Abstract
New-onset diabetes after transplantation is a common complication that reduces recipient survival. Research in renal transplant recipients has suggested that pancreatic β-cell dysfunction, as opposed to insulin resistance, may be the key pathologic process. In this study, clinical and genetic factors associated with new-onset diabetes after transplantation were identified in a white population. A joint analysis approach, with an initial genome-wide association study in a subset of cases followed by de novo genotyping in the complete case cohort, was implemented to identify single-nucleotide polymorphisms (SNPs) associated with the development of new-onset diabetes after transplantation. Clinical variables associated with the development of diabetes after renal transplantation included older recipient age, female sex, and percentage weight gain within 12 months of transplantation. The genome-wide association study identified 26 SNPs associated with new-onset diabetes after transplantation; this association was validated for eight SNPs (rs10484821, rs7533125, rs2861484, rs11580170, rs2020902, rs1836882, rs198372, and rs4394754) by de novo genotyping. These associations remained significant after multivariate adjustment for clinical variables. Seven of these SNPs are associated with genes implicated in β-cell apoptosis. These results corroborate recent clinical evidence implicating β-cell dysfunction in the pathophysiology of new-onset diabetes after transplantation and support the pursuit of therapeutic strategies to protect β cells in the post-transplant period.
One-year graft survival after renal transplantation is now excellent, exceeding 93% for organs donated after brain death and 96% for those from living donors.1–3 Technical advancements in surgery, improved understanding of immunology, and innovative developments in pharmacology have altered the landscape of renal transplantation. The goal of preventing early graft loss has largely been achieved and arguably the greatest challenge now is the avoidance of late graft failure. Although there has been a considerable improvement in 1-year renal transplant survival, the rate of graft attrition after the first year remains frustratingly constant.2,4
New-onset diabetes after transplantation (NODAT) is a common and serious disorder that curtails recipient survival.5–7 NODAT is associated with cardiovascular complications8–11 and develops in 2%–50%12 of renal transplant recipients. Approximately 50% of recipients with NODAT require insulin therapy.6–8,13–15 A number of clinical variables have been associated with NODAT, including black ethnicity, older recipient age, female sex, obesity, immunosuppression, and viral infections.5,6,8,13,16,17
Until recently, the pathophysiology of NODAT was considered to be analogous to type 2 diabetes mellitus. Renal transplant recipients have increased insulin resistance compared with transplant-naïve persons with normal renal function.18 In a nondiabetic renal transplant population, the main determinants of insulin resistance are obesity and corticosteroid therapy.19 Insulin resistance improves in renal transplant recipients after successful transplantation20,21 and recipients have enhanced insulin sensitivity compared with dialysis patients.22 At 1 year, there is no significant difference in insulin resistance between renal transplant recipients with NODAT and those with normal glucose tolerance.18,23 Furthermore, insulin resistance indices before transplantation and in the early post-transplant period do not predict NODAT development.11
Pancreatic β-cell dysfunction may prove to be the main pathologic contributor to NODAT. In glucose clamp studies, a deficit in insulin secretion was common to renal transplant recipients with NODAT.18,20,21,24 There are a number of possible mechanisms for β-cell toxicity in renal transplantation, including hyperglycemia,25 elevated free fatty acids,26 and the effect of immunosuppressive medication.27 A recent proof-of-concept clinical trial demonstrated that aggressive management of post-transplant hyperglycemia with insulin significantly reduced the 1-year incidence of NODAT.28 This provides further evidence that post-transplant hyperglycemia plays a key role in NODAT development.
This study investigates clinical and genetic factors associated with NODAT in a relatively large, white renal transplant population. Clinical variables were identified in a carefully phenotyped, ethnically homogeneous cohort. Initial exploratory analysis was conducted via a genome-wide association study (GWAS) in a subgroup of NODAT cases patients and controls to identify genetic variants associated with NODAT. De novo genotyping was then performed in a larger cohort of NODAT patients and controls to validate the findings.
Results
Patient Cohort
There were 707 first, deceased donor kidney transplants performed at Belfast City Hospital (Belfast, UK) between May 1986 and May 2005. Over 99% of both recipients and donors were white; genetic analysis was restricted to those of recorded white ancestry. The average age of recipients was 37 years (range, 2–77 years) and the average age of donors was 42 years (range, 1–75 years). There were 439 male recipients (62.1%) and 428 male donors (59.1%). All recipients had their primary renal diagnosis classified according to the European Dialysis and Transplantation Association coding system. Diagnoses were categorized as glomerular disease (21%), pyelonephritis/interstitial nephritis (20%), autosomal dominant polycystic kidney disease (15%), diabetic nephropathy (9%), other specified miscellaneous etiologies (22%), and CKD not defined (13%). The median follow-up time was 12.2 years (range, 0–26.0 years).
There were changes to the routine post-transplant immunosuppression during the study period. Before 1989, all recipients received dual therapy with prednisolone and azathioprine. Subsequently, calcineurin inhibitor (CNI)–based maintenance therapy was introduced. Mycophenolate mofetil became available in 1998 and from this time, approximately 25% of patients had CNI-free maintenance regimens. All patients received prednisolone for at least 1 year after transplantation.
In our study, the NODAT clinical phenotype was strictly defined as a new requirement for oral hypoglycemic agents or insulin for management of hyperglycemia after transplantation. NODAT status was available for 605 recipients; 58 of 605 recipients (9.6%) developed NODAT during the follow-up period.
Clinical Analyses
At 12 months, 529 adult renal transplant recipients had a functioning graft; 57 of these patients developed NODAT during the follow-up period.
The median graft survival was 10.4 years. During the follow-up period, there were 162 cases of death-censored graft failure. A further 159 recipients died with a functioning graft. Biopsy-proven acute rejection (P<0.001), donor age (P=0.001), and CNI usage (P=0.004) were associated with death-censored graft failure after 1 year. In this cohort, HLA mismatching did not significantly influence death-censored graft survival (P=0.85). This is likely to reflect the policy of favorable HLA matching at our center.29,30
NODAT
Comparison of clinical variables between NODAT cases and controls is shown in Table 1. The median time to NODAT was 100 months (interquartile range, 113 months). Clinical variables associated with NODAT were age, female sex, and percentage weight change in the first year after transplantation. There was no association between CNI therapy and NODAT. In multivariate analysis, the associations with recipient age, female sex, and percentage weight change in the first year remained significant. The association between weight at transplantation and NODAT was also significant after adjustment for confounding (Table 2).
Table 1.
Comparison of clinical variables between NODAT cases and controls
| Variable | NODAT (n=57) | Control (n=370) | P Value |
|---|---|---|---|
| Recipient age (yr) | 49.1±13.2 | 42.4±14.0 | 0.001 |
| Recipient sex | |||
| Male | 30 (53) | 249 (67) | |
| Female | 27 (47) | 121 (33) | 0.04 |
| Donor age (yr) | 37.1±14.8 | 37.6±16.1 | 0.80 |
| Donor sex | |||
| Male | 33 (58) | 213 (58) | |
| Female | 24 (42) | 157 (42) | 1.0 |
| Primary renal disease | |||
| GN | 7 (12) | 89 (24) | 0.07 |
| Interstitial/pyelonephritis | 15 (26) | 79 (21) | 0.50 |
| ADPKD | 16 (28) | 60 (16) | 0.05 |
| Other | 10 (18) | 84 (23) | 0.40 |
| Unknown | 9 (16) | 58 (16) | 0.70 |
| Decade of transplantation | |||
| 1986–1995 | 24 (42) | 174 (47) | |
| 1996–2005 | 33 (58) | 196 (53) | 0.60 |
| HLA mismatch (n) | 2.1±1.1 | 2.2±1.1 | 0.60 |
| Acute rejection | 9 (16) | 48 (13) | 0.90 |
| Immunosuppression | |||
| Calcineurin inhibitors | 41 (72) | 299 (81) | 0.40 |
| Azathioprine | 35 (61) | 224 (61) | 1.0 |
| Mycophenolate mofetil | 28 (49) | 185 (50) | 0.90 |
| mTOR inhibitor | 7 (12) | 34 (9) | 0.60 |
| Weight at transplant (kg) | 72.2±13.3 | 68.5±14.3 | 0.07 |
| Weight change at 1 yra | |||
| Weight loss | 9 (17) | 71 (21) | |
| Weight gain<10% | 11 (20) | 122 (36) | |
| Weight gain>10% | 34 (63) | 149 (43) | 0.04b |
Data are expressed as n (%) or mean±SD. ADPKD, autosomal dominant polycystic kidney disease; mTOR, mammalian target of rapamycin.
Values for weight change at 1 year were available for 396 of 427 recipients.
Linear by linear.
Table 2.
Multivariate logistic regression analysis for NODAT
| Variable | OR (95% Confidence Interval) | P Value |
|---|---|---|
| Recipient agea | 1.4 (1.1 to 1.8) | 0.003 |
| Recipient sex | 2.2 (1.2 to 4.3) | 0.02 |
| Weight at transplantation | 1.03 (1.01 to 1.05) | 0.01 |
| Weight change in first year | 2.0 (1.3 to 3.1) | 0.002 |
Per decade.
Genetic Analyses
GWAS
There were 561,233 single-nucleotide polymorphisms (SNPs) genotyped in 256 individuals consisting of 26 NODAT patients and 230 controls. After quality control, the average genotyping rate was >99%. Twenty-six SNPs were provisionally associated with NODAT (P<10−5); these SNPs were taken forward for second-stage genotyping (Supplemental Table 1).
Reported NODAT SNPs
A literature review identified 27 SNPs previously reported to be associated with NODAT with an additional 10 SNPs investigated for association (Table 3).31–45 Nine of these SNPs were genotyped in the GWAS and proxy SNPs were identified for 17 SNPs that were not directly genotyped (r2>0.80). No association was identified between these previously reported SNPs and NODAT in our population (Supplemental Table 2). Proxy SNPs were unavailable for the remaining 11 reported SNPs. Only one of these SNPs (rs1050450) had previously been associated with NODAT in a white population.
Table 3.
