Abstract
The effects of ceftizoxime (CZX), piperacillin (PIP), and PIP-tazobactam (PT) concentrations on the antibacterial activity and selection of resistant mutants of Bacteroides fragilis and Enterobacter cloacae were investigated in vitro in a mixed-culture anaerobic time-kill study and in vivo in a mixed-infection abscess model. Mixed cultures were incubated for 24 h with 0.125 to 512 μg of CZX per ml or 0.125 to 2,048 μg of PIP or PT per ml. Mice were treated every 2 h for 24 h with CZX at 6 to 1,536 mg/kg/day or with PIP or PT at 24 to 6,144 mg/kg/day starting 30 min before inoculation with different B. fragilis-E. cloacae combinations. There was a good correlation between the in vitro and in vivo activities of the antibiotics and their MICs obtained with high inocula (108 CFU/ml). The respective 50% effective doses (milligrams per kilogram per day) with B. fragilis and E. cloacae 22491 were 771 and 521 for CZX, 416 and 643 for PIP, and 85 and 554 for PT, and with the B. fragilis-E. cloacae 032349 combination, they were 81 and 21 for CZX and 77 and 766 for PT. Resistant mutants of E. cloacae 22491 were preferentially selected in vitro with 2 to 64 μg of CZX per ml and in vivo with CZX at 12 to 384 mg/kg/day. There was no preferential selection of CZX-resistant B. fragilis or E. cloacae 032349. For CZX-resistant E. cloacae 22491, we found a 16- to 512-fold increase in the MIC of CZX and increased MICs of other expanded-spectrum cephalosporins, owing in part to the production of a stably derepressed cephalosporinase. In vitro and in vivo, PT did not select resistant mutants of E. cloacae and B. fragilis. Results demonstrate the adverse microbiological outcome of choosing an expanded-spectrum cephalosporin like CZX for empirical treatment of mixed infections involving a susceptible Enterobacter strain.
Development of resistance in gram-negative bacteria during β-lactam therapy is a continuing dilemma (43) and can lead to therapeutic failure (13), increased hospital stays and costs, and increased mortality (5). The intensity of β-lactam use is proportional to the resistance levels of organisms isolated from patients (25, 43), although differences in the ability of β-lactams to select these resistant strains during therapy have been detected (9).
The most important mechanism of β-lactam resistance in Enterobacter and Bacteroides strains is the production of chromosomally encoded β-lactamases (10, 11). Most Bacteroides fragilis enzymes are constitutive cephalosporinases or penicillinases that are inhibited by clavulanic acid (18), while the chromosomally encoded cephalosporinases of Enterobacter strains are inducible enzymes that are not inhibited by clavulanic acid (11). The AmpC β-lactamases of Enterobacter spp. are normally produced at low basal levels. However, mutations in the genes regulating the expression of these AmpC β-lactamases can cause stable derepression and constitutive production of large amounts of cephalosporinase, resulting in mutant strains that are resistant to expanded-spectrum cephalosporins and aztreonam (17).
There have been very few in vivo studies that have investigated the effects of antibiotic dosing regimens on the selection of antimicrobial resistance during treatment (21). Expanded-spectrum cephalosporins are more likely to select resistant strains of Enterobacteriaceae than are other β-lactams (23, 30, 35), while piperacillin (PIP)-tazobactam (TAZ) (the combination of PIP and TAZ is abbreviated PT) appears to suppress the selection of derepressed mutants (20). Very little data are available on the selection of β-lactam resistance in B. fragilis strains (10, 14, 18, 37).
The aim of the present study was to compare the effects of a wide range of antibiotic doses of PIP and PT with those of the expanded-spectrum cephalosporin ceftizoxime (CZX) on the selection of antibiotic-resistant mutants of B. fragilis and Enterobacter cloacae during the early development of mixed-infection subcutaneous abscesses (39). Antimicrobial therapy was started before bacterial inoculation to simulate the empirical use of antibiotics to treat infections resulting from complications following abdominal surgery. In vivo findings were compared to the in vitro activities of the antibiotics against mixed cultures of the strains in an anaerobic time-kill study and to MICs determined by using standard and high inocula.
(This report was presented in part at the 2002 12th European Congress of Clinical Microbiology and Infectious Diseases, Milan, Italy [Clin. Microbiol. Infect. 8(Suppl. 1)56, abstr. 0388].)
MATERIALS AND METHODS
Antibiotics and media.
CZX (Cefizox) was supplied by Fujisawa Holland B.V. (Houten, The Netherlands), and injectable PIP was supplied by Bipharma B.V. (Weesp, The Netherlands). PT was used in all in vitro and in vivo studies at a ratio of 8:1. Pure powders of PIP monohydrate, TAZ, and injectable PT (Tazocin) came from Wyeth Lederle (Louvain-la-Neuve, Belgium). Wilkens Chalgren (WC) broth, WC agar, eosin methylene blue (EM) agar, brain heart infusion broth (BHI), and DST agar were all supplied by Unipath Ltd. (Haarlem, The Netherlands). Columbia blood agar plates were from Becton Dickinson B.V. (Woerden, The Netherlands).
Bacterial strains.
B. fragilis ATCC 23745 and E. cloacae clinical isolates 22491 and 032349 were used. The B. fragilis strain produced a constitutive cephalosporinase that was susceptible to inhibition by clavulanic acid. The Enterobacter strains demonstrated variations in the level of induction of their AmpC β-lactamase by cefoxitin (42). E. cloacae 22491 was cefoxitin inducible, and E. cloacae 032349 was less inducible. All strains were first passaged in BALB/c mice by intraperitoneal injection of 108 to 109 CFU of overnight cultures. After 24 to 48 h, strains were recovered from the liver and spleen and cultured on blood agar plates and standardized bacterial suspensions were made in BHI containing 20% glycerol and stored at −80°C until required. Overnight cultures were obtained by inoculating 30-ml volumes of WC broth with 0.1 ml of the standardized frozen bacterial suspensions and incubating them aerobically (E. cloacae) or anaerobically (B. fragilis) at 37°C for 18 h.
Animals.
Female specific-pathogen-free BALB/c mice (IFFA Credo, l'Arbresle, France) 12 to 18 weeks old and weighing 20 to 25 g were used throughout the study. The cecal contents of male specific-pathogen-free Swiss mice (Charles River, Maastricht, The Netherlands) were used for the production of autoclaved cecal contents (ACC) (41). All animals received water and food ad libitum. The study was approved by the Institutional Animal Care and Use Committee of the Erasmus MC, Rotterdam, The Netherlands.
In vitro activity and emergence of resistance.
The MICs of CZX, PIP, and PT were determined by the standard broth microdilution method with inocula of 105 or 108 CFU/ml as previously described (41). The in vitro activities of the antibiotics against mixed cultures of B. fragilis and E. cloacae 22491 were measured by the anaerobic time-kill technique outlined previously (41). Briefly, 2-ml volumes of WC broth containing twofold increasing concentrations of CZX, PIP, or PT were placed in 5-ml polypropylene tubes (Greiner B.V., Alphen a/d Rijn, The Netherlands). Control samples contained 2 ml of WC broth alone. Tubes were inoculated with diluted overnight cultures of B. fragilis (1 ml) and E. cloacae (1 ml) to give mixed cultures with a final volume of 4 ml containing 108 CFU of each strain per ml. The final antibiotic concentrations were 0.125 to 512 μg/ml (CZX) and 0.125 to 2,048 μg/ml (PIP and PT). The in vitro activity of CZX against mixed cultures of B. fragilis and E. cloacae strain 032349 was similarly tested. Cultures were incubated anaerobically at 37°C for 24 h. Cultures were washed twice in phosphate-buffered saline (PBS), and total bacterial counts were determined by making duplicate serial 10-fold dilutions in PBS and plating 20 μl of each dilution onto EM agar (E. cloacae) or WC agar containing 100 mg of gentamicin per liter (B. fragilis). Plates were incubated at 37°C aerobically for 24 h (EM agar) or anaerobically for 48 h (WC agar). Counts were expressed as log10 CFU per milliliter, and the lower limit of detection was 2.5 log10 CFU/ml. Resistant E. cloacae mutants were isolated from the same mixed cultures on WC agar plates containing CZX, PIP, or PT at 1, 2, 4, 8, or 16 times the MIC that had been incubated aerobically for 48 h at 37°C. To isolate B. fragilis mutants, the WC agar plates, in addition to the CZX, PIP, and PT concentrations mentioned above, also contained 100 mg of gentamicin per liter, which inhibited the growth of the E. cloacae mutants. These plates were incubated anaerobically at 37°C for 72 h. Gentamicin had no synergistic or antagonistic effect on the activity of the other antibiotics present in the agar plates. The mutation frequency was expressed as the ratio of the number of CFU of resistant colonies found to the total number of CFU plated on the antibiotic-free agar.
