Abstract
Pregnancy is rare in women with ESRD and when it occurs, it is often accompanied by significant maternal and fetal morbidity and even mortality. Preliminary data from the Toronto Nocturnal Hemodialysis Program suggested that increased clearance of uremic toxins by intensified hemodialysis improves pregnancy outcomes, but small numbers and the absence of a comparator group limited widespread applicability of these findings. We compared pregnancy outcomes from 22 pregnancies in the Toronto Pregnancy and Kidney Disease Clinic and Registry (2000–2013) with outcomes from 70 pregnancies in the American Registry for Pregnancy in Dialysis Patients (1990–2011). The primary outcome was the live birth rate and secondary outcomes included gestational age and birth weight. The live birth rate in the Canadian cohort (86.4%) was significantly higher than the rate in the American cohort (61.4%; P=0.03). Among patients with established ESRD, the median duration of pregnancy in the more intensively dialyzed Toronto cohort was 36 weeks (interquartile range, 32–37) compared with 27 weeks (interquartile range, 21–35) in the American cohort (P=0.002). Furthermore, a dose response between dialysis intensity and pregnancy outcomes emerged, with live birth rates of 48% in women dialyzed ≤20 hours per week and 85% in women dialyzed >36 hours per week (P=0.02), with a longer gestational age and greater infant birth weight for women dialyzed more intensively. Pregnancy complications were few and manageable. We conclude that pregnancy may be safe and feasible in women with ESRD receiving intensive hemodialysis.
The first successful pregnancy reported in a patient on chronic hemodialysis occurred in 1970, and was considered an overwhelming success.1 Initial enthusiasm was tempered with subsequent studies noting pregnancy in women with ESRD to be uncommon due to decreased fertility2–6 and often associated with poor outcomes.7,8 However, recent data suggest that pregnancy while on intensive hemodialysis may result in better outcomes,9 providing a viable option for young women whose reproductive years are lost to ESRD.
Our group previously demonstrated that nocturnal hemodialysis, as an intensive dialysis modality, resulted in reduced maternal and fetal complications.9,10 In this report, we present our expanding clinical series of all pregnant patients supported by intensive dialysis in Toronto from 2000 to 2013, and compare the outcomes from this prospective cohort to the American Registry for Pregnancy in Dialysis Patients (ARPD). We hypothesized that intensive hemodialysis with enhanced multidisciplinary follow-up would be associated with improved pregnancy outcomes compared with conventional dialysis regimens.
Results
Cohort Characteristics
The Toronto Pregnancy and Kidney Disease (PreKid) Clinic and Registry included 22 pregnancies in 17 patients. Characteristics of this cohort are detailed in Table 1. Eighteen pregnancies occurred after the start of chronic dialysis, whereas hemodialysis was initiated during pregnancy in four women. The comparison group included 70 pregnancies from the ARPD (57 occurred in women already on hemodialysis and 13 occurred in women approaching ESRD). The two cohorts were comparable with respect to the causes of ESRD. However, the Toronto group was significantly older than the American patients (34±4 versus 27±6 years; P<0.001). The PreKid cohort was 59% Caucasian (10 Caucasians, 3 African Canadians, 3 Asians, and 1 South Asian); however, we do not have data on race for patients in the ARPD cohort. In the established chronic dialysis patients, there was no significant difference in dialysis vintage at the time of pregnancy, with a median of 41 months (interquartile range [IQR], 16–72) and 18 months (IQR, 7–48) months in the PreKid and ARPD cohorts, respectively. However in women approaching ESRD, Canadian patients were started on dialysis at an earlier gestational age (14±7 versus 24±7 weeks; P=0.03).
Table 1.
