Abstract
This study tested the effects of aging and race on responses to noxious stimuli using a wide range of stimulus modalities. The participants were 53 non-Hispanic Blacks and 138 non-Hispanic White adults, ages 45 to 76. The participants completed a single 3-hour sensory testing session where responses to thermal, mechanical, and cold stimuli were assessed. The results suggest that there are selected age differences, with the older group less sensitive to warm and painful heat stimuli than middle-aged participants, particularly at the knee. This site effect supports the hypothesis that the greatest decrement in pain sensitivity associated with aging occurs in the lower extremities. In addition, there were several instances where age and race effects were compounded, resulting in greater race differences in pain sensitivity among the older participants. Overall, the data suggest that previously reported race differences in pain sensitivity emerged in our older samples, and this study contributes new findings in that these differences may increase with age in non-Hispanic Blacks for temporal summation and both heat and cold immersion tolerance. We have added to the aging and pain literature by reporting several small to moderate differences in responses to heat stimuli between middle and older age adults.
Keywords: aging, race, threshold, temporal summation, conditioned pain modulation
It is often reported that older adults experience increased prevalence of pain, greater pain intensity, and pain at more sites compared to younger adults.(5,18,23,34,35,38,42) It has long been thought that the increase in the prevalence of pain among older adults is partly due to the progressive musculoskeletal degeneration that accompanies aging.(16,20,27) Another explanation for increased pain in older populations has been that aging is associated with greater sensitivity to painful stimuli that results from changes in the structure and function of the nociceptive system.(11)
The strongest data documenting age-cohort differences in laboratory-induced pain comes from thermal heat stimuli.(12) Stevens and Choo provided extensive body maps of thermal thresholds at various ages and found that the greatest decline in sensitivity occurs in the extremities, with a greater change at the calf and thigh than the forearm.(40) Using more sophisticated experimental pain testing methods, some but not all studies have found age differences in temporal summation of pain, suggesting an age-related increase in pain facilitation.(8,10,17,25) Moreover, loss of inhibition has been demonstrated among older adults using conditioned pain modulation models.(6,24,37,49) One study suggests that some pain inhibitory mechanisms start declining at middle-age, although this has not been replicated, and is one of the aims of this study.(24)
Race and ethnicity are another set of psychosocial factors that are associated with increased pain across most settings and all types of pain.(13,36,39) As with age, it has been hypothesized that increased sensitivity to pain across race groups may contribute to the greater severity of clinical pain among minority adults.(3,9,32) Typically the largest differences in experimental pain sensitivity between non-Hispanic Blacks and non-Hispanic White healthy young adults occur for pain tolerance, suprathreshold pain ratings, and temporal summation.(32) A potential weakness of laboratory-based pain studies of aging is that the samples were often not racially/ethnically diverse. Consequently, studies reporting on age differences in pain have not directly tested the confluence of race and aging on pain processing.
Therefore, the primary hypothesis of this study is that older adults will show higher pain thresholds, increased temporal summation, and decreased pain inhibition compared to a middle-aged cohort. We also expect that the effects of aging will be stronger in the knee (distal site) compared to the forearm. Given that age and race are both risk factors for increased pain sensitivity, we hypothesize an interaction between age and race such that race differences become more pronounced with older age. It is also expected that non-Hispanic Blacks will exhibit decreased pain tolerance, increased suprathreshold pain ratings, and greater temporal summation of pain. The relatively large and racially diverse sample, allowing us to test groups of middle-aged and older adults across a range of stimulus modalities, sites, and pain measures, are strengths of this study.
METHODS
Subjects
The participants were 53 non-Hispanic Black (NHB) and 138 non-Hispanic White (NHW) adults between the ages of 45 and 76, with a mean age of 57.6 (SD = 7.9). By age, there were 89 participants between the ages of 45–56 and 102 who were 57–76. Other demographic and health variables are presented in Table 1. All subjects were recruited as part of a larger multi-site study examining race differences in knee osteoarthritic pain (Understanding Pain and Limitations in Osteoarthritic Disease, UPLOAD), and received the same protocol.
Table 1.
Demographic and health variables by age and race.
| Variable) | Middle-aged n = 89 |
Older aged n = 102 |
NHW n = 138 |
NHB n = 53 |
|---|---|---|---|---|
|
| ||||
| Mean (SD) or n (%) | Mean (SD) or n (%) | Mean (SD) or n (%) | Mean (SD) or n (%) | |
| Female | 61 (69%) | 69 (68%) | 94 (67%) | 36 (68%) |
| Attended at least some college (missing = 7) | 57 (67%) | 76 (77%) | 102 (77%) a | 32 (60%) a |
| CES-D | 5.6 (SD=5.0) | 6.5 (SD=5.4) | 5.6 (SD=5.0) a | 7.7 (SD=6.5) a |
| PANAS (negative affect) | 15.0 (SD=6.6) | 14.2 (SD=3.8) | 14.9 (SD=5.9) | 13.8 (SD=3.3) |
| PANAS (positive affect) | 37.5 (SD=7.6) | 36.8 (SD=6.9) | 37.0 (SD=6.3) | 37.3 (SD=9.1) |
| STAXI (state) | 16.2 (SD=3.7) | 16.2 (SD=4.7) | 16.4 (SD=4.9) | 15.7 (SD=1.8) |
| STAXI (trait) | 14.4 (SD=3.9) | 14.5 (SD=3.7) | 14.8 (SD=3.9) | 13.7 (SD=3.3) |
| Knee OA | 35 (39%) | 40 (39%) | 53 (38%) | 22 (42%) |
| GCPS score=1 | 58 (65%) | 69 (68%) | 88 (64%) | 39 (73%) |
Means differed at p < .05
These data were collected at the Clinical Research Centers of the University of Florida and University of Alabama at Birmingham. The Institutional Review Board at each participating center approved the study and written informed consent was obtained from each participant prior to enrollment as per the Declaration of Helsinki for the involvement of humans in research. Participants were compensated for their participation.
Recruitment and sampling
A total of 693 individuals responded to study recruitment material. The same methods were used for recruiting NHB and NHW participants: local media ads, posted ads, word of mouth. The recruitment of NHB participants was open for longer to fulfill the targeted stratification. Three hundred and fifty-three were not eligible following a telephone screening or in-person health assessment leaving 340 who completed the study experimental testing session. The sampling design included a non-knee osteoarthritic (OA) control group (n=116). For this set of analyses, 75 participants with mild levels of knee OA who scored as Grade 1 (low pain – low disability) on the Graded Chronic Pain Scale were included to increase the generalizability of our findings as many adults of this age range experience knee pain. Forty-five percent of the non-knee OA control group also received a Grade of 1 which requires having had “any pain” in the past 6 months which resulted in no more than mild interference with daily activities. An earlier manuscript from UPLOAD has demonstrated that individuals in the low symptomatic OA exhibited experimental pain responses similar to the non-knee pain control group.(21)
Study Procedures
Potential participants who passed the telephone screening completed a health assessment session that included vital signs and a comprehensive health history. In addition, participants completed the study questionnaires described below. Demographic characteristics, socioeconomic status, and health data were self-reported as part of the health history. The study inclusion criteria included self-identification as “Black or African American” or “White, Caucasian, or European”, and non-Hispanic with a chronological age between 45 and 85 years. We selected the arbitrary cut-point of 57 years of age to keep our middle-aged group similar to that used by Larivière(24) (40–55 years of age) and allow direct comparisons to older samples from Larivière (60–75 years of age), as well as the studies by Edwards and Fillingim,(7) where the older group was 55–67 years of age and the study by Lautenbacher(25) (mean age 65, age range not reported). In addition, this cut point resulted in a 50–50 split for the NHB sample. The results did not differ substantively when cut points of 55 and 60 years of age were tested.
