Abstract
Microscopic polyangiitis (MPA) is an ANCA-associated vasculitis that affects small vessels, especially renal glomeruli. We recently demonstrated that the abnormal formation and impaired degradation of neutrophil extracellular traps (NETs) may be crucially involved in the generation of myeloperoxidase (MPO)-ANCA and subsequent development of MPA. This study assessed the formation and regulation of NETs in patients with MPO-ANCA–associated MPA. Peripheral blood samples were obtained from 38 patients with MPO-ANCA–associated MPA, 23 patients with systemic lupus erythematosus (SLE), and 8 healthy controls. IgG eluted from MPO-ANCA–associated MPA sera demonstrated the highest ability to induce NETs, and this ability correlated with disease activity and paralleled ANCA affinity for MPO. Moreover, addition of recombinant human MPO to these IgG samples reduced NET induction. Additionally, MPO-ANCA–associated MPA sera exhibited lower rates of NET degradation that recovered partially upon depletion of IgG. The activity of DNase I, an important regulator of NETs, was also lower in MPO-ANCA–associated MPA and SLE sera. IgG depletion from MPO-ANCA–associated MPA sera partially restored the rate of NET degradation, and addition of DNase I synergistically enhanced this restoration. Addition of anti-MPO antibodies did not inhibit DNase I activity, and some MPO-ANCA–associated MPA sera contained anti-NET antibodies at levels not correlated with MPO-ANCA titers, suggesting the involvement of unidentified autoantibodies as well. The collective evidence suggests a vicious cycle involving MPO-ANCA and the regulation of NETs could be critically involved in the pathogenesis of MPO-ANCA–associated MPA.
Keywords: ANCA, vasculitis, immunology, pathology
Microscopic polyangiitis (MPA) is a systemic necrotizing vasculitis that affects small vessels predominantly in the kidney.1 Autoantibody against myeloperoxidase (MPO), which is detected as perinuclear ANCA by immunofluorescent staining (IF), is frequently present in the serum. Although MPO is an intracytoplasmic granule mainly in neutrophils, it can be released through the plasma membrane when these cells are activated by proinflammatory cytokines, such as TNF-α. It is considered that pathogenic ANCA can bind to the cell surface MPO of the proinflammatory cytokine–primed neutrophils, which leads to excessive activation of neutrophils and subsequent destruction of small vasculature.2 This concept, called ANCA-cytokine sequence theory, is commonly accepted as the critical part in the pathogenesis of MPO-ANCA–associated MPA.3 However, why such pathogenic MPO-ANCA is produced remains unknown.
Some patients receiving the antithyroid drug regimen propylthiouracil (PTU) are known to develop MPA with production of MPO-ANCA.4 Recently, we demonstrated that the process of abnormal formation and impaired degradation of neutrophil extracellular traps (NETs) induced by PTU was critically involved in the generation of MPO-ANCA and subsequent development of MPA.5–7 NETs represent the unique death of neutrophils, in which there is extracellular release of chromatin fibers and antibacterial cytoplasmic proteins, including MPO.8 The process is an innate defense mechanism to trap and kill invading microbes.9 Interestingly, NETs have been detected in glomerular crescents in patients diagnosed with MPA regardless of the absence of infectious agents.10 On the basis of the collective evidence, we hypothesize that the persistent MPO in the PTU-induced protracted NETs could be recognized as an autoantigen by the immune system.5 This hypothesis corresponds to the finding that NETs mediate the transfer of MPO to myeloid dendritic cells toward ANCA induction.11 However, most patients with MPA develop the disease without administration of PTU or related drugs. Thus, we consider that there are mechanisms unrelated to PTU that influence the formation and regulation of NETs in MPA.12
In this study, we first focused on the ability of serum IgG to induce NETs and then demonstrated the high ability of MPO-ANCA–associated MPA IgG for NET induction. The ability for NET induction was correlated to disease activity and parallel to the ANCA affinity to MPO. Furthermore, the NET induction ability was absorbed by addition of recombinant human MPO in the IgG samples. These findings indicated that the NET induction factor in MPO-ANCA–associated MPA serum was MPO-ANCA. Next, we examined the NET degradation ability of MPO-ANCA–associated MPA serum. NET degradation ability of serum was generally low in MPO-ANCA–associated MPA, and it was partially but not completely recovered by depletion of IgG from the sera. This finding suggested the presence of serum factors that precluded NET degradation in MPO-ANCA–associated MPA in addition to IgG. Correspondingly, low activity of DNase I, an important regulator of NETs in the serum, was determined in MPO-ANCA–associated MPA. Furthermore, the presence of anti-NET antibodies, which could possibly interfere with the degradation of NETs by DNase I, was demonstrated in some patients with MPO-ANCA–associated MPA. These findings showed the high ability for NET induction and low ability for NET degradation of serum in patients with MPO-ANCA–associated MPA. The collective evidence suggests that a vicious cycle through NETs and MPO-ANCA could be involved in the pathogenesis of MPO-ANCA–associated MPA.
