Abstract
Despite optimal treatment, including renin-angiotensin system (RAS) inhibitors, patients with type 2 diabetic nephropathy have high cardiorenal morbidity and mortality related to residual albuminuria. We evaluated whether or not atrasentan, a selective endothelin A receptor antagonist, further reduces albuminuria when administered concomitantly with maximum tolerated labeled doses of RAS inhibitors. We enrolled 211 patients with type 2 diabetes, urine albumin/creatinine ratios of 300–3500 mg/g, and eGFRs of 30–75 ml/min per 1.73 m2 in two identically designed, parallel, multinational, double-blind studies. Participants were randomized to placebo (n=50) or to 0.75 mg/d (n=78) or 1.25 mg/d (n=83) atrasentan for 12 weeks. Compared with placebo, 0.75 mg and 1.25 mg atrasentan reduced urine albumin/creatinine ratios by an average of 35% and 38% (95% confidence intervals of 24 to 45 and 28 to 47, respectively) and reduced albuminuria≥30% in 51% and 55% of participants, respectively. eGFR and office BP measurements did not change, whereas 24-hour systolic and diastolic BP, LDL cholesterol, and triglyceride levels decreased significantly in both treatment groups. Use of atrasentan was associated with a significant increase in weight and a reduction in hemoglobin, but rates of peripheral edema, heart failure, or other side effects did not differ between groups. However, more patients treated with 1.25 mg/d atrasentan discontinued due to adverse events. After stopping atrasentan for 30 days, measured parameters returned to pretreatment levels. In conclusion, atrasentan reduced albuminuria and improved BP and lipid spectrum with manageable fluid overload–related adverse events in patients with type 2 diabetic nephropathy receiving RAS inhibitors.
Despite an armamentarium of different intervention strategies, patients with type 2 diabetic nephropathy still have a high risk for comorbid events, such as cardiovascular and renal morbidity and mortality. In particular, renin-angiotensin system (RAS) inhibitors, including angiotensin-converting enzyme (ACE) inhibitors or angiotensin receptor blockers (ARBs), have been effective in reducing cardiovascular and renal risk. However, despite optimal treatment according to guidelines used for metabolic control, blood pressure control, and lipid management; patients with diabetic nephropathy still have a very high renal and cardiovascular risk.1
RAS inhibitors have renal and cardiovascular protective properties beyond their BP-lowering capacity, which has been partly attributed to their albuminuria-lowering effect. Indeed, several post hoc analyses show that the albuminuria-lowering effect of RAS inhibitors is related to renal and cardiovascular protection.2–6 However, the high residual risk in diabetic nephropathy is related to the residual albuminuria in these patients.2
Novel treatment options are needed, especially drugs that lower residual risk factors without increasing adverse events (AEs). Selective endothelin A (ETA) receptor antagonists are a promising class of drugs that have been shown to lower albuminuria in patients with diabetic nephropathy.7 However, they also have some potentially limiting side effects, such as fluid retention, with an increased risk for heart failure in patients with type 2 diabetes with nephropathy.8 Atrasentan is a highly selective ETA receptor antagonist that has been shown to lower albuminuria with renoprotective properties.9
In this study, we tested the efficacy and safety of two low doses of atrasentan (0.75 and 1.25 mg/d) on albuminuria and other renal risk–related parameters in patients with diabetic nephropathy who were concomitantly treated with stable RAS inhibitor therapy, and particularly evaluated the balance between albuminuria-lowering effects and fluid retention side effects.
Results
Patient Disposition
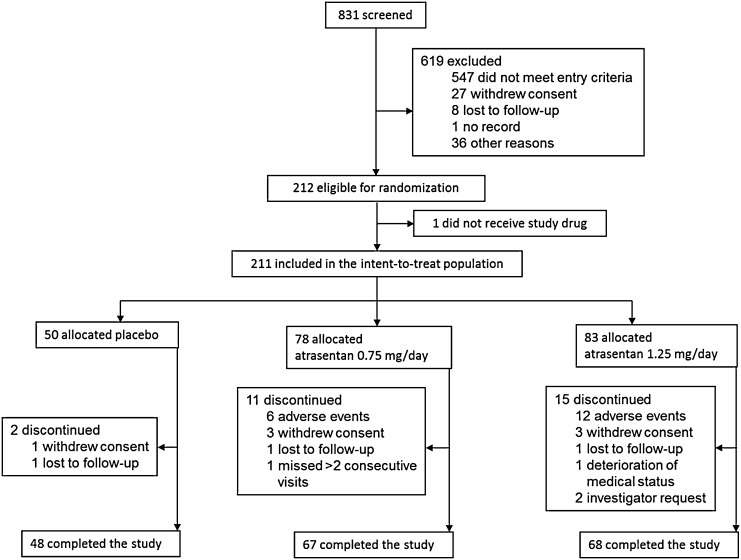
Figure 1 shows the disposition of patients. Of the 831 individuals screened, 212 were eligible for randomization and 211 received the study drug (placebo, n=50; 0.75 mg/d atrasentan, n=78; and 1.25 mg/d atrasentan, n=83). During the 12-week study, 28 participants (13.3%) discontinued (placebo, n=2; 0.75 mg/d, n=11; and 1.25 mg/d, n=15). As shown in Table 1, the most common reason for discontinuation was for AEs in 18 participants (8.5%). Six patients (8%) receiving 0.75 mg/d atrasentan discontinued due to AEs (peripheral edema, n=2; fatigue, n=1; Helicobacter pylori infection, n=1; acute appendicitis, n=1; and lung hemorrhage, n=1). In addition, 12 patients (15%) receiving 1.25 mg/d atrasentan discontinued due to AEs, mostly related to fluid overload (edema, n=6; anemia, n=2). Other reasons for discontinuation included fatigue (n=2), hyperkalemia (n=1), hypoglycemia (n=1), coronary artery stenosis (n=1), and thyroid cancer (n=1). Seven patients changed their dose or prescription of RAS inhibitors (placebo, n=2; 0.75 mg/d, n=1; and 1.25 mg/d, n=4) and one patient discontinued because of hyperkalemia at the lowest dose of RAS inhibitors. Overall, 13 patients changed the dose of diuretics (2 [4%], 4 [5.1%], and 7 [8.4%] participants in the placebo, 0.75 mg/d, and 1.25 mg/d groups, respectively). Edema was the most common reason for increasing the diuretic dose in the 1.25 mg/d atrasentan group (n=4), compared with the placebo group (n=1) and the 0.75 mg/d group (n=1).
Figure 1.
Consolidated Standards of Reporting Trials diagram. This is a summary of the disposition of study participants.
Table 1.
