011.05.507. [DOI] [PubMed] [Google Scholar]
- 84. Hermanson GT. Bioconjugate Techniques. 2nd ed. Amesterdam: Elsevier Inc. ; 2008.
- 85.Shin IS, Jang BS, Danthi SN, Xie J, Yu S, Le N. et al. Use of antibody as carrier of oligomers of peptidomimetic alphavbeta3 antagonist to target tumor-induced neovasculature. Bioconjug Chem. 2007;18:821–8. doi: 10.1021/bc0603485. [DOI] [PubMed] [Google Scholar]
- 86.Schellenberger EA, Sosnovik D, Weissleder R, Josephson L. Magneto/optical annexin V, a multimodal protein. Bioconjug Chem. 2004;15:1062–7. doi: 10.1021/bc049905i. [DOI] [PubMed] [Google Scholar]
- 87.Schellenberger EA, Reynolds F, Weissleder R, Josephson L. Surface-functionalized nanoparticle library yields probes for apoptotic cells. Chembiochem. 2004;5:275–9. doi: 10.1002/cbic.200300713. [DOI] [PubMed] [Google Scholar]
- 88.Strehblow C, Schuster M, Moritz T, Kirch HC, Opalka B, Petri JB. Monoclonal antibody-polyethyleneimine conjugates targeting Her-2/neu or CD90 allow cell type-specific nonviral gene delivery. J Control Release. 2005;102:737–47. doi: 10.1016/j.jconrel.2004.10.033. [DOI] [PubMed] [Google Scholar]
- 89.Yang LJ, Sui YF, Chen ZN. Preparation and activity of conjugate of monoclonal antibody HAb18 against hepatoma F(ab’)(2) fragment and staphylococcal enterotoxin A. World J Gastroenterol. 2001;7:216–21. doi: 10.3748/wjg.v7.i2.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hoppmann S, Miao Z, Liu S, Liu H, Ren G, Bao A. et al. Radiolabeled affibody-albumin bioconjugates for HER2-positive cancer targeting. Bioconjug Chem. 2011;22:413–21. doi: 10.1021/bc100432h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Pereira M, Lai EP. Capillary electrophoresis for the characterization of quantum dots after non-selective or selective bioconjugation with antibodies for immunoassay. J Nanobiotechnology. 2008;6:10. doi: 10.1186/1477-3155-6-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Frasco MF, Chaniotakis N. Bioconjugated quantum dots as fluorescent probes for bioanalytical applications. Anal Bioanal Chem. 2009;396:229–40. doi: 10.1007/s00216-009-3033-0. [DOI] [PubMed] [Google Scholar]
- 93.Chan WC, Maxwell DJ, Gao X, Bailey RE, Han M, Nie S. Luminescent quantum dots for multiplexed biological detection and imaging. Curr Opin Biotechnol. 2002;13:40–6. doi: 10.1016/s0958-1669(02)00282-3. [DOI] [PubMed] [Google Scholar]
- 94.Chang YP, Pinaud F, Antelman J, Weiss S. Tracking bio-molecules in live cells using quantum dots. J Biophotonics. 2008;1:287–98. doi: 10.1002/jbio.200810029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Xing Y, Chaudry Q, Shen C, Kong KY, Zhau HE, Chung LW. et al. Bioconjugated quantum dots for multiplexed and quantitative immunohistochemistry. Nat Protoc. 2007;2:1152–65. doi: 10.1038/nprot.2007.107. [DOI] [PubMed] [Google Scholar]
- 96.Medintz IL, Uyeda HT, Goldman ER, Mattoussi H. Quantum dot bioconjugates for imaging, labelling and sensing. Nat Mater. 2005;4:435–46. doi: 10.1038/nmat1390. [DOI] [PubMed] [Google Scholar]
- 97.Wang Y, Chen L. Quantum dots, lighting up the research and development of nanomedicine. Nanomedicine. 2011;7:385–402. doi: 10.1016/j.nano.2010.12.006. [DOI] [PubMed] [Google Scholar]
- 98.Wagner MK, Li F, Li J, Li XF, Le XC. Use of quantum dots in the development of assays for cancer biomarkers. Anal Bioanal Chem. 2010;397:3213–24. doi: 10.1007/s00216-010-3847-9. [DOI] [PubMed] [Google Scholar]