Reported SNPs investigated for association with NODAT
| Gene | SNP | Population | Reference |
|---|---|---|---|
| ATF6 | rs2340721a | White | Fougeray et al.31 |
| CAPN10 | rs5030952 | White | Kurzawski et al.32 |
| CCL5 | rs2107538 | Korean | Jeong et al.33 |
| rs2280789 | Korean | Jeong et al.33 | |
| rs3817655 | Korean | Jeong et al.33 | |
| ENPP1 | rs1044498a | Hispanic | Yang et al34 |
| GPX1 | rs1050450 | White | Dutkiewicz et al.35 |
| HNF1A | rs1169288a | Hispanic | Yang et al.34 |
| rs1800574a | Hispanic | Yang et al.34 | |
| HNF4A | rs2144908 | Hispanic | Yang et al.34 |
| rs1884614 | Hispanic | Yang et al.34 | |
| rs1800961a | Hispanic | Yang et al.34 | |
| IFNγ | rs2430561 | White | Babel et al.36 |
| IL-17E | rs1124053 | Korean | Kim et al.37 |
| IL-17RA | rs2229151 | Korean | Kim et al.37 |
| rs4819554 | Korean | Kim et al.37 | |
| IL-17RB | rs1043261 | Korean | Kim et al.37 |
| rs1025689 | Korean | Kim et al.37 | |
| IL-1B | rs3136558 | Korean | Kim et al.37 |
| IL-2 | rs2069762 | Korean | Kim et al.37 |
| rs2069763 | Korean | Kim et al.37 | |
| IL-4 | rs2243250 | Korean | Kim et al.37 |
| rs2070874 | Korean | Kim et al.37 | |
| IL-6 | rs1800795 | White | Bamoulid et al.38 |
| IL-7R | rs1494558 | Korean | Kim et al.37 |
| rs2172749 | Korean | Kim et al.37 | |
| IRS1 | rs1801278 | Hispanic | Yang et al.34 |
| KCNJ11 | rs5219 | Spanish | Tavira et al.39 |
| Hispanic | Yang et al.34 | ||
| KCNQ1 | rs2237895 | Spanish | Tavira et al.40 |
| NFATC4 | rs10141896 | Hispanic | Chen et al.41 |
| PPARG | rs1801282a | Hispanic | Yang et al.34 |
| PPARGC1 | rs8192678a | Hispanic | Yang et al.34 |
| SUR1 | rs1799854a | Hispanic | Yang et al.34 |
| rs1801261a | Hispanic | Yang et al.34 | |
| SLC30A8 | rs13266634 | Korean | Kang et al.42 |
| TCF7L2 | rs7903146 | White | Kurzawski et al.43 |
| rs12255372a | White | Ghisdal et al.44 | |
| Korean | Kang et al.45 | ||
| Hispanica | Yang et al.34 | ||
| Hispanic | Yang et al.34 |
Nonsignificant association.
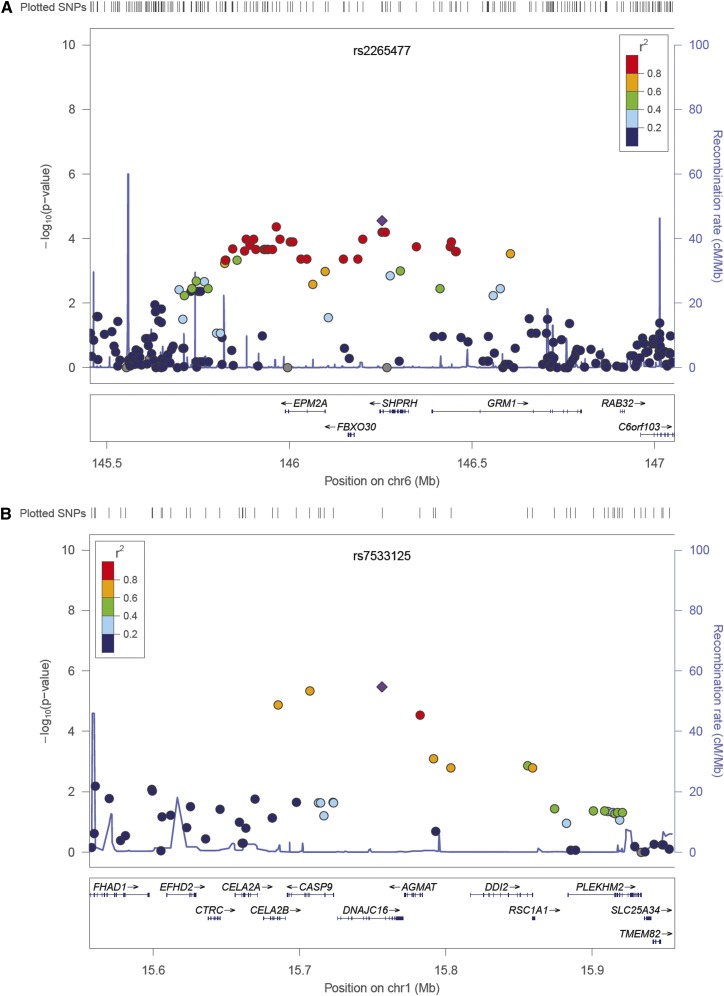
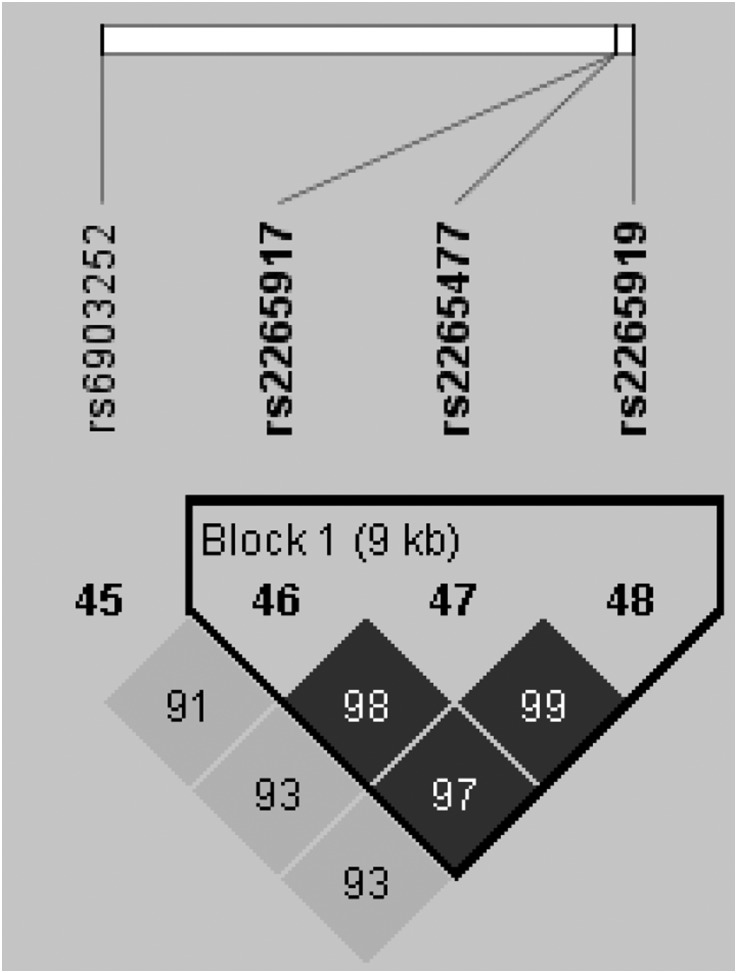
Linkage disequilibrium was assessed for all SNPs (Supplemental Figures 1–50) and is displayed for the top-ranked SNPs on chromosomes 1 and 6 (Figures 1 and 2).
Figure 1.
Regional association plots illustrating linkage disequilibrium between SNPs associated with NODAT. (A) On chromosome 6. (B) On chromosome 1.
Figure 2.
Haplotype block on chromosome 6.
Second-Stage Genotyping
De novo genotyping was undertaken in all NODAT patients and 383 controls. The top-ranked SNPs associated with NODAT in the GWAS (n=26), previously published NODAT SNPs investigated in the GWAS (n=26), and the SNP with a reported association in a white population for which a proxy was unavailable (n=1) were genotyped. Sequenom iPLEX (Hamburg, Germany) technology was used to genotype 44 SNPs; TaqMan (Applied Biosystems, Warrington, UK) was used to genotype six SNPs (Supplemental Figures 51–100, Supplemental Table 3). It was not possible to genotype three SNPs due to problems with primer design (Supplemental Table 4). The average genotyping success rate was >99% and no SNP demonstrated deviation from the Hardy–Weinberg equilibrium (HWE).
In logistic regression analysis, eight SNPs were associated with NODAT (P≤0.001). After adjustment for the clinical variables known to be associated with NODAT (Table 2), adjusted P values, adjusted odds ratios (ORs), and 95% confidence intervals for ORs were calculated (Table 4). Using Haploview,46 a single haplotype block was identified between adjacent SNPs on chromosome 6 (Figure 2). Haplotype analysis did not increase the strength of the association between these SNPs and NODAT (P=0.01).
Table 4.