Summary of incidence of AEs
| Adverse Event | Placebo (n=50) | Atrasentan | |
|---|---|---|---|
| 0.75 mg/d (n=78) | 1.25 mg/d (n=83) | ||
| Any adverse event | 34 (68) | 55 (71) | 61 (74) |
| Any serious adverse event | 4 (8) | 7 (9) | 6 (7) |
| Peripheral edema | 21 (42) | 27 (35) | 35 (42) |
| Facial edema | — | — | 3 (3.6) |
| Congestive heart failure | 1 (2.0) | 1 (1.3) | — |
| Weight increase | 1 (2.0) | — | 3 (3.6) |
| Headache | 2 (4.0) | 1 (1.3) | 5 (6.0) |
| Nasal congestion and rhinitis | 2 (4.0) | — | 1 (1.2) |
| Diarrhea and constipation | 7 (14) | 10 (13) | 17 (21) |
| Deaths | — | — | — |
| Adverse event leading to discontinuation of study drug | — | 6 (8) | 12 (15) |
| Peripheral edema | — | 2 (2.6) | 4 (4.8) |
| Facial edema | — | — | 2 (2.4) |
| Anemia | — | — | 2 (2.4) |
| Fatigue | — | 1 (1.3) | 2 (2.4) |
| Hyperkalemia | — | — | 1 (1.2) |
| H. pylori infection | — | 1 (1.3) | — |
| Hypoglycemia | — | — | 1 (1.2) |
| Coronary artery stenosis | — | — | 1 (1.2) |
| Acute appendicitis | — | 1 (1.3) | — |
| Lung hemorrhage | — | 1 (1.3) | — |
| Thyroid cancer | — | — | 1 (1.2) |
Data are presented as n (%).
Patient Characteristics
The baseline demographics, clinical and biochemical characteristics, and concomitant medications were similar between the three groups (Table 2).
Table 2.
Demographics and baseline characteristics of the intent-to-treat population
| Characteristic | Placebo (n=50) | Atrasentan | |
|---|---|---|---|
| 0.75 mg/d (n=78) | 1.25 mg/d (n=83) | ||
| Age, yr | 64.3 (9.0) | 65.0 (9.8) | 64.5 (8.8) |
| Sex, n (%) | |||
| Male | 40 (80) | 63 (81) | 57 (69) |
| Female | 10 (20) | 15 (19) | 26 (31) |
| Race, n (%) | |||
| White | 23 (46) | 36 (46) | 38 (46) |
| Black | 2 (4) | 14 (18) | 13 (16) |
| Asian | 24 (48) | 25 (32) | 28 (34) |
| Other | 1 (2) | 3 (4) | 4 (5) |
| Ethnicity, n (%) | |||
| Hispanic or Latino | 30 (60) | 36 (46) | 42 (51) |
| Other | 20 (40) | 42 (54) | 41 (49) |
| Weight, kg | 84.3 (20.2) | 87.1 (22.1) | 88.3 (18.4) |
| Known duration of diabetes, yr | 14.5 (9.5) | 15.3 (9.3) | 16.9 (9.4) |
| BP, mmHg | |||
| SBP | 136 (14) | 138 (14) | 136 (15) |
| DBP | 72 (10) | 75 (10) | 74 (9) |
| Serum albumin, g/L | 40.1 (4.2) | 40.3 (3.7) | 40.5 (3.2) |
| Serum creatinine, mg/dl | 1.50 (0.38) | 1.60 (0.44) | 1.40 (0.35) |
| eGFR, ml/min per 1.73 m2 | 49.3 (13.3) | 47.9 (14.6) | 50.6 (13.6) |
| Hemoglobin, g/L | 12.7 (1.8) | 12.9 (1.5) | 12.9 (1.8) |
| Hemoglobin A1c, % | 7.4 (1.3) | 7.5 (1.5) | 7.7 (1.4) |
| Cholesterol, mg/dl | |||
| Total | 182 (48) | 172 (42) | 172 (39) |
| LDL | 100 (40) | 91 (34) | 88 (30) |
| HDL | 47 (12) | 46 (14) | 45 (12) |
| Triglycerides, mg/dl | 165 (83) | 182 (129) | 193 (112) |
| Serum potassium, mmol/L | 4.62 (0.49) | 4.54 (0.53) | 4.50 (0.51) |
| UACR, median (Q1 to Q3), mg/g creatinine | 671 (410–1536) | 878 (515–1682) | 826 (481–1389) |
| Antihypertensives, n (%) | |||
| RAS inhibitors | 50 (100) | 78 (100) | 83 (100) |
| β-Blockers | 16 (32) | 31 (40) | 38 (46) |
| Calcium channel blockers | 27 (54) | 42 (54) | 46 (55) |
| Diuretics, n (%) | |||
| Loop diuretics | 19 (38) | 29 (37) | 27 (33) |
| Thiazides | 29 (58) | 42 (54) | 43 (52) |
| Glucose-lowering therapies, n (%) | |||
| Insulin glargine | 12 (24) | 25 (32) | 23 (28) |
| Metformin | 13 (26) | 19 (24) | 22 (27) |
| Sulphonylurea | 27 (54) | 33 (42) | 32 (39) |
| Statins, n (%) | 38 (76) | 58 (74) | 68 (82) |
| Coronary artery disease, n (%) | 8 (16) | 13 (16) | 9 (10) |
| Stroke, n (%) | 10 (20) | 8 (10) | 8 (9) |
Data are presented as the mean (SD) unless otherwise noted.
Primary Endpoint
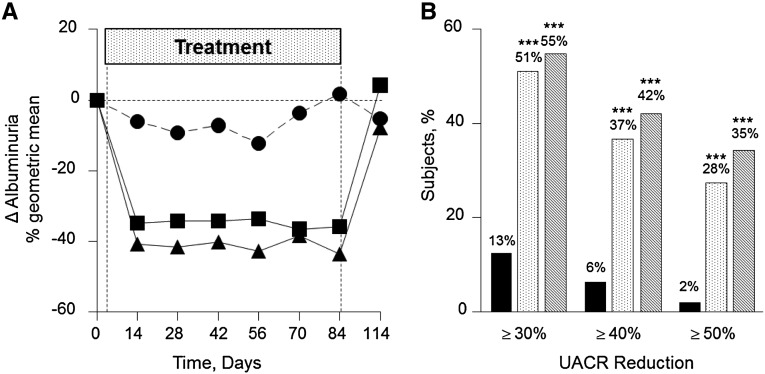
Repeated-measures analysis showed a significant decrease in albuminuria for the 0.75 mg/d atrasentan (−35.5% average reduction over 12 weeks) and 1.25 mg/d atrasentan (−38.6% average reduction over 12 weeks) groups compared with the placebo group. Figure 2A shows the geometric mean change in the urinary albumin/creatinine ratio (UACR) from baseline to each postbaseline visit. Patients receiving 0.75 mg/d atrasentan had an absolute median UACR of 878±908 mg/g at baseline, which was reduced to 573±787 mg/g (−34.7%) after 2 weeks of treatment, and remained stable ending at 521±816 mg/g (−35.8%) at 12 weeks (P<0.001 for both). In patients treated with 1.25 mg/d atrasentan, the UACR decreased from 826±690 mg/g at baseline to 515±472 mg/g (−40.7%) at 2 weeks and 470±547 mg/g (−43.6%) at week 12 (P<0.001 for both). However, for participants receiving placebo, baseline UACR was 671±941 mg/g, and it was not significantly changed over time (Table 3).