Potential participants were excluded if they had uncontrolled hypertension (greater than 150/95), a history of acute myocardial infarction, peripheral neuropathy, systemic rheumatic disorders, daily opioid use, cognitive impairment (Mini Mental Status Exam score of ≤ 22), excessive anxiety regarding protocol procedures, or hospitalization within the preceding year for psychiatric illness.
Within four weeks of the health assessment session, participants were scheduled for a 3-hour testing session where responses to thermal, mechanical, and cold stimuli were assessed. The experimental session started with insertion of an intravenous cannula followed by a 10-minute rest period, during which blood pressure and heart rate were monitored. Stimulus modalities and tests were balanced and ordered such that protocols with greater stimulus strength were given later in the session to reduce carry-over effects.
Pain Testing Procedures
Thermal and mechanical (blunt pressure and punctate) stimuli were delivered first in counterbalanced order. Before each pain testing procedure, standardized recorded instructions were played for all participants.
Thermal testing
All thermal stimuli were delivered using a computer-controlled Medoc Pathway Thermal Sensory Analyzer (Ramat Yishai, Israel). The first thermal procedure assessed warm detection threshold (WTh), heat pain threshold (HPTh) and heat pain tolerance (HPTo) using the ascending method of limits. From a baseline of 32°C, probe temperature increased at a rate of 0.5°C/s until the participant responded by pressing a button to indicate when they first felt warmth, when they first felt pain, and when they no longer felt able to tolerate the pain. These procedures were performed at both the ventral forearm, three sites an inch above the ventral wrist and an inch below the antecubital space; followed by the knee assessed once each at the medial joint line, the patella, and the tibial tuberosity distal to the joint in order to avoid either sensitization or response suppression of cutaneous heat nociceptors. Three trials of warm detection, heat pain threshold, and heat pain tolerance at the forearm and the knee were recorded with the average temperature for the trials serving as dependent measures. The forearm is a commonly tested site for quantitative sensory testing and allowed for comparisons with Edwards(7) and Lautenbacher.(25) The knee was selected to provide a lower extremity site, and it allowed a symptomatic vs. non-symptomatic comparison among knee osteoarthritic participants in the larger study.
The temporal summation of heat pain procedure (HPTS) was administered to the right dorsal forearm using 44°C, 46°C, and 48°C. This was followed by a series of temporal summation procedures using 44°C, 46°C, and 48°C at the knee. The site locations were as described above. Following a practice trial, participants rated thermal pain intensity of 5 repetitive heat pulses delivered for less than 1s, with a 2.5s inter-pulse interval during which the temperature of the contact returned to a baseline of 32°C. Participants rated the peak pain for each of the 5 heat pulses on a scale from 0 (no sensation) to 100 (the most intense pain imaginable). The pain rating for the first pulse subtracted from the most painful rating at any other pulse served as the measure of temporal summation of heat pain (HPTS). For the HPTS and conditioned pain modulation analysis (CPM), the lowest temperature that resulted in a maximum rating equal or greater than 50 for any pulse was used so that temperatures could be individualized for each participant. Suprathreshold pain ratings (SPR) were calculated using the pain rating from the first trial of each temperature series to eliminate any effects of temporal summation.
Mechanical testing
A handheld Medoc digital pressure algometer (Ramat Yishai, Israel) was used for the first set of mechanical procedures using an application rate of 30 kilopascals (kPa) per second. To assess pressure pain threshold (PPTh), the examiner applied a constant rate of pressure and the participant was instructed to press a button when they first felt pain at which time the device recorded the pressure in kPa. The average of the three trials was computed separately for the medial and lateral knee, and for the ipsilateral forearm to form overall pressure pain sensitivity scores for the knee and the ipsilateral forearm. Also, punctate mechanical stimuli (PMP) were delivered to the patella (knee) and the dorsal surface of the hand using a 300g nylon monofilament. This test involved delivering one initial stimulus and a series of 10 repeated stimuli. Temporal summation of punctate mechanical pain (PMPTS) was calculated by subtracting the single-trial pain rating from the peak pain rating from the series of 10 stimuli.
Cold water immersion
Cold pain was assessed by having participants immerse their right hand up to the wrist in cold water with their fingers spread as far as possible for up to 1 minute. Each participant observed a 10-minute rest before the cold water immersion procedures.
Trials were performed at 16°C, 12°C, and 8°C in des cending order, with data from the 12°C, and 8°C trials presented in this report, beca use these were the temperatures reported as frankly painful by most participants. The water temperature was maintained (± 1°C) by a refrigeration unit (Neslab, Portsmouth, NH), and was constantly circulated to prevent local warming around the submerged hand. Participants reported when they first felt pain (CPTh) and were prompted to rate the unpleasantness or intensity of the cold pain (CP) using the 0–100 scale, 55 seconds after immersion. Participants who removed their hand prior to the targeted 60-seconds of immersion provided a pain rating at that time. This was followed by a 20-minute rest period.
Conditioned pain modulation protocol
Conditioned pain modulation (CPM) was assessed by determining the change in heat pain on the left forearm following immersion of the contralateral hand in the cold-water bath. The CPM series began with a HPTS procedure (CPM1) and pain ratings (as described above) at the forearm to serve as the baseline, after which the participant immersed their contralateral hand in the cold water for up to 60 seconds. After 60 seconds of immersion, a second temporal summation series was applied to the left arm (CPM2). After a five-minute rest, the TS procedure was performed again (CPM3). This was followed by a second cold-water immersion and two additional TS series (CPM4, CPM5). The average heat pain rating achieved during each of the series was used as the CPM dependent variable. If the participant missed a rating for one of the pulses, the average rating of the other four pulses was substituted. The temperatures from the temporal summation procedure and cold pressor task that resulted in a pain rating of approximately 50 were used in the CPM protocol.