- 99.Chan LW, Wang YN, Lin LY, Upton MP, Hwang JH, Pun SH. Synthesis and characterization of anti-EGFR fluorescent nanoparticles for optical molecular imaging. Bioconjug Chem. 2013;24:167–75. doi: 10.1021/bc300355y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Girgis MD, Federman N, Rochefort MM, McCabe KE, Wu AM, Nagy JO. et al. An engineered anti-CA19-9 cys-diabody for positron emission tomography imaging of pancreatic cancer and targeting of polymerized liposomal nanoparticles. J Surg Res. 2013;185:45–55. doi: 10.1016/j.jss.2013.05.095. [DOI] [PubMed] [Google Scholar]
- 101.Wang Y, Liu P, Duan Y, Yin X, Wang Q, Liu X. et al. Specific cell targeting with APRPG conjugated PEG-PLGA nanoparticles for treating ovarian cancer. Biomaterials. 2014;35:983–92. doi: 10.1016/j.biomaterials.2013.09.062. [DOI] [PubMed] [Google Scholar]
- 102.Feng G, Li K, Liu J, Ding D, Liu B. Bright single-chain conjugated polymer dots embedded nanoparticles for long-term cell tracing and imaging. Small. 2014;10:1212–9. doi: 10.1002/smll.201302161. [DOI] [PubMed] [Google Scholar]
- 103.Shan X, Yuan Y, Liu C, Tao X, Sheng Y, Xu F. Influence of PEG chain on the complement activation suppression and longevity in vivo prolongation of the PCL biomedical nanoparticles. Biomed Microdevices. 2009;11:1187–94. doi: 10.1007/s10544-009-9336-2. [DOI] [PubMed] [Google Scholar]
- 104.Owens DE, 3rd, Peppas NA. Opsonization, biodistribution, and pharmacokinetics of polymeric nanoparticles. Int J Pharm. 2006;307:93–102. doi: 10.1016/j.ijpharm.2005.10.010. [DOI] [PubMed] [Google Scholar]
- 105.Moghimi SM, Szebeni J. Stealth liposomes and long circulating nanoparticles: critical issues in pharmacokinetics, opsonization and protein-binding properties. Prog Lipid Res. 2003;42:463–78. doi: 10.1016/s0163-7827(03)00033-x. [DOI] [PubMed] [Google Scholar]
- 106.Szebeni J, Bedocs P, Rozsnyay Z, Weiszhar Z, Urbanics R, Rosivall L. et al. Liposome-induced complement activation and related cardiopulmonary distress in pigs: factors promoting reactogenicity of Doxil and AmBisome. Nanomedicine. 2011;8:176–84. doi: 10.1016/j.nano.2011.06.003. [DOI] [PubMed] [Google Scholar]
- 107.Moghimi SM, Andersen AJ, Hashemi SH, Lettiero B, Ahmadvand D, Hunter AC. et al. Complement activation cascade triggered by PEG-PL engineered nanomedicines and carbon nanotubes: the challenges ahead. J Control Release. 2010;146:175–81. doi: 10.1016/j.jconrel.2010.04.003. [DOI] [PubMed] [Google Scholar]
- 108.Chanan-Khan A, Szebeni J, Savay S, Liebes L, Rafique NM, Alving CR. et al. Complement activation following first exposure to pegylated liposomal doxorubicin (Doxil): possible role in hypersensitivity reactions. Ann Oncol. 2003;14:1430–7. doi: 10.1093/annonc/mdg374. [DOI] [PubMed] [Google Scholar]
- 109.Cui L, Cohen JA, Broaders KE, Beaudette TT, Frechet JM. Mannosylated dextran nanoparticles: a pH-sensitive system engineered for immunomodulation through mannose targeting. Bioconjug Chem. 2011;22:949–57. doi: 10.1021/bc100596w. [DOI] [PubMed] [Google Scholar]
- 110.Hamelers IH, Staffhorst RW, Voortman J, de Kruijff B, Reedijk J, van Bergen en Henegouwen PM . et al. High cytotoxicity of cisplatin nanocapsules in ovarian carcinoma cells depends on uptake by caveolae-mediated endocytosis. Clin Cancer Res. 2009;15:1259–68. doi: 10.1158/1078-0432.CCR-08-1702. [DOI] [PubMed] [Google Scholar]