Patient characteristics, pregnancy outcomes, and complications in the Toronto PreKid Clinic and Registry
| Patient | Age (yr)a | Gravida Para | Cause of ESRD | Comorbidities | GA (wk) | Weight (g) | Complications |
|---|---|---|---|---|---|---|---|
| 1 | 39 | G2P1 | IgAN | HTN, DVT (recurrent in pregnancy) | 26+1 | 980 | Short cervix, preterm delivery |
| 2 | 34 | G1P0 | Medullary cystic disease | HTN, parathyroidectomy, PCOS | 37+2 | 2015 | Transient polyhydramnios |
| 3 | |||||||
| Pregnancy 1 | 36 | G1P0 | Liver transplant/CNI toxicity | Liver transplant ×2 for sclerosing cholangitis, Burkitt's lymphoma | 37+4 | 2930 | None |
| Pregnancy 2 | 38 | G2P1 | 38+3 | 2520 | None | ||
| 4 | |||||||
| Pregnancy 1 | 30 | G1P0 | Liver transplant/CNI toxicity | Liver transplant for sclerosing cholangitis | 37+0 | 3175 | Short cervix |
| Pregnancy 2 | 35 | G2P1 | 22+2 | 460 | Preterm delivery, neonatal death | ||
| 5 | 34 | G1P0 | SLE | SLE, juvenile scleroderma, HTN, congenital deafness | 32+3 | 1120 | Preeclampsia, IUGR |
| 6 | 33 | G2P1 | MPGN | Failed transplant due to rejection—CMV, EBV infections, cardiac arrest in prior pregnancy | 36+1 | 2415 | None |
| 7 | |||||||
| Pregnancy 1 | 30 | G1P0 | IgAN | HTN | 38+0 | 3000 | None |
| Pregnancy 2 | 32 | G2P1 | 37+4 | 2785 | None | ||
| 8 | 37 | G4P0 | Hypoplastic kidneys | Failed transplants (×2), HTN (retinal bleed), 3 prior spontaneous abortions (GA 5–8 wk) | 38+5 | 2750 | None |
| 9 | 27 | G1P0 | Hereditary nephritis | Hyperparathyroidism | 36+5 | 2690 | None |
| 10 | 34 | G1P0 | PCKD | HTN, failed transplant with nephrectomy | 30+0 | 1260 | Preterm delivery, cause unclear |
| 11 | 25 | G1P0 | Hypertensive nephrosclerosis | Failed transplant, HTN | 32+1 | 1850 | Rescue cerclage (22 wk), chorioamnionitis, PPROM |
| 12 | |||||||
| Pregnancy 1 | 34 | G2P1 | IgAN | Preeclampsia in prior pregnancy, HTN | 36+6 | 2900 | None |
| Pregnancy 2 | 36 | G3P2 | 36+2 | 3030 | None | ||
| 13 | |||||||
| Pregnancy 1 | 35 | G1P0 | PAN | Failed transplant due to rejection, bronchiectasis due to recurrent pneumonia | 35+2 | 1680 | Late-onset IUGR |
| Pregnancy 2 | 37 | 37+2 | 2202 | None | |||
| 14 | 33 | G1P0 | IgAN | None | 37+6 | 2866 | None |
| 15 | 38 | G2P0 | IgAN | Preeclampsia in prior pregnancy, HTN, pyelonephritis | 27+3 | 360 | IUGR, intrauterine fetal demise |
| 16 | 37 | G4P2 | Chronic GN NYD | Preeclampsia in prior pregnancy, HTN | 12+0 | NA | First-trimester loss, severe hypertension |
| 17 (twin pregnancy) | 31 | G5P2 | Chronic GN NYD | HTN in prior pregnancy, sickle cell trait | 33+4 | 2000, 1600 | Short cervix, PPROM, preterm delivery |
GA, gestational age; IgAN, IgA nephropathy; HTN, hypertension; DVT, deep vein thrombosis; PCOS, polycystic ovarian syndrome; CNI, calcineurin inhibitor; IUGR, intrauterine growth restriction; MPGN, membranoproliferative GN; CMV, cytomegalovirus; EBV, Epstein–Barr virus; PCKD, polycystic kidney disease; PPROM, preterm premature rupture of membranes; PAN, polyarteritis nodosa; GN NYD, GN not yet diagnosed; NA, not available.
Age is at conception.
Pregnancy Outcomes
Of the 22 pregnancies followed in the Toronto PreKid Clinic, 19 (86.4%) resulted in a live birth, which was significantly higher than the live birth rate of 61.4% reported to the ARPD (P=0.03; see Table 2). In the Toronto cohort, there was one first-trimester loss, one intrauterine fetal demise and one neonatal death resulting in a poor outcome in 14% of pregnancies compared with 39% in the United States registry. All poor pregnancy outcomes occurred in women with established ESRD, whereas the women who initiated dialysis during pregnancy all had live births in both cohorts. In patients with established ESRD, the live birth rate, however, was higher in the PreKid cohort (83.3%) compared with the ARPD cohort (52.6%; P=0.02).
Table 2.