Results
High Ability of Serum IgG from Patients with MPO-ANCA–Associated MPA for NET Induction
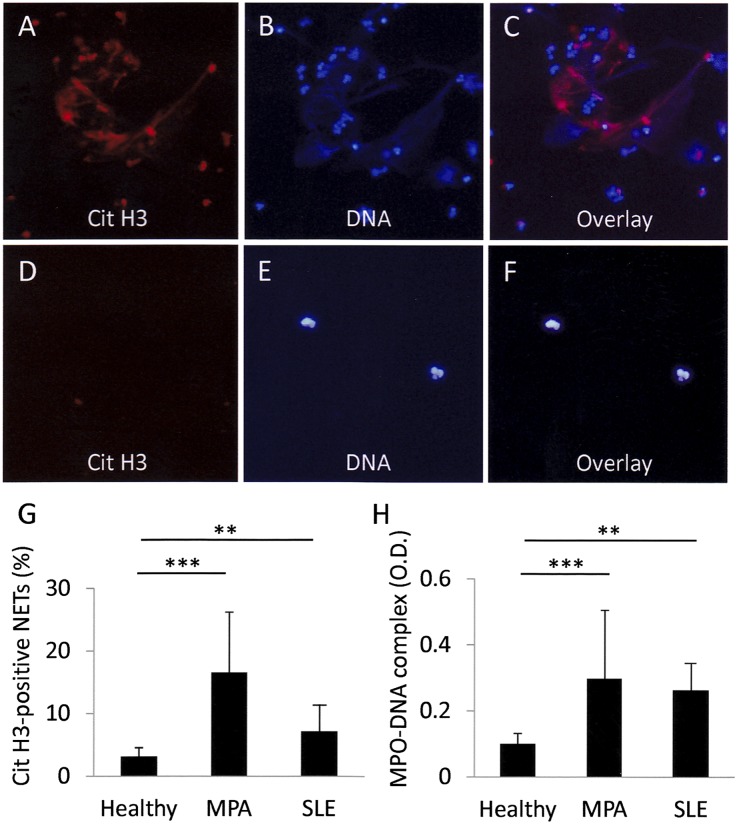
First, we confirmed the high ability of MPO-ANCA–associated MPA IgG for NET induction, which was reported previously.10 NET induction was quantified by two independent methods: IF for citrullinated histone 3 (Cit H3)–positive neutrophils (Figure 1, A–G) and ELISA for MPO-DNA complexes in culture supernatants (Figure 1H). Because deimination of histones is an essential process for the generation of NETs, the detection of Cit H3–positive neutrophils is regarded as an accurate method to identify NETs.13,14 The MPO-DNA complexes in the serum and culture supernatants reflected the amounts of NETs in the tissue10 and dish,5 respectively. There was a significant correlation between the values of IF and ELISA (R2=0.2485; P<0.01); thus, the results indicated the reliability of these two methods (Supplemental Figure 1). In this experiment, neutrophils from healthy volunteers were pretreated with TNF-α. When the TNF-α–primed neutrophils were incubated with serum IgG from patients with MPO-ANCA–associated MPA, the mean±SD rate of Cit H3–positive neutrophils was 16.6%±9.7% (Figure 1G). This value was significantly higher than when the neutrophils were incubated with serum IgG from healthy controls (3.2%±1.4%). Correspondingly, the amounts of MPO-DNA complexes in the supernatants were significantly higher when the TNF-α–primed neutrophils were incubated with serum IgG from patients with MPO-ANCA–associated MPA (OD, 0.29±0.21) than from healthy controls (OD, 0.13±0.03) (Figure 1H). Although the NET induction ability was also present in serum IgG from patients with systemic lupus erythematosus (SLE), the ability of SLE IgG was not as high (7.0%±3.5% in IF; OD, 0.25±0.10 in ELISA) compared with MPO-ANCA–associated MPA IgG (Figure 1, G and H).
Figure 1.
IgG eluted from MPO-ANCA–associated MPA sera demonstrates the highest ability to induce NETs. Neutrophils (1×106) from healthy volunteers were pretreated with 5 ng/ml TNF-α for 15 minutes at 37°C. The TNF-α–primed neutrophils were incubated with 250 μg/ml MPO-ANCA–associated MPA IgG (A–C) and control IgG (D–F) for 3 hours at 37°C. Representative figures are shown. (A and D) Cit H3. (B and E) DNA. (C and F) Overlay. Original magnification, ×400. (G) NET induction rate was evaluated by calculating the proportion of Cit H3–positive neutrophils on IF. (H) MPO-DNA complexes in the supernatants were measured by ELISA. Healthy, n=8; MPO-ANCA–associated MPA, n=38; SLE, n=23. **P<0.01; ***P<0.001.
MPO-ANCA as NET Induction Factor in MPO-ANCA–Associated MPA Serum
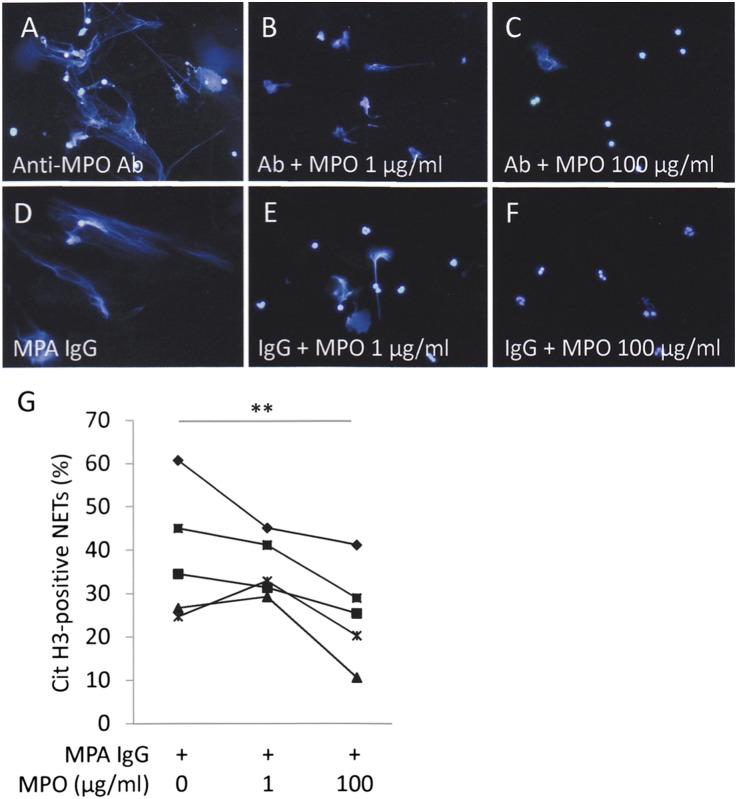
To determine that the NET induction ability of MPO-ANCA–associated MPA IgG could be caused by MPO-ANCA, recombinant human MPO was added to the IgG samples. When neutrophils were incubated with the antihuman MPO monoclonal antibody, NETs were induced (Figure 2A). On the other hand, the NET induction was inhibited by addition of recombinant MPO dose-dependently (Figure 2, B and C). Similarly, the NET induction ability of MPO-ANCA–associated MPA IgG was absorbed by the addition of MPO (Figure 2, D–G). These findings indicated that the NET induction factor in MPO-ANCA–associated MPA serum was MPO-ANCA.