Other measures
Graded Chronic Pain Scale (GCPS)
The GCPS is a 7-item scale that asks about pain severity and disability that results from pain over the past 6 months and was designed to assess pain in the general population.(47)
Center for Epidemiological Studies – Depression Scale (CES-D)
The CES-D is a 20-item measure of symptoms of depression that has been shown to be reliable and valid in both general and clinical populations.(32)
Positive and Negative Affect Schedule (PANAS)
The PANAS is a 20-item scale measuring both positive and negative affect. Each item indicates the extent to which the respondent has felt this way in the indicated time frame.(50)
State-Trait Anger Expression Inventory (STAXI)
The STAXI is a 44 item inventory that measures the intensity of anger as an emotional state and the disposition toward anger as a personality trait.(40)
Statistical methods
The dependent variables for this study were warm detection, thermal pain threshold and tolerance temperatures; pressure pain thresholds; temporal summation of heat and mechanical pain ratings; cold pressor pain threshold times and suprathreshold ratings. Functional pain range was calculated by subtracting each participant’s pain threshold temperature from their pain tolerance temperature. Our primary hypotheses involved testing for differences between two naturally occurring groups categorized as middle-aged, 45–56 years of age and older adults, 57–76 years. Self-reported race was included as an independent variable, with main and interaction effects with age tested. ANCOVA models were used to adjust for preexisting differences in non-equivalent (intact) groups. When families of dependents were highly correlated (r > 0.50), MANCOVA models were used to reduce the overall number of tests performed. No other adjustments were made for the number of tests performed. When time or trial was involved, repeated measures ANOVAs were used. Mauchly’s Test of Sphericity assessed whether the assumption of sphericity had been violated;(29) and if so, we applied the Greenhouse-Geisser correction.(14) Gender was used as a covariate in all analyses. GCPS, CES-D, PANAS, and STAXI scores were tested as covariates but were subsequently dropped as they did not significantly contribute to any of the tested models. For the HPTS and CPM analyses, the temperatures for the heat probe and water bath (for CPM) were also added as covariates. Participants who reported no pain during the first trial of HPTS or CPM1 were dropped from the respective analyses.
RESULTS
Thermal stimuli
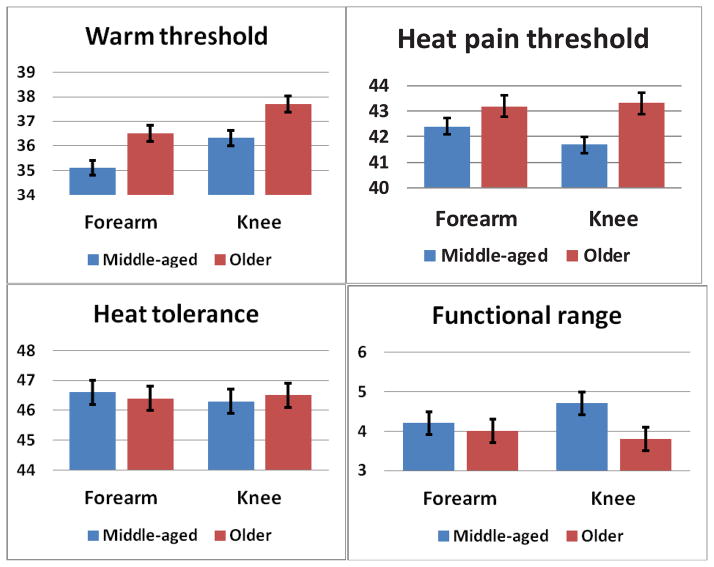
Table 2 presents means and standard deviations for the heat pain responses for the age and race groups (also see Figure 1). WTh at the arm and knee were weakly correlated (r = .17, p = .019) and were tested individually. A significant main effect of Age was found for WTh at the forearm [F (1,186) = 8.690, p = .004] and at the knee [F (1,186) = 7.404, p = .007], with older adults showing higher warm thresholds compared to the middle-aged group. Other effects were not significant. HPTh at the arm and knee were correlated (r = .58, p < .001) and a MANOVA model was used. The multivariate main effects for Age [F (2,185) = 5.160, p = .007] and Race [F (2,185) = 3.641, p = .028] were significant. Tests of means indicated that the age effect was driven by significant differences at the knee (p=.003) and not the forearm with pain threshold higher for older adults compared to the middle-aged group. The race effect was driven by significant differences at the forearm (p = .008) and not the knee with pain threshold lower for NHB compared to the NHW.
Table 2.
Heat pain responses for the age and race groups.
| Pain measure (site) | Middle-aged (45–56) n = 89 |
Older-aged (57–79) n = 102 |
NHW n = 138 |
NHB n = 53 |
|---|---|---|---|---|
|
| ||||
| Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | |
| WTh (forearm) | 35.2°C (1.8) a | 36.3°C (2.6) a | 35.6°C (2.4) | 35.9°C (2.2) |
| WTh (knee) | 36.5°C (2.6) a | 37.6°C (2.4) a | 37.1°C (2.7) | 37.0°C (2.2) |
| HPTh (forearm) | 42.2°C (2.6) | 42.5°C (2.8) | 43.0°C (2.6) | 41.8°C (2.8) |
| HPTh (knee) | 41.6°C (2.7) a | 43.0°C (2.6) a | 42.7°C (2.7) | 41.9°C (3.2) |
| HPTo (forearm) | 46.6°C (1.6) | 46.4°C (2.1) | 47.3°C (2.0) b | 45.7°C (1.4) b |
| HPTo (knee) | 46.3°C (1.7) | 46.5°C (1.8) | 47.1°C (1.8) b | 45.7°C (2.0) b |
| Pain range (forearm) | 4.2°C (2.2) | 4.0°C (2.3) | 4.2°C (2.1) | 3.9°C (2.6) |
| Pain range (knee) | 4.7°C (2.2) a | 3.8°C (2.1) a | 4.4°C (2.2) | 4.5°C (2.7) |
| SPR rating (forearm) | ||||
| 44°C | 26.5 (22.5) | 22.6 (20.8) | 20.2 (19.2) | 28.9 (25.9) |
| 46°C | 30.2 (23.6) | 28.6 (24.3) | 24.5 (21.6) | 34.2 (27.9) |
| 48°C | 36.6 (26.3) | 31.2 (24.7) | 29.2 (23.2) | 38.7 (29.6) |
| SPR rating (knee) | ||||
| 44°C | 20.9 (19.9) | 23.2 (21.3) | 16.2 (17.5) | 28.1 (25.2) |
| 46°C | 29.0 (22.8) | 28.6 (25.1) | 21.9 (19.5) | 36.7 (28.4) |
| 48°C | 35.2 (26.7) | 32.2 (24.3) | 26.0 (22.1) | 41.4 (30.8) |
| HPTS (forearm) | ||||
| Rating for trial 1 | 34.1 (21.6) | 30.2 (19.6) | 30.8 (18.3) | 33.5 (24.6) |
| Max rating any trial | 47.9 (21.8) | 50.8 (24.6) | 42.5 (21.7) | 56.2 (22.3) |
| ΔHPTS | 13.7 (16.4) a | 20.6 (17.9) a | 11.7 (14.6) b | 20.5 (22.7) b |
| HPTS (knee) | ||||
| Rating for trial 1 | 30.6 (23.0) | 27.3 (22.8) | 26.3 (19.3) | 33.6 (26.8) |
| Max rating any trial | 44.4 (26.6) | 40.9 (25.6) | 35.5 (23.8) | 49.9 (26.3) |
| ΔHPTS | 13.8 (16.3) | 11.9 (13.0) | 9.1 (16.5) b | 16.5 (13.6) b |
| CPM (ratings) | ||||
| CPM1 (baseline) | 45.8 (22.5) | 41.4 (25.5) | 40.5 (25.0) | 51.0 (24.5) |
| CPM2 (post CP #1) | 51.4 (25.5) | 42.5 (25.6) | 41.2 (25.1) | 60.2 (23.0) |
| CMP3 (pre CP #2) | 49.5 (24.2) | 43.8 (25.4) | 41.9 (24.5) | 58.0 (22.6) |
| CPM4 (post CP #2) | 46.5 (23.4) | 41.7 (25.5) | 39.7 (23.9) | 54.9 (23.2) |
| CPM5 (post CP #2) | 48.0 (24.7) | 41.2 (26.8) | 39.0 (24.2) | 58.3 (25.5) |
Adjusted means differed at p < .05 across age
Adjusted means differed at p < .05 across race
Abbreviations: Warm detection threshold (WTh), Heat pain threshold (HPTh), Heat pain tolerance (HPTo), Suprathreshold pain ratings (SPR), Heat pain temporal summation (HPTS), Conditioned pain modulation (CPM), Cold pressor (CP).