Association results for de novo genotyped SNPs with NODAT
| SNP | Gene | Location | MAF | P Value | Padj | ORadj (95% Confidence Interval) |
|---|---|---|---|---|---|---|
| rs10484821 | ATP5F1P6 | 6:139868910 | 0.14 | 1.5×10−7 | 0.000002 | 3.5 (2.1 to 5.8) |
| rs7533125 | DNAJC16 | 1:15883744 | 0.23 | 0.001 | 0.001 | 2.4 (1.5 to 3.6) |
| rs2861484 | CELA2B | 1:15812665 | 0.20 | 0.001 | 0.0002 | 2.4 (1.5 to 3.7) |
| rs11580170 | AGMAT | 1:15909744 | 0.26 | <0.00 | 0.0002 | 2.2 (1.4 to 3.4) |
| rs2020902 | CASP9 | 1:15834360 | 0.18 | <0.001 | 0.0003 | 2.3 (1.5 to 3.6) |
| rs1836882 | NOX4 | 11:89232161 | 0.12 | 0.001 | 0.001 | 2.7 (1.5 to 4.8) |
| rs198372 | NPPA | 1:11909514 | 0.13 | <0.001 | 0.001 | 2.5 (1.5 to 4.2) |
| rs4394754 | INPP5A | 10:134343062 | 0.28 | 0.001 | 0.001 | 2.1 (1.4 to 3.2) |
| rs7145618 | PPP2R5C | 14:102329098 | 0.08 | 0.01 | 0.002 | 3.1 (1.5 to 6.4) |
| rs17722392 | KIDINS220 | 2:8940154 | 0.06 | 0.02 | 0.003 | 3.2 (1.5 to 6.9) |
| rs2265919 | SHPRH | 6:146221753 | 0.42 | 0.002 | 0.003 | 2.0 (1.3 to 3.1) |
| rs1783606 | SHANK2 | 11:70576651 | 0.12 | 0.02 | 0.003 | 2.4 (1.3 to 4.1) |
| rs2265477 | SHPRH | 6:146212338 | 0.44 | 0.01 | 0.01 | 1.8 (1.2 to 2.8) |
| rs2069763a | IL-2 | 4:123377482 | 0.28 | 0.01 | 0.01 | 0.48 (0.3 to 0.8) |
| rs6903252 | Intergenic | 6:145922777 | 0.47 | 0.01 | 0.01 | 1.8 (1.2 to 2.8) |
| rs2265917 | SHPRH | 6:146212285 | 0.42 | 0.01 | 0.01 | 1.8 (1.2 to 2.8) |
| rs10899444 | SHANK2 | 11:70606500 | 0.12 | 0.05 | 0.01 | 2.2 (1.2 to 3.9) |
| rs16936667 | PRDM14 | 8:70975726 | 0.14 | 0.02 | 0.01 | 2.2 (1.2 to 3.8) |
| rs341497 | DIAPH3 | 13:60429001 | 0.06 | 0.004 | 0.01 | 2.6 (1.3 to 5.2) |
| rs1016429 | GRIN3A | 9:104402364 | 0.07 | 0.01 | 0.01 | 2.5 (1.2 to 5.0) |
| rs10117679 | GRIN3A | 9:104378479 | 0.04 | 0.01 | 0.02 | 2.8 (1.2 to 6.4) |
| rs1871184 | ITGA1 | 5:52234323 | 0.15 | 0.01 | 0.02 | 1.9 (1.1 to 3.1) |
| rs3212574 | ITGA2 | 5:52366779 | 0.22 | 0.01 | 0.02 | 1.7 (1.1 to 2.7) |
| rs2240747 | ZNRF4 | 19:5456930 | 0.18 | 0.01 | 0.03 | 1.8 (1.1 to 3.1) |
| rs6793265 | ITPR1 | 3:4735533 | 0.12 | 0.08 | 0.07 | 1.7 (0.9 to 3.0) |
| rs2070874a | IL-4 | 5:132009710 | 0.13 | 0.10 | 0.10 | 0.56 (0.3 to 1.1) |
| rs17657199 | NDST1 | 5:149950246 | 0.05 | 0.10 | 0.10 | 2.2 (0.8 to 5.6) |
| rs2069762a | IL-2 | 4:123377980 | 0.33 | 0.10 | 0.10 | 1.4 (0.9 to 2.1) |
| rs7903146a | TCF7L2 | 10:114758349 | 0.31 | 0.20 | 0.20 | 1.4 (0.9 to 2.1) |
| rs1043261a | IL-17RB | 3:53899276 | 0.07 | 0.20 | 0.20 | 0.54 (0.2 to 1.4) |
| rs2280789a | CCL5 | 17:34207003 | 0.11 | 0.30 | 0.20 | 0.61 (0.3 to 1.3) |
| rs2172749a | IL-7R | 5:35855264 | 0.32 | 0.30 | 0.30 | 0.77 (0.5 to 1.2) |
| rs1494558a | IL-7R | 5:35861068 | 0.32 | 0.40 | 0.30 | 0.77 (0.5 to 1.2) |
| rs1800795a | IL-6 | 7:22766645 | 0.39 | 0.60 | 0.30 | 1.3 (0.8 to 2.0) |
| rs12255372a | TCF7L2 | 10:114808902 | 0.30 | 0.30 | 0.30 | 1.3 (0.8 to 1.9) |
| rs5219a | KCNJ11 | 11:17409572 | 0.35 | 0.70 | 0.30 | 0.80 (0.5 to 1.3) |
| rs2107538a | CCL5 | 17:34207780 | 0.17 | 0.40 | 0.40 | 0.77 (0.4 to 1.4) |
| rs1801282a | PPARG | 3:12393125 | 0.13 | 0.50 | 0.40 | 0.75 (0.4 to 1.5) |
| rs3817655a | CCL5 | 17:34199641 | 0.17 | 0.40 | 0.40 | 0.79 (0.4 to 1.4) |
| rs1799854 | ABCC8 | 11:17448704 | 0.43 | 0.30 | 0.50 | 1.2 (0.8 to 1.8) |
| rs4819554a | IL-17RA | 22:17565035 | 0.22 | 0.50 | 0.50 | 0.83 (0.5 to 1.4) |
| rs1169288a | HNF1A | 12:121416650 | 0.32 | 0.60 | 0.60 | 1.1 (0.7 to 1.8) |
| rs2340721a | ATF6 | 1:161849385 | 0.32 | 0.40 | 0.60 | 1.1 (0.7 to 1.7) |
| rs1025689a | IL-17RB | 3:53883722 | 0.36 | 0.70 | 0.60 | 1.1 (0.7 to 1.8) |
| rs1124053a | IL-17E | 14:22914819 | 0.26 | 0.50 | 0.70 | 1.1 (0.7 to 1.7) |
| rs2144908a | HNF4A | 20:42985717 | 0.14 | 0.70 | 0.70 | 0.88 (0.4 to 1.8) |
| rs8192678a | PPARGC1A | 4:23815662 | 0.34 | 0.40 | 0.70 | 0.93 (0.6 to 1.5) |
| rs13266634a | SLC30A8 | 8:118184783 | 0.31 | 0.60 | 0.70 | 1.1 (0.7 to 1.7) |
| rs1044498a | ENPP1 | 6:132172368 | 0.15 | 0.90 | 0.80 | 0.93 (0.5 to 1.7) |
| rs1800961a | HNF4A | 20:43042364 | 0.03 | 0.99 | NA | NA (NA) |
NA, no rs1800961 minor alleles present in the NODAT cases.
SNPs previously reported to be associated with NODAT.
Gene Enrichment and Pathway Analyses
Gene enrichment and pathway analyses were undertaken for the top-ranked SNPs from the GWAS using Partek pathway within Genomics Suite 6.6. The top hit was the “response to stimulus” gene set (P=0.01) (Supplemental Figure 101, Supplemental Table 5). In pathway analysis, the P13K-AKT signaling pathway had the highest enrichment score (P<0.001) (Table 6, Supplemental Figure 102).
Table 6.
Top-ranked (P<0.05) gene pathways from GWAS results
| Pathway Name | Enrichment Score | Enrichment P Value |
|---|---|---|
| PI3K-Akt signaling pathway | 8.0 | <0.001 |
| Glutamatergic synapse | 6.8 | 0.001 |
| Regulation of actin cytoskeleton | 5.0 | 0.01 |
| Phosphatidylinositol signaling system | 4.8 | 0.01 |
| Extracellular matrix–receptor interaction | 4.7 | 0.01 |
| Arrhythmogenic right ventricular cardiomyopathy | 4.6 | 0.01 |
| Hypertrophic cardiomyopathy | 4.2 | 0.02 |
| Small cell lung cancer | 4.1 | 0.02 |
| Dilated cardiomyopathy | 4.1 | 0.02 |
| Oocyte meiosis | 4.0 | 0.02 |
| Hematopoietic cell lineage | 4.0 | 0.02 |
| Dopaminergic synapse | 3.7 | 0.03 |
P13K-Akt, phosphatidylinositol 3-kinase and protein kinase B.
Discussion
NODAT is a common and serious complication of solid organ transplantation.5,6 The growing prevalence of obesity among renal transplant recipients combined with improved access to transplantation for older patients is likely to increase the incidence of NODAT.59–61 Therapeutic strategies for NODAT have focused on altering immunosuppressive regimens with limited success.13,17,62 It is unlikely that NODAT can be avoided by tailoring immunosuppression without a detrimental effect on graft outcomes.
This is the first exploratory GWAS to be undertaken for NODAT. The secondary validation phase confirmed associations between the top-ranked loci; candidate genes, the top-ranked gene enrichment set, and the most significantly associated pathway are implicated in β-cell apoptosis (Tables 5 and 6, Supplemental Table 5).47–58 β-Cell dysfunction may be the primary pathologic process in NODAT18,20,21 and is discussed further in view of the genetic associations identified. Insulin resistance may also contribute to NODAT pathogenesis but this has not been conclusively proven.11,18,20,24,28
Table 5.
Candidate genes and association with diabetes for top-ranked SNPs
| SNP | Candidate Genesa | Gene Function | Other SNP Associations | Gene Association with Diabetes Mellitus |
|---|---|---|---|---|
| rs10484821 | ATP5F1P6b | Mitochondrial pseudogene | None | None known |
| rs7533125 | DNAJC16, CASP9, AGMAT, CELA2B, DDI2 | Component of heat shock protein 40 | None | Differential expression in pancreatic islets of type 2 patients with diabetes47 |
| rs2861484 | CELA2B, CASP9, CELA2A, DNAJC16, CTRC, EFHD2, AGMAT | Pancreatic elastase | None | None known |
| rs11580170 | AGMAT, DNAJC16, CASP9, DDI2, CELA2B | Agmatine | None | Reduces apoptosis,48 increases insulin secretion,49 and insulin sensitivity50 in rats |
| rs2020902 | CASP9, DNAJC16, CELA2B, CELA2A, AGMAT, CTRC, EFHD2 | Caspase 9 | Non-Hodgkin’s lymphoma | Component of intrinsic apoptotic pathway in pancreatic β cells51 |
| rs1836882 | NOX4 | Subunit of NADPH oxidase complex | None | Increases ROS generation, reduces insulin gene expression and increases β-cell apoptosis in mice and in vitro,52 blockade replenishes islet insulin stores in animal models53 |
| rs198372 | NPPA, NPPB, CLCN6, METHFR, KIAA2013, PLOD1 | Natriuretic peptide | None | Inhibits adipokine and cytokine production,54 reduced natriuretic peptide levels are associated with the development of diabetes55 |
| rs4394754 | INPP5A | Membrane associated type 1 inositol 1,4,5-triphosphate 5-phosphatase | None | Reduces β-cell proliferation in vitro,56 stimulates calcium induced insulin signaling and exocytosis in mice,57 regulates hepatic gluconeogenesis in mice58 |
Where there is more than one candidate, the gene in closest proximity to the SNP was chosen (underlined).
