Abstract
ESRD treated with dialysis is associated with increased left ventricular hypertrophy, which, in turn, is related to high mortality. Mineralocorticoid receptor antagonists improve survival in patients with chronic heart failure; however, the effects in patients undergoing dialysis remain uncertain. We conducted a multicenter, open-label, prospective, randomized trial with 158 patients receiving angiotensin-converting enzyme inhibitor or angiotensin type 1 receptor antagonist and undergoing peritoneal dialysis with and without (control group) spironolactone for 2 years. As a primary endpoint, rate of change in left ventricular mass index assessed by echocardiography improved significantly at 6 (P=0.03), 18 (P=0.004), and 24 (P=0.01) months in patients taking spironolactone compared with the control group. Rate of change in left ventricular ejection fraction improved significantly at 24 weeks with spironolactone compared with nontreatment (P=0.02). The benefits of spironolactone were clear in patients with reduced residual renal function. As secondary endpoints, renal Kt/V and dialysate-to-plasma creatinine ratio did not differ significantly between groups during the observation period. No serious adverse effects, such as hyperkalemia, occurred. In this trial, spironolactone prevented cardiac hypertrophy and decreases in left ventricular ejection fraction in patients undergoing peritoneal dialysis, without significant adverse effects. Further studies, including those to determine relative effectiveness in women and men and to evaluate additional secondary endpoints, should confirm these data in a larger cohort.
ESRD treated by hemodialysis or peritoneal dialysis (PD) is associated with a high prevalence of increased left ventricular (LV) mass (left ventricular hypertrophy [LVH]), fibrosis, and capillary loss.1,2 CKD exposes the heart to three major mechanisms that facilitate the development of cardiomyopathy and induce LV failure: pressure overload, volume overload, and CKD-related nonhemodynamic factors that alter the myocardium.3 The persistence and severity of LVH are closely associated with cardiovascular and all-cause mortality.3–6 Terminal events in LVH are known to include pump failure and sudden arrhythmic death.3,7–9 Furthermore, ischemic disease, as exemplified by coronary atherosclerosis, is a less important factor in cardiovascular mortality and morbidity from CKD and ESRD.1 Therefore, a new paradigm of therapy for ESRD that prioritizes prevention and reversal of LVH and cardiac fibrosis is needed.1
Mineralocorticoid receptor (MR) antagonists are reported to improve the survival of patients with chronic heart failure and heart failure that occurs after myocardial infarction10–12 and to reduce LV mass;13 however, in patients undergoing dialysis, the few reports on the effects of MR blockade have typically focused on small cohorts that were underpowered to allow conclusions to be drawn.14–17 We explored the effects of concurrent spironolactone and angiotensin-converting enzyme inhibitor (ACEI) or angiotensin type 1 receptor blocker (ARB) on cardiac complications (LV mass index [LVMI] and LV ejection fraction [LVEF]), peritoneal membrane, and residual renal functions in a multicenter, open-label, prospective controlled trial of patients undergoing PD.
Results
Patient Characteristics at Baseline
A total of 158 patients under treatment with ACEI or ARB and undergoing peritoneal dialysis were randomly assigned to receive spironolactone (n=78) or not receive spironolactone (control group, n=80) (Figure 1). None of the patients were receiving both an ACEI and an ARB at the start of the study. Primary causes of renal failure were as follows: diabetes mellitus (39.2%), chronic GN (31.5%), nephrosclerosis (21.5%), other (3.8%), or unknown (4.4%). Patients were younger than the mean age of 66.2 years seen in Japanese dialysis patients (mean age±SD, 57.4±12.27 and 55.6±14.37 years in the treatment and control groups, respectively).18 Duration of PD treatment at baseline was relatively short (6 and 7.5 months, respectively), and urine volume was preserved (1000 and 955 ml, respectively). Baseline values for rate of use of icodextrin solution, LVMI, LVEF, BP, dialysate-to-plasma creatinine ratio (D/P Cr) by peritoneal equilibration test, renal Kt/V urea, urine volume, serum brain natriuretic peptide, and aldosterone levels were similar between the groups. Other demographic and baseline characteristics in the randomized population were similar, except for the rate of furosemide use (Table 1).
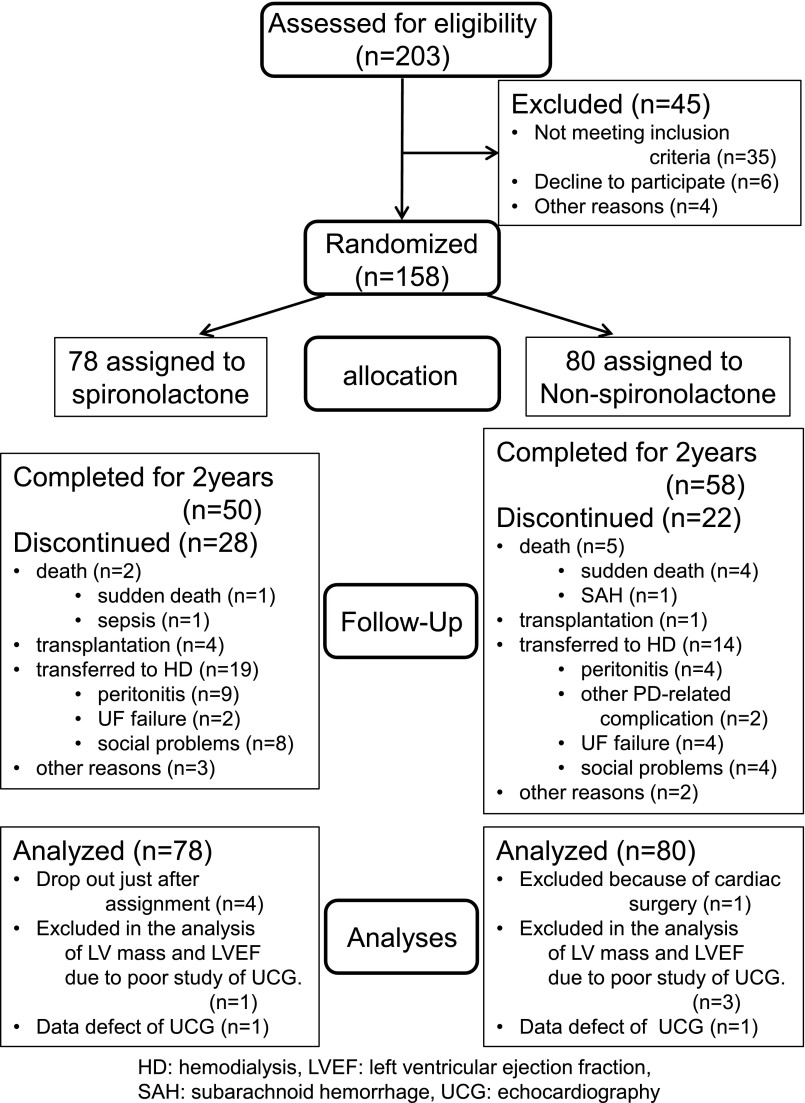
Figure 1.