Mouse model.
The subcutaneous-abscess model described previously (40) was used. Inocula were prepared by diluting overnight cultures of B. fragilis and E. cloacae 22491 (or E. cloacae 032349) in WC broth, which were then mixed together with ACC in a volume ratio of 1:1:2. The final inocula contained 107 CFU of B. fragilis, 107 CFU of E. cloacae, and 4 mg (dry weight) of ACC in a total volume of 0.25 ml. Mice were injected subcutaneously in both flanks. Abscesses were allowed to develop for 24 h, after which time mice were killed by CO2 asphyxiation and the abscesses were dissected, weighed, and homogenized in 1 ml of PBS for 10 s (Pro 200; B.V. Centraal Magazijn, Abcoude, The Netherlands). The resulting suspensions were used to determine the bacterial counts as outlined above for the in vitro studies.
Antibiotic treatment and emergence of resistance in early abscess development.
The antibiotic dosing regimens used in this study were chosen to produce a maximum effect in this model and took into consideration the short half-life, approximately 0.2 h, of both CZX and PIP in mice (3, 26). Groups of two mice were treated subcutaneously with total CZX doses of 6 to 1,536 mg/kg/day or PIP or PT doses of 24 to 6,144 mg/kg/day. These total daily doses were divided into12 CZX doses of 0.5 to 128 mg/kg or PIP or PT doses of 2 to 512 mg/kg given every 2 h. Treatment was started 30 min before bacterial inoculation and was continued for 24 h. Mice inoculated with the B. fragilis-E. cloacae 032349 combination were treated only with CZX or PT. The efficacy of the treatment was assessed by a decrease in total bacterial counts (CFU per abscess) compared to those of untreated controls analyzed on the same day. The number of resistant mutant bacteria in each of the treated and untreated abscesses was determined on agar plates containing CZX, PIP, or PT as outlined above for the in vitro studies. A total of four abscesses per treatment group were analyzed.
Population analysis profiles.
The population analysis profile (PAP) areas under the curves (AUCs) were used as a method to indicate the frequency of microorganisms isolated in relation to various drug MICs (45). The number of B. fragilis and E. cloacae 22491 CFU on each antibiotic-containing plate was plotted as a function of the concentration of CZX, PIP, or PT (GraphPad Prism version 3.0 for Windows; GraphPad Software, San Diego, Calif.). These plots were then used to calculate the AUC that characterized the relative frequency of microorganisms isolated in relation to various drug MICs. The PAP AUCs from the in vitro study were compared to those obtained from the abscesses.
Characterization of resistant mutants.
To assess the stability of the resistant mutants isolated from each of the untreated and treated abscesses, four colonies per treatment group were randomly selected from plates containing the highest antibiotic concentration on which mutant cells could be cultured. After multiple (at least five) subcultures on antibiotic-free agar, the MICs were retested by using an inoculum of 105 CFU/ml. The total number of mutant strains evaluated were 29 CZX-resistant E. cloacae 22491 mutants, 15 CZX-resistant E. cloacae 032349 mutants, 32 PIP-resistant E. cloacae 22491 mutants, 22 PT-resistant E. cloacae 22491 mutants, and 24 CZX-resistant B. fragilis mutants (isolated from abscesses with E. cloacae strain 22491). The susceptibility profiles of all Enterobacter mutants were determined by VITEK 2 (BioMérieux, Marcy l'Étoile, France) in accordance with the manufacturer's instructions.
The CZX-resistant E. cloacae 22491 isolates were further characterized by determining the β-lactamase activity of the mutants under cefoxitin-induced and noninduced conditions. Overnight cultures of each of the isolates in BHI were diluted 1:20 in 5 ml of fresh BHI and incubated with shaking at 37°C. A duplicate set of cultures was also inoculated, and after 2 h of incubation, 10 μg of cefoxitin per ml (0.2 times the MIC) was added to induce β-lactamase production. After 4 h, the bacteria were harvested from both the induced and noninduced cultures by centrifugation at 1,500 × g for 10 min at 4°C. The pellets were resuspended in 500 μl of 50 mM Tris-HCl (pH 7.4), and the β-lactamase was extracted by the lysozyme-based method outlined by Paterson et al. (31). The protein concentrations of the enzyme extractions were measured by using a dye-binding assay (Bio-Rad, Marnes la Coquette, France). Enzyme activity was measured by using the technique of O'Callaghan described previously (29), with slight modifications. Briefly, 100 μl of 1 mM nitrocefin solution was added to 100 μl of the enzyme extract and for 10 min the nitrocefin destruction was measured at a wavelength of 490 nm (Bio-Rad model 550 microplate reader). The enzyme activity was defined as micromoles of nitrocefin destroyed per minute per milliliter of added enzyme extract and calculated as follows: Δextinction/minute × 0.1 μmol of nitrocefin × 10 × dilution factor of enzyme extract. Enzyme specific activity was expressed as micromoles per minute per milligram of protein.
Statistical analysis.
The effect (E) of the antibiotics on the total bacterial counts of the in vitro mixed cultures and the abscesses was expressed as the difference, after 24 h, between the log10 CFU per milliliter (log10 CFU per abscess) in the absence and presence of the antibiotics as described by the equation E = (Emax × C)/(EC50 + C), where Emax is the maximum observed effect, C is the concentration (daily dose), and EC50 (50% effective dose [ED50]) is the concentration (daily dose) at which 50% of the maximum effect is reached. The EC50 (ED50) was estimated by using nonlinear least-squares regression techniques (GraphPad Prism version 3.0 for Windows; GraphPad Software). The 95% confidence intervals (CIs) were used to determine significant differences between the antibiotics and between the different abscess combinations. In the majority of cases, this simple Emax model gave a better fit than the more complex Hill model and was therefore used for all analyses.
RESULTS
Inoculum effect on MIC.
An inoculum effect was found with CZX, PIP, and PT against all three strains when the inoculum was increased from 105 to 108 CFU/ml (Table 1). The 4- to 8-fold increase in the MICs of PIP and PT against B. fragilis was noticeably lower than the 128-fold increase found with CZX and the 32- to 256-fold increase found with all of the antibiotics against the E. cloacae strains.
TABLE 1.
MICs of CZX, PIP, and PT with different inocula
| Bacterial strain and inoculum (CFU/ml) | MIC (μg/ml)
|
||
|---|---|---|---|
| CZX | PIP | PT | |
| B. fragilis ATCC 23745 | |||
| 105 | 1 | 2 | 1 |
| 108 | 128 | 16 | 4 |
| E. cloacae 22491 | |||
| 105 | 0.25 | 4 | 4 |
| 108 | 64 | 512 | 256 |
| E. cloacae 032349 | |||
| 105 | 0.125 | 2 | 2 |
| 108 | 4 | 512 | 256 |
In vitro activity.