Cohort-specific pregnancy outcomes
| Pregnancy Outcomes | Toronto PreKid Cohort | United States ARPD Cohort | P Value |
|---|---|---|---|
| Live birth rate (entire cohort) | 19 (86.4) | 43 (61.4) | 0.03 |
| Spontaneous abortion, first trimester | 1 (4.5) | 5 (7.1) | |
| Spontaneous abortion, second trimester | 0 (0) | 14 (20.0) | |
| Neonatal death | 1 (4.5) | 5 (7.1) | |
| Still birth | 1 (4.5) | 3 (4.3) | |
| Live birth rate (ESRD patients only) | 15 (83.3) | 30 (52.6) | 0.02 |
| Among patients with established ESRD | |||
| Dialysis time (h/wk) | 43±6 | 17±5 | <0.001 |
| Gestational age (wk) | 36 (32–37) | 27 (21–35) | 0.002 |
| Among patients with renal failure during pregnancy | |||
| Dialysis time (h/wk) | 33±6 | 15±4 | <0.001 |
| Gestational age (wk) | 34 (29–37) | 33 (31–37) | NS |
| All pregnancies (except first- and second-trimester spontaneous abortions | |||
| Dialysis time (h/wk) | 42±7 | 17±5 | <0.001 |
| Birth weight (g) | 2118±857 | 1748±949 | NS |
| Among surviving infants in established ESRD patients | |||
| Normal birth weight | 8 (50.0) | 10 (32.3) | NS |
| Low birth weight (<2500 g) | 7 (43.8) | 12 (38.7) | |
| Very low birth weight (<1500 g) | 1 (6.3) | 9 (29.0) |
Values are presented as n (%), mean±SD, or median (interquartile range). Values for gestational age are rounded to the nearest week.
Among women with established ESRD, the duration of pregnancy was longer in the PreKid cohort at 36 weeks (IQR, 32–37) compared with 27 weeks (IQR, 21–35) in the ARPD cohort (P=0.002). The Canadian ESRD patients received 43±6 hours of dialysis compared with the 17±5 hours provided in the United States (P<0.001). Of interest, there was no difference in the duration of pregnancy in women who started dialysis during pregnancy (34 [IQR, 29–37] versus 33 [IQR, 31–37]; P=NS) despite earlier initiation of dialysis (14±7 versus 24±7 weeks; P=0.03) and the provision of more dialysis (33±6 versus 15±4 hours; P<0.001) in the Canadian cohort. After excluding first- and second-trimester losses wherein birth weight was not available, gestational weight tended to be higher in the PreKid cohort compared with the ARPD cohort (2118±857 and 1748±949 g, respectively). Among surviving infants of established ESRD patients, 6% of infants in the Canadian cohort were classified as very low birth weight compared with 29% of infants in the US cohort (Table 1).
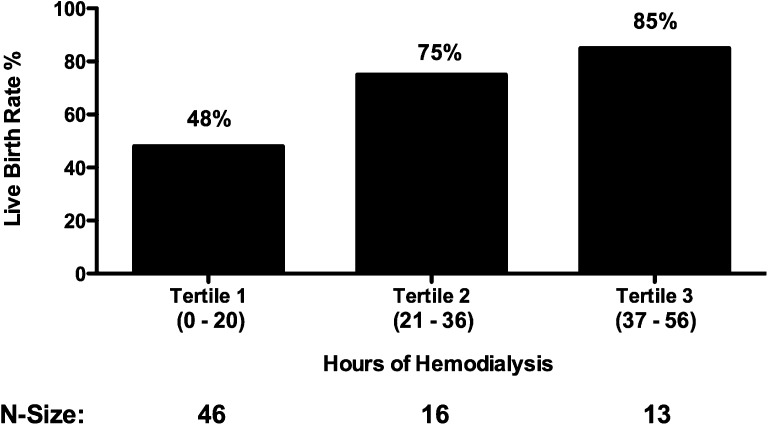
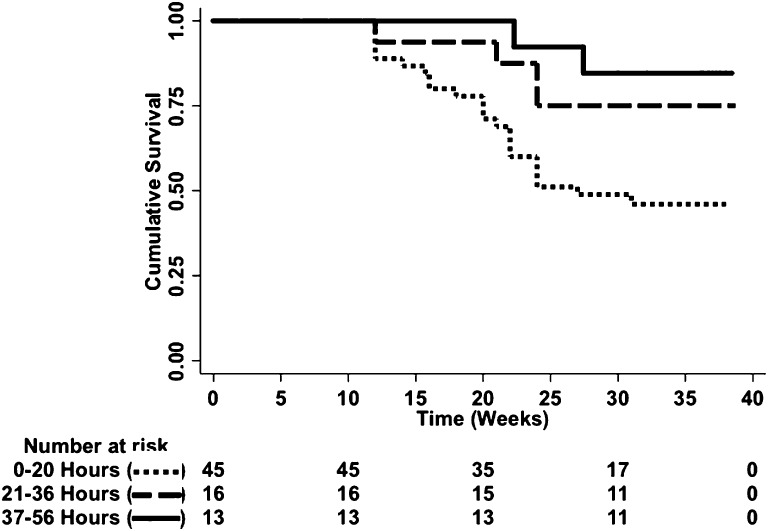
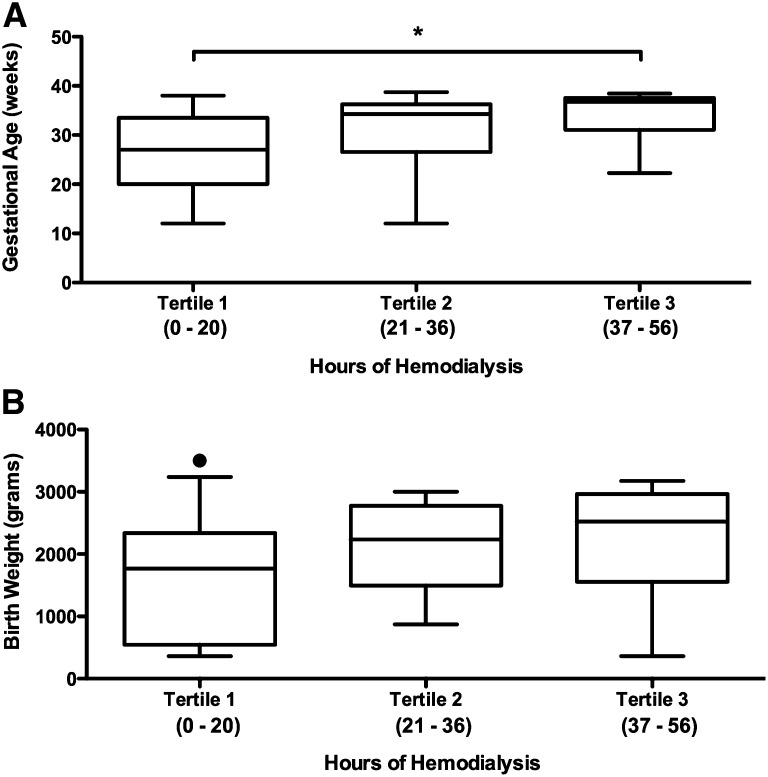
With respect to the assessment of dialysis intensity on pregnancy outcomes in women with ESRD, there was a dose-response relationship. The live birth rate improved in each successive tertile of hours of hemodialysis provided, improving from 48% in women receiving ≤20 hours to 85% in women receiving ≥37 hours of hemodialysis weekly (P=0.02; Figure 1). This relationship between dialysis intensity and outcome was confirmed by a time-to-event analysis (P=0.01; Figure 2). Furthermore, gestational age increased significantly with higher levels of hemodialysis (P=0.002; Figure 3A) along with a trend toward larger babies (Figure 3B). As such, correlations between dialysis intensity and gestational age (R2=0.15; P<0.001) as well as birth weight (R2=0.08; P=0.04) were observed.