Number of patients enrolled, randomly assigned, and analyzed as part of the study. HD, hemodialysis; SAH, subarachnoid hemorrhage; UCG, echocardiography; UF, ultrafiltration.
Table 1.
Baseline characteristics of participants
| Characteristic | Spironolactone Group (n=78) | Control Group (n=80) | P Value |
|---|---|---|---|
| Age (yr) | 57.4±12.27 | 55.6±14.37 | 0.40a |
| Women, n (%) | 23 (29.5) | 22 (27.5) | 0.86b |
| Diabetes mellitus, n (%) | 33 (42.3) | 29 (36.3) | 0.52b |
| Use of icodextrin, n (%) | 22 (28.2) | 23 (28.8) | 1b |
| Time receiving PD (mo) | 6 (2.0–21.0) | 7.5 (2.0–22.5) | 0.57c |
| NYHA-FC, n (%) | |||
| I | 77 (98.7) | 80 (100) | 0.49b |
| II | 1 (1.3) | 0 (0) | |
| Height (m2.7) | 3.8±0.55 | 3.8±0.47 | 0.69a |
| Systolic BP (mmHg) | 137.4±15.42 | 135±16.56 | 0.16a |
| Diastolic BP (mmHg) | 76.3±10.39 | 78.7±11.60 | 0.18a |
| Hemoglobin (g/dl) | 10.4±1.31 | 10.3±1.24 | 0.85a |
| Total protein (g/dl) | 6.3±0.64 | 6.3±0.60 | 0.66a |
| Serum albumin (g/dl) | 3.3±0.51 | 3.4±0.49 | 0.36a |
| TC (mg/dl) | 184 (162.0–200.0) | 182.5 (152.5–209.0) | 0.74 |
| LDL-C (mg/dl) | 106 (87.0–125.0) | 106 (81.5–135.0) | 0.59 |
| Na (mEq/L) | 137.8±3.65 | 138.4±3.40 | 0.33a |
| K (mEq/L) | 4.3±0.66 | 4.3±0.65 | 0.69a |
| P (mg/dl) | 5.4±1.54 | 5.2±1.28 | 0.44a |
| Ca (mg/dl) | 8.8±0.88 | 8.7±1.13 | 0.58a |
| Blood urea (mg/dl) | 52.9 (42.9–64.9) | 54 (45.7–63.6) | 0.58c |
| Serum creatinine (mg/dl) | 8.3 (6.6–10.8) | 8.5 (6.7–11.2) | 0.67c |
| β2-MG (μg/ml) | 20.6±5.66 | 22.6±8.02 | 0.08a |
| Urine volume (ml/d) | 1000 (500.0–1650.0) | 955 (550.0–1400.0) | 0.30c |
| Weekly renal Kt/V urea | 0.81 (0.41–1.08) | 0.66 (0.41–1.06) | 0.45c |
| Weekly PD Kt/V urea | 1.13 (0.70–1.40) | 1.17 (0.75–1.53) | 0.39c |
| D/P Cr 4 h | 0.66 (0.59–0.75) | 0.66 (0.58–0.76) | 0.57a |
| Serum aldosterone (pg/ml) | 53.6 (20.0–100.0) | 58.1 (24.4–120.0) | 0.58c |
| Serum BNP (pg/ml) | 83.1 (27.6–233.0) | 68.6 (29.0–214.1) | 0.62c |
| LV ejection fraction (%) | 64.9±10.77 | 65.5±9.89 | 0.70a |
| LVMI (g/m2.7) | 51.6±21.08 | 52.3±18.10 | 0.82a |
| Women | 45.4±19.79 | 51.2±14.28 | 0.30a |
| Men | 53.9±21.26 | 52.8±19.41 | 0.79a |
| Medications | |||
| ARBs, n (%) | 68 (87.2) | 73 (91.2) | 0.15b |
| ACEIs, n (%) | 10 (12.8) | 7 (8.8) | 0.60b |
| Calcium-channel blocker, n (%) | 54 (74.0) | 57 (71.3) | 0.72b |
| α-Blocker, n (%) | 13 (17.8) | 18 (22.5) | 0.55b |
| β-Blocker, n (%) | 17 (23.3) | 21 (26.3) | 0.71b |
| Diuretics (furosemide), n (%) | 53 (72.6) | 39 (50.6) | 0.01 |
| Statin, n (%) | 24 (32.9) | 21 (26.3) | 0.38 |
| P binder, n (%) | 44 (60.3) | 49 (61.3) | 1 |
| EPO dose (U/wk) | 24,000 (12,000–24,000) | 24,000 (12,000–24,000) | 0.79c |
Values are expressed as number (percentage), mean±SD, and median (interquartile range). TC, total cholesterol; LDL-C, LDL cholesterol; β2-MG, β2-macroglobulin; BNP, brain natriuretic peptide; EPO, erythropoietin.
Welch t test.
Fisher exact test.
Mann–Whitney U test.
Treatment Characteristics
Systolic and diastolic BP pressure did not significantly differ between the groups during the study period (P=0.65 and P=0.76 at 24 months, respectively) (Supplemental Figure 1).