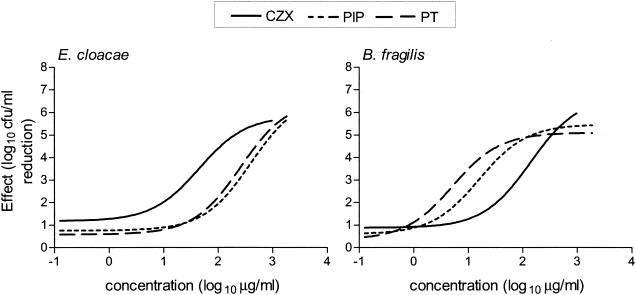
The in vitro effects (log10 CFU per milliliter reduction compared to controls) of CZX, PIP, and PT on mixed cultures of B. fragilis and E. cloacae 22149 after 24 h of incubation are shown in Fig. 1. The maximum decrease (Emax) in B. fragilis and E. cloacae 22149 bacterial counts was ≥5.1 log10 CFU/ml. There were no significant differences between the Emaxs of CZX, PIP, or PT against the two strains. However, the concentrations of CZX, PIP, and PT required to produce 50% of these maximum effects (EC50) were significantly different (Table 2). There was a 13-fold and 40-fold respective difference between the EC50 of CZX against B. fragilis compared to those of PIP and PT (P < 0.05). Against E. cloacae, the EC50 of CZX was eightfold and fivefold lower than those of PIP and PT, respectively. The EC50s of PIP and PT against the two bacterial strains were similar. Interestingly, when the EC50/MIC ratios were compared, all EC50s corresponded to the MICs that were determined by using an inoculum of 108 CFU/ml but not to those obtained with standard inocula (Table 2). Consistent with our previous studies, these MICs (108 CFU/ml) provided a better correlation with the in vitro activity of the antibiotics against the mixed cultures (40).
FIG. 1.
In vitro effects (log10 CFU per milliliter reduction compared to controls) of CZX, PIP, and PT on mixed cultures of B. fragilis ATCC 23745 and E. cloacae 22491 after 24 h of anaerobic incubation.
TABLE 2.
In vitro activities of CZX, PIP, and PT against mixed cultures of B. fragilis ATCC 23745 and E. cloacae 22491 after 24 h of anaerobic incubationa
| Antibiotic(s) and bacterial strain | Emax (log10 CFU/ml)b | EC50 (μg/ml)c | EC50/MIC ratiod
|
|
|---|---|---|---|---|
| 105 | 108 | |||
| CZX | ||||
| B. fragilis | 8.0 | 202e | 202 | 1.6 |
| E. cloacae | 6.1 | 51f | 204 | 0.8 |
| PIP | ||||
| B. fragilis | 5.5 | 16 | 8 | 1.0 |
| E. cloacae | 6.8 | 407 | 102 | 0.8 |
| PT | ||||
| B. fragilis | 5.1 | 5 | 5 | 1.25 |
| E. cloacae | 6.6 | 263 | 66 | 1.0 |
Parameters were calculated from the average log10 CFU per milliliter reduction in bacterial counts from two or three experiments.
Maximum decrease in bacterial counts compared to those of control cultures after 24 h of incubation.
Antibiotic concentration producing 50% of the maximum effect.
Ratios based on MICs determined with inocula of 105 or 108 CFU/ml.
Significant difference between the EC50 of CZX (95% CI, 41 to 984) against B. fragilis and those of PIP (95% CI, 7 to 35) and PT (95% CI, 3 to 10).
Significant difference between the EC50 of CZX (95% CI, 21 to 125) against E. cloacae and those of PIP (95% CI, 203 to 815) and PT (95% CI, 174 to 399).
In mixed cultures containing the same B. fragilis strain and another E. cloacae strain (032349), the EC50 of CZX against E. cloacae 032349 (5.9 μg/ml) was significantly lower than the EC50 for E. cloacae strain 22491 (results not shown). The EC50 of CZX against the anaerobe (152 μg/ml) was similar to the EC50 in the other mixed culture.
In vitro emergence of resistance.
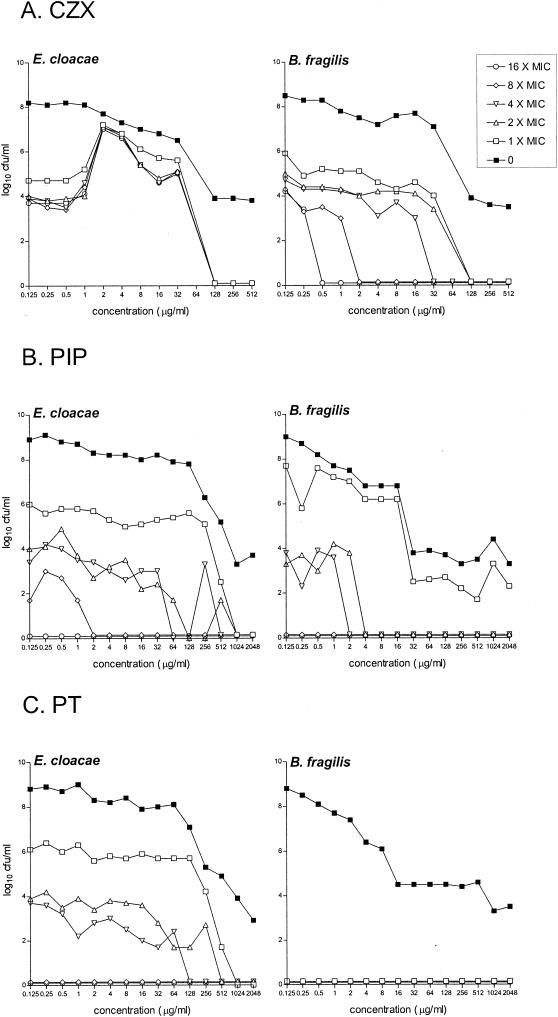
CZX-resistant colonies of B. fragilis and E. cloacae 22491 were isolated from antibiotic-free control mixed cultures at a frequency of 10−4 to 10−7 on agar plates containing CZX at up to 16 times the MIC. When the mixed cultures were incubated with increasing CZX concentrations, the numbers of resistant B. fragilis mutant bacteria remained stable (Fig. 2A). However, when exposed to a CZX concentration of 2 μg/ml, there was a 3 log10 CFU/ml increase in the number of CZX-resistant E. cloacae 22491 mutant bacteria isolated on all antibiotic plates. With concentrations of 2 to 64 μg/ml, the mutant frequency increased to 10−1 to 10−3. No CZX-resistant mutant bacteria were isolated from cultures containing ≥128 μg/ml. PIP-resistant mutant bacteria were isolated from control cultures after 24 h of incubation at a frequency of 10−4 to 10−7 (E. cloacae 22491) and 10−2 to 10−6 (B. fragilis) on plates containing up to eight times the MIC. Increasing PIP concentrations did not affect the total numbers of resistant mutants recovered, although with concentrations of ≥4 μg/ml, resistant B. fragilis colonies were not detected on plates containing more than the MIC (Fig. 2B). In mixed cultures incubated with PT, the mutant frequencies of PT-resistant E. cloacae 22491 were not significantly different from those of PIP-resistant E. cloacae 22491; however, PT-resistant B. fragilis mutants were completely suppressed (Fig. 2C).
FIG. 2.
In vitro effects of increasing concentrations of CZX, PIP, or PT on the emergence of resistant mutants from mixed cultures of B. fragilis ATCC 23745 and E. cloacae 22491 after 24 h of anaerobic incubation. The effects of the antibiotics on the total bacterial counts were determined on antibiotic-free media (0). Resistant mutants were isolated on agar plates containing CZX, PIP, or PT at 1, 2, 4, 8, or 16 times the MIC.
In contrast to the mixed cultures with E. cloacae 22491 reported above, increasing CZX concentrations did not preferentially select resistant mutants of E. cloacae 032349 from mixed cultures with B. fragilis (results not shown).
In vivo efficacy.