Figure 1.
Live birth rates by dialysis intensity. In women with established ESRD, there is a significant dose-response relationship between hemodialysis intensity and the live birth rate (P=0.02), improving from 48% in women receiving ≤20 hours to 75% in women receiving between 21 and 36 hours to 85% in women receiving ≥37 hours of hemodialysis weekly.
Figure 2.
Time-to-event analysis by dialysis intensity. In women with established ESRD, there is a significant pregnancy survival advantage among women with high delivered doses of dialysis (log-rank test; P=0.01).
Figure 3.
Gestational age and birth weight by dialysis intensity. In women with established ESRD, gestational age increased significantly with higher levels of hemodialysis (A) along with a trend toward larger babies (B). The circle represents an outlier. *P=0.002.
Pregnancy Complications in the Toronto PreKid Clinic and Registry Cohort
Pregnancy complications for the PreKid cohort are also summarized in Table 1. The majority of the patients had unremarkable pregnancies or manageable complications. All but two women delivered vaginally. Four patients were noted to have short cervices. Patient 1 was diagnosed with a short cervix after 24 weeks of gestation, delivering preterm at 26 weeks. Patient 4 (first pregnancy) was diagnosed after 20 weeks of gestation, but her cervical length remained stable on bed rest. She subsequently delivered at 37 weeks of gestation. In her second pregnancy, a normal cervical length was noted at her anatomy scan at 20 weeks of gestation, but she delivered precipitously at a previable gestational age of 22 weeks. Patient 11 required a rescue cerclage at 22 weeks and delivered at 32 weeks due to preterm premature rupture of membranes. Finally, the twin pregnancy was also noted to have a shortened cervix and delivered at almost 34 weeks gestation. Only a single patient (patient 2) had transient polyhydramnios. Evidence of placental insufficiency was noted in three pregnancies. The pregnancy in patient 5 was complicated presumably by preeclampsia (diagnosed on the basis of worsening hypertension, decreased fetal growth rate, and elevated umbilical artery pulsatility indices), requiring a caesarean section just beyond 32 weeks of gestation. Late-onset intrauterine growth restriction was noted in patient 13 during her first pregnancy, whereas early severe intrauterine growth restriction resulting in intrauterine demise was noted in patient 15. Of note, this pregnancy was conceived with ovarian stimulation and intrauterine insemination. Patient 10 was admitted for preterm labor at 30 weeks of gestation. The only first-trimester loss occurred in a patient with difficult to manage hypertension due to noncompliance. Otherwise, the mean systolic and diastolic BP was maintained within normal physiologic ranges during the full period of gestation, with only four patients requiring antihypertensive therapy.