Figure 2.
Atrasentan treatment significantly decreases albuminuria. (A) UACR change in the percent geometric mean from baseline to recovery for the placebo (●), 0.75 mg/d atrasentan (▪), and 1.25 mg/d atrasentan (▲) groups. (B) Degree of UACR reduction from baseline to final in the placebo (squares), 0.75 mg/d atrasentan (open bars), and 1.25 mg/d atrasentan (filled bars) groups. ***P<0.001.
Table 3.
Changes in main physical and biochemical parameters induced by placebo or atrasentan at different time points
| Variable | Time (wk) | Placebo | Atrasentan | ||||
|---|---|---|---|---|---|---|---|
| 0.75 mg/d | 1.25 mg/d | ||||||
| n | Mean (SD) or Median | n | Mean (SD) or Median | n | Mean (SD) or Median | ||
| UACR, mg/g | Baseline | 50 | 671 | 78 | 878 | 83 | 826 |
| 2 | 48 | 696 | 75 | 573 | 82 | 515 | |
| 6 | 48 | 686 | 74 | 636 | 75 | 461 | |
| 12 | 48 | 797 | 70 | 521 | 69 | 470 | |
| Recovery | 45 | 737 | 68 | 1051 | 71 | 727 | |
| SBP, mmHg | Baseline | 50 | 136 (14) | 78 | 138 (14) | 83 | 136 (15) |
| 2 | 48 | 134 (16) | 77 | 135 (16) | 82 | 132 (16) | |
| 6 | 48 | 137 (12) | 74 | 137 (17) | 75 | 133 (17) | |
| 12 | 48 | 134 (16) | 70 | 136 (15) | 69 | 133 (18) | |
| Recovery | 44 | 134 (13) | 61 | 141 (19) | 70 | 135 (16) | |
| DBP, mmHg | Baseline | 50 | 72 (10) | 78 | 75 (10) | 83 | 74 (9) |
| 2 | 48 | 71 (9) | 77 | 71 (11) | 82 | 69 (10) | |
| 6 | 48 | 71 (10) | 74 | 70 (11) | 75 | 69 (9) | |
| 12 | 48 | 71 (10) | 70 | 72 (9) | 69 | 72 (12) | |
| Recovery | 44 | 71 (10) | 61 | 75 (11) | 70 | 73 (11) | |
| 24-h SBP, mmHg | Baseline | 38 | 140 (14) | 63 | 142 (15) | 66 | 140 (14) |
| 6 | 20 | 137 (14) | 33 | 137 (16) | 29 | 134 (16) | |
| 10 | 32 | 139 (16) | 39 | 134 (15) | 37 | 134 (15) | |
| 24-h DBP, mmHg | Baseline | 38 | 74 (9) | 63 | 76 (9) | 66 | 73 (8) |
| 6 | 20 | 72 (8) | 33 | 70 (8) | 29 | 69 (8) | |
| 10 | 32 | 73 (8) | 39 | 71 (9) | 37 | 69 (9) | |
| LDL cholesterol, mg/dl | Baseline | 49 | 101 (39) | 75 | 88 (34) | 77 | 87 (30) |
| 12 | 46 | 102 (38) | 63 | 77 (30) | 64 | 74 (26) | |
| Recovery | 44 | 98 (39) | 68 | 89 (36) | 67 | 84 (31) | |
| eGFR, ml/min per 1.73 m2 | Baseline | 50 | 49 (13) | 78 | 48 (15) | 83 | 51 (14) |
| 2 | 48 | 48 (14) | 74 | 47 (15) | 83 | 49 (14) | |
| 6 | 48 | 48 (15) | 74 | 46 (15) | 74 | 49 (15) | |
| 12 | 48 | 48 (14) | 68 | 47 (16) | 69 | 48 (15) | |
| Recovery | 47 | 45 (13) | 74 | 45 (17) | 77 | 47 (14) | |
| HbA1C, % | Baseline | 50 | 7.4 (1.3) | 78 | 7.5 (1.5) | 83 | 7.7 (1.4) |
| 12 | 48 | 7.3 (1.2) | 69 | 7.1 (1.4) | 75 | 7.2 (1.3) | |
| Hemoglobin, mg/dl | Baseline | 50 | 12.7 (1.8) | 78 | 12.9 (1.5) | 83 | 12.9 (1.8) |
| 2 | 47 | 12.5 (1.9) | 74 | 12.0 (1.5) | 80 | 11.8 (1.8) | |
| 6 | 48 | 12.6 (1.9) | 72 | 11.9 (1.7) | 73 | 11.6 (1.7) | |
| 12 | 47 | 12.6 (2.1) | 69 | 11.8 (1.6) | 68 | 11.8 (1.8) | |
| Recovery | 26 | 12.3 (1.9) | 39 | 12.4 (1.5) | 47 | 12.5 (1.9) | |
| Hematocrit, % | Baseline | 50 | 38.1 (5.4) | 78 | 38.7 (4.4) | 83 | 38.6 (5.3) |
| 2 | 47 | 37.5 (5.8) | 74 | 36.1 (4.6) | 80 | 35.4 (5.2) | |
| 6 | 48 | 37.8 (5.5) | 72 | 35.6 (5.0) | 73 | 35.1 (5.2) | |
| 12 | 47 | 37.9 (6.3) | 69 | 35.6 (4.8) | 68 | 35.6 (5.5) | |
| Recovery | 26 | 37.0 (6.2) | 39 | 37.2 (4.5) | 47 | 37.9 (5.7) | |
| Weight, kg | Baseline | 50 | 84.3 (20.2) | 78 | 87.1 (22.1) | 83 | 88.3 (18.4) |
| 2 | 48 | 83.2 (19.6) | 77 | 88.0 (22.2) | 82 | 89.4 (18.7) | |
| 6 | 48 | 83.3 (19.6) | 74 | 87.3 (21.8) | 75 | 89.6 (19.0) | |
| 12 | 48 | 82.8 (18.6) | 70 | 87.3 (22.3) | 69 | 89.0 (19.2) | |
| Recovery | 44 | 83 (20) | 61 | 85 (23) | 70 | 88 (19) | |
At the end of the 30-day follow-up period without study medication, the median UACRs were 737±912 mg/g, 1051±1206 mg/g, and 727±821 mg/g for the placebo, 0.75 mg/d, and 1.25 mg/d groups, respectively. The geometric mean changes from baseline to recovery were −5.3%, +4.3%, and −7.8% for the placebo, 0.75 mg/d, and 1.25 mg/d groups, respectively, thus showing that UACR values returned to baseline values.