Similar to the in vitro study, sigmoid dose-response curves showing the effect (log10 CFU per abscess reduction compared to controls) of CZX, PIP, and PT in early abscess development were created (not shown). Compared to untreated control abscesses, there was a ≥5.0 log10 CFU per abscess maximum reduction (Emax) in the bacterial counts of both strains after 24 h of treatment with all of the antibiotics tested (Table 3). Comparable doses of PIP and PT were required to reach the ED50 for E. cloacae strain 22491. Interestingly, the ED50 of CZX against E. cloacae 22491 was similar to those of PIP and PT. There was, however, a significant ninefold and fivefold respective difference between the ED50 of PT against B. fragilis and those of CZX and PIP against the anaerobe (P < 0.05).
TABLE 3.
In vivo efficacies of CZX, PIP, and PT against different B. fragilis ATCC 23745-E. cloacae mixed infections in early abscess developmenta
| Mixed infection and antibiotic | Bacterium | Emax (log10 CFU/ abscess)b | ED50 (mg/kg/ day)c | ED50/MIC ratiod
|
|
|---|---|---|---|---|---|
| 105 | 108 | ||||
| B. fragilis-E. cloacae 22491 | |||||
| CZX | B. fragilis | 7.5 | 771 | 771 | 6 |
| E. cloacae | 8.4 | 521 | 2,084 | 8 | |
| PIP | B. fragilis | 5.9 | 416 | 208 | 26 |
| E. cloacae | 5.0 | 643 | 161 | 1.3 | |
| PT | B. fragilis | 6.1 | 85e | 85 | 21 |
| E. cloacae | 6.1 | 554 | 139 | 2.2 | |
| B. fragilis-E. cloacae 032349 | |||||
| CZX | B. fragilis | 5.5 | 81f | 81 | 0.6 |
| E. cloacae | 7.6 | 21g | 168 | 5 | |
| PT | B. fragilis | 5.7 | 77 | 77 | 19 |
| E. cloacae | 5.2 | 766 | 383 | 3 | |
Treatment (CZX every 2 h at 6 to 1,536 mg/kg/day or PIP or PT every 2 h at 24 to 6,144 mg/kg/day) was started 30 min before inoculation and continued for 24 h. Parameters were calculated from the average log10 CFU per abscess reduction in bacterial counts of four abscesses.
Maximum decrease in abscess bacterial counts after treatment for 24 h compared to those of untreated control abscesses.
Daily dose of antibiotic producing 50% of the maximum effect.
Ratios based on MICs determined with inocula of 105 or 108 CFU/ml.
Significant difference between the ED50s of PT (95% CI, 44 to 166) against B. fragilis and those of CZX (95% CI, 314 to 1,892) and PIP (95% CI, 180 to 961) against the anaerobe in the E. cloacae 22491 combination.
Significant difference between the ED50s of CZX against B. fragilis in the E. cloacae 22491 (95% CI, 314 to 1,892) and E. cloacae 032349 (95% CI, 46 to 143) abscess combinations.
Significant difference between the ED50 of CZX against E. cloacae 032349 (95% CI, 13 to 34) and the ED50 of PT (95% CI, 421 to 1,394) and those of CZX (95% CI, 228 to 1,188) and PT (95% CI, 207 to 1,485) against E. cloacae strain 22491.
To determine whether the antibiotics would have a similar bactericidal effect against another clinical isolate of E. cloacae, abscesses containing mixed infections of the same B. fragilis strain with E. cloacae 032349 were also treated with CZX or PT (Table 3). The Emaxs of both antimicrobials were comparable to those found in the other mixed infection. However, compared to E. cloacae 22491, a significant 25-fold lower dose of CZX was required to reach the ED50 for E. cloacae 032349 (P < 0.05). Furthermore, the ED50 of CZX against B. fragilis was significantly 10-fold lower when the anaerobe was in the mixed infection with E. cloacae 032349 (P < 0.05). The ED50s of PT against the two E. cloacae strains were similar, as were the ED50s of PT against the B. fragilis strain in both abscess combinations.
Consistent with the in vitro study, MICs obtained with the higher inocula correlated better with in vivo activity than did standard MICs. When the ED50/MIC ratios (inocula of 108 CFU/ml) of the two combinations were compared, similar ratios were found between the two Enterobacter strains with both CZX and PT (Table 3). Similarly, all of the PIP and PT ratios of B. fragilis in both abscess combinations were comparable although they were 4- or 30-fold higher than those of CZX. This may have been due to the higher protein binding of PIP in vivo compared to that of CZX (6, 12, 26) and was more apparent with the anaerobe owing to the lower MIC (high inoculum) compared to that for the Enterobacter strains. The CZX ratios of B. fragilis in the two mixed infections differed 10-fold.
Emergence of resistance in early abscess development.
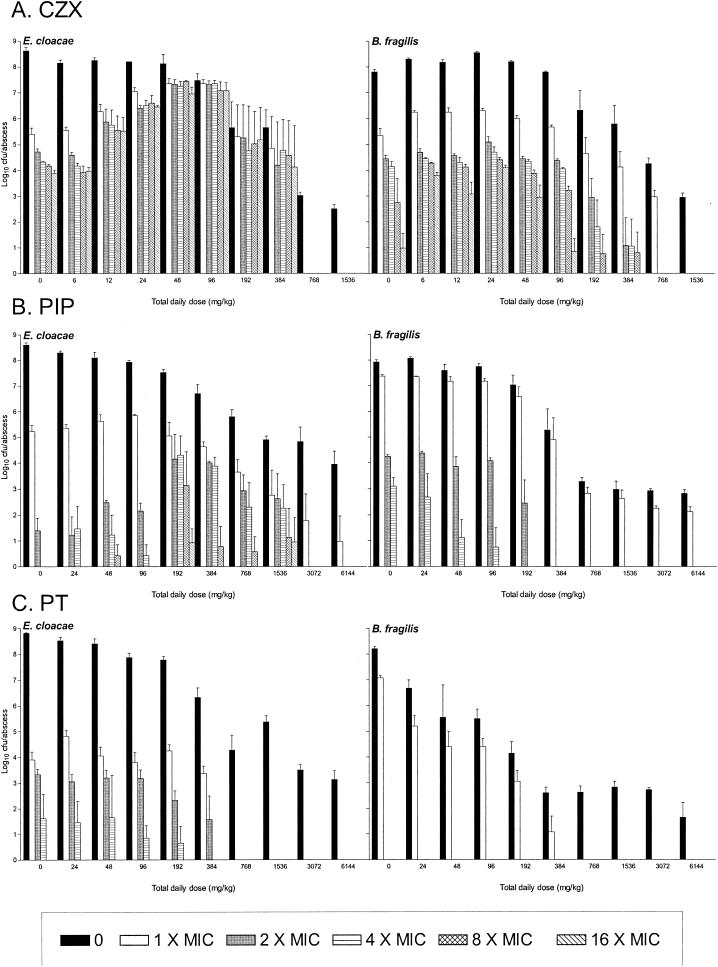
CZX-resistant mutants were isolated from untreated control B. fragilis-E. cloacae 22491 abscesses 24 h after inoculation at a frequency of 10−3 to 10−6 for both strains on plates containing drug concentrations of up to 16 times the MICs (Fig. 3A). With increasing doses of CZX, the numbers of resistant B. fragilis colonies remained stable. However, with doses of 12 to 96 mg/kg/day, there was an increase, up to 1,000-fold, in the number of CZX-resistant E. cloacae bacteria, with almost all susceptible bacteria being replaced by resistant mutants. No CZX-resistant mutants were cultured from abscesses treated with CZX at >768 mg/kg/day. PIP resistance occurred in untreated B. fragilis-E. cloacae 22491 mixed infections at frequencies of 10−4 to 10−8 on plates containing PIP at two to four times the MIC (Fig. 3B). The B. fragilis mutant frequency was unaffected by increasing PIP doses. However, with doses of 192 to 1,536 mg/kg/day, there was an increase in the degree of resistance of the E. cloacae mutants recovered since they could also be isolated on plates containing PIP at 8 and 16 times the MIC. PT-resistant E. cloacae 22491 mutants were isolated at a frequency of 10−5 to 10−6 on plates containing PT at up to four times the MIC (Fig. 3C). Increasing PT doses did not alter this frequency. B. fragilis mutants were not isolated on plates containing PT at concentrations greater than the MIC.