Primary Outcomes
Changes in LVMI
Rate of change in LVMI assessed by echocardiography was significantly improved in the spironolactone group at 6 (P=0.03 versus control), 18 (P=0.004 versus control), and 24 (P=0.01 versus control) months compared with the nontreatment control group (Figure 2A). Because we observed interactions between women and men (P=0.01), we separately analyzed the effects of spironolactone. The effect of spironolactone on rate of changes in LVMI was similar in men with LVMI≤50 g/m2.7 (normal range for men) and LVMI>50 g/m2.7 (LVH)19,20 at baseline (P for interaction=0.21). The rate of change in LVMI for men with LVMI≤50 g/m2.7 (normal range) at baseline did not change during the follow-up period in the group given spironolactone but increased significantly in the control group at 12 (P=0.02 versus baseline; P=0.01 versus spironolactone treatment group), 18 (P<0.001 versus baseline; P<0.001 versus spironolactone treatment group), and 24 (P<0.001 versus baseline; P<0.001 versus spironolactone treatment group) months (Figure 2B). The rate of change in LVMI for men with LVMI>50 g/m2.7 (LVH) at baseline did not significantly change during the observation period in the control group, but decreased significantly after 6 months when compared with baseline, and was further suppressed at 18 (P=0.001 versus baseline; P=0.003 versus control) and 24 (P<0.001 versus baseline; P=0.02 versus control) months by spironolactone (Figure 2C). In contrast, in women, rate of change in LVMI did not differ significantly during the observation period in patients with LVMI≤47 g/m2.7 (normal range for women) or in those with LVMI>47 g/m2.7 (LVH)19,20 at baseline (Supplemental Figure 2).
Figure 2.
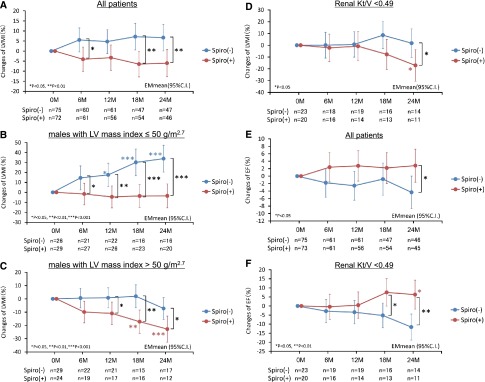
Spironolactone prevented cardiac hypertrophy and decreases in LVEF in patients on PD. Effects of spironolactone are clear in men and in patients with reduced residual renal function. Effects of spironolactone on LVMI and function in patients undergoing PD. LVMI in all patients (A), in men with LVMI≤50 g/m2.7 (B) or >50 g/m2.7 (C), and in PD patients with renal Kt/V<0.49 (D). Ejection fraction in all patients (E) and in patients with renal Kt/V<0.49 (F). 95% C.I., 95% confidence interval; Spiro (−), control group; Spiro (+), spironolactone treatment group.
Residual renal function was reported to affect LVMI in patients undergoing PD.20–22 Patients were divided into tertiles according to baseline renal Kt/V: tertile 1, <0.49; tertile 2, ≥0.49 and <0.94; and tertile 3, ≥0.94. The effects of spironolactone were obvious in patients with renal Kt/V<0.49 (tertile 1) (Figure 2D), but were not significant in tertiles 2 and 3.
Changes in LVEF
Rate of change in LVEF was significantly improved at 24 weeks compared with the nontreatment control group (P=0.02) (Figure 2E). On analysis of changes of LVEF in tertiles of renal Kt/V, spironolactone improved LVEF at 18 (P=0.02 versus control) and 24 (P=0.002 versus control) months in the patients with Kt/V<0.49 (Figure 2F), but in tertiles 2 and 3, these effects were not detected.
Secondary Outcomes
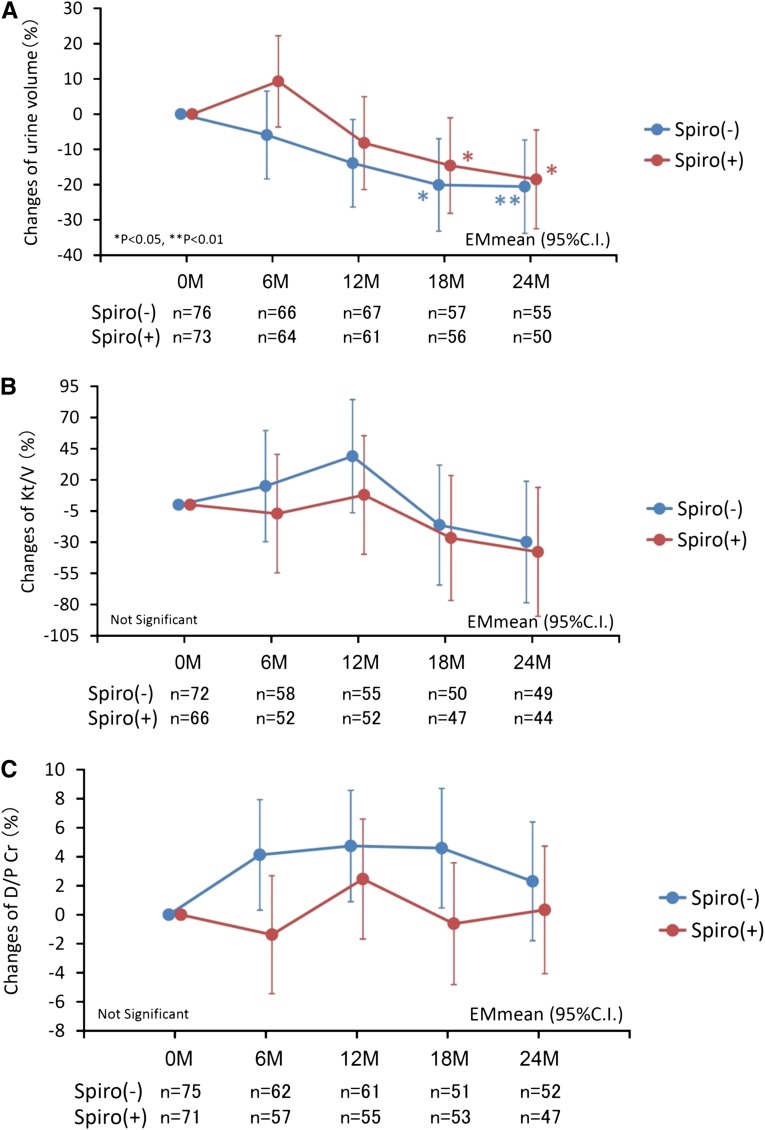
Both urine volume and renal Kt/V decreased with time, but rate of change did not significantly differ between the treatment and control groups (Figure 3, A and B). Significant effects of spironolactone were not detected in the rate of change in D/P Cr ratio between the groups (Figure 3C).
Figure 3.