Candidate genes within 100 kB of SNP recorded in order of proximity.
Pseudogene.
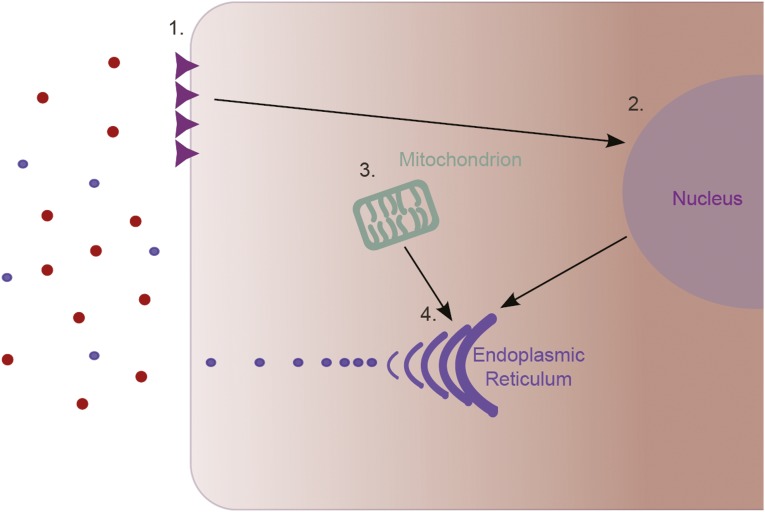
Glucose-Stimulated Insulin Secretion
Hyperglycemia upregulates Fas receptor expression on the β cell surface (Figure 3); initially, this promotes cell proliferation and insulin secretion.63,64 The SNP rs4394754 is upstream from the 5′-untranslated region of INPP5A. The inositol polyphosphate 5-phosphatases are implicated in insulin signaling and exocytosis and inhibit the proliferation of insulin-producing cells in vitro.56,57,65
Figure 3.
Glucose-stimulated insulin secretion. (1) Hyperglycemia induces Fas receptor expression on the β-cell surface. (2) Fas receptor expression and IL-1β production increase transcription of insulin genes and β-cell proliferation. (3) Mitochondrial generation of ATP is stimulated by hyperglycemia. (4) Insulin production in the ER is enhanced and increased insulin secretion restores normoglycemia.
Hyperglycemia stimulates ATP generation in mitochondria, causing membrane depolarization, calcium ion influx, and increased insulin exocytosis.63,66
Glucotoxicity
Hyperglycemia initiates a train of events that begins with enhanced insulin secretion but, unchecked, will result in β-cell apoptosis. After gene enrichment, the top-ranked gene set associated with NODAT was “response to stimulus.” β Cells demonstrate increased apoptosis and reduced proliferation after 2 days of exposure to moderately elevated glucose concentrations in vitro.67 Within 4 days, human β cells have almost completely lost their secretory function.63 The period of exposure to elevated glucose concentrations is more important than the degree of hyperglycemia.68,69 In the immediate post-transplantation period, hyperglycemia is virtually universal among renal transplant recipients; 87% of nondiabetic recipients at the Mayo Clinic recorded a blood glucose≥200 mg/dl during this period.28,70 In a recent clinical trial, all renal transplant recipients recorded a blood glucose ≥140 mg/dl during hospitalization.28 Factors contributing to post-transplant hyperglycemia include the catecholamine stress response to surgery,71,72 corticosteroid treatment,73 and restoration of normal renal degradation and excretion of insulin.74,75
Hyperglycemia in the immediate post-transplant period is associated with a 4-fold increase in NODAT.10,76 A recent proof-of-concept trial suggests that aggressive management of hyperglycemia in the postoperative period can reduce the incidence of NODAT. Hecking et al. compared insulin therapy when blood glucose exceeded 140 mg/dl to standard diabetic treatment in a cohort of kidney transplant recipients. At 1 year, there were no cases of NODAT in the treatment group compared with an incidence of 28% in the control group.28 It is plausible that early management of hyperglycemia with insulin prevents β-cell glucotoxicity and apoptosis.
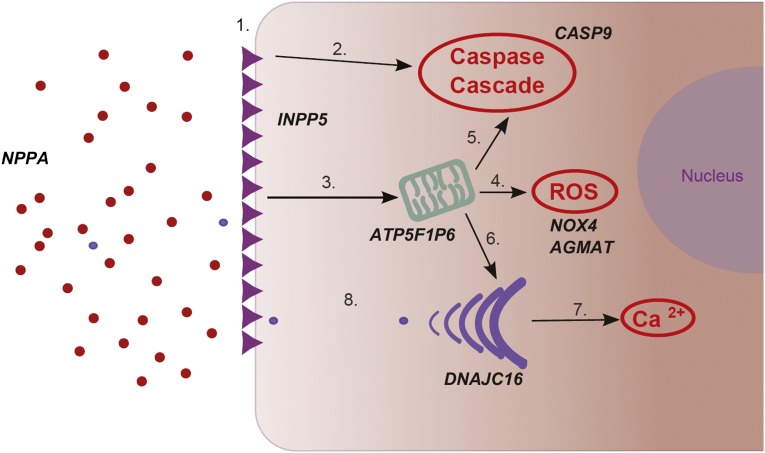
Hyperglycemia-induced β-cell apoptosis occurs via multiple pathways (Figure 4). Prolonged hyperglycemia attenuates the expression of Fas-associated protein with death domain–like IL-1β–converting enzyme inhibitory protein.63,64,77 In the absence of Fas-associated protein with death domain–like IL-1β–converting enzyme inhibitory protein, Fas receptors induce apoptosis via both caspase-dependent and independent pathways.69,77–79 Protracted stimulation of IL-1β promotes apoptosis by downregulating other protective proteins within the β cell.64,80 In addition, both Fas activation and IL-1β induce mitochondrial dysfunction77 and in vitro studies of β cells in NODAT have demonstrated a role for mitochondrial dysfunction.81 During prolonged hyperglycemia, generation of reactive oxygen species (ROS) by both mitochondria and the NADPH pathway exceed the oxidative stress threshold of the β cell.66,82,83 ATP5F1P6 (rs10484821) is an inferred mitochondrial ATP synthase pseudogene.84 NOX4 (rs1836882) encodes an enzyme of the NADPH oxidase complex.85 In animal models of type 2 diabetes, NADPH oxidase blockade reduces oxidative stress and replenishes insulin stores.53 Agmatine, a protein encoded by AGMAT (rs11580170), acts as a ROS scavenger to protect the mitochondrial membrane.48 In animal models, agmatine also enhances insulin secretion.49 Damaged mitochondria release cytochrome C and induce apoptosis via the activation of caspase 977; rs2020902 is located in the CASP9 gene.49
Figure 4.
β-Cell glucotoxicity. (1) Prolonged hyperglycemia results in large numbers of Fas receptors being expressed on the β-cell membrane. (2) Fas activation induces β-cell apoptosis via both caspase-dependent and independent pathways. (3) Fas activation and elevated IL-1β concentrations induce mitochondrial dysfunction. (4) Excessive mitochondrial stimulation generates ROS that cause oxidative damage to the β cell. (5) Damaged mitochondria release cytochrome C, which activates the caspase cascade. (6) Reduced mitochondrial generation of ATP limits the production of insulin by the ER. (7) Prolonged insulin demands result in ER stress and intracytoplasmic accumulation of calcium ions, which stimulates apoptosis. (8) There is inadequate insulin exocytosis to restore normoglycemia.
Sustained demands on the endoplasmic reticulum (ER) during hyperglycemia result in cytoplasmic accumulation of calcium,86,87 causing activation of the caspase cascade86 and mitochondrial damage.48 In β cells, ER stress upregulates heat shock protein 40 expression; this correlates with increased apoptosis.47 The transcription product of the DNAJC16 (rs7533125) gene forms part of the heat shock protein 40 complex. NODAT is associated with elevated serum proinsulin concentrations15,20 and the exocytosis of this inactive precursor reflects ER stress.88
Lipotoxicity
Body weight and, in this study, percentage weight gain in the first year after transplantation are associated with NODAT.5,6 Leptin is secreted by adipose tissues and is associated with both pretransplantation body fat and post-transplantation weight gain.89,90 Leptin enhances IL-1β production in pancreatic islets promoting apoptosis.91 Elevated leptin concentrations correlate with increased serum free fatty acids. These directly induce β-cell apoptosis by caspase activation,77 induction of ER stress,87 and ROS generation.66 The natriuretic peptides, encoded by NPPA (rs198372), inhibit leptin secretion and low natriuretic peptide levels are associated with the development of diabetes mellitus.54,55
Immunosuppression
The contribution of corticosteroids and CNIs to the development of NODAT is well recognized.17,92 Corticosteroids increase insulin resistance and promote β-cell apoptosis by exacerbating mitochondrial and ER stress.13,73,93,94 The calcineurin/nuclear factor of activated T cell pathway modulates insulin exocytosis and β-cell proliferation. In animal models and in vitro, calcineurin/nuclear factor of activated T-cell blockade reduces insulin exocytosis and β-cell mass.27,95,96 However, the evidence for an association between CNIs and NODAT is mixed; a recent meta-analysis found only a weak association between CNI therapy and NODAT.17 In this study, a statistically significant association was not demonstrated, which may reflect the low incidence of NODAT in our population. Mycophenolate also induces β-cell death by upregulation of the caspase cascade and induction of ER stress.27,77 The contribution of each of these immunosuppressants explains the disappointing results of limiting one group in reducing NODAT incidence.
Strengths and Limitations
This study identified clinical and genetic variables associated with NODAT in an ethnically homogeneous population. It is the first to utilize an exploratory GWAS with confirmation by de novo genotyping; all previous studies have focused on candidate genes. The majority of SNPs previously reported to be associated with NODAT have been identified in a single population with failure of replication in other ethnic groups.33,34,37 The TCF7L2 SNP rs7903146 is an exception to this; rs7903146 variants have been associated with NODAT in Korean45 (P=0.02), white European44 (P=0.002), and Polish Caucasian43 (P=0.02) populations, although not all reports demonstrate an association.34,97,98 This study had a comparable cohort size and rs7903146 minor allele frequency to the other studies but did not find an association between rs7903146 and NODAT. This may reflect the use of an extreme NODAT phenotype, which could prevent identification of genetic variants associated with milder disease. It is also possible that there may be an interaction between rs7903146 and CNIs in the risk for NODAT. Recipients were receiving CNIs in all three studies reporting an association, whereas 25% of this cohort had CNI-free immunosuppression.