Figure 2.
NET induction ability of MPO-ANCA–associated MPA IgG is absorbed by MPO. Neutrophils (1×106) from healthy volunteers were pretreated with 5 ng/ml TNF-α for 15 minutes at 37°C. The TNF-α–primed neutrophils were incubated with 250 μg/ml mouse antihuman MPO monoclonal antibody (A–C) and MPO-ANCA–associated MPA IgG (D–F) with or without recombinant human MPO for 3 hours at 37°C. The concentrations of recombinant human MPO were 0 µg/ml (A and D), 1 µg/ml (B and E), and 100 µg/ml (C and F). NET formation was evaluated as DAPI-positive extracellular chromatin fibers. Representative figures are shown. Original magnification, ×400. (G) The NET induction ability of MPO-ANCA–associated MPA serum (n=5) was significantly decreased by addition of 100 µg/ml recombinant human MPO. **P<0.01.
Association of NET Induction Ability with Vasculitis Activity and MPO-ANCA Affinity
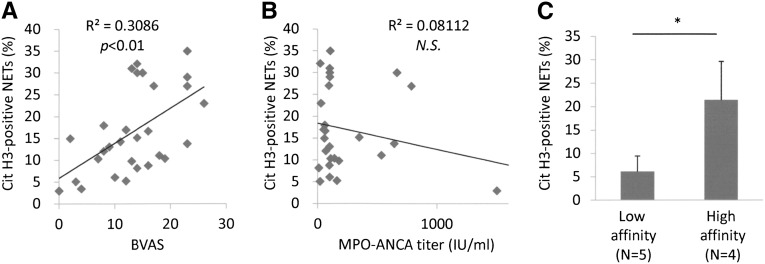
Next, we determined that the NET induction ability of MPO-ANCA–associated MPA IgG was correlated with disease activity, as represented by the Birmingham Vasculitis Activity Score (BVAS) (Figure 3A). The result was in contrast to the finding in patients with SLE that the NET induction ability did not show a significant correlation to the disease activity as represented by the SLE disease activity index (Supplemental Figure 2). Although MPO-ANCA is a useful diagnostic marker of MPA, it is known that the disease activity sometimes dissociates from the ANCA titer.15 Correspondingly, the NET induction ability of MPO-ANCA–associated MPA IgG did not show a significant correlation to the MPO-ANCA titer in our patients (Figure 3B). On the other hand, good association between MPO-ANCA affinity and disease activity was reported with clinical evidence.16 According to this finding, we determined whether the NET induction ability of MPO-ANCA–associated MPA IgG would be correlated to the MPO-ANCA affinity, which was measured by the competitive inhibitory ELISA as previously described.16 Among nine patients with MPO-ANCA–associated MPA included in this study, four had high-affinity ANCA, whereas five patients had low-affinity ANCA (Supplemental Figure 3A). Consistent with the earlier report,16 BVASs were significantly higher in patients with high-affinity MPO-ANCA than in those with low-affinity MPO-ANCA (Supplemental Figure 3B). As expected, NET induction ability of serum IgG from patients with high-affinity MPO-ANCA was significantly higher than that from patients with low-affinity MPO-ANCA (Figure 3C).
Figure 3.
NET induction ability of MPO-ANCA–associated MPA IgG correlates with BVAS and ANCA affinity for MPO. (A) Correlation between NET induction ability and BVAS. (B) No correlation between NET induction ability and MPO-ANCA titer. (C) Comparison of NET induction ability between patients with low-affinity MPO-ANCA (n=5) and with high-affinity MPO-ANCA (n=4). *P<0.05. NS, not significant.