Both urine volume and renal Kt/V decreased with time, but rate of change did not significantly differ between the treatment and control groups. Effects of spironolactone on residual renal function and peritoneal membrane function. (A) Changes in urine volume. Rate of change in urine volume was significantly higher in both groups after 18 months compared with baseline values but did not significantly differ between groups during the observation period. (B) Changes in residual renal function Kt/V. Changes in renal Kt/V were not significantly different compared with baseline values and did not significantly differ between groups during the observation period. (C) Peritoneal membrane function assessed by D/P Cr. Rate of change in D/P Cr did not differ between groups. 95% C.I., 95% confidence interval; Spiro (−), control group; Spiro (+), spironolactone treatment group.
Adverse Effects
A total of two patients in the spironolactone group (2.6%) and five patients in the control group (6.3%) died (Figure 1). Kaplan–Meier estimates of mortality and technical survival rate at 24 months were not significantly different (Supplemental Figure 3, A and B; P=0.34 and 0.34, respectively). Kaplan–Meier values for cardiovascular death and technical survival from cardiovascular causes did not significantly differ between the groups during the study period (Supplemental Figure 3, C and D; P=0.15 and 0.71, respectively). There were no significant differences between the two groups in occurrence of cerebral bleeding (P>0.99), cerebral infarction (P>0.99), myocardial infarction (P>0.76), and hypotension (P=0.76) (Table 2).
Table 2.
Adverse events
| Adverse Event | Patients, n (%) | P Value | |
|---|---|---|---|
| Spironolactone Group (n=78) | Control Group (n=80) | ||
| Cerebral bleeding | 1 (1.3) | 2 (2.5) | >0.99 |
| Cerebral infarction | 4 (5.1) | 4 (5.0) | >0.99 |
| Acute myocardial infarction | 2 (2.6) | 8 (10.0) | 0.10 |
| Hypotension (BP<100 mmHg) | 6 (7.7) | 5 (6.3) | 0.76 |
| Hyperkalemia (K>6.0 mEq/L) | 2 (2.6) | 1 (1.3) | 0.62 |
| Hypokalemia (P<3.0 mEq/L) | 12 (15.4) | 20 (25.0) | 0.17 |
| Gynecomastia | 11 (14.1) | 2 (2.5) | 0.01 |
| Peritonitis | 16 (20.5) | 17 (21.3) | >0.99 |
Serum potassium levels were significantly higher in patients receiving spironolactone at 6 and 12 months (Supplemental Figure 4). Serious hyperkalemia (potassium≥6.0 mEq/L) occurred in two (2.6%) patients (maximum level, 6.0 mEq/L) who received spironolactone and in one (1.3%) (maximum level, 6.2 mEq/L) in the control group during the observation period (P=0.62) (Table 2). Gynecomastia developed in 11 (14.1%) and 2 (2.5%) patients in the spironolactone and control groups, respectively (P=0.01) (Table 2). Spironolactone was switched to eplerenone in two patients, and the doses of spironolactone were reduced from 25 to 12.5 mg/d in two other patients. The remaining seven patients continued with spironolactone. There were no other clinically significant differences between the two groups with respect to laboratory variables, reported adverse events, or adverse events leading to permanent withdrawal of the study medicine.
Discussion
Left ventricular hypertrophy is present in almost 75% of patients starting dialysis23 and is strongly associated with poor outcomes in patients with CKD and ESRD.1 Recently, much attention has focused on the mediators and signaling pathways for LVH. Activation of the renin-angiotensin system (RAS), oxidative stress, elevated asymmetric dimethyl arginine, low-grade inflammation with increased circulating cytokines, and dyslipidemia are reported to be involved in the pathogenesis of cardiovascular diseases in patients with CKD.24 Persistent hyperaldosteronemia and/or activation of MR can promote cardiac fibrosis, possibly through generation of signals promoting profibrotic TGF-β production.1,25,26 In animal models, spironolactone ameliorates cardiac fibrosis in nephrectomized uremic rats.27,28
We evaluated the effects of adding spironolactone to ACEI or ARB in patients undergoing PD, and we found that LVMI was significantly suppressed in the spironolactone group (Figure 2). LVMI in patients receiving PD was reported to be linked to a loss of residual renal function2,20 and to a high peritoneal membrane permeability.29 Thus, the absence of significant effects from spironolactone on renal Kt/V and D/P Cr in this study (Figure 3) supports the notion that these results were mainly due to the effects of spironolactone. In subgroup analysis, the effects of spironolactone were more significant in men (Figure 2). On the other hand, we observed no effect from spironolactone in women (Supplemental Figure 2). In previous studies, in which about 30% of enrolled patients were women, spironolactone was effective in both male and female patients with severe heart dysfunction who were not undergoing dialysis.10 Because of the low number of women in our study, a comparison of the effects of spironolactone between men and women in a larger cohort will be necessary to confirm our data. We did not observe significant interactions between the effect of spironolactone and residual renal functions on rate of changes in LVMI (P for interaction=0.43) or LVEF (P for interaction=0.18); however, we analyzed the relationship with residual renal function on the basis of its clinical importance for PD patients.20–22 Interestingly, the effects of LVMI and LVEF tended to be predominant in PD patients with decreased levels of residual renal function (renal Kt/V<0.49) (Figure 2, D and F). These important points need to be confirmed in future studies.
As for secondary outcomes, we were unable to identify the beneficial effects of spironolactone on residual renal function and peritoneal membrane functions. In most clinical studies on MR blockade in patients with CKD, the primary endpoint has been reduction of proteinuria and/or albuminuria.30–32 The effects of MR blockade in prevention of deterioration of renal function remain unclear.30,31 In our studies, we were unable to identify the effects on prevention of decline in urine volume and residual renal function in patients undergoing PD. MR protein and mRNA were expressed in rat peritoneum, mesothelial cells, and fibroblasts.33,34 Spironolactone and ARB effectively ameliorated the peritoneal thickening and D/P Cr in the rat scraped peritoneal fibrosis model.33 In addition, ARB was reported to suppress the deterioration of peritoneal membrane function in PD patients.35 In this study, RAS was suppressed at baseline in patients by administration of ACEI or ARB for >3 months. Therefore, in these cases, it may be difficult to detect the effects of spironolactone on peritoneal membrane function.