Secondary Endpoints
Albuminuria
The geometric mean change from baseline to final UACR was significantly changed in the 0.75 mg/d (−36.2%) and 1.25 mg/d (−43.9%) groups compared with the placebo group (+2.0%; P<0.001 for both). In the 0.75 and 1.25 mg/d groups, 51.3% and 55.1%, respectively, of participants achieved a ≥30% reduction in UACR, respectively (P<0.001 versus placebo) (Figure 2B). The differences were also statistically significantly different compared with placebo for the proportion of patients that achieved ≥40% and ≥50% reductions in UACR (36.8% and 27.6% for the 0.75 mg/d group versus 42.3% and 34.6% for 1.25 mg/d group, respectively; P<0.001 versus placebo).
The response to atrasentan was tested for the different baseline parameters. In particular, change from baseline to final on-treatment UACR was not significantly influenced by whether baseline UACR was ≤1000 mg/g (−39% and −46%) or >1000 mg/g (−32% and −41%) for the 0.75 and 1.25 mg/d groups, respectively. Similarly, baseline differences in age (<65 versus ≥65 years), sex, race (white versus nonwhite), or country (United States versus non–United States) did not significantly influence the effect of atrasentan on UACR reduction.
Renal Function
Serum creatinine showed no significant change in any of the three groups. Baseline eGFR was in the low to normal ranges (49.3±13.3, 47.9±14.6, and 50.1±13.6 ml/min per 1.73 m2 for the placebo, 0.75 mg/d atrasentan, and 1.25 mg/d atrasentan arms, respectively). For the 0.75 mg/d atrasentan group, the placebo-corrected changes in eGFR (in milliliters per minute per 1.73 m2) were 0.44 at 2 weeks (P=0.35), −0.99 at 6 weeks (P=0.77), −1.08 at 12 weeks (P=0.79), and 1.16 at recovery (P=0.22). For the 1.25 mg/d atrasentan group, the placebo-corrected changes in eGFR (in milliliters per minute per 1.73 m2) were −0.79 at 2 weeks (P=0.75), −0.93 at 6 weeks (P=0.75), −2.05 at 12 weeks (P=0.94), and 0.63 for recovery (P=0.34) (Supplemental Figure 1).
Safety Results
BP
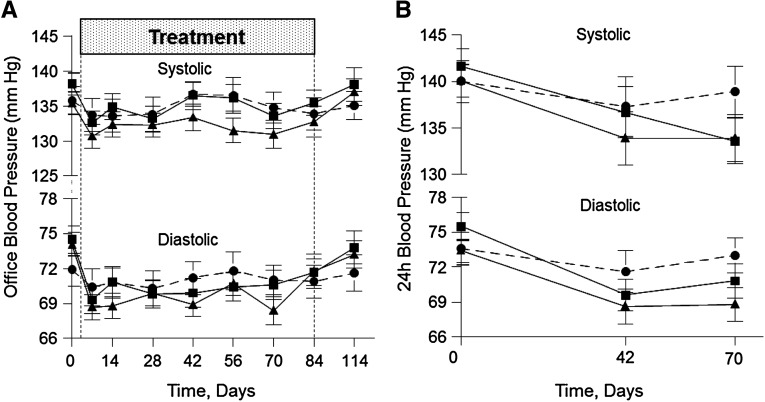
After an initial reduction, overall office systolic BP (SBP) was not significantly different from baseline in either atrasentan group (Figure 3A). However, overall diastolic BP (DBP) was significantly changed (−1.9 mmHg for 0.75 mg/d, P=0.07; and −2.7 mmHg for 1.25 mg/d, P=0.01) compared with placebo.
Figure 3.
Atrasentan treatment modulates office and 24-hour ambulatory blood pressure. Mean office SBP/DBP over 12 weeks and after recovery (A) and mean 24-hour ambulatory SBP/DBP over 6 and 10 weeks (B) for the placebo (circles), 0.75 mg/d atrasentan (squares), and 1.25 mg/d atrasentan (triangles) groups. Overall P values are as follows: 0.63 and 0.23 for 0.75 and 1.25 mg/d atrasentan, respectively, for SBP; 0.07 and 0.01 for 0.75 and 1.25 mg/d atrasentan, respectively, for DBP; 0.03 and 0.01 for 0.75 and 1.25 mg/d atrasentan, respectively, for 24-hour SBP; and <0.001 for 0.75 and 1.25 mg/d atrasentan, respectively, for 24-hour DBP.
In contrast, 24-hour ambulatory SBP fell significantly for the 0.75 mg/d (−4.5 mmHg, P=0.03) and 1.25 mg/d (−5.4 mmHg, P=0.01) atrasentan groups (Figure 3B). Similarly, overall 24-hour DBP was significantly changed in both groups compared with placebo (−4.2 and −4.6 mmHg for 0.75 and 1.25 mg/d atrasentan groups, respectively; P<0.001 for both).
Serum Lipids
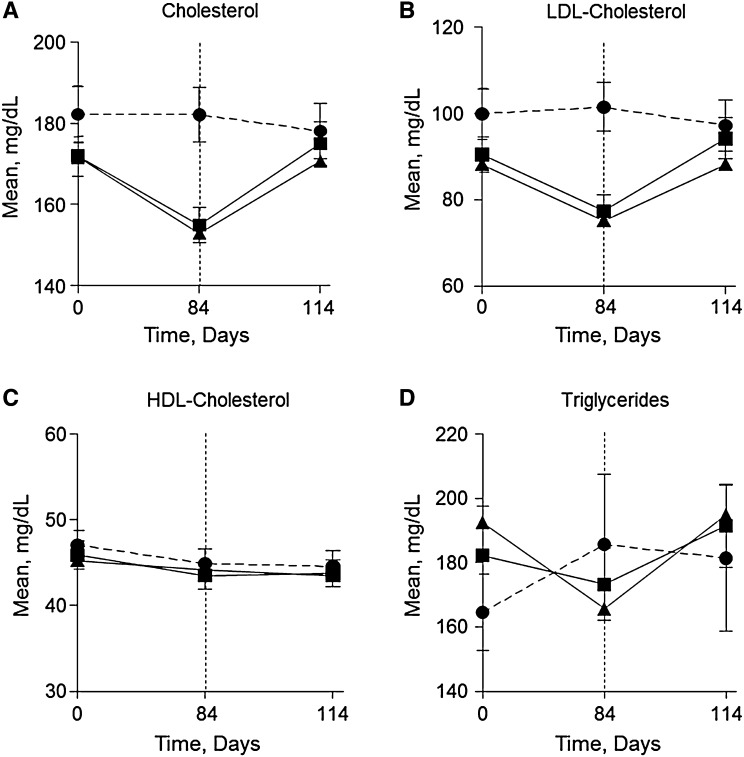
Intriguingly, atrasentan lowered mean total cholesterol, LDL cholesterol, and triglycerides over 12 weeks of treatment (Figure 4, A, B, and D). Total cholesterol was significantly lowered by 16.8 and 18.6 mg/dl for the 0.75 and 1.25 mg/d atrasentan groups, respectively, compared with placebo (P<0.001 for both versus placebo). LDL cholesterol decreased significantly by 14.6 mg/dl for both atrasentan doses compared with placebo (P<0.001 for both versus placebo). Similarly, 0.75 mg/d atrasentan reduced triglycerides by 30.2 mg/dl (P=0.11), whereas 1.25 mg/d atrasentan significantly lowered triglycerides by 47.9 mg/dl (P=0.01) compared with placebo; however, HDL cholesterol did not change (Figure 4C). Lipid profiles recovered 30 days post-treatment with atrasentan and similar lipid-lowering effects were observed in patients receiving statins versus those patients not receiving statins (data not shown).