Low Ability of MPO-ANCA–Associated MPA Serum for NET Degradation
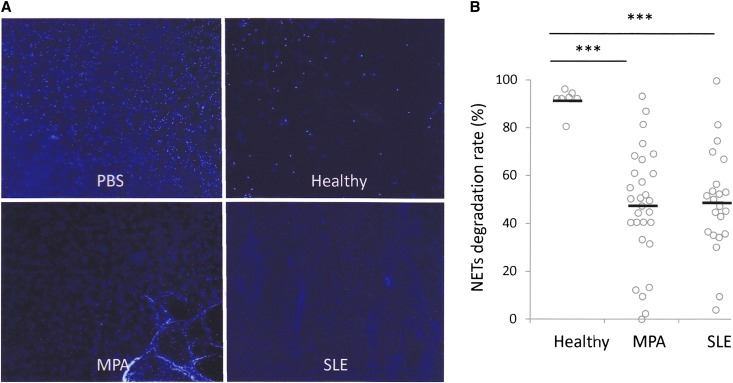
It was reported that sera from healthy individuals could digest NETs effectively, but the sera from patients with SLE had low ability for NET degradation.17 We determined whether the sera from patients with MPO-ANCA–associated MPA would be impotent to digest NETs, as was seen in patients with SLE. For this purpose, 10% serum was added to NETs, which were induced on healthy donor neutrophils by phorbol myristate acetate (PMA) (Supplemental Figure 4). Sera from healthy controls could digest the PMA-induced NETs almost completely (NET degradation rates>80%). In contrast, most MPO-ANCA–associated MPA and SLE sera examined in this study showed impaired degradation of the PMA-induced NETs (NET degradation rates<80%) (Figure 4, A and B). The NET degradation rates of MPO-ANCA–associated MPA and SLE sera were 47.4%±24.2% and 48.8%±21.7%, respectively. Both rates were significantly lower than that of healthy controls (91.5%±5.1%) (Figure 4B).
Figure 4.
MPO-ANCA–associated MPA sera exhibit lower rates of NET degradation than healthy controls. (A) Neutrophils (1×106) from healthy volunteers were treated with 100 nM PMA for 3 hours at 37°C. The PMA-induced NET were incubated with 10% serum from healthy controls, patients with MPO-ANCA–associated MPA, and patients with SLE, or with PBS for 6 hours at 37°C. The residual NETs were recognized as DAPI-positive extracellular chromatin fibers. Representative figures are shown. Original magnification, ×40. (B) Comparison of NET degradation ability of serum among healthy controls (n=8), patients with MPO-ANCA–associated MPA (n=38), and patients with SLE (n=23). ***P<0.001.
Low DNase I Activity in MPO-ANCA–Associated MPA Serum
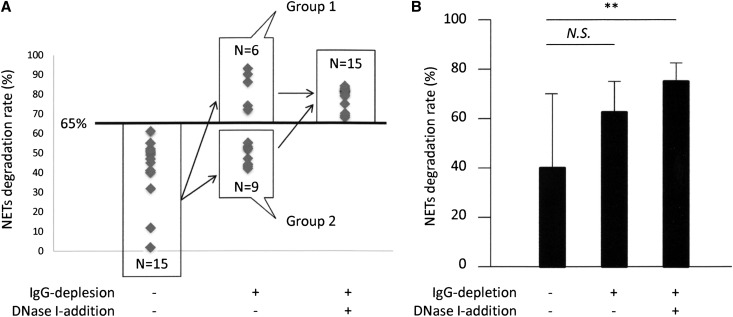
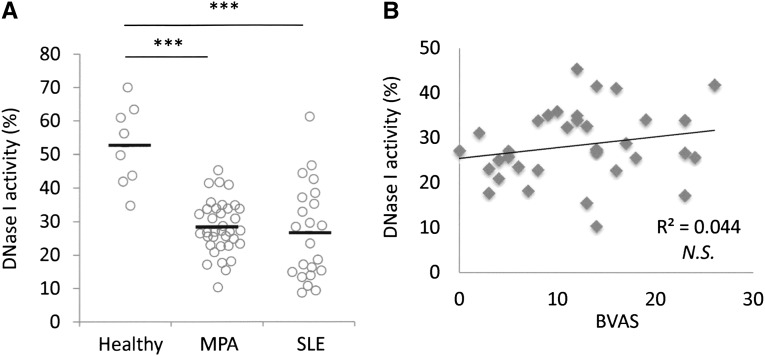
To eliminate the NET induction effects by MPO-ANCA–associated MPA IgG on NET digestion, we examined NET degradation ability of IgG-depleted serum. Results indicated that the NET degradation ability was recovered in some samples by IgG depletion (6 of 15 samples) (Figure 5A, group 1). At this time, although the mean value of NET degradation ability tended to increase by IgG depletion, there was no significant difference between the sera with and without IgG depletion (Figure 5B). These findings suggested the presence of other serum factors that precluded NET degradation in MPO-ANCA–associated MPA besides IgG. In particular, NETs are known to be physiologically digested by serum DNase I. It has been reported that DNase I activity in the serum was generally low in SLE18 and that the low DNase I activity could induce the abnormal regulation of apoptotic cells in SLE.19 As was seen in patients with SLE, the DNase I activity in sera from patients with MPO-ANCA–associated MPA was significantly lower than that in healthy controls (controls, 52.6%±12.1%; patients with MPO-ANCA–associated MPA, 28.3%±7.8%; patients with SLE, 26.7%±14.3%) (Figure 6A). Interestingly, the DNase I activity in MPO-ANCA–associated MPA serum was not correlated to disease activity as represented by BVAS (Figure 6B), a feature similar to that seen with SLE (Supplemental Figure 5). To demonstrate that the low activity of DNase I in MPO-ANCA–associated MPA serum contributed to the low ability for NET degradation, we determined whether the addition of DNase I to the MPO-ANCA–associated MPA sera with depletion of IgG would recover NET degradation ability. As shown in Figure 5A, all samples (n=15, including 9 samples in group 2) recovered NET degradation ability by the combination of IgG depletion and addition of DNase I. Correspondingly, the mean value of NET degradation ability in MPO-ANCA–associated MPA serum was significantly increased by the combination of IgG depletion and DNase I addition (Figure 5B).
Figure 5.