Discussion
Our comparative analysis demonstrates that intensive hemodialysis is feasible and optimizes pregnancy outcomes in patients with ESRD. The proportion of live infants was higher in the Canadian cohort compared with controls derived from the ARPD cohort. Furthermore, median gestational age was higher, and maternal and neonatal complications were few and manageable. Because a dose-response relationship between dialysis dose and pregnancy outcomes including survival, gestational age, and birth weight was noted, we speculate that an intensified dialysis dose may play an important role by providing a healthy maternal environment for normal placental development, a prerequisite for normal fetal growth and decreased pregnancy complications. In women with established ESRD, it would appear that more intensive hemodialysis is necessary to optimize outcome, because women receiving the highest amounts of hemodialysis had significantly better live birth rates, longer gestational ages, and thus bigger babies. However, our data again reiterated the importance of residual renal function in women who started dialysis after pregnancy, with all women in both cohorts having live births. In fact, dialysis intensity should likely be adjusted to account for the degree of residual renal function, because the intensive routine dialysis regimen in the Toronto patients may have been unnecessarily burdensome. Taken together, our growing experience of intensively dialyzed pregnant patients may allow us to titrate the optimal dialysis dose in women with ESRD both with and without residual renal function.
Pregnancy in women with ESRD is typically complicated by increased maternal and fetal morbidity and mortality. In the 1970s, only 23% of pregnancies in women on dialysis resulted in live infants.7 One might presume that the high rates of termination in this early study influenced the poor live birth rate, but subsequent data from the United States and Saudi Arabian Registry, wherein termination was unlikely, did not reflect a much better outcome, with a live birth rate of only 37%.11,12 Even in these early data the importance of the relationship between time on dialysis and outcome began to emerge as those cases that progressed beyond 28 weeks had their dialysis time increased from an average of 9.4±2.3 to 12.0±2.6 hours.8
More recently, live birth rates have improved because it has become standard practice to increase the dose of delivered dialysis after conception. In the largest series to date, 52 pregnancies with an 87% overall successful birth rate was described wherein the standard practice was to increase dialysis time to 12–18 hours per week.13 Despite remarkably better live birth rates, the mean gestational age was noted to be only 32.7±3.1 weeks with a mean gestational weight of 1554±663 g. Furthermore, significant rates of complications including preeclampsia (19%), polyhydramnios (40%), transfusions (25%), and hypertension (70%) were reported. In our study, the amount of delivered dialysis was significantly higher in the Toronto cohort, and although the live birth rate was similar (86%), gestation was prolonged considerably compared with both the reported literature as well as our ARPD comparator cohort. Complications were few, transfusions were unnecessary, and hypertension was either absent or easily managed with only three women manifesting evidence of placental dysfunction. Only one patient had transient polyhydramnios, which was managed by lowering the dry weight. Four patients developed a short cervix detected on the level II ultrasound with untoward outcomes, and the exact reason for the high incidence of short cervix in our cohort remains unknown.
There are a number of potential mechanisms wherein intensive dialysis regimens might improve pregnancy outcome. Studies in nocturnal hemodialysis patients have revealed beneficial effects, including clinical (BP, left ventricular hypertrophy), biochemical (urea and phosphate clearance, anemia), and biologic parameters (endothelial function, inflammation),14 which may all contribute to improved pregnancy outcomes. However, the success of intensified regimens appears to be directly related to enhanced clearance of urea and likely other solutes. In a series of 28 pregnant women receiving hemodialysis with 18 surviving infants, a significant negative relationship was noted between BUN and birth weight (r=−0.53, P=0.02) as well as gestational age (r=−0.50, P=0.02).15 A birth weight of at least 1500 g was achieved at a BUN<49 mg/dl (urea 17.9 mmol/L) and a gestational age of at least 32 weeks was achieved at a BUN<48 mg/dl (urea 17.1 mmol/L). Furthermore, residual renal function has been demonstrated to improve pregnancy outcomes, with significant discrepancies in live birth rates that favored women who conceived before the initiation of dialysis compared with established dialysis patients.8,16 Our study confirmed the importance of enhanced clearance with improved live birth rates among women with residual renal function and intensified dialysis regimens. Taken together, dialysis dose should be titrated to maintain a normal metabolic state, with women with more residual kidney function requiring less intensive dialysis.