Figure 1.
Differences in pain ratings (0–100) between the middle-aged and older group were found for warm threshold (p = .045), pain threshold (p = .013) and functional pain range (p = .007) at the knee and warm threshold at the forearm (p = .005). No differences were found for heat tolerance at either site.
HPTo at the arm and knee were highly correlated (r = .82, p < .001) and a MANOVA model was used. An overall effect of Race was found [F (2,185) = 16.214, p < .001], with NHB showing lower HPTo at both the arm and knee. In addition, the Age × Race interaction was significant [F (2,185) = 3.578, p = .030], with the effect significant only at the forearm (p = .033). Pair-wise comparisons revealed that the older NHB exhibited the lowest HPTo (M = 45.3, SD = 1.8) compared to the other three groups, with no differences between the middle-aged NHB (M = 47.1, SD = 1.8), the middle-aged NHW (M = 47.1, SD = 1.1), and older NHW (M = 47.5, SD = 21.0).
Thermal pain range (HPTo - HPTh) at the arm and knee were only moderately correlated (r = .35, p < .001) and separate ANOVA models were used. For the knee, a significant main effect for age [F (1,186) = 8.785, p = .003] was found for the thermal pain range, with a smaller range for the older group compared to the middle-aged group. There were no significant effects for pain range at the forearm.
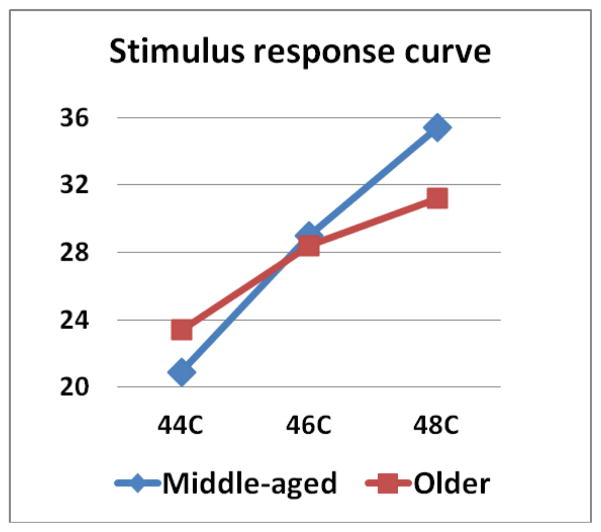
For tests of thermal SPR, repeated-measures ANOVAs were used. At the forearm, there was a significant main effect of Race [F (1,183) = 6.365, p = .012] with NHB showing overall higher pain ratings than NHW. Similarly, when testing at the knee, there was a significant main effect of Race [F (1,181) = 18.564), p < .001] with NHB showing higher overall pain ratings than HW. There was also a significant Age × Temperature interaction [F (1,181) = 4.531), p = .035] when fitting a linear model. The middle-aged group showed a greater increase in pain ratings across the three temperatures than the older group (see Figure 2). No other effects were significant.
Figure 2.
Stimulus response curves for suprathreshold pain ratings (0–100) at 44°C, 46°C, 48°C tested at the knee for the middle-aged and older groups. The pain ratings for the middle-aged group increased significantly more across the three temperatures than the older group.
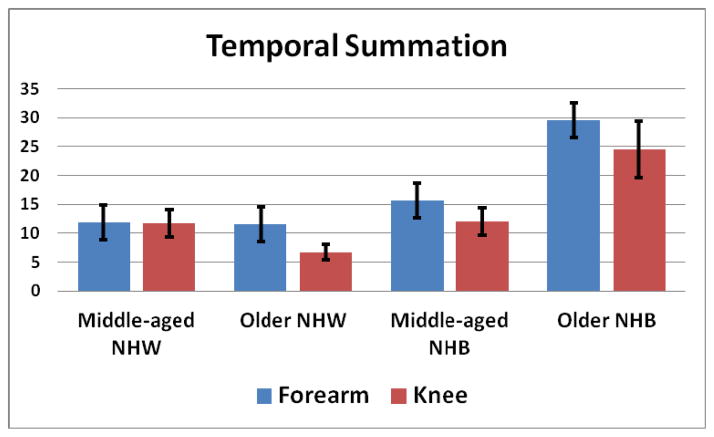
When testing HPTS at the forearm, the Trial × Age [F (1,182) = 5.928, p = .016], Trial × Race [F (1,182) = 14.607, p < .001], and Trial × Age × Race interactions [F (1,182) = 6.753, p = .010] were significant. The older group showed a greater increase from the first to the maximum painful trial compared to the middle-aged group. In addition, the NHB group showed a greater increase than NHW. Inspection of Figure 3 shows that the older NHB experienced greater HPTS than the other three groups at the forearm. Tests of HPTS at the knee resulted in significant Trial × Race [F (1,174) = 7.192, p = .008] and Trial × Age × Race [F (1,174) = 6.864, p = .010] interactions. The NHB group showed a greater increase than NHW. Examination of Figure 3 shows that the older NHB experienced greater HPTS than the other three groups at the knee. In addition, the older NHW experienced less HPTS at the knee compared to the other three groups.
Figure 3.
Age × Race interactions for temporal summation calculated as the difference in pain ratings between the maximum rated pulse and first pulse. At the forearm, Older NHB (M = 29.6, SD = 23.9) experienced greater temporal summation than the other groups, whereas there was no difference between middle-aged NHB (M = 15.6, SD = 18.5), middle-aged NHW (M = 11.9, SD = 18.7), and older NHW (M = 11.5, SD = 14.5). At the knee, older NHB (M = 24.5, SD = 19.9) experienced greater temporal summation than the other 3 groups. In addition, older NHW (M = 6.5, SD = 8.7) experienced less temporal summation at the knee that the middle-aged NHB (M = 12.0, SD = 15.9) and middle-aged NHW (M = 11.7, SD = 19.3) groups.
The main effect of time for the conditioned pain modulation analysis was not significant [F (3.320, 320.362) = 1.253, p = .288] indicating that there was no effect of the CPM manipulation.
Mechanical and cold stimuli
Table 3 presents means and standard deviations for the responses to the mechanical and cold stimuli for age and race groups. PPTh at the medial and lateral knee (r = .71) and at the quadriceps, trapezius, and forearm (r = .56-.74) were significantly correlated and MANOVA models were used. There were no significant effects for PPTh at the knee. Similarly, there were no significant effects for PPTh at the quadriceps, trapezius, and forearm.
Table 3.