IgG depletion from MPO-ANCA–associated MPA sera partially restores the rate of NET degradation, and addition of DNase I synergistically enhances the restoration. (A) In this study, 15 MPO-ANCA–associated MPA serum samples that showed low ability for NET degradation (NET degradation rates<65%) were included. IgG was removed from the sera using the immunoabsorbent column. Thereafter, 10 U/ml DNase I was added to the IgG-depleted serum. After these preparations, the NET degradation ability of each sample was evaluated. Fifteen samples were classified into two groups: group 1, ability for NET degradation was recovered by IgG depletion; group 2, ability for NET degradation was not recovered by IgG depletion. (B) The NET degradation ability of MPO-ANCA–associated MPA serum (n=15) was significantly increased by the combination of IgG depletion and DNase I addition but not by the IgG depletion alone. **P<0.01. NS, not significant.
Figure 6.
DNase I activity in MPO-ANCA–associated MPA sera is lower than in healthy controls. (A) Comparison of serum DNase I activity among healthy controls (n=8), patients with MPO-ANCA–associated MPA (n=38), and patients with SLE (n=23). (B) No correlation between DNase I activity and BVAS in MPO-ANCA–associated MPA. ***P<0.001. NS, not significant.
Presence of Anti-NET Antibodies in MPO-ANCA–Associated MPA
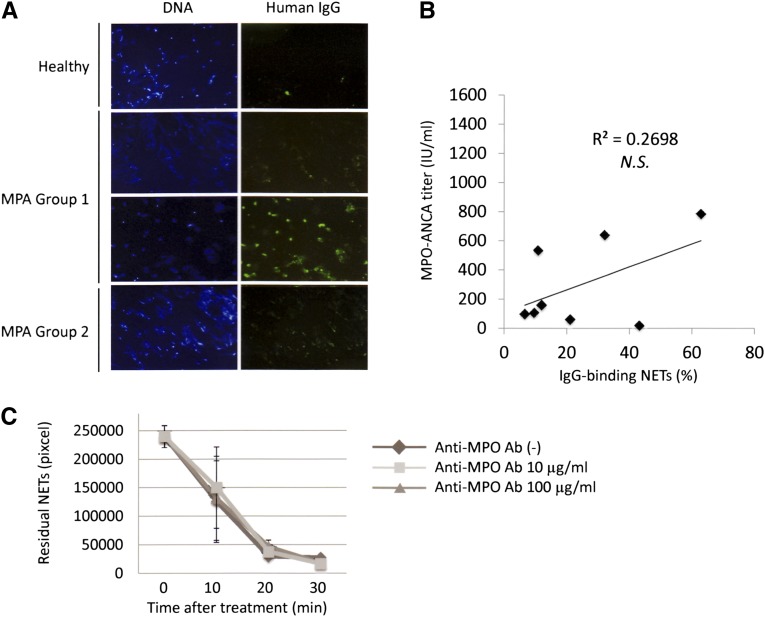
The NET degradation ability in sera was increased by depletion of IgG in some patients with MPO-ANCA–associated MPA (Figure 5A, group 1). Although this could be interpreted by the removal effect of the NET-inducible MPO-ANCA, another possibility is the presence of inhibitory antibodies for NET degradation in MPO-ANCA–associated MPA serum. On the basis of this consideration, we determined whether anti-NET antibodies would be present in MPO-ANCA–associated MPA serum. As a result, the presence of anti-NET antibodies was demonstrated in some patients with MPO-ANCA–associated MPA in group 1, but not in group 2 or in healthy controls (Figure 7A). The amount of anti-NET antibodies was not correlated to the titer of MPO-ANCA in the serum (Figure 7B). In addition, the intervention of anti-MPO antibody did not inhibit NET degradation by DNase I (Figure 7C).
Figure 7.
Some MPO-ANCA–associated MPA sera contain anti-NET antibodies at levels not correlated with MPO-ANCA titers. (A) Neutrophils from healthy volunteers were seeded on chamber slides (1×106/ml) and then treated with 20 nM PMA for 2 hours at 37°C. After fixation with 4% PFA, the samples were washed with PBS and then incubated with 250 µg/ml IgG eluted from MPO-ANCA–associated MPA sera or healthy control sera for 1 hour at 37°C. After removal of unbound IgG, the samples were next allowed to react with 1:5000 dilution of FITC-conjugated antihuman IgG antibodies (Santa Cruz Biotechnology, Dallas, TX) for 1 hour at 37°C. In some patients in group 1, but not in group 2 or healthy controls, human IgG bound to the NETs was seen. Representative figures are shown. Original magnification, ×100. (B) The amount of anti-NET antibodies was represented as the rate of IgG-binding area in NETs. No significant correlation between the amount of anti-NET antibodies and the MPO-ANCA titer. NS, not significant. (C) The PMA-induced NETs were treated by 1 U/ml DNase I with 10 µg/ml or 100 µg/ml antihuman MPO antibody (Ab) at room temperature. Before and 10, 20, and 30 minutes after the treatment, the residual NETs were determined using ImageJ software. No inhibitory effect of the anti-MPO antibody on the DNase I activity was seen.
Discussion
Although NETosis is an essential defense mechanism, its disorder could be related to autoimmune diseases, including SLE20,21 and rheumatoid arthritis.22 In the present study, we demonstrated three diverse disorders in MPO-ANCA–associated MPA serum, which were related to the formation or regulation of NETs.