Our study has a number of important limitations. Data for the Canadian cohort were collected prospectively, whereas the survey data from the American cohort were retrospectively reported. The difficulty in finding a control population necessitated including patients from 1990 onward in the American cohort, but this cutoff was chosen because it reflects widespread availability of erythropoietin-stimulating agents and biocompatible membranes. Furthermore, recently published data from the Australia and New Zealand Dialysis and Transplant Registry, which included 49 pregnancies spanning 1966–2008, failed to demonstrate an effect of era on the live birth rate.17 The Canadian and American cohorts were mismatched with respect to age, but the older age of the Canadian patients should have translated into worse pregnancy outcomes. Although the Canadian patients also had extensive comorbidities, diabetes, which in itself can result in very poor pregnancy outcomes, was notably absent. The maternal mortality ratio and the infant mortality rate are established markers of the overall quality of obstetric care, and both are consistently higher in the United States than in Canada by a small fraction.18 All patients in the Greater Toronto Area were managed in one of two obstetrics centers wherein the two designated obstetricians are experienced with respect to the management of this high-risk patient population. We lacked data on potentially important characteristics that could have factored toward worse pregnancy outcomes including race and access to health care, which was known and standardized only in the PreKid high-risk obstetrics clinic. Finally, there were missing data in the ARPD, but every effort was made to ensure that our imputation strategies provided the most conservative effect size estimates.
In conclusion, intensive hemodialysis is associated with a higher proportion of live births compared with standard care in pregnant ESRD patients. In addition, maternal and neonatal complications tended to be few and manageable. We propose that intensive hemodialysis should be considered as a viable and feasible option for dialysis patients of childbearing age who want to become pregnant or who are pregnant. However, given that maternal and neonatal complications are still more frequent in pregnant ESRD patients than in the pregnant non-ESRD population, these patients require meticulous follow-up by a dedicated high-risk obstetrician in close collaboration with a nephrology team. Ongoing international collaborative research remains necessary to continue to improve pregnancy outcomes in women with ESRD.
Concise Methods
The research ethics boards at both the University of Toronto and Loyola University Medical Center approved the collection of study data as per the Declaration of Helsinki for Medical Research Involving Human Subjects. Both registries meet the criteria for privacy compliance per the Health Services Research and the Health Insurance Portability and Accountability Act Privacy Rules.
Toronto PreKid Clinic and Registry Patients
The Toronto PreKid Clinic was established in 2000 capturing all young women with ESRD who conceived on hemodialysis or required hemodialysis during pregnancy for CKD progressing to ESRD. Therapeutic terminations were excluded, as were women with peripartum AKI who recovered enough function to come off hemodialysis in the postpartum period. Data for the Toronto PreKid Clinic Registry were collected prospectively, including information on the dialysis prescription, laboratory data, as well as pregnancy follow-up and outcome data. In all cases, antenatal care was performed in the PreKid Clinic by an obstetrician specializing in high-risk pregnancy (J.K. or D.H.) and supported by a designated nephrologist (M.H.). Both the dialysis prescription and the obstetric follow-up were standardized in all patients, as previously described in detail.19
In stable hemodialysis patients, the dialysis intensity was increased to 6–8 hours for 6 to 7 days per week either before or immediately after conception. Patients who were not on dialysis before pregnancy initiated this same intensive dialysis regimen. As previously described,9 the intensified dialysis regimen resulted in normalization of predialysis and postdialysis urea levels. No dietary restrictions were required and phosphate supplementation was required for all patients. Ultrafiltration was determined by clinical assessment of BP and volume status. The degree of anemia and iron deficiency increased during the pregnancy with increasing needs for erythropoietin-stimulating agents and intravenous iron administration, typically administered weekly to maintain a target hemoglobin of 10–11 g/dl.
With respect to the obstetric care, a first-trimester screen that included nuchal translucency was offered between 11 and 14 weeks of gestation to detect fetal aneuploidy. The maternal serum screen or the quadruple test was done between 15 and 20 weeks of gestation. Between 18 and 20 weeks of gestation, a level II ultrasound was performed to exclude fetal anomalies and to measure cervical length. Between 22 and 24 weeks of gestation, a placental ultrasound was performed to assess placental dimensions along with uterine and umbilical artery Doppler waveforms to detect impending placental insufficiency.20 From 26 weeks of gestation onward, ultrasounds were scheduled every 2 weeks to follow measurements of fetal well-being, biometry, amniotic fluid index, cervical length, and umbilical artery pulsatility indices, which occurred weekly as the patient neared term or when pregnancy complications were encountered. Routinely, induction of labor was booked before 39 weeks of gestation mainly for logistical reasons to coordinate in-center hemodialysis and nephrology care.