Figure 4.
Atrasentan treatment exerts lipid-lowering effects. Mean changes in total cholesterol (A), LDL cholesterol (B), HDL cholesterol (C), and triglycerides (D) after placebo (●), 0.75 mg/d atrasentan (▪), and 1.25 mg/d atrasentan (▲) treatment for 12 weeks and after recovery.
We tested whether the multiple effects of atrasentan on albuminuria, BP, and lipids could be related to each other. Only a minor correlation was found between changes in SBP and changes in albuminuria for the 0.75 mg/d atrasentan group (SBP: r=0.24, P=0.04; DBP: r=0.05; P=0.66) and the 1.25 mg/d atrasentan group (SBP: r=0.30, P=0.01; DBP: r=0.24, P=0.03). Similarly, the correlation between albuminuria changes and lipid changes were minimal for the 0.75 mg/d atrasentan group (r=0.25, P=0.04) and absent for the 1.25 mg/d atrasentan group (r=0.01, P=0.95).
AEs
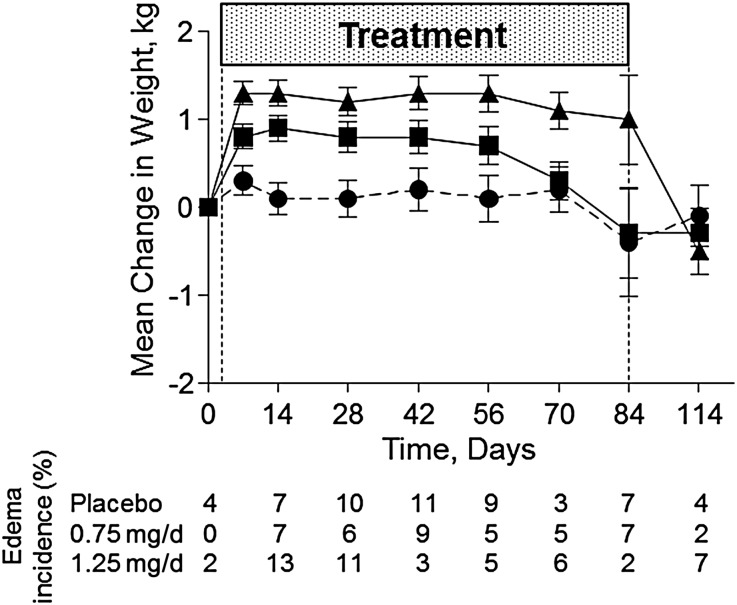
Overall mean change in body weight did not change significantly after low-dose (0.75 mg/d) atrasentan treatment (0.4 kg; P=0.14 versus placebo), whereas high dose (1.25 mg/d) atrasentan significantly increased weight by 0.9 kg (P<0.001) compared with placebo over 12 weeks (Figure 5). An initial and significant increase in weight was observed for both doses of atrasentan compared with baseline (Figure 5). In patients receiving 0.75 mg/d atrasentan, the differences were no longer significant at week 10, and weight returned to baseline values at week 12. However, weight did not return to baseline levels until 30 days post-treatment for participants receiving 1.25 mg/d atrasentan. Signs of hemodilution were observed in both atrasentan groups as indicated by a small but significant decrease in hemoglobin and hematocrit (Table 3).
Figure 5.
Atrasentan treatment affects body weight. Mean changes in weight and new incidence of edema after placebo (●), 0.75 mg/d atrasentan treatment (▪), and 1.25 mg/d atrasentan treatment (▲) and after recovery.
At baseline, the prevalence of edema was 47%, 31%, and 27% for the placebo, 0.75 mg/d, and 1.25 mg/d groups. After 12 weeks of treatment, the overall rate of new or worsening edema was comparable for the placebo (42%), 0.75 mg/d (35%), and 1.25 mg/d (42%) groups and was mostly mild in severity. Figure 5, bottom, shows the weekly incidence of new or worsening edema during the study. Moreover, the change in the dose of diuretics was not different between treatment groups during the study (4%, 5%, and 8% for the placebo, 0.75 mg/d, and 1.25 mg/d groups, respectively). The mean durations of edema were 24.1, 29.5, and 33.8 days for the placebo, 0.75 mg/d, and 1.25 mg/d groups, respectively.
Two patients developed congestive heart failure in the placebo and 0.75 mg/d groups (n=1 each) (Table 1). The participant in the placebo group was diagnosed at week 12 with a previous event of edema at week 6, and the patient receiving 0.75 mg/d atrasentan was diagnosed after an appendectomy at week 9 (Table 1). In addition, brain natriuretic peptide levels did not change significantly over 12 weeks in the 0.75 mg/d (+1.0±9.2 pg/ml) and 1.25 mg/d (+18.8±9.0 pg/ml) groups compared with the placebo group (+29.5±11.5 pg/ml). Table 1 shows a summary of AEs and AEs leading to discontinuation. Although it was not statistically significant, a higher number of patients (n=8) discontinued the study due to fluid retention–related events (edema, facial edema, and anemia) after treatment with the highest dose of atrasentan (1.25 mg/d) compared with the 0.75 mg/d dose (n=2) or placebo (n=0).
Discussion
The low dose (0.75 mg/d) of the ETA receptor antagonist, atrasentan, lowered albuminuria by 36% without major side effects in patients with type 2 diabetes with nephropathy who were treated with the maximal tolerated labeled dose of ACE inhibitors or ARBs. Moreover, atrasentan had a mild BP-lowering effect and significantly improved lipid profiles. Conversely, the higher dose of atrasentan (1.25 mg/d) had a similar albuminuria-lowering effect but elicited more fluid retention.