First, the high ability of serum IgG for NET induction in MPO-ANCA–associated MPA was shown. Although the feature of MPA IgG has been reported previously,10 as far as we know, this is the first evidence that the NET induction ability of MPO-ANCA–associated MPA IgG is correlated to disease activity and reflects the ANCA affinity to MPO. In addition, the NET induction ability of MPO-ANCA–associated MPA IgG was absorbed by addition of recombinant human MPO in the IgG samples. These findings indicate that the NET induction factor in MPO-ANCA–associated MPA serum is MPO-ANCA. On the other hand, it has not been revealed what factors contribute to the NET induction in SLE serum. Although one or more autoantibodies seem likely to play a role in the NET induction, further studies are needed to identify the factors.
Second, as reported in SLE,17 NET degradation ability in serum was significantly lower in patients with MPO-ANCA–associated MPA than healthy controls. Serum DNase I activity is generally low in SLE,18 and the low levels of DNase I activity in the serum could cause the disordered elimination of apoptotic cells in SLE.19 Further investigation is needed to clarify whether the disorder of scavenger system for apoptotic cells is involved in the pathogenesis of MPO-ANCA–associated MPA, as well as in SLE. Although we should take into account the possibility that the low level of DNase I activity in the sera of these patients could be caused by the consumption of DNase I in order to eliminate NETs, it seems unlikely to be relevant because the serum DNase I activity in these patients is not correlated to disease activity. Rather, it is considered that the low level of DNase I activity in the serum, which may be defined genetically, is a common feature in MPO-ANCA–associated MPA and SLE. In addition, the low levels of serum DNase I activity in patients with SLE and those with MPO-ANCA–associated MPA could induce impaired degradation of NETs and consequent breakdown of tolerance against NET components, which result in the production of anti-DNA antibodies17 and anti-MPO antibodies (MPO-ANCA),5,12 respectively.
Third, the presence of anti-NET antibodies was demonstrated in some patients with MPO-ANCA–associated MPA. The amount of anti-NET antibodies was not correlated to the MPO-ANCA titer, and the intervention of the anti-MPO antibody did not inhibit the DNase I activity. In addition, most patients with MPA did not produce anti-DNA antibodies. These findings suggested that the anti-NET antibodies in MPO-ANCA–associated MPA were regarded as autoantibodies other than MPO-ANCA or anti-DNA antibodies. In SLE, the presence of anti-NET antibodies, including anti-DNA antibody, was shown, and these anti-NET antibodies could interfere with DNase I, the important regulator of NETs.17 As seen in SLE, the anti-NET antibodies could possibly contribute to the low ability for NET degradation in MPO-ANCA–associated MPA. The components of anti-NET antibodies in MPO-ANCA–associated MPA IgG should be assessed in future studies.
Although these three mechanisms have individual variations, the comprehensive feature of MPO-ANCA–associated MPA serum could cause the excessive formation and persistence of NETs. Because such dysregulation of NETs could induce NET producers, including MPO-ANCA, and inhibitors for NET regulators, including anti-NET antibodies, it is considered that a vicious cycle through NET and MPO-ANCA, namely an NET-ANCA vicious cycle, is critically involved in the pathogenesis of MPO-ANCA–associated MPA.
On the basis of involvement of this vicious cycle in the pathogenesis of MPO-ANCA–associated MPA, novel therapies can be considered. It is known that NADPH oxidase23 and peptidylarginine deiminase 4 (PAD4)13 are essential for the generation of NETs. Blockades for NET generation, such as NADPH oxidase inhibitors24 and PAD4 inhibitors,25 could be candidates to cause interference in the NET-ANCA vicious cycle in the pathogenesis of MPA. Further study using animal models and prospective clinical trials is needed to establish novel therapeutic strategies for the treatment of MPO-ANCA–associated MPA.
Concise Methods
Patients and Blood Samples
Patients enrolled in this study included 38 patients with MPA and 23 with SLE diagnosed and treated in Hokkaido University Hospital between January 2008 and May 2012. Among the 38 patients with MPA, there were 31 women and 7 men (mean age±SD, 66.7±13.3 years; range, 38–82 years). All patients with MPA enrolled in this study were positive for MPO-ANCA but negative for proteinase 3–ANCA. After patients provided written informed consent, peripheral blood samples were obtained without anticoagulants, and sera were stored at −80°C until use. At the time of blood sampling, BVAS and SLE disease activity index were recorded for patients with MPA and SLE, respectively. For controls, peripheral blood samples were obtained from 8 healthy volunteers.
NET Induction by IgG Eluted from Serum
IgG was eluted from sera using an immunoadsorbent column (Protein G HP SpinTrap; GE Healthcare, Tokyo, Japan). Contamination of endotoxin in the IgG samples was ruled out using the Limulus test kit (Wako Pure Chemical, Osaka, Japan). Neutrophils were extracted from peripheral blood samples of healthy volunteers, as described elsewhere.5 The neutrophils were seeded on chamber slides (1×106/ml), pretreated with 5 ng/ml TNF-α for 15 minutes at 37°C, and then treated with 250 μg/ml eluted IgG from patients with MPO-ANCA–associated MPA (n=38), patients with SLE (n=23), and healthy controls (n=8). After incubation for 3 hours at 37°C, the supernatants were collected and the remaining samples on the slides were fixed with 4% paraformaldehyde (PFA).
IF for Cit H3–Positive Neutrophils
After fixation with 4% PFA, the samples were made to react with 1:100 dilution of anti-Cit H3 antibody (rabbit IgG) (Abcam, Tokyo, Japan) for 60 minutes at room temperature. After removal of unbound antibody by washing with PBS, the samples were next allowed to react with 1:500 dilution of Alexa Fluor 594–conjugated goat antirabbit IgG (Invitrogen, Tokyo, Japan) for 60 minutes at room temperature. After washing with PBS, DNA was stained using 4′,6-diamidino-2-phenylindole (DAPI; Sigma-Aldrich, St. Louis, MO), which was contained in mounting solution. The Cit H3–positive and DAPI-positive NETs were counted, and then the data were standardized by the number of DAPI-positive neutrophils.