ARPD Patients
The ARPD collects retrospective data through physician surveys. Data include maternal characteristics (age, the cause of ESRD where available, and dialysis vintage), the hours of hemodialysis provided during pregnancy, as well as pregnancy outcomes (gestational age and birth weight). Eligible controls were selected from 394 pregnancies reported to the ARPD. All cases before 1990 were excluded in an attempt to provide more contemporaneous controls. Pregnancies in which the woman was treated with peritoneal dialysis and pregnancies that were electively terminated were also excluded. From the remaining pregnancies, we selected those in which the number of hours of dialysis per week and the pregnancy outcome were known. We used only pregnancies in which we had information either on gestational age or birth weight. In 10 patients, the precise gestational age was missing for patients losing their infants in the first or second trimester. To provide the most conservative estimate possible, a gestational age of 12 weeks was assigned for a first-trimester loss and 24 weeks for a second-trimester loss. Birth weight was not reported in four patients with either a stillbirth or a neonatal death, along with an additional four patients with surviving infants. Hence, the mean birth weight for the particular gestational age was imputed to again provide the most conservative estimate.
Compared Outcomes
Outcomes were ascertained by review of the relevant medical records and defined as follows: surviving infants, infants who were alive at discharge from the hospital; neonatal death, death before discharge from hospital or within the first month of life; intrauterine fetal demise, intrauterine fetal death after the first trimester before the onset of labor; and spontaneous abortion, spontaneous loss of pregnancy before viability or 24 weeks of gestation. Spontaneous abortions were further classified into first- and second-trimester losses. Low birth weight was defined as neonatal weight<2500 g, whereas very low birth weight was defined as neonatal weight<1500 g. Pregnancy outcomes were analyzed separately in established hemodialysis patients and those who required dialysis initiation during pregnancy. Only women who conceived after dialysis initiation were included in the analysis of dialysis intensity on pregnancy outcomes due to the potential confounding effects of residual kidney function. Dialysis intensity was assessed continuously as well as categorically after dividing the hours of dialysis into clinically relevant tertiles (0–20, 21–36, and 37–56 hours) to capture the typically prescribed dialysis regimens in the two countries.
Statistical Analyses
Descriptive statistics were calculated for all variables of interest. Continuous variables were assessed for normality both visually (normal probability plot) and by inferential statistics (Shapiro–Wilk W and Kolmogorov–Smirnov tests). Normally distributed variables were expressed as the mean±SD and nonparametric variables were expressed as the median and interquartile range. Categorical measures were summarized using counts and percentages. The t test or the Mann–Whitney U test was used to compare the two groups. Proportions were compared using the chi-squared test statistic. The time to event (neonatal death, intrauterine fetal demise, or spontaneous abortion) was analyzed using the Kaplan–Meier product limit method. Survival functions were compared using the log-rank test. The Kruskal–Wallis test with a pairwise Mann–Whitney U test and a Bonferroni correction for between-group differences was used to compare gestational age by dialysis dose, whereas an ANOVA was used to compare birth weight by dialysis dose along with a linear test for trend. Correlation was used to assess the relationship between dialysis intensity and gestational age as well as birth weight. A P value <0.05 was deemed statistically significant. All analyses were carried out or supervised by M.H. using Stata Version 13 software (Stata Press).
Disclosures
None.