Strengths of this study include the availability of high-quality clinical follow-up data over 25 years, the identification and correction for clinical variables in the same population, the use of multiple genotyping technologies, and second-stage typing of additional DNA samples to validate discovery findings. Stringent diagnostic criteria were used to militate against phenotypic heterogeneity and to enrich the population for risk alleles. The reported prevalence of NODAT in renal transplant recipients varies between 2% and 50%, reflecting differences in diagnostic criteria and reporting.12 Although the diagnostic criteria used in this study will have potentially underestimated the NODAT incidence, we are confident that all recipients who developed a “severe diabetic phenotype” were identified. The small number of NODAT cases is a weakness of this study, resulting in the possibility that SNPs associated with a milder phenotype or that are less strongly associated with NODAT may not have been identified. However, GWAS have been successfully utilized to identify loci associated with extreme phenotypes in a number of complex diseases.99–102
As a consequence of the small number of NODAT cases, these results did not reach the conventional threshold for genome-wide significance. As described, the stringent diagnostic criteria were used to generate phenotypic homogeneity with reduced case numbers as a consequence. A joint analysis approach was implemented to maximize power in this study; SNPs of interest were identified in the exploratory GWAS and de novo genotyping was used in the complete cohort of NODAT patients to validate these findings. This study has <30% power to identify a risk allele, with a minor allele frequency (MAF) of 10% and an OR of 1.5 with 95% confidence; however, the strong effects observed confirm that the smaller sample size utilized was sufficient to identify risk alleles for NODAT. Larger studies are required to identify genetic variants that confer a smaller effect on the risk of developing NODAT. The individuals genotyped for this study provided at least 60%, 80%, and 90% power to identify a risk allele at MAFs of 10%, 20%, or 30% respectively and OR of 2.0 with 95% confidence. The top-ranked SNPs associated with NODAT are in biologically plausible pathways that influence -cell apoptosis and corroborate current clinical evidence regarding NODAT pathogenesis. The associated genes or their transcription products have been associated with diabetes in vitro as well as in animal models and in longitudinal follow-up studies.
In conclusion, NODAT is a serious complication of solid organ transplantation that is detrimental to recipient survival.5–7 Recent clinical studies suggest that β-cell toxicity, predominantly mediated by hyperglycemia, may be the primary pathologic process in NODAT28,76 and that aggressive management of elevated serum glucose in the post-transplant period may prevent NODAT.28 This study is the first exploratory GWAS with secondary validation for NODAT undertaken in a population of solid organ transplant recipients. SNPs associated with NODAT were associated with β-cell apoptotic pathways. This provides further evidence that β-cell dysfunction and death are key components of NODAT pathogenesis.
Concise Methods
Patient Cohort
All kidney transplant procedures in Northern Ireland are performed at the Belfast City Hospital. Consecutive renal transplant recipients who received first, deceased donor kidney transplants between May 1986 and May 2005 inclusive were included in this study. Donor and recipient clinical variables, graft outcomes, and survival are prospectively recorded in the Northern Ireland Kidney Transplant Database. Available data include donor age and sex, recipient age and sex, primary renal disease, HLA match, immunosuppression, acute rejection episodes, weight, incidence of NODAT, allograft survival, recipient survival, and cause of death. There were significant advances in transplantation during the study period. To allow for this, the variable “decade of transplantation” was considered. The first decade terminated on December 31, 1995, with the second decade commencing on January 1, 1996. Follow-up was continued until August 2012.
NODAT
NODAT was defined as a new requirement for oral hypoglycemic therapy or insulin after kidney transplantation. Controls were renal transplant recipients who did not have diabetes mellitus at transplantation and did not develop a requirement for oral hypoglycemic therapy or insulin during the follow-up period.
Clinical Analyses
All adults (aged≥16 years) who received a first, deceased donor transplant between May 1986 and May 2005 inclusive and who had a functioning renal transplant at 1 year were included. One-year graft survival was a prerequisite to investigating the effect of weight gain in the first year on NODAT incidence. Clinical variables investigated for association with NODAT were recipient age, recipient sex, primary renal disease, donor age, donor sex, HLA mismatch, acute rejection, immunosuppressive regimen, weight at transplantation, percent weight gain in the first year after transplantation, and decade of transplantation. SPSS for Windows (version 21.0; SPSS, Inc., Chicago, IL) was utilized for all analyses, with P values<0.05 considered significant.
Genetic Analyses
GWAS
DNA from individuals in the patient cohort was genotyped using the Illumina 660K array as part of the Wellcome Trust Case Control Consortium 3 Study into Renal Transplant Dysfunction (http://www.wtccc.org.uk/ccc3/). No sample was excluded for poor DNA quality, quantity, or poor genotype concordance with previous genotypes during a fingerprint evaluation stage. There were 26 NODAT patients and 230 controls successfully genotyped. Standard quality control was utilized with exclusion if the genotyping call rate was <90%, if MAF was <1%, if there was deviation from HWE (P<10−6), or if the sample call rate was <95%. Extreme heterozygosity and cryptic relatedness were not observed in this cohort. Known copy number variation and mitochondrial SNPs were excluded from analyses. These quality control steps and association analysis was performed using PLINK.103 The level of statistical significance was set at P<10−5 considering the relatively small number of cases available; second-stage genotyping was conducted for validation and supporting data for all SNPs of interest. Linkage disequilibrium was calculated and results were plotted using Haploview46 with regional association plots generated in LocusZoom.104
In addition, there are 37 SNPs previously published as associated with NODAT. The association between these SNPs and NODAT was investigated in our GWAS cohort. Where reported SNPs were not genotyped in the GWAS, proxy SNPs were identified (r2>0.80) and association with NODAT was investigated.
Second-Stage Genotyping
All SNPs associated with NODAT with a P value<10−5 from the GWAS were selected for further analysis. We included previously reported NODAT SNPs as well as a single SNP (rs1050450) that was not genotyped in the GWAS and for which a proxy was unavailable. De novo genotyping for the above SNPs was undertaken in 58 NODAT patients and 383 controls from the patient cohort using Sequenom iPLEX and TaqMan technologies. DNA samples were randomly arranged in a 384-well format with four father-mother-proband trios and four negative controls per plate. Assays were performed according to the manufacturers’ instructions. The genotyping success rate and HWE were calculated for all SNPs using PLINK. Haploview (version 4.2; http://www.broadinstitute.org/haploview)46 was used to identify linkage disequilibrium between SNPs and visualize haplotype blocks.
Statistical analysis was performed using PLINK.103 A logistic regression multivariate model was utilized to investigate the confounding effect of clinical variables significantly associated with NODAT and the SNP associations. We included those clinical covariates that remained significantly associated with NODAT in multivariate logistic regression analysis (Table 2).
Gene Set Enrichment and Pathway Analyses
Gene set enrichment and pathway analysis were performed on the top hits from the GWAS using Partek software (version 6.6; Partek, Inc., St. Louis, MO). For gene set enrichment and pathway analysis, Fisher’s exact test was utilized, gene sets were restricted to those with two or more genes included, and genes were analyzed according to the hg19 genome build.
Ethics Statement
Ethical approval for this study was granted by the Office for Research Ethics Committees, Northern Ireland (http://www.hscbusiness.hscni.net/orecni.htm, 08/NIR03/79, 12/NI/0178). This study adhered to the Declaration of Istanbul.
Disclosures
None.
Acknowledgments
The authors acknowledge the assistance of Stuart Hacking in the preparation of figures for this article. The initial GWAS typing was generated by the United Kingdom and Ireland Renal Transplant Consortium, led by Professor Graham Lord, as part of the Wellcome Trust Case Control Consortium 3 Study into Renal Transplant Dysfunction (http://www.wtccc.org.uk/ccc3/projects/ccc3_rtd.html).
This work was supported by the Northern Ireland Kidney Research Fund. J.A.M. is a recipient of a Kidney Research UK Clinical Research Training Fellowship.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2013040383/-/DCSupplemental.