Mechanical and cold pain responses for age and race groups.
| Pain measure (site) | Middle-aged (45–56) n=89 |
Older-aged (57–79) n=102 |
NHW N=138 |
NHB n=53 |
|---|---|---|---|---|
|
| ||||
| Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | |
| Mechanical | ||||
| PPTh in kPa (medial joint line) | 347.1 (155.2) | 374.7 (162.7) | 380.9 (157.8) | 327.1 (158.7) |
| PPTh in kPa (lateral joint line) | 374.7 (152.2) | 411.4 (172.1) | 410.9 (162.8) | 352.9 (164.5) |
| PPTh in kPa (quadricep) | 524.0 (231.7) | 509.0 (238.7) | 519.5 (225.4) | 507.2 (251.7) |
| PPTh in kPa (trapezius) | 335.0 (189.1) | 329.2 (170.5) | 339.0 (177.3) | 311.0 (183.8) |
| PPTh in kPa (epicondyle) | 289.6 (151.0) | 296.0 (189.4) | 288.7 (173.4) | 300.7 (170.2) |
| PMP (knee) first stimuli | 9.7 (13.6) | 7.7 (12.1) | 6.0 (9.4) b | 15.8 (17.4) b |
| PMP (knee) tenth stimuli | 24.9 (26.3) | 20.3 (23.2) | 16.4 (19.6) b | 41.2 (27.3) b |
| PMP (hand) first stimuli | 5.3 (8.4) | 4.4 (10.6) | 3.4 (6.4) b | 10.7 (14.0) b |
| PMP (hand) tenth stimuli | 16.1 (20.6) | 14.1 (20.1) | 9.1 (13.4) b | 31.0 (26.2) b |
| Cold water immersion of the hand | ||||
| CPTh in sec @ 12°C | 17.2 (14.1) | 19.3 (13.1) | 19.1 (13.5) | 16.0 (13.5) |
| Withdrew early | 8% (n=14) | 13% (n=20) | 28% (n=15) b | 50% (n=19) b |
| CP ratings for 12°C | ||||
| Intensity @ withdrawal | 35.5 (13.3) | 33.9 (15.8) | 35.3 (14.5) | 34.0 (15.1) |
| Intensity @ 30-sec | 41.1 (31.2) | 42.4 (31.5) | 37.8 (29.3) | 52.9 (34.0) |
| Intensity @ 55-sec | 51.1 (33.3) | 52.5 (32.2) | 48.8 (31.0) | 61.0 (35.5) |
| Unpl. @ 55-sec | 52.6 (31.0) | 57.5 (32.6) | 51.9 (30.8) | 64.8 (33.0) |
| CPTh in sec @ 8°C | 12.0 (10.8) | 12.6 (10.4) | 12.6 (9.9) | 11.3 (12.0) |
| Withdrew early | 32% (n=30) | 25% (n=32) | 37% (n=28) b | 61% (n=24) b |
| CP ratings for 8°C | ||||
| Intensity @ withdrawal | 29.4 (16.0) | 29.8 (13.9) | 34.0 (14.5) | 22.7 (12.9) |
| Intensity @ 30-sec | 57.2 (31.3) | 57.4 (32.4) | 54.4 (30.8) | 65.3 (33.9) |
| Intensity @ 55-sec | 64.9 (29.9) | 64.6 (30.9) | 62.6 (29.2) | 70.4 (33.1) |
| Unpl. @ 55-sec | 68.0 (29.7) | 68.9 (29.7) | 66.1 (29.0) | 73.8 (31.0) |
Adjusted means differed at p < .05 across age
Adjusted means differed at p < .05 across race
Abbreviations: Kilopascals (kPa), Pressure pain threshold (PPTh), Punctate mechanical pain (PMP), Cold pain threshold (CPTh), Cold pressor (CP), Unpleasantness (Unpl).
PMP at the hand and patella using a single stimulus were significantly correlated (r = .84) so the MANOVA model was used. A significant effect of Race was found [F (2,184) = 13.192, p < .001], with NHB showing higher pain ratings at both the hand and patella. No other effects were significant. PMPTS at the hand and patella were significantly correlated (r = .73) so again, the MANOVA model was used. A significant effect of Race was found [F (2,184) = 27.792, p < .001], with NHB showing greater temporal summation of punctate pain at both the hand and patella. No other effects were significant.
The time to CPTh for the hand using 12°C and 8°C were significantly correlated (r = .75) and a MANOVA model was used. There were no significant effects for CPTh. CP ratings at 12°C and 8°C were analyzed using repeated-measures ANOVAs. There were no significant effects for CP pain ratings. As the distribution for CPTo was skewed, this pain tolerance variable was dichotomized into withdrew during the test (20%, coded=1) or did not withdraw (80% coded=0). The results of logistic regression for the 8°C and 12°C data indicated that Race and the Age × Race interactions were significant (see Figure 4). When the race subgroups were dummy coded, the middle-aged NHW (8%, OR = 0.1, p < .001), older NHW (13%, OR = 0.1, p = .007), and middle-aged NHB (28%, OR = 0.4, p = .048) (all coded = 1) were significantly less likely to withdraw before the completion of the 12°C test than the older NHB group (50%, coded = 0). Similarly, for the 8°C test, the middle-aged NHW (32%, OR = 0.1, p = .002), older NHW (25%, OR = 0.2, p = .003), and middle aged NHB (37%, OR = 0.4, p = .007) (all coded = 1) were significantly less likely to withdraw before 60-seconds than the older NHB group (61%, coded = 0).
Figure 4.
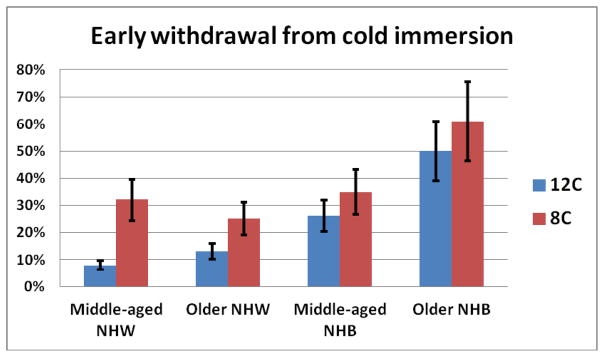
Percentages of middle-aged and older participants stratified by race who withdrew from the cold water bath before the targeted 60-seconds. Older NHB were significantly more likely to withdraw than the other 3 groups at both 12°C and 8°C. Furthermore, middle-aged NHB were more likely to withdraw at 12°C than the middle-aged and older NHW.
DISCUSSION
This is the first study to test for age differences in response to noxious stimuli between middle-aged and older adults using a wide range of stimulus modalities, while testing for differences across race. Testing at the forearm and knee allowed us to compare the effects of aging on pain at different levels of spinal innervation. The results suggest that there are selected age differences, with the older group less sensitive to warm and painful heat stimuli than middle-aged participants, particularly at the knee. In addition, there were several instances where age and race effects were compounded, resulting in greater race differences among the older participants.