Acknowledgments
The authors thank the staff of the combined high-risk obstetrics-nephrology clinic at Mount Sinai Hospital and Sunnybrook Heath Sciences Centre as well as the staff of the respective Greater Toronto Area hemodialysis units. Dr. Christopher T. Chan holds the R. Fraser Elliott Chair in Home Dialysis.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
References
- 1.Confortini P, Galanti G, Ancona G, Giongo A, Bruschi E, Lorenzini E: Full-term pregnancy and successful delivery in a patient on chronic haemodialysis. Proc Eur Dial Transplant Assoc 8: 74–80, 1971 [Google Scholar]
- 2.Holley JL, Schmidt RJ, Bender FH, Dumler F, Schiff M: Gynecologic and reproductive issues in women on dialysis. Am J Kidney Dis 29: 685–690, 1997 [DOI] [PubMed] [Google Scholar]
- 3.Lim VS, Henriquez C, Sievertsen G, Frohman LA: Ovarian function in chronic renal failure: Evidence suggesting hypothalamic anovulation. Ann Intern Med 93: 21–27, 1980 [DOI] [PubMed] [Google Scholar]
- 4.Mantouvalos H, Metallinos C, Makrygiannakis A, Gouskos A: Sex hormones in women on hemodialysis. Int J Gynaecol Obstet 22: 367–370, 1984 [DOI] [PubMed] [Google Scholar]
- 5.Gómez F, de la Cueva R, Wauters JP, Lemarchand-Béraud T: Endocrine abnormalities in patients undergoing long-term hemodialysis. The role of prolactin. Am J Med 68: 522–530, 1980 [DOI] [PubMed] [Google Scholar]
- 6.Hou SH, Grossman S, Molitch ME: Hyperprolactinemia in patients with renal insufficiency and chronic renal failure requiring hemodialysis or chronic ambulatory peritoneal dialysis. Am J Kidney Dis 6: 245–249, 1985 [DOI] [PubMed] [Google Scholar]
- 7.Report from the Registration Committee of the European Dialysis and Transplant Association : Successful pregnancies in women treated by dialysis and kidney transplantation. Br J Obstet Gynaecol 87: 839–845, 1980 [DOI] [PubMed] [Google Scholar]
- 8.Bagon JA, Vernaeve H, De Muylder X, Lafontaine JJ, Martens J, Van Roost G: Pregnancy and dialysis. Am J Kidney Dis 31: 756–765, 1998 [DOI] [PubMed] [Google Scholar]
- 9.Barua M, Hladunewich M, Keunen J, Pierratos A, McFarlane P, Sood M, Chan CT: Successful pregnancies on nocturnal home hemodialysis. Clin J Am Soc Nephrol 3: 392–396, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gangji AS, Windrim R, Gandhi S, Silverman JA, Chan CT: Successful pregnancy with nocturnal hemodialysis. Am J Kidney Dis 44: 912–916, 2004 [PubMed] [Google Scholar]
- 11.Hou SH: Frequency and outcome of pregnancy in women on dialysis. Am J Kidney Dis 23: 60–63, 1994 [DOI] [PubMed] [Google Scholar]
- 12.Souqiyyeh MZ, Huraib SO, Saleh AG, Aswad S: Pregnancy in chronic hemodialysis patients in the Kingdom of Saudi Arabia. Am J Kidney Dis 19: 235–238, 1992 [DOI] [PubMed] [Google Scholar]
- 13.Luders C, Castro MC, Titan SM, De Castro I, Elias RM, Abensur H, Romão JE, Jr: Obstetric outcome in pregnant women on long-term dialysis: A case series. Am J Kidney Dis 56: 77–85, 2010 [DOI] [PubMed] [Google Scholar]
- 14.Perl J, Chan CT: Home hemodialysis, daily hemodialysis, and nocturnal hemodialysis: Core Curriculum 2009. Am J Kidney Dis 54: 1171–1184, 2009 [DOI] [PubMed] [Google Scholar]
- 15.Asamiya Y, Otsubo S, Matsuda Y, Kimata N, Kikuchi K, Miwa N, Uchida K, Mineshima M, Mitani M, Ohta H, Nitta K, Akiba T: The importance of low blood urea nitrogen levels in pregnant patients undergoing hemodialysis to optimize birth weight and gestational age. Kidney Int 75: 1217–1222, 2009 [DOI] [PubMed] [Google Scholar]
- 16.Okundaye I, Abrinko P, Hou S: Registry of pregnancy in dialysis patients. Am J Kidney Dis 31: 766–773, 1998 [DOI] [PubMed] [Google Scholar]
- 17.Shahir AK, Briggs N, Katsoulis J, Levidiotis V: An observational outcomes study from 1966-2008, examining pregnancy and neonatal outcomes from dialyzed women using data from the ANZDATA Registry. Nephrology (Carlton) 18: 276–284, 2013 [DOI] [PubMed] [Google Scholar]
- 18.World Health Organization: Trends in Maternal Mortality: 1990 to 2010, Geneva, Switzerland, World Health Organization, 2012 [Google Scholar]
- 19.Hladunewich M, Hercz AE, Keunen J, Chan C, Pierratos A: Pregnancy in end stage renal disease. Semin Dial 24: 634–639, 2011 [DOI] [PubMed] [Google Scholar]
- 20.Toal M, Chan C, Fallah S, Alkazaleh F, Chaddha V, Windrim RC, Kingdom JC: Usefulness of a placental profile in high-risk pregnancies. Am J Obstet Gynecol 196: 363.e361–363.e367, 2007 [DOI] [PubMed] [Google Scholar]