References
- 1.Hariharan S, Johnson CP, Bresnahan BA, Taranto SE, McIntosh MJ, Stablein D: Improved graft survival after renal transplantation in the United States, 1988 to 1996. N Engl J Med 342: 605–612, 2000 [DOI] [PubMed] [Google Scholar]
- 2.Lamb KE, Lodhi S, Meier-Kriesche HU: Long-term renal allograft survival in the United States: A critical reappraisal. Am J Transplant 11: 450–462, 2011 [DOI] [PubMed] [Google Scholar]
- 3.NHS Blood and Transplant : Survival rates following transplantation. In: Organ Donation and Transplantation Activity Report 2011-2012, Hertfordshire, UK, NHS Blood and Transplant, 2012, pp 79–99 [Google Scholar]
- 4.Meier-Kriesche HU, Schold JD, Kaplan B: Long-term renal allograft survival: Have we made significant progress or is it time to rethink our analytic and therapeutic strategies? Am J Transplant 4: 1289–1295, 2004 [DOI] [PubMed] [Google Scholar]
- 5.Kasiske BL, Snyder JJ, Gilbertson D, Matas AJ: Diabetes mellitus after kidney transplantation in the United States. Am J Transplant 3: 178–185, 2003 [DOI] [PubMed] [Google Scholar]
- 6.Cole EH, Johnston O, Rose CL, Gill JS: Impact of acute rejection and new-onset diabetes on long-term transplant graft and patient survival. Clin J Am Soc Nephrol 3: 814–821, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Valderhaug TG, Hjelmesæth J, Hartmann A, Røislien J, Bergrem HA, Leivestad T, Line PD, Jenssen T: The association of early post-transplant glucose levels with long-term mortality. Diabetologia 54: 1341–1349, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wauters RP, Cosio FG, Suarez Fernandez ML, Kudva Y, Shah P, Torres VE: Cardiovascular consequences of new-onset hyperglycemia after kidney transplantation. Transplantation 94: 377–382, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yates CJ, Fourlanos S, Hjelmesaeth J, Colman PG, Cohney SJ: New-onset diabetes after kidney transplantation-changes and challenges. Am J Transplant 12: 820–828, 2012 [DOI] [PubMed] [Google Scholar]
- 10.Chakkera HA, Knowler WC, Devarapalli Y, Weil EJ, Heilman RL, Dueck A, Mulligan DC, Reddy KS, Moss AA, Mekeel KL, Mazur MJ, Hamawi K, Castro JC, Cook CB: Relationship between inpatient hyperglycemia and insulin treatment after kidney transplantation and future new onset diabetes mellitus. Clin J Am Soc Nephrol 5: 1669–1675, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nagaraja P, Ravindran V, Morris-Stiff G, Baboolal K: Role of insulin resistance indices in predicting new-onset diabetes after kidney transplantation. Transpl Int 26: 273–280, 2013 [DOI] [PubMed] [Google Scholar]
- 12.Montori VM, Basu A, Erwin PJ, Velosa JA, Gabriel SE, Kudva YC: Posttransplantation diabetes: A systematic review of the literature. Diabetes Care 25: 583–592, 2002 [DOI] [PubMed] [Google Scholar]
- 13.Luan FL, Steffick DE, Ojo AO: New-onset diabetes mellitus in kidney transplant recipients discharged on steroid-free immunosuppression. Transplantation 91: 334–341, 2011 [DOI] [PubMed] [Google Scholar]
- 14.Shabir S, Jham S, Harper L, Ball S, Borrows R, Sharif A: Validity of glycated haemoglobin to diagnose new onset diabetes after transplantation. Transpl Int 26: 315–321, 2013 [DOI] [PubMed] [Google Scholar]
- 15.Zelle DM, Corpeleijn E, Deinum J, Stolk RP, Gans RO, Navis G, Bakker SJ: Pancreatic β-cell dysfunction and risk of new-onset diabetes after kidney transplantation. Diabetes Care 36: 1926–1932, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chadban S: New-onset diabetes after transplantation—should it be a factor in choosing an immunosuppressant regimen for kidney transplant recipients. Nephrol Dial Transplant 23: 1816–1818, 2008 [DOI] [PubMed] [Google Scholar]
- 17.Sharif A, Shabir S, Chand S, Cockwell P, Ball S, Borrows R: Meta-analysis of calcineurin-inhibitor-sparing regimens in kidney transplantation. J Am Soc Nephrol 22: 2107–2118, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ekstrand AV, Eriksson JG, Grönhagen-Riska C, Ahonen PJ, Groop LC: Insulin resistance and insulin deficiency in the pathogenesis of posttransplantation diabetes in man. Transplantation 53: 563–569, 1992 [DOI] [PubMed] [Google Scholar]
- 19.Oterdoom LH, de Vries AP, Gansevoort RT, van Son WJ, van der Heide JJ, Ploeg RJ, de Jong PE, Gans RO, Bakker SJ: Determinants of insulin resistance in renal transplant recipients. Transplantation 83: 29–35, 2007 [DOI] [PubMed] [Google Scholar]
- 20.Nam JH, Mun JI, Kim SI, Kang SW, Choi KH, Park K, Ahn CW, Cha BS, Song YD, Lim SK, Kim KR, Lee HC, Huh KB: beta-Cell dysfunction rather than insulin resistance is the main contributing factor for the development of postrenal transplantation diabetes mellitus. Transplantation 71: 1417–1423, 2001 [DOI] [PubMed] [Google Scholar]
- 21.Hagen M, Hjelmesaeth J, Jenssen T, Morkrid L, Hartmann A: A 6-year prospective study on new onset diabetes mellitus, insulin release and insulin sensitivity in renal transplant recipients. Nephrol Dial Transplant 18: 2154–2159, 2003 [DOI] [PubMed] [Google Scholar]
- 22.Shimizu M, Iino Y, Terashi A: [Improvement of insulin sensitivity after renal transplantation measured by a glucose clamp technique]. Nippon Ika Daigaku Zasshi 65: 50–54, 1998 [DOI] [PubMed] [Google Scholar]
- 23.Hjelmesaeth J, Hagen M, Hartmann A, Midtvedt K, Egeland T, Jenssen T: The impact of impaired insulin release and insulin resistance on glucose intolerance after renal transplantation. Clin Transplant 16: 389–396, 2002 [DOI] [PubMed] [Google Scholar]
- 24.Midtvedt K, Hartmann A, Hjelmesaeth J, Lund K, Bjerkely BL: Insulin resistance is a common denominator of post-transplant diabetes mellitus and impaired glucose tolerance in renal transplant recipients. Nephrol Dial Transplant 13: 427–431, 1998 [DOI] [PubMed] [Google Scholar]
- 25.Yki-Järvinen H: Glucose toxicity. Endocr Rev 13: 415–431, 1992 [DOI] [PubMed] [Google Scholar]
- 26.Poitout V, Robertson RP: Minireview: Secondary beta-cell failure in type 2 diabetes—a convergence of glucotoxicity and lipotoxicity. Endocrinology 143: 339–342, 2002 [DOI] [PubMed] [Google Scholar]
- 27.Johnson JD, Ao Z, Ao P, Li H, Dai LJ, He Z, Tee M, Potter KJ, Klimek AM, Meloche RM, Thompson DM, Verchere CB, Warnock GL: Different effects of FK506, rapamycin, and mycophenolate mofetil on glucose-stimulated insulin release and apoptosis in human islets. Cell Transplant 18: 833–845, 2009 [DOI] [PubMed] [Google Scholar]
- 28.Hecking M, Haidinger M, Döller D, Werzowa J, Tura A, Zhang J, Tekoglu H, Pleiner J, Wrba T, Rasoul-Rockenschaub S, Mühlbacher F, Schmaldienst S, Druml W, Hörl WH, Krebs M, Wolzt M, Pacini G, Port FK, Säemann MD: Early basal insulin therapy decreases new-onset diabetes after renal transplantation. J Am Soc Nephrol 23: 739–749, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Courtney AE, McNamee PT, Maxwell AP: The evolution of renal transplantation in clinical practice: For better, for worse? QJM 101: 967–978, 2008 [DOI] [PubMed] [Google Scholar]
- 30.Courtney AE, McNamee PT, Middleton D, Heggarty S, Patterson CC, Maxwell AP: Association of functional heme oxygenase-1 gene promoter polymorphism with renal transplantation outcomes. Am J Transplant 7: 908–913, 2007 [DOI] [PubMed] [Google Scholar]
- 31.Fougeray S, Loriot MA, Nicaud V, Legendre C, Thervet E, Pallet N: Increased body mass index after kidney transplantation in activating transcription factor 6 single polymorphism gene carriers. Transplant Proc 43: 3418–3422, 2011 [DOI] [PubMed] [Google Scholar]
- 32.Kurzawski M, Dziewanowski K, Kedzierska K, Gornik W, Banas A, Drozdzik M: Association of calpain-10 gene polymorphism and posttransplant diabetes mellitus in kidney transplant patients medicated with tacrolimus. Pharmacogenomics J 10: 120–125, 2010 [DOI] [PubMed] [Google Scholar]
- 33.Jeong KH, Moon JY, Chung JH, Kim YH, Lee TW: Significant associations between CCL5 gene polymorphisms and post-transplantational diabetes mellitus in Korean renal allograft recipients. Am J Nephrol 32: 356–361, 2010 [DOI] [PubMed] [Google Scholar]
- 34.Yang J, Hutchinson II, Shah T, Min DI: Genetic and clinical risk factors of new-onset diabetes after transplantation in Hispanic kidney transplant recipients. Transplantation 91: 1114–1119, 2011 [DOI] [PubMed] [Google Scholar]
- 35.Dutkiewicz G, Domanski L, Pawlik A, Binczak-Kuleta A, Safranow K, Ciechanowicz A, Dziedziejko V, Pietrzak-Nowacka M, Ciechanowski K: Polymorphisms of superoxide dismutase, glutathione peroxidase and catalase genes in patients with post-transplant diabetes mellitus. Arch Med Res 41: 350–355, 2010 [DOI] [PubMed] [Google Scholar]
- 36.Babel N, Cherepnev G, Kowalenko A, Horstrup J, Volk HD, Reinke P: Nonimmunologic complications and gene polymorphisms of immunoregulatory cytokines in long-term renal transplants. Kidney Int 66: 428–432, 2004 [DOI] [PubMed] [Google Scholar]
- 37.Kim YG, Ihm CG, Lee TW, Lee SH, Jeong KH, Moon JY, Chung JH, Kim SK, Kim YH: Association of genetic polymorphisms of interleukins with new-onset diabetes after transplantation in renal transplantation. Transplantation 93: 900–907, 2012 [DOI] [PubMed] [Google Scholar]