Endothelin receptor antagonists have been available since the early 1990s. Many preclinical studies with animal models have suggested that selective blockade of the ETA receptor is associated with renal protection when used in addition to independent proven therapies, such as RAS interventions.9 Although the mechanism of their renoprotective effect is still debated, three important pathways have clearly emerged. First, ETA receptor blockade has vascular effects, which causes glomerular vasodilation. This alters the glomerular permeability for albumin, thus lowering the tubular load of albumin.9 Both the hemodynamic and albuminuria-lowering effects of atrasentan have been associated with renal protection. Second, endothelin has been associated with (renal) inflammation, and, thus, ETA receptor blockade reduces renal inflammation by mitigating the inflammatory effects of albuminuria reabsorption and degradation in the proximal tubule and interstitium.10 Third, the endothelin system has been implicated in the deposition of collagen and fibrosis,11 and, thus, ETA receptor antagonists may be beneficial for reducing fibrosis in the kidney.7
This study was not designed to explore the mechanism by which atrasentan lowers albuminuria. In addition, the current data did not resolve the question of whether the BP-lowering effect of atrasentan is associated with a renal hemodynamic effect that controls albuminuria. This study also did not resolve the question of whether the albuminuria-lowering effect is linked to improvement of the lipid profile.
One may wonder why endothelin antagonists have not yet emerged in clinical practice, despite their clear, preclinical renoprotective profile. The clinical effects of endothelin antagonists are similar to the preclinical: systemic and renal vasodilation and albuminuria-lowering effects. However, observations from clinical studies indicate that these compounds also elicit volume-related side effects. This was particularly evident with the highest dose of avosentan, another ETA antagonist, during a large multidose phase IIa study in patients with diabetes.12 Despite this finding, a hard renal outcome study was started with high doses of avosentan, which was prematurely terminated because of a 3-fold increase in heart failure compared with placebo.8 This unfortunate outcome nearly ended the future of endothelin antagonists in the renal arena. Besides the side effect of fluid retention, some endothelin receptor antagonists (especially those with a sulfonamide group) have been linked to an increased risk of liver toxicity.13,14 Taken together, these studies show that endothelin antagonists have a narrow therapeutic window, and working within this window may be the key to success.
An important observation that was drawn from past clinical studies is that the dose response associated with fluid retention is different from the dose for albuminuria-lowering. One possibility is that fluid retention is driven by the endothelin B receptor blocking capacity of the drugs, although there are also reports of sodium retention induced by ETA receptor blockade. Even though most clinical endothelin receptor blockers are developed with high ETA receptor selectivity, high doses of such drugs could still block the endothelin B receptor. If so, lower doses of highly selective endothelin receptor antagonists could be the way forward for effective clinical use of this drug class, and atrasentan is an example of such a selective receptor blocker.15 Recent dose response studies by Kohan et al. showed that atrasentan has a remarkable capacity to lower albuminuria when used in addition to ACE inhibitor/ARB therapy without overt signs of fluid retention at lower doses.16 However, the sample size of the study was too small to draw any final conclusion, which prompted this study. Indeed, we confirm the very effective albuminuria-lowering capacity of the two atrasentan doses that were tested, and, importantly, atrasentan did not cause a higher incidence of heart failure. However, both atrasentan doses were associated with signs and/or symptoms of fluid overload. The highest dose (1.25 mg/d) promoted weight gain; thus, an optimal dose is critical to achieve the maximal albuminuria-lowering effect with minimal fluid retention. In addition, 12 patients receiving 1.25 mg/d atrasentan (15%) discontinued use due to AEs, more than half of which were related to fluid retention (8 patients had either edema or anemia). Compared with placebo (none), more patients receiving 0.75 mg/d atrasentan discontinued (n=6, 8%), but only two patients discontinued due to edema and the other causes were not likely linked to fluid overload–related events. Even though weight increased in participants receiving 0.75 mg/d atrasentan in the first weeks of the study, it returned to baseline values by week 10, without a significant increase in the use of diuretics (n=4, 5.1%) compared with placebo (n=2, 4%). Lower doses of atrasentan have been tested for efficacy and safety,16 and although the 0.25 mg/d dose was well tolerated, it showed a weak albuminuria-lowering effect. In another phase IIb study,17 0.5 mg/d atrasentan did not significantly reduce albuminuria compared with placebo (ClinicalTrials.gov identifier NCT01399580). Taken together, 0.75 mg/d atrasentan per day has demonstrated the best efficacy (albuminuria-lowering) and safety (AE profile) effect thus far. For this reason, the 0.75 mg/d dose has been selected for future studies.
Intriguingly, atrasentan also improved lipid profiles (total and LDL cholesterol). This study did not explore potential mechanisms mediating the lipid-lowering effects of atrasentan, which could be an indirect effect of the albuminuria-lowering effect of atrasentan. Similar effects have been observed with drugs that intervene in the RAS, which significantly reduce proteinuria.18
In this study, the efficacy of atrasentan was independent of the baseline characteristics of the patients; in particular, neither baseline levels of albuminuria, nor age, sex, or geographic location determined the albuminuria-lowering response. This study was not powered to detect differences between participating countries (e.g., Japan versus the United States and Canada); however, when both studies were analyzed separately, we did not find differences in key variables, such as albuminuria reduction or increase in weight (data not shown). In contrast, sitaxentan (another endothelin receptor blocker) exerted a greater albuminuria-lowering effect in patients with high baseline albuminuria.19 However, whether this is due to differences in the two drugs or due to the relatively small sample size of the sitaxentan study remains unclear.
It is noteworthy that this study was designed to address the effects of atrasentan in a patient population with the highest unmet medical need. The enrolled patients had CKD stages 2 and 3, all had macroalbuminuria and were receiving maximum tolerated labeled doses of RAS inhibitors, and most of them received diuretics. Despite these characteristics, albuminuria reduction was clinically highly relevant. Another strength of the design of this study is that blood/urine was collected 30 days after stopping therapy, and we observed that a majority of efficacy and safety variables returned to baseline, indicating the hemodynamic nature of the drug response.