ELISA for MPO-DNA Complexes
The MPO-DNA complexes in supernatants were measured using the capture ELISA system as previously described.10
Absorption of MPO-ANCA by Recombinant MPO
Recombinant human MPO (1 µg/ml or 100 µg/ml) (Creative BioMart, Shirley, NY) was added to the IgG samples obtained from patients with MPO-ANCA–associated MPA, and then the NET induction assay was performed as described earlier. For control, mouse antihuman MPO monoclonal antibody (AbD Serotec, Düsseldorf, Germany) was used instead of the IgG samples.
Measurement of MPO-ANCA Affinity
The MPO-ANCA affinity was determined by the competitive inhibition assay in the ELISA system as previously described.16
NET Degradation by Serum
Peripheral blood neutrophils obtained from healthy volunteers were seeded on chamber slides (1×106/ml) and then treated with 100 nM PMA for 3 hours at 37°C. After washing with PBS, the samples were incubated in 10% serum for 6 hours at 37°C. For negative control, PBS was used instead of sera. To stop the serum nuclease activity, 2 mM EDTA was added, and then the samples were fixed with 4% PFA followed by mounting with the solution containing DAPI. The DAPI-positive residual NETs were quantified using ImageJ software. NET degradation rate (%) was calculated as follows:
 |
Measurement of DNase I Activity
Serum DNase I activity was measured using the ELISA kit (Orgentec GmbH, Mainz, Germany) according to the instruction of the manufacturer. Briefly, sera were allowed to react with the specific substrate coated on the ELISA plate. After incubation for 1 hour at 37°C, horseradish peroxidase–conjugated antibodies to the residual DNase I substrate were added. Then, the developing color of the tetramethylbenzidine substrate, which showed a negative correlation to the amount of active DNase I in the serum, was measured using a spectrophotometer.
Interference with DNase I by Anti-MPO Antibody
NETs were induced by PMA as described earlier. The NETs were treated by 1 U/ml DNase I with 10 µg/ml or 100 µg/ml antihuman MPO antibody (AbD Serotec) at room temperature. Before and 10, 20, and 30 minutes after the treatment, the residual NETs were determined using ImageJ software.
Statistical Analyses
A t test was applied for comparison of mean values between the two groups. A Pearson test was applied to evaluate the correlation of values between the two groups. A P value<0.05 was regarded to represent a statistically significant difference.
Study Approval
This study was permitted by the Institutional Clinical Research Committee in Hokkaido University Hospital (No. 12–0423).
Disclosures
None.
Acknowledgments
The authors would like to thank Drs. Sekiya Shibasaki, Yasunobu Ishikawa, Tasuku Nakagaki, Akiko Sato, Takayuki Toyoyama, Junya Yamamoto, and Naoko Matsuoka for collecting the serum samples. We also thank Mariko Sasaki for technical assistance.
This study was supported by Grants-in-Aid for Intractable Vasculitis Research (H23-004) and Progressive Renal Diseases Research (H23-033) from the Ministry of Health, Labour and Welfare of Japan.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2013060606/-/DCSupplemental.
References
- 1.Jennette JC, Falk RJ, Bacon PA, Basu N, Cid MC, Ferrario F, Flores-Suarez LF, Gross WL, Guillevin L, Hagen EC, Hoffman GS, Jayne DR, Kallenberg CG, Lamprecht P, Langford CA, Luqmani RA, Mahr AD, Matteson EL, Merkel PA, Ozen S, Pusey CD, Rasmussen N, Rees AJ, Scott DG, Specks U, Stone JH, Takahashi K, Watts RA: 2012 revised International Chapel Hill Consensus Conference Nomenclature of Vasculitides. Arthritis Rheum 65: 1–11, 2013 [DOI] [PubMed] [Google Scholar]
- 2.Falk RJ, Terrell RS, Charles LA, Jennette JC: Anti-neutrophil cytoplasmic autoantibodies induce neutrophils to degranulate and produce oxygen radicals in vitro. Proc Natl Acad Sci U S A 87: 4115–4119, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Csernok E: Anti-neutrophil cytoplasmic antibodies and pathogenesis of small vessel vasculitides. Autoimmun Rev 2: 158–164, 2003 [DOI] [PubMed] [Google Scholar]
- 4.Chen M, Gao Y, Guo XH, Zhao MH: Propylthiouracil-induced antineutrophil cytoplasmic antibody-associated vasculitis. Nat Rev Nephrol 8: 476–483, 2012 [DOI] [PubMed] [Google Scholar]
- 5.Nakazawa D, Tomaru U, Suzuki A, Masuda S, Hasegawa R, Kobayashi T, Nishio S, Kasahara M, Ishizu A: Abnormal conformation and impaired degradation of propylthiouracil-induced neutrophil extracellular traps: Implications of disordered neutrophil extracellular traps in a rat model of myeloperoxidase antineutrophil cytoplasmic antibody-associated vasculitis. Arthritis Rheum 64: 3779–3787, 2012 [DOI] [PubMed] [Google Scholar]
- 6.Radic M, Kaplan MJ: Jumbled NETs promote vasculitis. Arthritis Rheum 64: 3498–3501, 2012 [DOI] [PubMed] [Google Scholar]
- 7.Ray K: Autoimmunity: disordered NETs implicated in pathogenesis of MPO-ANCA–associated vasculitis. Nat Rev Rheumatol 8: 501, 2012 [DOI] [PubMed] [Google Scholar]