- 38.Bamoulid J, Courivaud C, Deschamps M, Mercier P, Ferrand C, Penfornis A, Tiberghien P, Chalopin JM, Saas P, Ducloux D: IL-6 promoter polymorphism -174 is associated with new-onset diabetes after transplantation. J Am Soc Nephrol 17: 2333–2340, 2006 [DOI] [PubMed] [Google Scholar]
- 39.Tavira B, Coto E, Torres A, Díaz-Corte C, Díaz-Molina B, Ortega F, Arias M, Díaz JM, Selgas R, López-Larrea C, Ruiz-Ortega M, Ortiz A, González E, Campistol JM, Alvarez V, Pharmacogenetics of tacrolimus REDINREN study group : Association between a common KCNJ11 polymorphism (rs5219) and new-onset posttransplant diabetes in patients treated with tacrolimus. Mol Genet Metab 105: 525–527, 2012 [DOI] [PubMed] [Google Scholar]
- 40.Tavira B, Coto E, Díaz-Corte C, Ortega F, Arias M, Torres A, Díaz JM, Selgas R, López-Larrea C, Campistol JM, Ruiz-Ortega M, Alvarez V, Pharmacogenetics of Tacrolimus REDINREN Study Group : KCNQ1 gene variants and risk of new-onset diabetes in tacrolimus-treated renal-transplanted patients. Clin Transplant 25: E284–E291, 2011 [DOI] [PubMed] [Google Scholar]
- 41.Chen Y, Sampaio MS, Yang JW, Min D, Hutchinson IV: Genetic polymorphisms of the transcription factor NFATc4 and development of new-onset diabetes after transplantation in Hispanic kidney transplant recipients. Transplantation 93: 325–330, 2012 [DOI] [PubMed] [Google Scholar]
- 42.Kang ES, Kim MS, Kim YS, Kim CH, Han SJ, Chun SW, Hur KY, Nam CM, Ahn CW, Cha BS, Kim SI, Lee HC: A polymorphism in the zinc transporter gene SLC30A8 confers resistance against posttransplantation diabetes mellitus in renal allograft recipients. Diabetes 57: 1043–1047, 2008 [DOI] [PubMed] [Google Scholar]
- 43.Kurzawski M, Dziewanowski K, Kędzierska K, Wajda A, Lapczuk J, Droździk M: Association of transcription factor 7-like 2 (TCF7L2) gene polymorphism with posttransplant diabetes mellitus in kidney transplant patients medicated with tacrolimus. Pharmacol Rep 63: 826–833, 2011 [DOI] [PubMed] [Google Scholar]
- 44.Ghisdal L, Baron C, Le Meur Y, Lionet A, Halimi JM, Rerolle JP, Glowacki F, Lebranchu Y, Drouet M, Noël C, El Housni H, Cochaux P, Wissing KM, Abramowicz D, Abramowicz M: TCF7L2 polymorphism associates with new-onset diabetes after transplantation. J Am Soc Nephrol 20: 2459–2467, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kang ES, Kim MS, Kim YS, Hur KY, Han SJ, Nam CM, Ahn CW, Cha BS, Kim SI, Lee HC: A variant of the transcription factor 7-like 2 (TCF7L2) gene and the risk of posttransplantation diabetes mellitus in renal allograft recipients. Diabetes Care 31: 63–68, 2008 [DOI] [PubMed] [Google Scholar]
- 46.Barrett JC: Haploview: Visualization and analysis of SNP genotype data. Cold Spring Harb Protoc 2009: pdb ip71, 2009 [DOI] [PubMed] [Google Scholar]
- 47.Laybutt DR, Preston AM, Akerfeldt MC, Kench JG, Busch AK, Biankin AV, Biden TJ: Endoplasmic reticulum stress contributes to beta cell apoptosis in type 2 diabetes. Diabetologia 50: 752–763, 2007 [DOI] [PubMed] [Google Scholar]
- 48.Arndt MA, Battaglia V, Parisi E, Lortie MJ, Isome M, Baskerville C, Pizzo DP, Ientile R, Colombatto S, Toninello A, Satriano J: The arginine metabolite agmatine protects mitochondrial function and confers resistance to cellular apoptosis. Am J Physiol Cell Physiol 296: C1411–C1419, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sener A, Lebrun P, Blachier F, Malaisse WJ: Stimulus-secretion coupling of arginine-induced insulin release. Insulinotropic action of agmatine. Biochem Pharmacol 38: 327–330, 1989 [DOI] [PubMed] [Google Scholar]
- 50.Su CH, Liu IM, Chung HH, Cheng JT: Activation of I2-imidazoline receptors by agmatine improved insulin sensitivity through two mechanisms in type-2 diabetic rats. Neurosci Lett 457: 125–128, 2009 [DOI] [PubMed] [Google Scholar]
- 51.Tibbetts MD, Zheng L, Lenardo MJ: The death effector domain protein family: Regulators of cellular homeostasis. Nat Immunol 4: 404–409, 2003 [DOI] [PubMed] [Google Scholar]
- 52.Jiao J, Dou L, Li M, Lu Y, Guo HB, Man Y, Wang S, Li J: NADPH oxidase 2 plays a critical role in dysfunction and apoptosis of pancreatic β-cells induced by very low-density lipoprotein. Mol Cell Biochem 370: 103–113, 2012 [DOI] [PubMed] [Google Scholar]
- 53.Nakayama M, Inoguchi T, Sonta T, Maeda Y, Sasaki S, Sawada F, Tsubouchi H, Sonoda N, Kobayashi K, Sumimoto H, Nawata H: Increased expression of NAD(P)H oxidase in islets of animal models of type 2 diabetes and its improvement by an AT1 receptor antagonist. Biochem Biophys Res Commun 332: 927–933, 2005 [DOI] [PubMed] [Google Scholar]
- 54.Moro C, Klimcakova E, Lolmède K, Berlan M, Lafontan M, Stich V, Bouloumié A, Galitzky J, Arner P, Langin D: Atrial natriuretic peptide inhibits the production of adipokines and cytokines linked to inflammation and insulin resistance in human subcutaneous adipose tissue. Diabetologia 50: 1038–1047, 2007 [DOI] [PubMed] [Google Scholar]
- 55.Magnusson M, Jujic A, Hedblad B, Engström G, Persson M, Struck J, Morgenthaler NG, Nilsson P, Newton-Cheh C, Wang TJ, Melander O: Low plasma level of atrial natriuretic peptide predicts development of diabetes: The prospective Malmo Diet and Cancer study. J Clin Endocrinol Metab 97: 638–645, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Grempler R, Leicht S, Kischel I, Eickelmann P, Redemann N: Inhibition of SH2-domain containing inositol phosphatase 2 (SHIP2) in insulin producing INS1E cells improves insulin signal transduction and induces proliferation. FEBS Lett 581: 5885–5890, 2007 [DOI] [PubMed] [Google Scholar]
- 57.Dyachok O, Gylfe E: Ca(2+)-induced Ca(2+) release via inositol 1,4,5-trisphosphate receptors is amplified by protein kinase A and triggers exocytosis in pancreatic beta-cells. J Biol Chem 279: 45455–45461, 2004 [DOI] [PubMed] [Google Scholar]
- 58.Wang Y, Li G, Goode J, Paz JC, Ouyang K, Screaton R, Fischer WH, Chen J, Tabas I, Montminy M: Inositol-1,4,5-trisphosphate receptor regulates hepatic gluconeogenesis in fasting and diabetes. Nature 485: 128–132, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lentine KL, Delos Santos R, Axelrod D, Schnitzler MA, Brennan DC, Tuttle-Newhall JE: Obesity and kidney transplant candidates: How big is too big for transplantation? Am J Nephrol 36: 575–586, 2012 [DOI] [PubMed] [Google Scholar]
- 60.Friedman AN, Miskulin DC, Rosenberg IH, Levey AS: Demographics and trends in overweight and obesity in patients at time of kidney transplantation. Am J Kidney Dis 41: 480–487, 2003 [DOI] [PubMed] [Google Scholar]
- 61.Abecassis M, Bridges ND, Clancy CJ, Dew MA, Eldadah B, Englesbe MJ, Flessner MF, Frank JC, Friedewald J, Gill J, Gries C, Halter JB, Hartmann EL, Hazzard WR, Horne FM, Hosenpud J, Jacobson P, Kasiske BL, Lake J, Loomba R, Malani PN, Moore TM, Murray A, Nguyen MH, Powe NR, Reese PP, Reynolds H, Samaniego MD, Schmader KE, Segev DL, Shah AS, Singer LG, Sosa JA, Stewart ZA, Tan JC, Williams WW, Zaas DW, High KP: Solid-organ transplantation in older adults: Current status and future research. Am J Transplant 12: 2608–2622, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pascual J, Royuela A, Galeano C, Crespo M, Zamora J: Very early steroid withdrawal or complete avoidance for kidney transplant recipients: A systematic review. Nephrol Dial Transplant 27: 825–832, 2012 [DOI] [PubMed] [Google Scholar]
- 63.Donath MY, Ehses JA, Maedler K, Schumann DM, Ellingsgaard H, Eppler E, Reinecke M: Mechanisms of beta-cell death in type 2 diabetes. Diabetes 54[Suppl 2]: S108–S113, 2005 [DOI] [PubMed] [Google Scholar]
- 64.Maedler K, Schumann DM, Sauter N, Ellingsgaard H, Bosco D, Baertschiger R, Iwakura Y, Oberholzer J, Wollheim CB, Gauthier BR, Donath MY: Low concentration of interleukin-1beta induces FLICE-inhibitory protein-mediated beta-cell proliferation in human pancreatic islets. Diabetes 55: 2713–2722, 2006 [DOI] [PubMed] [Google Scholar]
- 65.Ooms LM, Horan KA, Rahman P, Seaton G, Gurung R, Kethesparan DS, Mitchell CA: The role of the inositol polyphosphate 5-phosphatases in cellular function and human disease. Biochem J 419: 29–49, 2009 [DOI] [PubMed] [Google Scholar]
- 66.Ma ZA, Zhao Z, Turk J: Mitochondrial dysfunction and β-cell failure in type 2 diabetes mellitus. Exp Diabetes Res 2012: 703538, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Favaro E, Miceli I, Bussolati B, Schmitt-Ney M, Cavallo Perin P, Camussi G, Zanone MM: Hyperglycemia induces apoptosis of human pancreatic islet endothelial cells: Effects of pravastatin on the Akt survival pathway. Am J Pathol 173: 442–450, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gleason CE, Gonzalez M, Harmon JS, Robertson RP: Determinants of glucose toxicity and its reversibility in the pancreatic islet beta-cell line, HIT-T15. Am J Physiol Endocrinol Metab 279: E997–E1002, 2000 [DOI] [PubMed] [Google Scholar]
- 69.Maedler K, Spinas GA, Lehmann R, Sergeev P, Weber M, Fontana A, Kaiser N, Donath MY: Glucose induces beta-cell apoptosis via upregulation of the Fas receptor in human islets. Diabetes 50: 1683–1690, 2001 [DOI] [PubMed] [Google Scholar]
- 70.Chakkera HA, Weil EJ, Castro J, Heilman RL, Reddy KS, Mazur MJ, Hamawi K, Mulligan DC, Moss AA, Mekeel KL, Cosio FG, Cook CB: Hyperglycemia during the immediate period after kidney transplantation. Clin J Am Soc Nephrol 4: 853–859, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ewaldsson CA, Hahn RG: Beta 2-adrenergic responsiveness in vivo during abdominal surgery. Br J Anaesth 81: 343–347, 1998 [DOI] [PubMed] [Google Scholar]