Warm and pain thresholds
Our findings for increased warm thresholds with increasing age were similar to other studies.(2,25,28,41) Age differences were also found in thermal pain thresholds at the knee, but not the forearm. Stevens and Choo reported that the greatest decrement in sensitivity with aging occurs in the lower extremities,(41) and this effect is consistent with our data. Dysfunctional primary afferents are a possible mechanism for changes in thermal sensitivity.(19,44) A recent meta-analysis reported small effect sizes using contact thermal stimuli with older samples demonstrating increased pain threshold, with greater differences as a function of the mean differences in the groups’ ages.(27) Nevertheless, recent studies have continued to report mixed findings for measures of pain threshold using various stimulus modalities or sites. For example, Lautenbacher failed to find age differences in heat pain threshold, but reported differences in pressure pain threshold at the forearm.(25) Edwards, on the other hand, did not find age differences for pressure pain threshold at the trapezius and masseter muscles, but reported marginal significance for heat at the forearm.(7) The differential effects in thermal, but not pressure sensitivity supports the idea that decline in large myelinated afferents mediating mechanical detection may start during middle age. Variation in pain thresholds may have been more apparent if we had included a younger comparison group as changes in pain threshold were already in the process of increasing in our middle-aged sample.
Race differences in heat pain thresholds only emerged at the forearm, with NHB reporting lower thresholds than NHW. In the present study, there were no instances where threshold differences across age were larger in NHB or race differences greater with older age. From a biologic perspective, pain threshold represents the acuity of the pain system as a warning signal. However, the correct balance between early warning and discrimination of clearly innocuous stimuli within the lower boundary of painful sensations is necessary. This may become a greater issue should elevated pain thresholds place individuals at increased risk for injury through lack of sensitivity resulting in overuse or delayed withdrawal.
Suprathreshold stimuli
Pain tolerance is less often tested in studies of pain and aging, nevertheless; age differences in pain tolerance have been reported for pressure pain,(51) ischemic pain,(8) electrical stimulation,(31) and cold-water immersion.(48) We did not uncover an age effect for heat pain tolerance. However, we did find NHB, and particularly older NHB, reached heat pain tolerance at lower temperatures than NHW, and this overall effect is consistent with several other reports.(3,9,15,30) Similarly, older NHB were the most likely to fail to reach the targeted 60-second time-limit for the cold water immersion tests. Pain tolerance can be considered a complex behavioral index of the motivational capacity of the person influenced by sensory, cognitive, and secondary affective responses.(43,45)
If the pain threshold increases with aging, and some studies show tolerance decreases to varying degrees,(12) then the purposeful reserve between the onset of pain and the beginning of injury is reduced.(11) To test for differences in this functional pain range, we subtracted each participant’s pain threshold temperature from their pain tolerance temperature. We found an age effect for heat pain range at the knee, which was driven by the higher threshold in the older sample. This effect did not appear to be influenced by race. To what extent differences in the functional pain range across the life-span could account for differences in clinical pain is not known. It is possible that a reduced pain range is caused by a dampened sensory component of pain in older adults (i.e., increased threshold), paired with a heightened pain unpleasantness by which tolerance is reduced.(11) Another intriguing interpretation is that the longer duration stimuli needed for assessing pain tolerance activates additional excitatory processes, and results in an age-related imbalance between excitatory and inhibitory mechanisms.(26)
Ratings of the graded heat pulses allowed us to determine stimulus response curves for the thermal heat stimuli. A scaling difference was found with the middle-aged group showing a greater increase in pain across temperatures than the older participants, but only at the knee. This difference, along with similar levels of tolerance, supports Gibson’s hypothesis that older adults may reach tolerance at lower sensory but higher unpleasantness scores.(11) Unfortunately, we did not assess unpleasantness levels during the thermal tolerance test. There were also overall race differences in sensitivity to the heat pulses for both sites with NHB rating all temperatures as more painful than NHW. There was no evidence of an interaction between age and race on these tests.
Tests of pain facilitation or inhibition
Temporal summation of second pain results from repetitive stimulation of peripheral C-fibers and is thought to be associated with increased central sensitization.(46) Whereas both Edwards(8) and Lautenbacher(25) have reported greater temporal summation among older adults at the forearm, Harkins found that at the foot, older individuals reported less summation of pain than their younger counterparts.(17) Our results for greater temporal summation among NHB, and especially older NHB, at both the forearm and knee have not been reported. That older NHW experienced the least temporal summation at the knee parallels Harkins findings. The loss of sensory modulation among the older NHW is consistent with the previously reported distal changes in the aging somatosensory system where older adults likely depend more on unmyelinated afferents (versus myelinated) for sensory transmission, but is not consistent with the findings of greater temporal summation for older NHB.(19,44)
Punctate mechanical temporal summation was also tested, but no significant effects involved age; however, NHB showed greater summation of pain at both sites tested. Punctate mechanical stimuli are thought to evoke pain through a higher contribution of A-fibers relative to C-fiber nociceptors.(52) This methodology has not been used to test for differences between NHB and NHW; the only study reporting race differences to punctate stimuli compared participants of Belgian and Japanese descent.(22)
To date, four studies have tested the effects of aging on CPM and all have reported a decline in pain inhibition associated with aging.(6,24,37,49) Larivière demonstrated that some loss of pain inhibitory functions are already occurring in middle-age with less pain reduction in this group than for participants 20–35 years of age.(24) Not surprisingly, we also did not find a significant overall CPM effect in either age group. Interestingly, there is only one other study that tested for race differences in models of CPM and reported that NHB experienced less pain reduction than NHW.(4) These studies are difficult to compare, as substantially different methodology was used. On the other hand, the lack of effects from tests of central pain inhibition in older age provides an explanation for our null findings between older NHB and NHW.
Limitations of this study include considerable diversity within the racial categories of “Black” and “White” that we were unable to address. The study lacked a younger control group which would have allowed a better comparison to earlier studies. We addressed a specific set of questions related to sampling weaknesses of previous studies using experimental pain to test for age or race differences. Although the results do not directly explain clinical differences observed in pain, they do suggest areas for mechanistic inquiry with clinical relevance (i.e., differential impact of age on pain sensitivity in upper vs. lower extremities, age-related changes in primary sensory afferent function and that certain groups may experience a greater affective component of pain). Clinically, earlier assessment of somatosensory dysfunction may identify persons at increased risk for developing chronic pain.
Summary and implications
This study found several age differences in heat pain at the knee, but not the forearm, which supports the hypothesis that the greatest decrement in sensitivity with aging occurs in the lower extremities. Overall, the data suggest that previously reported race differences observed in younger adults also emerged in our older samples, and this study contributes new findings in that these race differences may increase with age for temporal summation of heat pain and for both heat and cold pain tolerance. We have also added to the aging and pain literature by reporting several small to moderate differences to heat stimuli between middle and older age, furthering the findings of Larivière.(24) This field of inquiry needs to move to more systematic designs testing mechanism-driven hypotheses (sites, stimulus modalities, more sophisticated tests) and demonstrate linkages between laboratory findings and more clinically relevant pain outcomes.
PERSPECTIVE.
This study found that the greatest decline in pain sensitivity with aging occurs in the lower extremities. In addition, race differences in pain sensitivity observed in younger adults were also found in our older sample.
Acknowledgments
The current study was supported by NIH/NIA grant R01AG033906 and R01AG039659. This publication was also made possible by the UF Clinical and Translational Science Institute (UL1TR000064) and the UAB Center for Clinical and Translational Science (UL1TR000165).