- 8.Brinkmann V, Reichard U, Goosmann C, Fauler B, Uhlemann Y, Weiss DS, Weinrauch Y, Zychlinsky A: Neutrophil extracellular traps kill bacteria. Science 303: 1532–1535, 2004 [DOI] [PubMed] [Google Scholar]
- 9.Fuchs TA, Abed U, Goosmann C, Hurwitz R, Schulze I, Wahn V, Weinrauch Y, Brinkmann V, Zychlinsky A: Novel cell death program leads to neutrophil extracellular traps. J Cell Biol 176: 231–241, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kessenbrock K, Krumbholz M, Schönermarck U, Back W, Gross WL, Werb Z, Gröne HJ, Brinkmann V, Jenne DE: Netting neutrophils in autoimmune small-vessel vasculitis. Nat Med 15: 623–625, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sangaletti S, Tripodo C, Chiodoni C, Guarnotta C, Cappetti B, Casalini P, Piconese S, Parenza M, Guiducci C, Vitali C, Colombo MP: Neutrophil extracellular traps mediate transfer of cytoplasmic neutrophil antigens to myeloid dendritic cells toward ANCA induction and associated autoimmunity. Blood 120: 3007–3018, 2012 [DOI] [PubMed] [Google Scholar]
- 12.Nakazawa D, Tomaru U, Ishizu A: Possible implication of disordered neutrophil extracellular traps in the pathogenesis of MPO-ANCA–associated vasculitis. Clin Exp Nephrol 17:631–633, 2013. [DOI] [PubMed] [Google Scholar]
- 13.Li P, Li M, Lindberg MR, Kennett MJ, Xiong N, Wang Y: PAD4 is essential for antibacterial innate immunity mediated by neutrophil extracellular traps. J Exp Med 207: 1853–1862, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nakazawa D, Tomaru U, Yamamoto C, Jodo S, Ishizu A: Abundant neutrophil extracellular traps in thrombus of patient with microscopic polyangiitis. Front Immunol 3: 333, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.De’Oliviera J, Gaskin G, Dash A, Rees AJ, Pusey CD: Relationship between disease activity and anti-neutrophil cytoplasmic antibody concentration in long-term management of systemic vasculitis. Am J Kidney Dis 25: 380–389, 1995 [DOI] [PubMed] [Google Scholar]
- 16.Yoshida M, Sasaki M, Nakabayashi I, Akashi M, Tomiyasu T, Yoshikawa N, Kojima T, Ohno N, Yamada M: Two types of myeloperoxidase-antineutrophil cytoplasmic autoantibodies with a high affinity and a low affinity in small vessel vasculitis. Clin Exp Rheumatol 27[Suppl 52]: S28–S32, 2009 [PubMed] [Google Scholar]
- 17.Hakkim A, Fürnrohr BG, Amann K, Laube B, Abed UA, Brinkmann V, Herrmann M, Voll RE, Zychlinsky A: Impairment of neutrophil extracellular trap degradation is associated with lupus nephritis. Proc Natl Acad Sci U S A 107: 9813–9818, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sallai K, Nagy E, Derfalvy B, Müzes G, Gergely P: Antinucleosome antibodies and decreased deoxyribonuclease activity in sera of patients with systemic lupus erythematosus. Clin Diagn Lab Immunol 12: 56–59, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shao WH, Cohen PL: Disturbances of apoptotic cell clearance in systemic lupus erythematosus. Arthritis Res Ther 13: 202, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Villanueva E, Yalavarthi S, Berthier CC, Hodgin JB, Khandpur R, Lin AM, Rubin CJ, Zhao W, Olsen SH, Klinker M, Shealy D, Denny MF, Plumas J, Chaperot L, Kretzler M, Bruce AT, Kaplan MJ: Netting neutrophils induce endothelial damage, infiltrate tissues, and expose immunostimulatory molecules in systemic lupus erythematosus. J Immunol 187: 538–552, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Leffler J, Martin M, Gullstrand B, Tydén H, Lood C, Truedsson L, Bengtsson AA, Blom AM: Neutrophil extracellular traps that are not degraded in systemic lupus erythematosus activate complement exacerbating the disease. J Immunol 188: 3522–3531, 2012 [DOI] [PubMed] [Google Scholar]
- 22.Khandpur R, Carmona-Rivera C, Vivekanandan-Giri A, Gizinski A, Yalavarthi S, Knight JS, Friday S, Li S, Patel RM, Subramanian V, Thompson P, Chen P, Fox DA, Pennathur S, Kaplan MJ: NETs are a source of citrullinated autoantigens and stimulate inflammatory responses in rheumatoid arthritis. Sci Transl Med 5: 178ra40, 2013. [DOI] [PMC free article] [PubMed]
- 23.Almyroudis NG, Grimm MJ, Davidson BA, Röhm M, Urban CF, Segal BH: NETosis and NADPH oxidase: At the intersection of host defense, inflammation, and injury. Front Immunol 4: 45, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vlahos R, Stambas J, Bozinovski S, Broughton BR, Drummond GR, Selemidis S: Inhibition of Nox2 oxidase activity ameliorates influenza A virus-induced lung inflammation. PLoS Pathog 7: e1001271, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chumanevich AA, Causey CP, Knuckley BA, Jones JE, Poudyal D, Chumanevich AP, Davis T, Matesic LE, Thompson PR, Hofseth LJ: Suppression of colitis in mice by Cl-amidine: A novel peptidylarginine deiminase inhibitor. Am J Physiol Gastrointest Liver Physiol 300: G929–G938, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]