- 72.Nilsson A, Persson MP, Hartvig P, Wide L: Effect of total intravenous anaesthesia with midazolam/alfentanil on the adrenocortical and hyperglycaemic response to abdominal surgery. Acta Anaesthesiol Scand 32: 379–382, 1988 [DOI] [PubMed] [Google Scholar]
- 73.European FK506 Multicentre Liver Study Group: Randomised trial comparing tacrolimus (FK506) and cyclosporin in prevention of liver allograft rejection. Lancet 344: 423–428, 1994 [PubMed] [Google Scholar]
- 74.Rabkin R, Ryan MP, Duckworth WC: The renal metabolism of insulin. Diabetologia 27: 351–357, 1984 [DOI] [PubMed] [Google Scholar]
- 75.Rubenstein AH, Mako ME, Horwitz DL: Insulin and the kidney. Nephron 15: 306–326, 1975 [DOI] [PubMed] [Google Scholar]
- 76.Rodrigo E, Santos L, Piñera C, Quintanar JA, Ruiz JC, Fernández-Fresnedo G, Palomar R, Gómez-Alamillo C, Arias M: Early prediction of new-onset diabetes mellitus by fifth-day fasting plasma glucose, pulse pressure, and proteinuria. Transplant Proc 43: 2208–2210, 2011 [DOI] [PubMed] [Google Scholar]
- 77.Hui H, Dotta F, Di Mario U, Perfetti R: Role of caspases in the regulation of apoptotic pancreatic islet beta-cells death. J Cell Physiol 200: 177–200, 2004 [DOI] [PubMed] [Google Scholar]
- 78.Lightfoot YL, Chen J, Mathews CE: Role of the mitochondria in immune-mediated apoptotic death of the human pancreatic β cell line βLox5. PLoS ONE 6: e20617, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Maedler K, Fontana A, Ris F, Sergeev P, Toso C, Oberholzer J, Lehmann R, Bachmann F, Tasinato A, Spinas GA, Halban PA, Donath MY: FLIP switches Fas-mediated glucose signaling in human pancreatic beta cells from apoptosis to cell replication. Proc Natl Acad Sci U S A 99: 8236–8241, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Maedler K, Sergeev P, Ris F, Oberholzer J, Joller-Jemelka HI, Spinas GA, Kaiser N, Halban PA, Donath MY: Glucose-induced beta cell production of IL-1beta contributes to glucotoxicity in human pancreatic islets. J Clin Invest 110: 851–860, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Rostambeigi N, Lanza IR, Dzeja PP, Deeds MC, Irving BA, Reddi HV, Madde P, Zhang S, Asmann YW, Anderson JM, Schimke JM, Nair KS, Eberhardt NL, Kudva YC: Unique cellular and mitochondrial defects mediate FK506-induced islet β-cell dysfunction. Transplantation 91: 615–623, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ortega-Camarillo C, Guzmán-Grenfell AM, García-Macedo R, Rosales-Torres AM, Avalos-Rodríguez A, Durán-Reyes G, Medina-Navarro R, Cruz M, Díaz-Flores M, Kumate J: Hyperglycemia induces apoptosis and p53 mobilization to mitochondria in RINm5F cells. Mol Cell Biochem 281: 163–171, 2006 [DOI] [PubMed] [Google Scholar]
- 83.Tsubouchi H, Inoguchi T, Inuo M, Kakimoto M, Sonta T, Sonoda N, Sasaki S, Kobayashi K, Sumimoto H, Nawata H: Sulfonylurea as well as elevated glucose levels stimulate reactive oxygen species production in the pancreatic beta-cell line, MIN6-a role of NAD(P)H oxidase in beta-cells. Biochem Biophys Res Commun 326: 60–65, 2005 [DOI] [PubMed] [Google Scholar]
- 84.Maglott D, Ostell J, Pruitt KD, Tatusova T: Entrez Gene: Gene-centered information at NCBI. Nucleic Acids Res 39[Database issue]: D52–D57, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Bedard K, Krause KH: The NOX family of ROS-generating NADPH oxidases: Physiology and pathophysiology. Physiol Rev 87: 245–313, 2007 [DOI] [PubMed] [Google Scholar]
- 86.Wang Y, Gao L, Li Y, Chen H, Sun Z: Nifedipine protects INS-1 β-cell from high glucose-induced ER stress and apoptosis. Int J Mol Sci 12: 7569–7580, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Cnop M, Ladrière L, Igoillo-Esteve M, Moura RF, Cunha DA: Causes and cures for endoplasmic reticulum stress in lipotoxic β-cell dysfunction. Diabetes Obes Metab 12[Suppl 2]: 76–82, 2010 [DOI] [PubMed] [Google Scholar]
- 88.Li Y, Xu W, Liao Z, Yao B, Chen X, Huang Z, Hu G, Weng J: Induction of long-term glycemic control in newly diagnosed type 2 diabetic patients is associated with improvement of beta-cell function. Diabetes Care 27: 2597–2602, 2004 [DOI] [PubMed] [Google Scholar]
- 89.Souza GC, Costa C, Scalco R, Gonçalves LF, Manfro RC: Serum leptin, insulin resistance, and body fat after renal transplantation. J Ren Nutr 18: 479–488, 2008 [DOI] [PubMed] [Google Scholar]
- 90.Teplan V, Malý J, Gürlich R, Teplan V, Jr, Kudla M, Pit’ha J, Racek J, Haluzík M, Senolt L, Stollová M: Muscle and fat metabolism in obesity after kidney transplantation: No effect of peritoneal dialysis or hemodialysis. J Ren Nutr 22: 166–170, 2012 [DOI] [PubMed] [Google Scholar]
- 91.Maedler K, Sergeev P, Ehses JA, Mathe Z, Bosco D, Berney T, Dayer JM, Reinecke M, Halban PA, Donath MY: Leptin modulates beta cell expression of IL-1 receptor antagonist and release of IL-1beta in human islets. Proc Natl Acad Sci U S A 101: 8138–8143, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Knight SR, Morris PJ: Steroid avoidance or withdrawal after renal transplantation increases the risk of acute rejection but decreases cardiovascular risk. A meta-analysis. Transplantation 89: 1–14, 2010 [DOI] [PubMed] [Google Scholar]
- 93.Ranta F, Avram D, Berchtold S, Düfer M, Drews G, Lang F, Ullrich S: Dexamethasone induces cell death in insulin-secreting cells, an effect reversed by exendin-4. Diabetes 55: 1380–1390, 2006 [DOI] [PubMed] [Google Scholar]
- 94.Linssen MM, van Raalte DH, Toonen EJ, Alkema W, van der Zon GC, Dokter WH, Diamant M, Guigas B, Ouwens DM: Prednisolone-induced beta cell dysfunction is associated with impaired endoplasmic reticulum homeostasis in INS-1E cells. Cell Signal 23: 1708–1715, 2011 [DOI] [PubMed] [Google Scholar]
- 95.Heit JJ, Apelqvist AA, Gu X, Winslow MM, Neilson JR, Crabtree GR, Kim SK: Calcineurin/NFAT signalling regulates pancreatic beta-cell growth and function. Nature 443: 345–349, 2006 [DOI] [PubMed] [Google Scholar]
- 96.Ajabnoor MA, El-Naggar MM, Elayat AA, Abdulrafee A: Functional and morphological study of cultured pancreatic islets treated with cyclosporine. Life Sci 80: 345–355, 2007 [DOI] [PubMed] [Google Scholar]
- 97.Kurzawski M, Dziewanowski K, Łapczuk J, Wajda A, Droździk M: Analysis of common type 2 diabetes mellitus genetic risk factors in new-onset diabetes after transplantation in kidney transplant patients medicated with tacrolimus. Eur J Clin Pharmacol 68: 1587–1594, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ling Q, Xie H, Lu D, Wei X, Gao F, Zhou L, Xu X, Zheng S: Association between donor and recipient TCF7L2 gene polymorphisms and the risk of new-onset diabetes mellitus after liver transplantation in a Han Chinese population. J Hepatol 58: 271–277, 2013 [DOI] [PubMed] [Google Scholar]
- 99.Zeggini E, Panoutsopoulou K, Southam L, Rayner NW, Day-Williams AG, Lopes MC, Boraska V, Esko T, Evangelou E, Hoffman A, Houwing-Duistermaat JJ, Ingvarsson T, Jonsdottir I, Jonnson H, Kerkhof HJ, Kloppenburg M, Bos SD, Mangino M, Metrustry S, Slagboom PE, Thorleifsson G, Raine EV, Ratnayake M, Ricketts M, Beazley C, Blackburn H, Bumpstead S, Elliott KS, Hunt SE, Potter SC, Shin SY, Yadav VK, Zhai G, Sherburn K, Dixon K, Arden E, Aslam N, Battley PK, Carluke I, Doherty S, Gordon A, Joseph J, Keen R, Koller NC, Mitchell S, O’Neill F, Paling E, Reed MR, Rivadeneira F, Swift D, Walker K, Watkins B, Wheeler M, Birrell F, Ioannidis JP, Meulenbelt I, Metspalu A, Rai A, Salter D, Stefansson K, Stykarsdottir U, Uitterlinden AG, van Meurs JB, Chapman K, Deloukas P, Ollier WE, Wallis GA, Arden N, Carr A, Doherty M, McCaskie A, Willkinson JM, Ralston SH, Valdes AM, Spector TD, Loughlin J, arcOGEN Consortium. arcOGEN Collaborators : Identification of new susceptibility loci for osteoarthritis (arcOGEN): A genome-wide association study. Lancet 380: 815–823, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Grassi MA, Tikhomirov A, Ramalingam S, Below JE, Cox NJ, Nicolae DL: Genome-wide meta-analysis for severe diabetic retinopathy. Hum Mol Genet 20: 2472–2481, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kerner B, Lambert CG, Muthén BO: Genome-wide association study in bipolar patients stratified by co-morbidity. PLoS ONE 6: e28477, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Zhang CK, Stein PB, Liu J, Wang Z, Yang R, Cho JH, Gregersen PK, Aerts JM, Zhao H, Pastores GM, Mistry PK: Genome-wide association study of N370S homozygous Gaucher disease reveals the candidacy of CLN8 gene as a genetic modifier contributing to extreme phenotypic variation. Am J Hematol 87: 377–383, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Purcell S, Neale B, Todd-Brown K, Thomas L, Ferreira MA, Bender D, Maller J, Sklar P, de Bakker PI, Daly MJ, Sham PC: PLINK: A tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet 81: 559–575, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Pruim RJ, Welch RP, Sanna S, Teslovich TM, Chines PS, Gliedt TP, Boehnke M, Abecasis GR, Willer CJ: LocusZoom: Regional visualization of genome-wide association scan results. Bioinformatics 26: 2336–2337, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]