Overall, spironolactone therapy was well tolerated. The use of MR antagonists has not been recommended in patients with CKD because of concerns about hyperkalemia, which often occurs when multiple renin-angiotensin-aldosterone blockers are used30,36,37; however, the risk of severe hyperkalemia was not significantly elevated among PD patients assigned to spironolactone (Table 2). Removal of potassium is greater in PD than in hemodialysis. Between 10% and 36% of patients receiving PD are reported to have hypokalemia, and adding potassium tablets is necessary for 10%–20% of PD patients.38–40 In our cohort, hypokalemia (potassium<3.0 mEq/L) was detected in 15.4% of the spironolactone treatment group and in 25% of the control group. On the basis of our findings, MR antagonists do not present serious problems with regard to serum potassium levels in patients undergoing PD. Antiandrogenic adverse effects appeared with high doses of spironolactone,30,41 and a significant incidence of gynecomastia was noted in the spironolactone group (14.1%; P=0.01); however, in these cases the spironolactone dose was reduced from 25 mg/d to 12.5 mg/d or the patients were switched to eplerenone. Use of the selective MR blocker eplerenone appears to minimize the risk of gynecomastia.
This study had several limitations. First, this study was open label. Second, we estimated sample size according to the effects of spironolactone on LVMI; thus, analyzing sex effects and other endpoints in this sample may be inappropriate. Third, all patients in the study were Japanese. Japanese patients are reported to have a better prognosis than comparable patients in the United States and Europe, and subclinical atherosclerosis, coronary disease mortality, and risk of coronary calcification are lower in Japanese persons.42 Fourth, an ACEI or ARB was administered for >3 months; therefore, the RAS was already suppressed at the start of this study in patients of both groups. In addition, several types of ACEI and ARB were used, and we did not evaluate their doses. Furthermore, the prescription rate for ARBs was much higher than that for ACEIs in this cohort; this is typical in Japan43 but is very different from patterns in the United States and European countries. The high incidence of cough among Asian individuals44 may be related to the lower use of ACEI in this cohort and in Japanese hypertensive patients. Fifth, most of the patients in this study were classified as New York Heart Association functional class (NYHA-FC) I, which may have contributed to the lower mortality rate. Finally, the effects of spironolactone in women were not studied in detail because the number of female patients enrolled was limited (<30%) in this cohort. Furthermore, there was an imbalance between the two groups with regard to baseline LVMI in women (Table 1) because randomization was performed before echocardiography.
In summary, the MR antagonist spironolactone may help to prevent cardiac hypertrophy and dysfunction in patients undergoing PD without significant adverse effects. Future studies are necessary in larger cohorts to confirm the effects of spironolactone with regard to relative effectiveness in women and men and the relationship between residual renal function and secondary endpoints.
Concise Methods
Study Design
The Nagoya Spiro Study is a multicenter, open-label, prospective, randomized controlled trial conducted in 12 hospitals. Patients were randomly assigned and followed for 2 years. This study was performed in accordance with the Declaration of Helsinki and Good Clinical Practice guidelines. The institutional review boards or ethics committees of all participating institutions approved the protocols. Written informed consent was obtained from all patients before enrollment. The trial was monitored by an independent data and safety monitoring committee. Safety was evaluated by adverse events reports and clinical laboratory values, including serum potassium levels. The Nagoya Spiro Study was registered at The University Hospital Medical Information Network Clinical Trials Registry as UMIN000492.
Participants
Patients with ESRD undergoing PD were recruited from 12 hospitals in the Tokai area of Japan, including Nagoya University Hospital (Nagoya, Japan), Handa Municipal Hospital (Handa, Japan), Daiyukai Daiichi Hospital (Ichinomiya, Japan), Yokkaichi Municipal Hospital (Yokkaichi, Japan), Anjo Kosei Hospital (Anjo, Japan), Aihoku-Konankosei Hospital (Konan, Japan), Nagoya Kyoritsu Hospital (Nagoya, Japan), Gifu Prefectural Hospital (Gifu, Japan), Tosei General Hospital (Seto, Japan), Ogaki Kita Clinic (Ogaki, Japan), and Kasugai Municipal Hospital (Kasugai, Japan). Inclusion criteria were as follows: age 18–80 years, treatment with PD among patients with NYHA-FC I and II for <5 years, concurrent administration of ARB or ACEI treatment for >3 months, and treatment with neutral-pH dialysate and/or icodextrin solution. Patients were excluded if any of the following criteria were present: severe anemia (hemoglobin<9.0 g/dl), NYHA-FC III and IV heart failure, treatment with acid-pH fluid dialysate, previous treatment with hemodialysis or transplantation within the preceding 3 years, and pregnancy or suspected pregnancy.
Treatment Procedures
The 25-mg once-daily dose of spironolactone was selected on the basis of previous results of clinical trials.10,32 If hyperkalemia or gynecomastia developed, the dose could be decreased to 12.5 mg/d because spironolactone at a dose 12.5–25 mg/d is reported to be pharmacologically effective in blocking the aldosterone receptors and atrial natriuretic peptide concentrations.10,45 In addition, antihypertensive agents, excluding other ACEIs, ARBs, and MR blockers, were allowed to control systolic BP (<130 mmHg). Doses of ACEIs and ARBs were not allowed to change unless adverse events occurred. Following the approval of eplerenone for use in clinical settings in Japan in July 2007, the Nagoya University Institutional Review Board approved the change to eplerenone, 50 mg/d, from spironolactone after demonstration of the safety of spironolactone on serum potassium levels in this study. Follow-up evaluation and laboratory measurements, including serum potassium, were conducted every 4 weeks for up to 2 years.
Outcome Measures
The primary outcome measures were LVMI and LVEF. Secondary outcome measures comprised residual renal function and peritoneal membrane function. We evaluated LVMI and LVEF by echocardiography, renal Kt/V, urine volume, and D/P Cr by peritoneal equilibration test every 6 months for up to 24 months.
Echocardiography
Standard two-dimensional (2D) and Doppler echocardiography was performed in each institute using a commercially available echocardiographic machine with a 2.0- to 3.5-MHz transducer by a single experienced echocardiography sonographer. All echocardiographic data were recorded in accordance with the guidelines of the American Society of Echocardiography (ASE).46,47
Interventricular septal thickness (IVS), posterior wall thickness (PWT), and LV internal dimension (LVID) were measured at end-diastole and end-systole according to the established standards of ASE. To obtain optimal medial–lateral beam orientation and accurate linear measurements, the protocols recommend recording and measuring IVS, PWT, and LVID from the parasternal long-axis acoustic window using 2D-targeted M-mode echocardiography. In addition, observation from the parasternal short-axis acoustic window is required. Measurements of LVID and wall thickness are recommended at the level of mitral valve leaflet tips. Scanned images of 2D long-axis and short-axis views of end-diastolic and end-systolic ventricle with M-mode scans are required to send to the evaluation center.