FIG. 3.
Effects of CZX, PIP, or PT doses on the emergence of resistant strains of B. fragilis ATCC 23745 and E. cloacae 22492 in early abscess development. Treatment (CZX every 2 h at 6 to 1,536 mg/kg/day or PIP or PT every 2 h at 24 to 6,144 mg/kg/day) was started 30 min before inoculation and continued for 24 h. Total bacterial counts were determined on antibiotic-free media (0), and those of resistant mutants were determined on agar plates containing CZX, PIP, or PT at 1, 2, 4, 8, or 16 times the MIC.
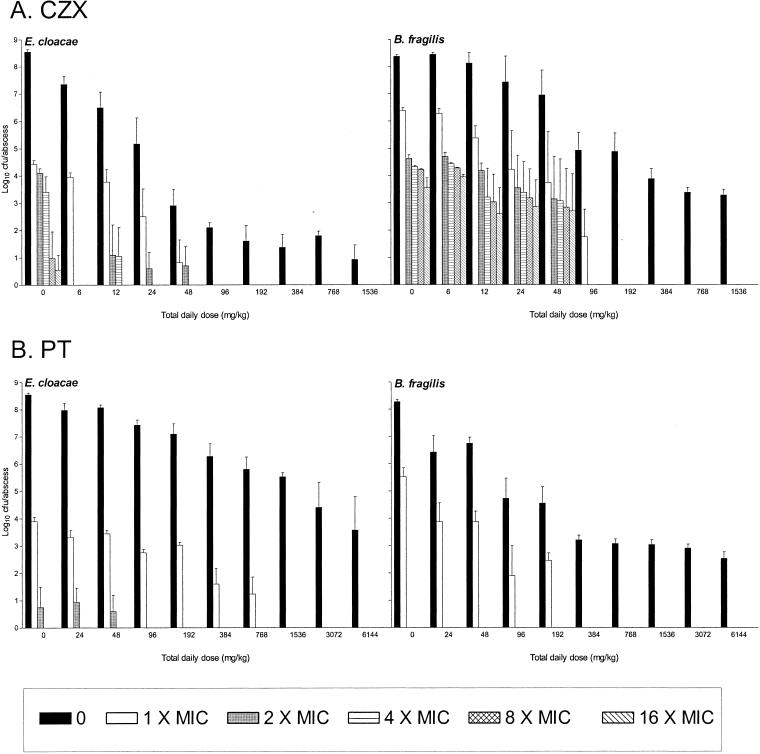
In B. fragilis-E. cloacae 032349 mixed infections, the number of CZX-resistant E. cloacae mutants isolated from untreated abscesses 24 h after inoculation was markedly lower than that of CZX-resistant E. cloacae mutants cultured from the mixed infections with E. cloacae 22491 (Fig. 4A). These mutant numbers were reduced even further by increasing CZX doses. The mutant frequencies of CZX-resistant B. fragilis mutants in both untreated and treated abscesses were similar in both abscess combinations. However, compared to the B. fragilis-E. cloacae 22491 abscesses, there was an eightfold reduction in the total daily doses of CZX at which resistant cells of both E. cloacae and B. fragilis could no longer be isolated, i.e., >96 mg/kg/day versus >768 mg/kg/day. Very few PT-resistant E. cloacae 032349 bacteria and no PT-resistant B. fragilis bacteria were isolated from untreated and PT-treated abscesses on plates containing PT concentrations greater than the MIC (Fig. 4B).
FIG. 4.
Effects of CZX or PT doses on the emergence of resistant strains of B. fragilis ATCC 23745 and E. cloacae 032349 in early abscess development. Treatment (CZX every 2 h at 6 to 1,536 mg/kg/day or PT every 2 h at 24 to 6,144 mg/kg/day) was started 30 min before inoculation and continued for 24 h. Total bacterial counts were determined on antibiotic-free media (0), and those of resistant mutants were determined on agar plates containing CZX, PIP, or PT at 1, 2, 4, 8, or 16 times the MIC.
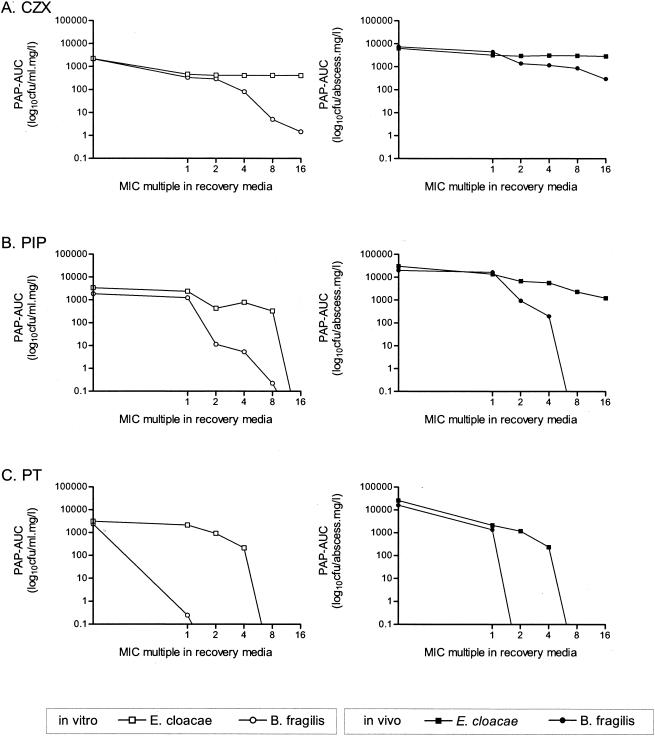
Comparison of in vitro and in vivo PAP AUCs.
The effects of the antibiotics on bacterial populations recovered from abscesses were compared to those on bacteria isolated from in vitro mixed cultures. Figure 5 shows that the in vitro profiles of both B. fragilis and E. cloacae 22491 were very similar to those found in vivo with each respective antibiotic. In all instances, B. fragilis behaved differently from the E. cloacae strain; the PAP AUCs of the anaerobe decreased with increasing antibiotic concentrations, while those of E. cloacae remained more stable. Furthermore, the superior effect of PT compared to those of CZX and PIP on the population profiles of the resistant B. fragilis mutants is evident.
FIG. 5.
Comparison of the in vitro and in vivo effects of CZX, PIP, and PT on the PAPs of B. fragilis and E. cloacae 22491 recovered from mixed cultures and mixed-infection abscesses. The PAP AUCs of bacteria recovered from plates containing each drug at 0, 1, 2, 4, 8, or 16 times the MIC are shown.
Stability and susceptibility profiles of resistant mutants.
After multiple subcultures on antibiotic-free agar, all B. fragilis mutants and one E. cloacae 22491 mutant isolated on plates containing CZX at 8 to 16 times the MIC were unstable, with the MICs of the cephalosporin for these mutants being the same as those for the parent strains. However, 28 of the 29 CZX-resistant E. cloacae 22491 mutants isolated from untreated or CZX-treated abscesses were stable after multiple subcultures, with 8- to 512-fold increases in the MICs for the mutants (inoculum, 105 CFU/ml) compared to that for the parent strain. The susceptibility profiles of the parent strain and the CZX-resistant mutants were determined by VITEK 2. The parent strain and mutants were all susceptible to the aminoglycosides, quinolones, sulfonamides, and carbapenems tested and resistant to amoxicillin-clavulanic acid, ampicillin, and narrow- and extended-spectrum cephalosporins. However, the CZX-resistant mutants differed from the parent strain in their susceptibility to broad-spectrum cephalosporins, as well as PIP and PT. On the basis of their susceptibilities to these antibiotics and independent of whether they were isolated from an untreated abscess or from any of the treated abscesses, the mutants could be divided into two groups with differing profiles (Table 4). Fifty percent of the isolates for which the MICs of CZX were 32 to 128 μg/ml (group A) had an intermediate susceptibility to PT and were resistant to PIP and all of the broad-spectrum cephalosporins tested, except for cefepime. The group B mutants (CZX MICs of 2 to 64 μg/ml) were resistant to cefpodoxime and susceptible to all of the other antibiotics.