Footnotes
Conflicts of Interests
Dr. Fillingim has received consulting fees, speaking fees, and/or honoraria from WebMD and Algynomics (less than $10,000 each) and owns stock or stock options in Algynomics.
Dr. Staud has received consulting fees, speaking fees, and/or honoraria from Pfizer (less than $10,000).
Disclosures
Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Auvray M, Myin E, Spence C. The sensory-discriminative and affective-motivational aspects of pain. Neurosci Biobehav Rev. 2010;34(2):214–23. doi: 10.1016/j.neubiorev.2008.07.008. [DOI] [PubMed] [Google Scholar]
- 2.Bartlett G, Stewart JD, Tamblyn R. Normal distributions of thermal and vibration sensory thresholds. Muscle Nerve. 1998;21:367–74. doi: 10.1002/(sici)1097-4598(199803)21:3<367::aid-mus11>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 3.Campbell CM, Edwards RR, Fillingim RB. Ethnic differences in responses to multiple experimental pain stimuli. Pain. 2006;13:22–6. doi: 10.1016/j.pain.2004.08.013. [DOI] [PubMed] [Google Scholar]
- 4.Campbell CM, France CR, Robinson ME, Logan HL, Geffken GR, Fillingim RB. Ethnic differences in nociceptive flexion reflex. Pain. 2007;134:91–6. doi: 10.1016/j.pain.2007.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Català E, Reig E, Artés M, Aliaga L, López JS, Segú JL. Prevalence of pain in the Spanish population: telephone survey in 5000 homes. Eur J Pain. 2006;6:133–140. doi: 10.1053/eujp.2001.0310. [DOI] [PubMed] [Google Scholar]
- 6.Edwards RR, Fillingim RB, Ness TJ. Age-related differences in endogenous pain modulation: a comparison of diffuse noxious inhibitory controls in healthy older and younger adults. Pain. 2003;101(1–2):155–65. doi: 10.1016/s0304-3959(02)00324-x. [DOI] [PubMed] [Google Scholar]
- 7.Edwards RR, Fillingim RB. Age-associated differences in responses to noxious stimuli. J Gerontol A Biol Sci Med Sci. 2001;56(3):M180–5. doi: 10.1093/gerona/56.3.m180. [DOI] [PubMed] [Google Scholar]
- 8.Edwards RR, Fillingim RB. Effects of age on temporal summation and habituation of thermal pain: clinical relevance in healthy older and younger adults. J Pain. 2001;2(6):307–17. doi: 10.1054/jpai.2001.25525. [DOI] [PubMed] [Google Scholar]
- 9.Edwards RR, Fillingim RB. Ethnic differences in thermal pain responses. Psychosom Med. 1999;61(3):346–54. doi: 10.1097/00006842-199905000-00014. [DOI] [PubMed] [Google Scholar]
- 10.Farrell M, Gibson SJ. Age interacts with stimulus frequency in the temporal summation of pain. Pain Med. 2007;8(6):514–20. doi: 10.1111/j.1526-4637.2007.00282.x. [DOI] [PubMed] [Google Scholar]
- 11.Gibson SJ, Farrell M. A review of age differences in the neurophysiology of nociception and the perceptual experience of pain. Clin J Pain. 2004;20(4):227–39. doi: 10.1097/00002508-200407000-00004. [DOI] [PubMed] [Google Scholar]
- 12.Gibson SJ, Helme RD. Age-related differences in pain perception and report. Clin Geriatr Med. 2001;17(3):433–56. doi: 10.1016/s0749-0690(05)70079-3. [DOI] [PubMed] [Google Scholar]
- 13.Green CR, Anderson KO, Baker TA, Campbell LC, Decker S, Fillingim RB, Kalauokalani DA, Lasch KE, Myers C, Tait RC, Todd KH, Vallerand AH. The unequal burden of pain: confronting racial and ethnic disparities in pain. Pain Med. 2003;4(3):277–94. doi: 10.1046/j.1526-4637.2003.03034.x. [DOI] [PubMed] [Google Scholar]
- 14.Greenhouse SW, Geisser S. On methods in the analysis of profile data. Psychometrika. 1959;24:95–112. [Google Scholar]
- 15.Grewen KM, Light KC, Mechlin B, Girdler SS. Ethnicity is associated with alterations in oxytocin relationships to pain sensitivity in women. Ethn Health. 2008;13:219–41. doi: 10.1080/13557850701837310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hamerman D. Aging and the musculoskeletal system. Annals of the Rheumatic Diseases. 1997;56:578–585. doi: 10.1136/ard.56.10.578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Harkins SW, Davis MD, Bush FM, Kasberger J. Suppression of first pain and slow temporal summation of second pain in relation to age. J Gerontol A Biol Sci Med Sci. 1996;51(5):M260–5. doi: 10.1093/gerona/51a.5.m260. [DOI] [PubMed] [Google Scholar]
- 18.Helme RD, Gibson SJ. The epidemiology of pain in elderly people. Clin Geriatr Med. 2001;17:417–3. doi: 10.1016/s0749-0690(05)70078-1. [DOI] [PubMed] [Google Scholar]
- 19.Jacobs JM, Love S. Qualitative and quantitative morphology of human sural nerve at different ages. Brain. 1985;108:897–24. doi: 10.1093/brain/108.4.897. [DOI] [PubMed] [Google Scholar]
- 20.Kalu DN, Masoro EJ. The biology of aging, with particular reference to the musculoskeletal system. Clin Geriatr Med. 1988;4(2):257–67. [PubMed] [Google Scholar]
- 21.King CD, Sibille KT, Goodin B, Glover TL, Riley JL, Staud R, Fillingim RB. Enhanced processing of experimental pain stimuli is related to the level of clinical pain in symptomatic knee osteoarthritis. Osteoarthritis and Cartilage. 2013;21(9):1243–52. doi: 10.1016/j.joca.2013.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Komiyama O, Kawara M, De Laat A. Ethnic differences regarding tactile and pain thresholds in the trigeminal region. J Pain. 2007;8:363–9. doi: 10.1016/j.jpain.2006.12.002. [DOI] [PubMed] [Google Scholar]
- 23.Krueger AB, Stone AA. Assessment of pain: A community-based diary survey in the USA. Lancet. 2008;371(9623):1519–25. doi: 10.1016/S0140-6736(08)60656-X. [DOI] [PubMed] [Google Scholar]
- 24.Larivière M, Goffaux P, Marchand S, Julien N. Changes in pain perception and descending inhibitory controls start at middle age in healthy adults. Clin J Pain. 2007;23(6):506–10. doi: 10.1097/AJP.0b013e31806a23e8. [DOI] [PubMed] [Google Scholar]
- 25.Lautenbacher S, Kunz M, Strate P, Nielsen J, Arendt-Nielsen L. Age effects on pain thresholds, temporal summation and spatial summation of heat and pressure pain. Pain. 2005;115(3):410–8. doi: 10.1016/j.pain.2005.03.025. [DOI] [PubMed] [Google Scholar]