LV mass was calculated according to the ASE-recommended formula47,48:
LV mass was indexed by height to minimize potential distortion by extracellular volume expansion, and LVMI was defined as LV mass/height2.7.19,20 LVH was defined as LVMI>50 g/m2.7 in men and 47 g/m2.7 in women.19,20 LVEF was obtained using the modified biplane Simpson method from the apical two- and four-chamber views. Echocardiography sonographers and interpreters were blinded to treatment assignment and protocol. All echocardiographic data were sent to the evaluation center and were reviewed in a blinded manner by a cardiologist (T.M.) to confirm the suitability and validity of echocardiography. In these procedures, data from four patients were omitted because of poor evaluation.
Sample Size
According to previous studies on LVH regression by eplerenone in hypertensive patients without advanced renal dysfunction,13 we estimated a −30% reduction in LVMI for 2 years by adding the spironolactone to an ACEI or ARB. It was estimated that a total of 105 patients was necessary to detect significant differences between the two groups with 90% power. The rate of PD dropout from all causes is 10%–20% per year in Japan. To compensate for dropout from this study, we planned to enroll and randomly assigned at least 145 patients.
Statistical Analyses
The trial population in this study is the full analysis set population. Comparison between two groups of continuous variables was performed using the Welch t test or the Mann–Whitney U test. The Fisher exact test was used for categorical variables. Death and technical survival were analyzed by the Kaplan–Meier method and log-rank test. We used estimated marginal means with the linear mixed-effect model with Bonferroni correction to assess group differences in percentage change in LVMI. For the linear mixed-effect model, we included comparison groups and times as fixed effects and patients as random effects. We separately analyzed the effects of spironolactone between men and women, and also between normal LV mass and LVH. Effects of spironolactone were assessed by tertile of residual renal function. Tests of between-subject effects with type III sum of squares were assessed for interactions between fixed effects. We adjusted all analyses with baseline use of diuretics (furosemide). Differences were considered to be statistically significant at P<0.05. All analyses were performed using SPSS Statistics 20 (IBM Corp., Armonk, NY).
Disclosures
None.
Acknowledgments
Other members of the Nagoya Spiro Study group are as follows: Investigator/Institute: M. Watanabe, H. Nishimura, M. Mizutani, H Kinashi (Handa Municipal Hospital, Handa); A. Dambara, Y. Saka, S. Toda, S. Kimu (Yokkaichi Municipal Hospital, Yokkaichi), K. Minoshima, M. Yamaha (Daiyukai Daiichi Hospital, Ichinomiya); R. Takahashi, K. Kimura (Nagoya Kyoritsu Hospital, Nagoya); T. Naruse (Kasugai Municipal Hospital, Kasugai); T. Matsuoka (Ogaki Kita Clinic, Ogaki, Japan); and D. Inaguma, K. Kurata (Tosei General Hospital, Seto).
We are grateful to Professor E. Imai (Nagoya University, Nagoya, Japan, and Nakayamadera Imai Clinic, Takarazuka, Japan) and Dr. K. Matsushita (Johns Hopkins Bloomberg School of Public Health, Baltimore, Maryland) for discussion and advice with regard to this manuscript.
This study was supported in part by a Grant-in-Aid for Progressive Renal Disease Research, Research on Rare and Intractable Diseases, from the Ministry of Health, Labor and Welfare of Japan.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2013030273/-/DCSupplemental.
References
- 1.Glassock RJ, Pecoits-Filho R, Barberato SH: Left ventricular mass in chronic kidney disease and ESRD. Clin J Am Soc Nephrol 4 [Suppl 1]: S79–S91, 2009 [DOI] [PubMed] [Google Scholar]
- 2.Krediet RT, Balafa O: Cardiovascular risk in the peritoneal dialysis patient. Nat Rev Nephrol 6: 451–460, 2010 [DOI] [PubMed] [Google Scholar]
- 3.Herzog CA, Asinger RW, Berger AK, Charytan DM, Díez J, Hart RG, Eckardt KU, Kasiske BL, McCullough PA, Passman RS, DeLoach SS, Pun PH, Ritz E: Cardiovascular disease in chronic kidney disease. A clinical update from Kidney Disease: Improving Global Outcomes (KDIGO). Kidney Int 80: 572–586, 2011 [DOI] [PubMed] [Google Scholar]
- 4.Zoccali C, Benedetto FA, Mallamaci F, Tripepi G, Giacone G, Stancanelli B, Cataliotti A, Malatino LS: Left ventricular mass monitoring in the follow-up of dialysis patients: Prognostic value of left ventricular hypertrophy progression. Kidney Int 65: 1492–1498, 2004 [DOI] [PubMed] [Google Scholar]
- 5.London GM, Pannier B, Guerin AP, Blacher J, Marchais SJ, Darne B, Metivier F, Adda H, Safar ME: Alterations of left ventricular hypertrophy in and survival of patients receiving hemodialysis: Follow-up of an interventional study. J Am Soc Nephrol 12: 2759–2767, 2001 [DOI] [PubMed] [Google Scholar]
- 6.Aoki J, Ikari Y, Nakajima H, Mori M, Sugimoto T, Hatori M, Tanimoto S, Amiya E, Hara K: Clinical and pathologic characteristics of dilated cardiomyopathy in hemodialysis patients. Kidney Int 67: 333–340, 2005 [DOI] [PubMed] [Google Scholar]
- 7.Stewart GA, Gansevoort RT, Mark PB, Rooney E, McDonagh TA, Dargie HJ, Stuart R, Rodger C, Jardine AG: Electrocardiographic abnormalities and uremic cardiomyopathy. Kidney Int 67: 217–226, 2005 [DOI] [PubMed] [Google Scholar]
- 8.Foley RN, Parfrey PS, Harnett JD, Kent GM, Murray DC, Barré PE: The prognostic importance of left ventricular geometry in uremic cardiomyopathy. J Am Soc Nephrol 5: 2024–2031, 1995 [DOI] [PubMed] [Google Scholar]