TABLE 4.
Susceptibility profiles of the parent strain and CZX-resistant mutants of E. cloacae 22491 isolated from mixed-infection abscesses
| Antibiotic(s) | MIC(s) (μg/ml)
|
||
|---|---|---|---|
| Parent straina | Group A mutantsb | Group B mutantsc | |
| CZX | 0.25 | 32-128 | 2-64 |
| Cefepime | ≤1 | ≤1 | ≤1 |
| Cefotaxime | ≤1 | ≥64 | 4-8 |
| Cefpodoxime | 2 | ≥8 | ≥8 |
| Ceftazidime | ≤1 | ≥64 | 4 |
| PIP | ≤4 | ≥128 | 8-16 |
| PT | ≤4 | 64 | ≤4 |
One mutant isolated from an untreated control abscess had the same susceptibility profile as the parent strain after multiple subcultures on antibiotic-free agar.
Fourteen of 29 mutants isolated in group A.
Fourteen of 29 mutants isolated in group B.
The PT-resistant E. cloacae 22491 mutants (n = 22) could be similarly divided into groups with differing susceptibility profiles. Thirty-two percent of the mutants were unstable after subculture and had the same profile as the parent strain, while 32 and 36%, respectively, had profiles similar to those in groups A and B in Table 4 (excluding CZX). Likewise, the PIP-resistant E. cloacae 22491 mutants (n = 32) could be categorized as unstable (19%), group A (41%), or group B (41%). Interestingly, the group A mutants were only isolated from abscesses treated with PIP at 192 to 1,536 mg/kg/day.
In contrast to E. cloacae 22491, E. cloacae 032349 was susceptible to all of the antibiotics tested, except cefalotin and cefuroxime. After subculture, the CZX MIC for the CZX-resistant E. cloacae 032349 mutants (n = 15) was either the same as or two- to fourfold higher than that for the parent strain. When examined by VITEK 2, 60% of the mutants had the same susceptibility profile as the parent strain and 40% were resistant to cefoxitin and ampicillin and had intermediate susceptibilities to cefpodoxime (results not shown).
β-Lactamase activities of CZX-resistant E. cloacae 22491 mutants.
The CZX-resistant E. cloacae 22491 mutants were further characterized by determination of their β-lactamase activities in the presence (induced) or absence (noninduced) of cefoxitin. Under normal noninduced conditions, the parent strain produced low-level β-lactamase activity (Table 5). This enzyme level could be increased 10-fold by induction with cefoxitin. The CZX-resistant mutants could be further divided according to their β-lactamase activities. In susceptibility profile group A, six mutants (for all of which the CZX MIC was 128 μg/ml) produced very high enzyme levels that could not be increased by induction, indicating that these were stably derepressed mutants. Seven of the group A mutants also produced higher base levels of the enzyme than the parent strain but could still be induced by cefoxitin. These mutants could be regarded as partially derepressed. Susceptibility profile group B mutants could be similarly divided into mutants that were stably (n = 4) or partially (n = 4) derepressed, albeit with lower enzyme activity than those of group A. Seven isolates (group A, n = 1; group B, n = 6) had an enzyme activity similar to that of the parent strain despite the fact that the CZX MICs for these isolates were 8- to 256-fold higher. This suggests that resistance mechanisms other than β-lactamase production might play an important role in the resistance of these mutants to CZX.
TABLE 5.
β-Lactamase activities of the parent strain and CZX-resistant mutants of E. cloacae 22492 isolated from mixed-infection abscesses
| Strain(s) | CZX MIC (μg/ml) | Avg sp act (μmol/min/mg of protein) ± SD
|
|
|---|---|---|---|
| Noninduced | Induced | ||
| Parent | 0.25 | 0.06 | 0.62 |
| Group A mutants | |||
| n = 6 | 128 | 5.15 ± 3.5 | 5.81 ± 3.0 |
| n = 7 | 32-128 | 0.4 ± 0.18 | 3.84 ± 2.62 |
| n = 1 | 64 | 0.08 | 1.26 |
| Group B mutants | |||
| n = 4 | 2-64 | 0.79 ± 0.34 | 0.84 ± 0.76 |
| n = 4 | 4-16 | 0.21 ± 0.06 | 1.58 ± 0.68 |
| n = 6 | 2-32 | 0.07 ± 0.02 | 1.39 ± 0.57 |
DISCUSSION
In this B. fragilis-E. cloacae subcutaneous mixed-infection abscess model, we have shown that treatment with PT can suppress the selection of resistant mutants of both the E. cloacae and B. fragilis strains during early abscess development. In contrast to PT, the importance of antibiotic doses for the selection of CZX-resistant E. cloacae has been clearly demonstrated. This has not been widely reported previously; indeed, the authors are unaware of any studies with an in vivo model that have so thoroughly documented this adverse aspect of expanded-spectrum cephalosporin therapy in comparison to treatment with a β-lactam-β-lactamase inhibitor combination (19, 21, 23, 28, 32). Furthermore, the resistant mutants selected during therapy comprised a heterogeneous population of not just stably derepressed mutants, as previously reported, but also mutants with resistance mechanisms other than β-lactamase production (4, 17, 34).
Although broad-spectrum cephalosporins should be avoided as primary agents against Enterobacter strains (8) and CZX would not be considered the most useful antianaerobic agent, broad-spectrum cephalosporins are still advised for empirical use against mixed infections (7). Furthermore, and despite recommendations to the contrary, expanded-spectrum cephalosporins are still widely used in many countries for surgical prophylaxis (16, 36). The murine model used in this study simulates the clinical situation in which PT or an expanded-spectrum cephalosporin would be empirically used to treat an infection resulting from complications following abdominal surgery, such as leakage of an intestinal suture (7). In this case, antibiotic treatment has to be started prior to reoperation. Since preoperative prophylaxis with a narrow-spectrum cephalosporin is standard practice in abdominal surgery (15, 16), there is a risk that these intraabdominal infections could involve Enterobacter strains unaffected by the antibiotic prophylaxis. Although antibiotic therapy is an adjunctive treatment for surgical abscess drainage, small undrainable intraabdominal abscesses could also develop.
Definite antibiotic therapy is chosen on the basis of in vitro susceptibility tests with low inocula (24). However, the present study has demonstrated the importance of using high inocula to identify the potential of an antibiotic to select resistance to itself and other antibiotics and to predict its in vivo efficacy in infections involving high bacterial numbers (38, 40, 44). In standard MIC tests with inocula of 105 CFU/ml, both the Enterobacter and B. fragilis strains would be regarded as susceptible to CZX, PIP, and PT (27), and yet differing antibacterial activities were found against mixtures of these strains both in vitro and in vivo. However, there was a good correlation between in vitro and in vivo efficacy and the MICs obtained with a higher inoculum. These results corroborate the findings of Soriano et al. (38), who also reported a better correlation of the in vivo efficacies of five different β-lactams in a murine E. coli infection model with the MICs of the antibiotics obtained with high inocula. Although there are several rigorous environmental conditions that could limit the activity of an antibiotic in abscesses, e.g., pH, anaerobiosis, and cell debris (22), the present study emphasizes how important the presence of high bacterial numbers can be to antibiotic efficacy (41). All EC50s corresponded to the higher MICs, and in vivo, low MICs were associated with lower ED50s. In both abscess combinations, the ED50s of CZX against all of the strains tested corresponded to the doses used when no or few resistant mutants were isolated from the mixed infections. The inferior in vivo killing of the anaerobe by CZX in E. cloacae 22491 abscesses compared to that in the E. cloacae 032349 combination may, in part, be due to hydrolysis of the antibiotic by the high β-lactamase concentrations produced by the resistant Enterobacter cells. Consequently, it appeared that the preferential selection of resistant mutants of one strain had compromised the efficacy of the antimicrobial against another member of the mixed infection. These findings indicate, therefore, that the problem of emergence of resistance during β-lactam therapy may be further compounded when the infections being treated comprise different bacterial strains. This phenomenon, however, may not be confined to β-lactams since we have also found that the in vitro and in vivo activities of a fluoroquinolone against the B. fragilis strain were similarly affected by the nature of the bacterial strain coexisting in the mixed culture (infection) (40, 41).