- 26.Lautenbacher S. Experimental approaches in the study of pain in the elderly. Pain Med. 2012;13 (Suppl 2):S44–50. doi: 10.1111/j.1526-4637.2012.01326.x. [DOI] [PubMed] [Google Scholar]
- 27.Leveille SG. Musculoskeletal aging. Curr Opin Rheumatol. 2004;16(2):114–8. doi: 10.1097/00002281-200403000-00007. [DOI] [PubMed] [Google Scholar]
- 28.Lin YH, Hsieh SC, Chao CC, Chang YC, Hsieh ST. Influence of aging on thermal and vibratory thresholds of quantitative sensory testing. J Peripher Nerv Syst. 2005;10(3):269–81. doi: 10.1111/j.1085-9489.2005.10305.x. [DOI] [PubMed] [Google Scholar]
- 29.Mauchly JW. Significance test for sphericity of n-variate normal population. Annals of Mathematical Statistics. 1940;11:204–9. [Google Scholar]
- 30.Mechlin B, Morrow AL, Maixner W, Girdler SS. African Americans show alterations in endogenous pain regulatory mechanisms and reduced pain tolerance to experimental pain procedures. Psychosom Med. 2005;67:948–56. doi: 10.1097/01.psy.0000188466.14546.68. [DOI] [PubMed] [Google Scholar]
- 31.Neri M, Agazzani E. Aging and right-left asymmetry in experimental pain measurement. Pain. 1984;19:43–48. doi: 10.1016/0304-3959(84)90063-0. [DOI] [PubMed] [Google Scholar]
- 32.Radloff LS. The CES-D scale: a self-report depression scale for research in the general population. Appl Psychol Meas. 1977;1:385–401. [Google Scholar]
- 33.Rahim-Williams FB, Fillingim RB, Riley JL., 3rd A quantitative and qualitative review of ethnic group differences in experimental pain sensitivity: A Meta-analysis. Pain Med. 2012;13(4):522–40. doi: 10.1111/j.1526-4637.2012.01336.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Riley JL, 3rd, Gilbert GH, Heft MW. Orofacial pain symptom prevalence:selective sex differences in the elderly? Pain. 1998;76(1–2):97–104. doi: 10.1016/s0304-3959(98)00030-x. [DOI] [PubMed] [Google Scholar]
- 35.Riley JL, 3rd, Gilbert GH, Heft MW. Orofacial pain: racial and sex differences among older adults. J Public Health Dent. 2002;62(3):132–9. doi: 10.1111/j.1752-7325.2002.tb03434.x. [DOI] [PubMed] [Google Scholar]
- 36.Riley JL, 3rd, Gilbert GH. Racial differences in orofacial pain. J Pain. 2002;3(4):284–91. doi: 10.1054/jpai.2002.124898. [DOI] [PubMed] [Google Scholar]
- 37.Riley JL, 3rd, King CD, Wong F, Fillingim RB, Mauderli AP. Lack of endogenous modulation but delayed decay of prolonged heat pain in older adults. Pain. 2010;150(1):153–60. doi: 10.1016/j.pain.2010.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rustøen T, Wahl AK, Hanestad BR, Lerdal A, Paul S, Miaskowski C. Age and the experience of chronic pain: differences in health and quality of life among younger, middle-aged, and older adults. Clin J Pain. 2005;21(6):513–23. doi: 10.1097/01.ajp.0000146217.31780.ef. [DOI] [PubMed] [Google Scholar]
- 39.Shavers VL, Bakos A, Sheppard VB. Race, ethnicity, and pain among the U.S. adult population. J Health Care Poor Underserved. 2010;21(1):177–220. doi: 10.1353/hpu.0.0255. [DOI] [PubMed] [Google Scholar]
- 40.Spielberger CD. Manual for the State-Trait Anger Expression Inventory (STAXI) Odessa, FL: Psychological Assessment Resources; 1988. [Google Scholar]
- 41.Stevens JC, Choo KK. Temperature sensitivity of the body surface over the life span. Somatosens Mot Res. 1998;15:13–28. doi: 10.1080/08990229870925. [DOI] [PubMed] [Google Scholar]
- 42.Thomas E, Peat G, Harris L, Wilkie R, Croft PR. The prevalence of pain and pain interference in a general population of older adults: cross-sectional findings from the North Staffordshire Osteoarthritis Project (NorStOP) Pain. 2004;110:361–8. doi: 10.1016/j.pain.2004.04.017. [DOI] [PubMed] [Google Scholar]
- 43.Van Damme S, Legrain V, Vogt J, Crombez G. Keeping pain in mind: a motivational account of attention to pain. Neurosci Biobehav Rev. 2010;34(2):204–13. doi: 10.1016/j.neubiorev.2009.01.005. [DOI] [PubMed] [Google Scholar]
- 44.Verdú E, Ceballos D, Vilches JJ, Navarro X. Influence of aging on peripheral nerve function and regeneration. Journal of the Peripheral Nervous System. 2000;5:191–208. doi: 10.1046/j.1529-8027.2000.00026.x. [DOI] [PubMed] [Google Scholar]
- 45.Verhoeven K, Crombez G, Eccleston C, Van Ryckeghem DM, Morley S, Van Damme S. The role of motivation in distracting attention away from pain: an experimental study. Pain. 2010;149(2):229–34. doi: 10.1016/j.pain.2010.01.019. [DOI] [PubMed] [Google Scholar]
- 46.Vierck CJ, Cannon RL, Fry G, Maixner W, Whitsel BL. Characteristics of temporal summation of second pain sensations elicited by brief contact of glabrous skin by a preheated thermode. J Neurophysiol. 1997;78:992–1002. doi: 10.1152/jn.1997.78.2.992. [DOI] [PubMed] [Google Scholar]
- 47.Von Korff M, Ormel J, Keefe FJ, Dworkin SF. Grading the severity of chronic pain. Pain. 1992;50:133–149. doi: 10.1016/0304-3959(92)90154-4. [DOI] [PubMed] [Google Scholar]
- 48.Walsh N, Schoenfeld L, Ramamurth S, Hoffman J. Normative model for the cold pressor test. Am J Phys Med Rehabil. 1989;68:6–11. doi: 10.1097/00002060-198902000-00003. [DOI] [PubMed] [Google Scholar]
- 49.Washington LL, Gibson SJ, Helme RD. Age-related differences in the endogenous analgesic response to repeated cold water immersion in human volunteers. Pain. 2000;89(1):89–96. doi: 10.1016/S0304-3959(00)00352-3. [DOI] [PubMed] [Google Scholar]
- 50.Watson D, Clark LA, Tellegen A. Development and validation of brief measures of positive and negative affect: The PANAS scales. Journal of Personality and Social Psychology. 1988;54(6):1063–70. doi: 10.1037//0022-3514.54.6.1063. [DOI] [PubMed] [Google Scholar]
- 51.Woodrow K, Friedman G, Siegelaub A, Collen M. Pain tolerance: differences according to age, sex, and race. Psychosom Med. 1972;34:548–56. doi: 10.1097/00006842-197211000-00007. [DOI] [PubMed] [Google Scholar]
- 52.Ziegler E, Magerl W, Meyer R, Treede R. Secondary hyperalgesia to punctate mechanical stimuli: central sensitization to A-fibre nociceptor input. Brain. 1999;122:2245–57. doi: 10.1093/brain/122.12.2245. [DOI] [PubMed] [Google Scholar]