- 9.Shamseddin MK, Parfrey PS: Sudden cardiac death in chronic kidney disease: Epidemiology and prevention. Nat Rev Nephrol 7: 145–154, 2011 [DOI] [PubMed] [Google Scholar]
- 10.Pitt B, Zannad F, Remme WJ, Cody R, Castaigne A, Perez A, Palensky J, Wittes J, Randomized Aldactone Evaluation Study Investigators : The effect of spironolactone on morbidity and mortality in patients with severe heart failure. N Engl J Med 341: 709–717, 1999 [DOI] [PubMed] [Google Scholar]
- 11.Pitt B, Remme W, Zannad F, Neaton J, Martinez F, Roniker B, Bittman R, Hurley S, Kleiman J, Gatlin M, Eplerenone Post-Acute Myocardial Infarction Heart Failure Efficacy and Survival Study Investigators : Eplerenone, a selective aldosterone blocker, in patients with left ventricular dysfunction after myocardial infarction. N Engl J Med 348: 1309–1321, 2003 [DOI] [PubMed] [Google Scholar]
- 12.Zannad F, McMurray JJ, Krum H, van Veldhuisen DJ, Swedberg K, Shi H, Vincent J, Pocock SJ, Pitt B, EMPHASIS-HF Study Group : Eplerenone in patients with systolic heart failure and mild symptoms. N Engl J Med 364: 11–21, 2011. 21073363 [Google Scholar]
- 13.Pitt B, Reichek N, Willenbrock R, Zannad F, Phillips RA, Roniker B, Kleiman J, Krause S, Burns D, Williams GH: Effects of eplerenone, enalapril, and eplerenone/enalapril in patients with essential hypertension and left ventricular hypertrophy: The 4E-left ventricular hypertrophy study. Circulation 108: 1831–1838, 2003 [DOI] [PubMed] [Google Scholar]
- 14.Taheri S, Mortazavi M, Pourmoghadas A, Seyrafian S, Alipour Z, Karimi S: A prospective double-blind randomized placebo-controlled clinical trial to evaluate the safety and efficacy of spironolactone in patients with advanced congestive heart failure on continuous ambulatory peritoneal dialysis. Saudi J Kidney Dis Transpl 23: 507–512, 2012 [PubMed] [Google Scholar]
- 15.Hausmann MJ, Liel-Cohen N: Aldactone therapy in a peritoneal dialysis patient with decreased left ventricular function. Nephrol Dial Transplant 17: 2035–2036, 2002 [DOI] [PubMed] [Google Scholar]
- 16.Covic A, Gusbeth-Tatomir P, Goldsmith DJ: Is it time for spironolactone therapy in dialysis patients? Nephrol Dial Transplant 21: 854–858, 2006 [DOI] [PubMed] [Google Scholar]
- 17.McGill RL, Biederman RW, Getts RT, Hazlett SM, Sharma SB, Duran J, Brandys DE, Sysak JC, Sureshkumar KK, Sandroni SE, Marcus RJ: Cardiac magnetic resonance imaging in hemodialysis patients. J Nephrol 22: 367–372, 2009 [PubMed] [Google Scholar]
- 18.Nakai S, Iseki K, Itami N, Ogata S, Kazama JJ, Kimata N, Shigematsu T, Shinoda T, Shoji T, Suzuki K, Taniguchi M, Tsuchida K, Nakamoto H, Nishi H, Hashimoto S, Hasegawa T, Hanafusa N, Hamano T, Fujii N, Masakane I, Marubayashi S, Morita O, Yamagata K, Wakai K, Wada A, Watanabe Y, Tsubakihara Y: An overview of regular dialysis treatment in Japan (as of 31 December 2010). Ther Apher Dial 16: 483–521, 2012 [DOI] [PubMed] [Google Scholar]
- 19.Zoccali C, Mallamaci F, Benedetto FA, Tripepi G, Parlongo S, Cataliotti A, Cutrupi S, Giacone G, Bellanuova I, Cottini E, Malatino LS, Creed Investigators : Cardiac natriuretic peptides are related to left ventricular mass and function and predict mortality in dialysis patients. J Am Soc Nephrol 12: 1508–1515, 2001 [DOI] [PubMed] [Google Scholar]
- 20.Wang AY, Lam CW, Wang M, Chan IH, Lui SF, Zhang Y, Sanderson JE: Diagnostic potential of serum biomarkers for left ventricular abnormalities in chronic peritoneal dialysis patients. Nephrol Dial Transplant 24: 1962–1969, 2009 [DOI] [PubMed] [Google Scholar]
- 21.Wang AY, Wang M, Woo J, Law MC, Chow KM, Li PK, Lui SF, Sanderson JE: A novel association between residual renal function and left ventricular hypertrophy in peritoneal dialysis patients. Kidney Int 62: 639–647, 2002 [DOI] [PubMed] [Google Scholar]
- 22.Perl J, Bargman JM: The importance of residual kidney function for patients on dialysis: A critical review. Am J Kidney Dis 53: 1068–1081, 2009 [DOI] [PubMed] [Google Scholar]
- 23.Foley RN, Parfrey PS, Sarnak MJ: Clinical epidemiology of cardiovascular disease in chronic renal disease. Am J Kidney Dis 32[Suppl 3]: S112–S119, 1998 [DOI] [PubMed] [Google Scholar]
- 24.Schiffrin EL, Lipman ML, Mann JF: Chronic kidney disease: Effects on the cardiovascular system. Circulation 116: 85–97, 2007 [DOI] [PubMed] [Google Scholar]
- 25.Ritz E: Left ventricular hypertrophy in renal disease: Beyond preload and afterload. Kidney Int 75: 771–773, 2009 [DOI] [PubMed] [Google Scholar]
- 26.Steigerwalt S, Zafar A, Mesiha N, Gardin J, Provenzano R: Role of aldosterone in left ventricular hypertrophy among African-American patients with end-stage renal disease on hemodialysis. Am J Nephrol 27: 159–163, 2007 [DOI] [PubMed] [Google Scholar]
- 27.Michea L, Villagrán A, Urzúa A, Kuntsmann S, Venegas P, Carrasco L, Gonzalez M, Marusic ET: Mineralocorticoid receptor antagonism attenuates cardiac hypertrophy and prevents oxidative stress in uremic rats. Hypertension 52: 295–300, 2008 [DOI] [PubMed] [Google Scholar]
- 28.Tian J, Shidyak A, Periyasamy SM, Haller S, Taleb M, El-Okdi N, Elkareh J, Gupta S, Gohara S, Fedorova OV, Cooper CJ, Xie Z, Malhotra D, Bagrov AY, Shapiro JI: Spironolactone attenuates experimental uremic cardiomyopathy by antagonizing marinobufagenin. Hypertension 54: 1313–1320, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tonbul Z, Altintepe L, Sözlü C, Yeksan M, Yildiz A, Türk S: The association of peritoneal transport properties with 24-hour blood pressure levels in CAPD patients. Perit Dial Int 23: 46–52, 2003 [PubMed] [Google Scholar]