The results presented here confirm that TAZ can potentiate the activity of PIP against B. fragilis, as shown by the reduced MICs, EC50s, ED50s, and PAP AUCs (2, 18, 33), but does not enhance the activity of PIP against the two E. cloacae strains (1, 33). However, TAZ does appear to play an important role in the suppression of resistance to PIP in both the Enterobacter and B. fragilis strains (19, 20). Although TAZ is a highly active inhibitor of the β-lactamase of B. fragilis (2, 18, 33), its activity against the AmpC β-lactamase of E. cloacae is limited (1). Nevertheless, TAZ seems to have some ability to potentiate the activity of PIP against derepressed mutants of E. cloacae (1). It has been suggested that suppression of resistance by PT is mediated by the inhibition of the AmpC β-lactamase of a subpopulation of moderately resistant mutants of E. cloacae (20). In this study, we have verified that during treatment with selective doses of PIP (192 to 1,536 mg/kg/day), E. cloacae mutants with a high degree of resistance to PIP can be suppressed by TAZ.
CZX-resistant B. fragilis mutants were isolated from untreated abscesses at a frequency similar to that of E. cloacae strain 22491, but these were not preferentially selected during treatment with the cephalosporin. Furthermore, it appeared that the resistance character of these strains could be lost by multiple subcultures on antibiotic-free agar. Resistance to β-lactams in anaerobic bacteria is attributed primarily to the constitutive production of high levels of β-lactamase (10). In a recent in vitro study, it was demonstrated that resistant Bacteroides strains selected with the cephalosporin cefoxitin displayed no enhanced β-lactamase activity compared to that of the parent strain but did have altered penicillin-binding proteins and outer membrane proteins (14). In addition, this resistance was lost after multiple subcultures on antibiotic-free agar, indicating that the alterations were only produced under antibiotic pressure. The resistant Bacteroides mutants isolated in the present study were not further characterized. Nevertheless, owing to their unstable character, it is possible that their initial resistance to CZX was mediated by a non-β-lactamase resistance mechanism.
In conclusion, the present animal model demonstrates the adverse microbiological outcome of choosing an expanded-spectrum cephalosporin such as CZX for the empirical treatment of mixed infections in which a susceptible Enterobacter strain could be present. Importantly, the selection of these resistant mutants was apparent within 24 h of treatment and was dose dependent. This preferential selection was not found after treatment with PT. In addition, our study confirms that clinicians could be misled by results of standard susceptibility tests of isolates from intraabdominal infections.
Acknowledgments
This study was financially supported by an unrestricted grant from Wyeth Lederle, Hoofddorp, The Netherlands.
We thank Liesbeth A. E. van der Zwaan for technical assistance.
REFERENCES
- 1.Akova, M., Y. Yang, and D. M. Livermore. 1990. Interactions of tazobactam and clavulanate with inducibly- and constitutively-expressed class 1 beta-lactamases. J. Antimicrob. Chemother. 25:199-208. [DOI] [PubMed] [Google Scholar]
- 2.Aldridge, K. E. 1994. Anaerobes in polymicrobial surgical infections: incidence, pathogenicity and antimicrobial resistance. Eur. J. Surg. Suppl. 573:31-37. [PubMed] [Google Scholar]
- 3.Beale, A. S., and J. Gisby. 1991. Comparative efficacies of ticarcillin, ticarcillin/clavulanic acid, piperacillin and cefoxitin against polymicrobial infections in mice caused by Escherichia coli and Bacteroides fragilis. Infection 19:101-105. [DOI] [PubMed] [Google Scholar]
- 4.Charrel, R. N., J.-M. Pagès, P. De Micco, and M. Mallea. 1996. Prevalence of outer membrane porin alteration in β-lactam-antibiotic-resistant Enterobacter aerogenes. Antimicrob. Agents Chemother. 40:2854-2858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cosgrove, S. E., K. S. Kaye, G. M. Eliopoulos, and Y. Carmeli. 2002. Health and economic outcomes of the emergence of third-generation cephalosporin resistance in Enterobacter species. Arch. Intern. Med. 162:185-190. [DOI] [PubMed] [Google Scholar]
- 6.Craig, W. A., and B. Suh. 1991. Protein binding and the antimicrobial effects: methods for the determination of protein binding, p. 367-402. In V. Lorian (ed.), Antibiotics in laboratory medicine, third edition. Williams & Wilkins, New York, N.Y.
- 7.Cunha, B. A., and J. H. Rex. 2003. Empiric therapy based on clinical syndrome, p. 18-134. In B. A. Cunha (ed.), Antibiotic essentials, 2nd ed. Physicians' Press, Royal Oak, Mich.
- 8.Cunha, B. A., P. E. Schoch, and J. H. Rex. 2003. Initial therapy of isolates pending susceptibility testing, p. 143-185. In B. A. Cunha (ed.), Antibiotic essentials, 2nd ed. Physicians' Press, Royal Oak, Mich.
- 9.de Man, P., B. A. N. Verhoeven, H. A. Verbrugh, M. C. Vos, and J. N. van den Anker. 2000. An antibiotic policy to prevent emergence of resistant bacilli. Lancet 355:973-978. [DOI] [PubMed] [Google Scholar]
- 10.Edwards, R. 1997. Resistance to beta-lactam antibiotics in Bacteroides spp. J. Med. Microbiol. 46:979-986. [DOI] [PubMed] [Google Scholar]
- 11.Ehrhardt, A. F., and C. C. Sanders. 1993. Beta-lactam resistance amongst Enterobacter species. J. Antimicrob. Chemother. 32:1-11. [DOI] [PubMed] [Google Scholar]
- 12.el-Komy, A. A. 1995. Disposition kinetics and bioavailability of piperacillin and cephapirin in mares. Dtsch. Tieraerztl. Wochenschr. 102:244-248. [PubMed] [Google Scholar]
- 13.Falagas, M. E., L. Barefoot, J. Griffith, R. Ruthazar, and D. R. Snydman. 1996. Risk factors leading to clinical failure in the treatment of intra-abdominal or skin/soft tissue infections. Eur. J. Clin. Microbiol. Infect. Dis. 15:913-921. [DOI] [PubMed] [Google Scholar]
- 14.Fang, H., C. Edlund, C. E. Nord, and M. Hedberg. 2002. Selection of cefoxitin-resistant Bacteroides thetaiotaomicron mutants and mechanisms involved in beta-lactam resistance. Clin. Infect. Dis. 35(Suppl. 1):S47-S53. [DOI] [PubMed] [Google Scholar]
- 15.Gardner, P., and B. A. Cunha. 2003. Antibiotic prophylaxis and immunizations, p. 268-292. In B. A. Cunha (ed.), Antibiotic essentials, first ed. Physicians' Press, Royal Oak, Mich.