What are the chances that 0.75 mg/d atrasentan per day will be renoprotective and delay hard endpoints? First, the albuminuria-lowering response, both average as well as proportion of responders, is exceptionally strong; thus far, there are no published reports of a drug added to RAS inhibitors that shows such a strong albuminuria-lowering effect. Second, the drug does not cause side effects, such as hyperkalemia or hypotension, that have eliminated dual RAS blockers. Third, it is unlikely that 0.75 mg/d atrasentan per day will cause major cardiovascular problems, as observed in the study to Assess the Effect of the Endothelin Receptor Antagonist Avosentan on Time to Doubling of Serum Creatinine, End Stage Renal Disease or Death in Patients With Type 2 Diabetes Mellitus and Diabetic Nephropathy (ASCEND) clinical trial.8 Finally, the BP- and lipid-lowering effects of the drug may help reduce vascular events. In that regard, a phase III clinical trial (Study of Diabetic Nephropathy with Atrasentan [SONAR]; ClinicalTrials.gov identifier NCT01858532) has just begun and will shed further light on the renal effects of atrasentan.20
In conclusion, 0.75 mg atrasentan per day markedly lowers albuminuria in patients with type 2 diabetes with nephropathy with manageable fluid overload–related side effects. This profile, combined with the lipid- and BP-lowering effects, appears to make this drug an interesting candidate for clinical development to reduce the unmet need in diabetic renal disease progression.
Concise Methods
Study Design
Data from two identically designed phase IIb, randomized, double-blind, parallel-designed, placebo-controlled, 12-week, multicenter studies were pooled for analysis. Both studies were identical in patient population, doses of atrasentan, and study procedures, but were conducted in different geographic regions (ClinicalTrials.gov identifiers NCT01356849 for study 1 and NCT01424319 for study 2). The efficacy and safety of two doses of atrasentan were evaluated based on the reduction of albuminuria in patients with type 2 diabetes and nephropathy who were simultaneously receiving the maximum tolerated labeled daily dose of a RAS inhibitor, 90% of whom were also receiving a diuretic. Patients were enrolled from 93 sites in the United States, Canada, Taiwan (study 1), and Japan (study 2). After screening, there was a run-in period of 4–12 weeks (4 weeks for patients already receiving the maximum tolerated labeled dose of RAS inhibitors and a diuretic; otherwise, participants were titrated up to the maximum tolerated labeled dose for 4–12 weeks). Eligible patients then entered a 12-week treatment period with a follow-up visit 4 weeks after discontinuation of the study drug. Individual demographics and baseline characteristics results for study 1 (NCT01356849) and study 2 (NCT01424319) are shown in Supplemental Tables 1 and 2, respectively. The study protocol was approved by an independent ethics committee and local and central review boards, and all participants signed written informed consent before any study-specific procedures commenced.
Eligibility Criteria and Recruitment
At screening, eligible participants had type 2 diabetes with nephropathy, were receiving hypoglycemic medication and a RAS inhibitor within the previous 12 months, and had a UACR≥300 and ≤3500 mg/g calculated from two first morning void (FMV) urine samples, eGFR of 30–75 ml/min per 1.73 m2 by Chronic Kidney Disease Epidemiology Collaboration formula, brain natriuretic peptide concentration<200 pg/ml, hemoglobin>9 g/dl, and serum albumin>3 g/dl. At the end of the run-in period, participants were eligible if their SBP was stable (110–160 mmHg), serum potassium was <5.5 mEq/L, and UACR was >200 mg/g. Women were nonpregnant and postmenopausal for at least 1 year or were surgically sterile. Participants were excluded from the trial based the following criteria: a history of moderate or severe edema, pulmonary edema, pulmonary hypertension, heart failure, receiving loop diuretic therapy (>120 mg/d furosemide, >3 mg/d bumetanide, >150 mg/d ethacrynic acid, or >60 mg/d torasemide), and recent coronary artery disease. Full inclusion and exclusion criteria are provided in the Supplemental Methods.
Randomization and Blinding
Participants were asked to return three consecutive FMV urine samples during the last run-in week and only those that met all of the inclusion criteria and none of the exclusion criteria were randomized. For study 1, participants were randomized using an interactive voice response system with two stratification factors: country where the participant was enrolled and the participant’s UACR level at week 1 (≤1000 mg/g [113 mg/mmol], or >1000 mg/g [113 mg/mmol]). For study 2, participants were randomized using a Central Case Registration and Randomization Center using UACR level at week 1 (≤1000 mg/g [113 mg/mmol], or >1000 mg/g [113 mg/mmol]) as a stratification factor. Participants were assigned to placebo, 0.75 mg/d atrasentan, or 1.25 mg/d atrasentan packaged identically in a 1:2:2 ratio in study 1 or in a 1:1:1 ratio in study 2. The study investigator, participant, and sponsor remained blinded to the participant’s treatment group assignment and UACR results for the duration of the study. Participants were allowed to modify the dose of RAS inhibitors or diuretics during the study only if medically necessary.
Procedures
Study visits were scheduled for week 1 and week 2 and occurred every other week thereafter. Physical examination, including measurement of BP and weight, and assessment of edema were performed at every visit. Full details on assessment of edema are provided in Supplemental Methods. Supplemental Tables 3 and 4 show individual summaries of adverse events for study 1 (NCT01356849) and study 2 (NCT01424319), respectively. Three FMVs were collected at each visit, with the exception of week 1. In addition, 24-hour ambulatory BP measurements were performed three times during the study: at baseline and at weeks 6 and 10. Blood samples were collected under fasting conditions at baseline, week 12 (or premature discontinuation), and at the 30-day follow-up visit.
Outcomes
The primary efficacy endpoint was the change from baseline to week 12 in log-transformed UACR. Secondary efficacy measures included the change from baseline to final on-treatment log-transformed UACR, the proportion of participants who achieved at least a 30%, 40%, and 50% reduction from baseline to final on-treatment UACR level, and the mean change in eGFR from baseline to each postbaseline measurement (up to week 12). The same endpoints were used to evaluate participants at 30 days after stopping treatment.
Statistical Analyses
The planned sample size was 30 for the placebo group and 60 for each atrasentan dose group for study 1 and 18 per group for study 2. This sample size would have provided at least 85% power to detect, at a one-sided level of significance of 0.05, a treatment group difference of 0.44 in the change from baseline to week 12 in log-transformed UACR between each dose group and placebo (assuming a SD of 0.67) for study 1, and at least 80% power to detect the overall treatment group difference in log-transformed UACR change from baseline over 12 weeks of treatment between each dose group and placebo with two-sided significance level of 0.05 for study 2. All efficacy and safety analyses were conducted on the data obtained from the intent-to-treat population, which consisted of data from all randomized participants who received at least one dose of study drug. Analyses were based on comparisons between placebo and atrasentan groups as assigned by randomization, regardless of whether participants followed through the protocol or were fully compliant with the protocol procedures. The primary analysis was a mixed-effects model repeated-measures analysis of change from baseline to each postbaseline measurement of log UACR. The model included the fixed effects of treatment, country (study 1 only), visit, and treatment-by-visit interaction, with covariates of baseline measurements and baseline-by-visit interaction. The contrasts between each atrasentan dose group and the placebo group at week 12 were compared using a one-sided significance level of 0.05; treatment differences at other time points were also analyzed. The within-group geometric mean change (%) is derived by 100×(exp(LS mean change)−1), and the same transformation is applied on the 95% confidence interval limits to obtain an approximate 95% confidence interval for the geometric mean change (%). The secondary efficacy analysis of change from baseline to final on-treatment log UACR was determined by an analysis of covariance model with treatment and country (study 1 only) as the main effects and baseline measurement as the covariate. Similar statistical models were used to assess treatment group differences in other efficacy and safety variables, such as eGFR, serum creatinine, SBP, DBP, weight, hemoglobin, and lipids (cholesterol, LDL cholesterol, HDL cholesterol, and triglycerides). Treatment group differences in the proportion of participants that achieved at least a 30%, 40%, or 50% reduction from baseline to final on-treatment UACR was analyzed by the Fisher’s exact test. Concomitant medications including RAS inhibitors, diuretics, β-blockers, calcium channel blockers, glucose-lowering drugs, and lipid-modifying drugs were summarized by treatment groups.