- 30.Bertocchio JP, Warnock DG, Jaisser F: Mineralocorticoid receptor activation and blockade: An emerging paradigm in chronic kidney disease. Kidney Int 79: 1051–1060, 2011 [DOI] [PubMed] [Google Scholar]
- 31.Shavit L, Lifschitz MD, Epstein M: Aldosterone blockade and the mineralocorticoid receptor in the management of chronic kidney disease: Current concepts and emerging treatment paradigms. Kidney Int 81: 955–968, 2012 [DOI] [PubMed] [Google Scholar]
- 32.Mehdi UF, Adams-Huet B, Raskin P, Vega GL, Toto RD: Addition of angiotensin receptor blockade or mineralocorticoid antagonism to maximal angiotensin-converting enzyme inhibition in diabetic nephropathy. J Am Soc Nephrol 20: 2641–2650, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nishimura H, Ito Y, Mizuno M, Tanaka A, Morita Y, Maruyama S, Yuzawa Y, Matsuo S: Mineralocorticoid receptor blockade ameliorates peritoneal fibrosis in new rat peritonitis model. Am J Physiol Renal Physiol 294: F1084–F1093, 2008 [DOI] [PubMed] [Google Scholar]
- 34.Okazaki A, Mori Y, Nakata M, Kimura T, Sonomura K, Sakoda C, Matsuoka E, Ishida M, Yamahara H, Kishimoto N, Nakagawa H, Matsubara H: Peritoneal mesothelial cells as a target of local aldosterone action: Upregulation of connective tissue growth factor expression via serum- and glucocorticoid-inducible protein kinase 1. Kidney Blood Press Res 32: 151–160, 2009 [DOI] [PubMed] [Google Scholar]
- 35.Suzuki H, Kanno Y, Sugahara S, Okada H, Nakamoto H: Effects of an angiotensin II receptor blocker, valsartan, on residual renal function in patients on CAPD. Am J Kidney Dis 43: 1056–1064, 2004 [DOI] [PubMed] [Google Scholar]
- 36.Schepkens H, Vanholder R, Billiouw JM, Lameire N: Life-threatening hyperkalemia during combined therapy with angiotensin-converting enzyme inhibitors and spironolactone: An analysis of 25 cases. Am J Med 110: 438–441, 2001 [DOI] [PubMed] [Google Scholar]
- 37.Juurlink DN, Mamdani MM, Lee DS, Kopp A, Austin PC, Laupacis A, Redelmeier DA: Rates of hyperkalemia after publication of the Randomized Aldactone Evaluation Study. N Engl J Med 351: 543–551, 2004 [DOI] [PubMed] [Google Scholar]
- 38.Oreopoulos DG, Khanna R, Williams P, Vas SI: Continuous ambulatory peritoneal dialysis - 1981. Nephron 30: 293–303, 1982 [DOI] [PubMed] [Google Scholar]
- 39.Spital A & Sterns RH: Potassium supplementation via the dialysate in continuous ambulatory peritoneal dialysis. Am J Kidney Dis 6 6: 173–176, 1985 [DOI] [PubMed]
- 40.Szeto CC, Chow KM, Kwan BC, Leung CB, Chung KY, Law MC, Li PK: Hypokalemia in Chinese peritoneal dialysis patients: Prevalence and prognostic implication. Am J Kidney Dis 46: 128–135, 2005 [DOI] [PubMed] [Google Scholar]
- 41.Navaneethan SD, Nigwekar SU, Sehgal AR, Strippoli GF: Aldosterone antagonists for preventing the progression of chronic kidney disease: A systematic review and meta-analysis. Clin J Am Soc Nephrol 4: 542–551, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sekikawa A, Ueshima H, Kadowaki T, El-Saed A, Okamura T, Takamiya T, Kashiwagi A, Edmundowicz D, Murata K, Sutton-Tyrrell K, Maegawa H, Evans RW, Kita Y, Kuller LH: Less subclinical atherosclerosis in Japanese men in Japan than in White men in the United States in the post-World War II birth cohort. Am J Epidemiol 165: 617–624, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kohro T, Yamazaki T, Sato H, Ohe K, Nagai R: The impact of a change in hypertension management guidelines on diuretic use in Japan: Trends in antihypertensive drug prescriptions from 2005 to 2011. Hypertens Res 36: 559–563, 2013 [DOI] [PubMed] [Google Scholar]
- 44.Woo KS, Norris RM, Nicholls G: Racial difference in incidence of cough with angiotensin-converting enzyme inhibitors (a tale of two cities). Am J Cardiol 75: 967–968, 1995 [DOI] [PubMed] [Google Scholar]
- 45.RALESInvestigators T : Effectiveness of spironolactone added to an angiotensin-converting enzyme inhibitor and a loop diuretic for severe chronic congestive heart failure (the Randomized Aldactone Evaluation Study [RALES]). Am J Cardiol 78: 902–907, 1996 [DOI] [PubMed] [Google Scholar]
- 46.Schiller NB, Shah PM, Crawford M, DeMaria A, Devereux R, Feigenbaum H, Gutgesell H, Reichek N, Sahn D, Schnittger I, et al. : Recommendations for quantitation of the left ventricle by two-dimensional echocardiography. American Society of Echocardiography Committee on Standards, Subcommittee on Quantitation of Two-Dimensional Echocardiograms. J Am Soc Echocardiogr 2: 358–367, 1989 [DOI] [PubMed] [Google Scholar]
- 47.Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, Picard MH, Roman MJ, Seward J, Shanewise JS, Solomon SD, Spencer KT, Sutton MS, Stewart WJ, Chamber Quantification Writing Group. American Society of Echocardiography’s Guidelines and Standards Committee. European Association of Echocardiography : Recommendations for chamber quantification: a report from the American Society of Echocardiography’s Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr 18: 1440–1463, 2005 [DOI] [PubMed] [Google Scholar]
- 48.Takasaki K, Miyata M, Imamura M, Yuasa T, Kuwahara E, Kubota K, Kono M, Ueya N, Horizoe Y, Chaen H, Mizukami N, Kisanuki A, Hamasaki S, Tei C: Left ventricular dysfunction assessed by cardiac time interval analysis among different geometric patterns in untreated hypertension. Circ J 76: 1409–1414, 2012 [DOI] [PubMed] [Google Scholar]