- 16.Geroulanos, S., K. Marathias, J. Kriaras, and B. Kadas. 2001. Cephalosporins in surgical prophylaxis. J. Chemother. 13:23-26. [DOI] [PubMed] [Google Scholar]
- 17.Goldstein, F. W. 2002. Cephalosporinase induction and cephalosporin resistance: a longstanding misinterpretation. Clin. Microbiol. Infect. 8:823-825. [DOI] [PubMed] [Google Scholar]
- 18.Hedberg, M., and C. E. Nord. 1996. Beta-lactam resistance in anaerobic bacteria: a review. J. Chemother. 8:3-16. [DOI] [PubMed] [Google Scholar]
- 19.Higashitani, F., K. Nishida, A. Hyodo, and M. Inque. 1995. Effects of tazobactam on the frequency of emergence of resistant strains from Enterobacter cloacae, Citrobacter freundii and Proteus vulgaris (beta-lactamases derepressed mutants). J. Antibiot. 48:1027-1033. [DOI] [PubMed] [Google Scholar]
- 20.Kadima, T. A., and J. H. Weiner. 1997. Mechanism of suppression of piperacillin resistance in enterobacteria by tazobactam. Antimicrob. Agents Chemother. 41:2177-2183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Marchou, B., M. Michea-Hamzehpour, C. Lucain, and J. C. Pechere. 1987. Development of beta-lactam-resistant Enterobacter cloacae in mice. J. Infect. Dis. 156:369-373. [DOI] [PubMed] [Google Scholar]
- 22.McClean, K. L., G. J. Sheehan, and G. K. M. Harding. 1994. Intraabdominal infection: a review. Clin. Infect. Dis. 19:100-116. [DOI] [PubMed] [Google Scholar]
- 23.Michea-Hamzehpour, M., and J. C. Pechere. 1989. How predictable is development of resistance after beta-lactam therapy in Enterobacter cloacae infection? J. Antimicrob. Chemother. 24:387-395. [DOI] [PubMed] [Google Scholar]
- 24.Mouton, J. W., M. N. Dudley, O. Cars, H. Derendorf, and G. L. Drusano. 2002. Standardization of pharmacokinetic/pharmacodynamic (PK/PD) terminology for anti-infective drugs. Int. J. Antimicrob. Agents 19:355-358. [DOI] [PubMed] [Google Scholar]
- 25.Mouton, R. P., J. H. Glerum, and A. C. van Loenen. 1976. Relationship between antibiotic consumption and frequency of antibiotic resistance of four pathogens—a seven year survey. J. Antimicrob. Chemother. 2:9-19. [DOI] [PubMed] [Google Scholar]
- 26.Murakawa, T., H. Sakamoto, S. Fukada, S. Nakamoto, T. Hirose, N. Itoh, and M. Nishida. 1980. Pharmacokinetics of ceftizoxime in animals after parenteral dosing. Antimicrob. Agents Chemother. 17:157-164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.National Committee for Clinical Laboratory Standards. 2002. Performance standards for antimicrobial susceptibility testing; twelfth informational supplement. NCCLS document M100-S12. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 28.Negri, M.-C., M. Lipsitch, J. Blázquez, B. R. Levin, and F. Baquero. 2000. Concentration-dependent selection of small phenotypic differences in TEM β-lactamase-mediated antibiotic resistance. Antimicrob. Agents Chemother. 44:2485-2491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.O'Callaghan, C. H., A. Morris, S. M. Kirby, and A. H. Shingler. 1972. Novel method for detection of β-lactamases by using a chromogenic cephalosporin substrate. Antimicrob. Agents Chemother. 1:283-288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Olson, B., R. A. Weinstein, C. Nathan, and S. A. Kabins. 1983. Broad-spectrum beta-lactam resistance in Enterobacter: emergence during treatment and mechanisms of resistance. J. Antimicrob. Chemother. 11:299-310. [DOI] [PubMed] [Google Scholar]
- 31.Paterson, D. L., L. B. Rice, and R. A. Bonomo. 2001. Rapid method of extraction and analysis of extended-spectrum beta-lactamases from clinical strains of Klebsiella pneumoniae. Clin. Microbiol. Infect. 7:709-711. [PubMed] [Google Scholar]
- 32.Pechere, J. C., and I. R. Vladoianu. 1992. Development of resistance during ceftazidime and cefepime therapy in a murine peritonitis model. J. Antimicrob. Chemother. 29:563-573. [DOI] [PubMed] [Google Scholar]
- 33.Perry, C. M., and A. Markham. 1999. Piperacillin/tazobactam. An updated review of its use in the treatment of bacterial infections. Drugs 57:805-843. [DOI] [PubMed] [Google Scholar]
- 34.Ryan, B. M., T. J. Dougherty, D. Beaulieu, J. Chuang, B. A. Dougherty, and J. F. Barret. 2001. Efflux in bacteria: what do we really know about it? Expert Opin. Investig. Drugs 10:1409-1422. [DOI] [PubMed] [Google Scholar]
- 35.Sanders, W. E., and C. C. Sanders. 1997. Enterobacter spp.: pathogens poised to flourish at the turn of the century. Clin. Microbiol. Rev. 10:220-241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sganga, G. 2001. New perspectives in antibiotic prophylaxis for intra-abdominal surgery. J. Hosp. Infect. 50(Suppl. A):S17-S21. [DOI] [PubMed] [Google Scholar]
- 37.Snydman, D. R., N. V. Jacobus, L. A. McDermott, S. Supran, G. J. Cuchural, S. Finegold, L. Harrell, D. W. Hecht, P. Iannini, S. Jenkins, C. Pierson, J. Rihs, and S. L. Gorbach. 1999. Multicenter study of in vitro susceptibility of the Bacteroides fragilis group, 1995 to 1996, with comparison of resistance trends from 1990 to 1996. Antimicrob. Agents Chemother. 43:2417-2422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Soriano, F., P. Garcia-Corbeira, C. Ponte, R. Fernandez-Roblas, and I. Gadea. 1996. Correlation of pharmacodynamic parameters of five β-lactam antibiotics with therapeutic efficacies in an animal model. Antimicrob. Agents Chemother. 40:2686-2690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stearne, L. E. T., S. L. Buijk, J. W. Mouton, and I. C. Gyssens. 2002. Effect of a single percutaneous abscess drainage puncture and imipenem therapy, alone or in combination, in the treatment of mixed-infection abscesses in mice. Antimicrob. Agents Chemother. 46:3712-3718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stearne, L. E. T., I. C. Gyssens, W. H. F. Goessens, J. W. Mouton, W. J. G. Oyen, J. W. M. van der Meer, and H. A. Verbrugh. 2001. In vivo efficacy of trovafloxacin against Bacteroides fragilis in mixed infection with either Escherichia coli or a vancomycin-resistant strain of Enterococcus faecium in an established-abscess murine model. Antimicrob. Agents Chemother. 45:1394-1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stearne, L. E. T., C. Kooi, W. H. F. Goessens, I. A. J. M. Bakker-Woudenberg, and I. C. Gyssens. 2001. In vitro activity of trovafloxacin against Bacteroides fragilis in mixed culture with either Escherichia coli or a vancomycin-resistant strain of Enterococcus faecium determined by an anaerobic time-kill technique. Antimicrob. Agents Chemother. 45:243-251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stoorvogel, J., M. H. van Gestel, P. A. Ketelaar-van Gaalen, R. P. Mouton, and J. A. M. van de Klundert. 1991. Variation in induction of chromosomal beta-lactamase expression in strains of Enterobacter cloacae. Chemotherapy 37:175-185. [DOI] [PubMed] [Google Scholar]
- 43.Tenover, F. C. 2001. Development and spread of bacterial resistance to antimicrobial agents: an overview. Clin. Infect. Dis. 33(Suppl. 3):S108-S115. [DOI] [PubMed] [Google Scholar]
- 44.Thomson, K. S., and E. S. Moland. 2001. Cefepime, piperacillin-tazobactam, and the inoculum effect in tests with extended-spectrum β-lactamase-producing Enterobacteriaceae. Antimicrob. Agents Chemother. 45:3548-3554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wootton, M., R. A. Howe, R. Hillman, T. R. Walsh, P. M. Bennett, and A. P. MacGowan. 2001. A modified population analysis profile (PAP) method to detect hetero resistance to vancomycin in Staphylococcus aureus in a UK hospital. J. Antimicrob. Chemother. 47:399-403. [DOI] [PubMed] [Google Scholar]