Disclosures
D.D.Z. is a consultant for and receives honoraria (to employer) from AbbVie, Astellas, AstraZeneca, Chemocentryx, Johnson & Johnson, Hemocue, Novartis, Reata Pharmaceuticals, Takeda, and Vitae. R.C.-R. has consultancy agreements with Roche, AbbVie, Amgen, Fresenius Medical Care, and Boehringer; in the last 2 years, he has been in the speakers’ bureau of Abbott, Amgen, Roche, and Sanofi. D.K. is a consultant for AbbVie, Bristol-Myers Squibb, Reata Pharmaceuticals, and Retrophin. H.J.L.H. is a consultant for and receives honoraria (to employer) from AbbVie, Astellas, Johnson & Johnson, Reata Pharmaceuticals, and Vitae. H.M. is a consultant for AbbVie and Astellas; receives speaker honoraria from Astellas, MSD, Takeda, and Tanabe-Mitsubishi; and receives grant support from Astellas, MSD, Daiichi Sankyo, Dainippon Sumitomo, Takeda, and Novo Nordisk. V.P. is a consultant for AbbVie, Astellas, AstraZeneca, Boehringer Ingelheim, Janssen, Roche, Servier, Merck, and Vitae; his employer receives funding/contracts for clinical trials from AbbVie, Baxter, Fresenius, Novartis, Pfizer, Resmed, Roche, Janssen, and Servier. Y.P. is a former employee of Abbott and owns AbbVie stocks. G.R. is a consultant for Alexion Pharmaceuticals, Reata Pharmaceuticals, Bayer Healthcare, Novartis Pharma, and AbbVie; all compensations are paid to his institution for research and educational activities. S.W.T. is a consultant for AbbVie and receives honoraria for academic talks from Servier and Valeant; he is an investigator on both contract and investigator-initiated research projects with AstraZeneca, Bristol-Myers Squibb, Mitsubishi, and Pfizer. R.T. serves on advisory boards for AbbVie, Boehringer Ingelheim, Amgen, Eli Lilly, Takeda, Reata Pharmaceuticals, and Merck & Co. G.V. is a consultant for GlaxoSmithKline, AbbVie, Mitsubishi Pharma, Daiichi Sankyo, Roche, and Eli Lilly. H.-H.P. is a consultant for AbbVie. B.C., D.A., J.J.B., H.T., and M.H. are all AbbVie employees and own AbbVie stock.
Acknowledgments
The authors greatly appreciate the participation of many individuals in these studies as well as the efforts of all of the investigators. D.D.Z., R.C.-R., D.K., H.J.L.H., H.M., V.P., G.R., S.W.T., R.T., G.V., and H.-H.P. are members of the steering committee. Rajiv Agarwal, David Webb, Rudolph Bilous, Mark Molitch, and Sheryl Kelsey are members of data safety and monitoring board.
This study was supported by AbbVie. The sponsor was involved in the design of the study, in the collection and analysis of the data, and in writing the report. All authors had access to study results, and the lead author vouches for the accuracy and completeness of the data reported. The lead author had the final decision to submit the publication. The study was overseen by a data review committee. Medical writing support was provided by Adebusola Ajibade, on behalf of AbbVie.
An abstract containing data from this study was submitted and accepted for presentation at the European Renal Association–European Dialysis and Transplant Association 2013 Congress, May 18-21, 2013, Istanbul, Turkey.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
See related editorial, “Endothelin Antagonists in Diabetic Nephropathy: Back to Basics,” on pages 869–871.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2013080830/-/DCSupplemental.
References
- 1.Heerspink HJ, de Zeeuw D: The kidney in type 2 diabetes therapy. Rev Diabet Stud 8: 392–402, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.de Zeeuw D, Remuzzi G, Parving HH, Keane WF, Zhang Z, Shahinfar S, Snapinn S, Cooper ME, Mitch WE, Brenner BM: Proteinuria, a target for renoprotection in patients with type 2 diabetic nephropathy: Lessons from RENAAL. Kidney Int 65: 2309–2320, 2004 [DOI] [PubMed] [Google Scholar]
- 3.Atkins RC, Briganti EM, Lewis JB, Hunsicker LG, Braden G, Champion de Crespigny PJ, DeFerrari G, Drury P, Locatelli F, Wiegmann TB, Lewis EJ: Proteinuria reduction and progression to renal failure in patients with type 2 diabetes mellitus and overt nephropathy. Am J Kidney Dis 45: 281–287, 2005 [DOI] [PubMed] [Google Scholar]
- 4.Cravedi P, Ruggenenti P, Remuzzi G: Proteinuria should be used as a surrogate in CKD. Nat Rev Nephrol 8: 301–306, 2012 [DOI] [PubMed] [Google Scholar]
- 5.Ibsen H, Olsen MH, Wachtell K, Borch-Johnsen K, Lindholm LH, Mogensen CE: Reduction in albuminuria translates to reduction in cardiovascular events in hypertensive patients with left ventricular hypertrophy and diabetes. J Nephrol 21: 566–569, 2008 [PubMed] [Google Scholar]
- 6.Schmieder RE, Mann JF, Schumacher H, Gao P, Mancia G, Weber MA, McQueen M, Koon T, Yusuf S, ONTARGET Investigators : Changes in albuminuria predict mortality and morbidity in patients with vascular disease. J Am Soc Nephrol 22: 1353–1364, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kohan DE: Endothelin, hypertension and chronic kidney disease: New insights. Curr Opin Nephrol Hypertens 19: 134–139, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mann JF, Green D, Jamerson K, Ruilope LM, Kuranoff SJ, Littke T, Viberti G, ASCEND Study Group : Avosentan for overt diabetic nephropathy. J Am Soc Nephrol 21: 527–535, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ko Kohan DE, Pollock DM: Endothelin antagonists for diabetic and non-diabetic chronic kidney disease. Br J Clin Pharmacol 76: 573–579, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gagliardini E, Corna D, Zoja C, Sangalli F, Carrara F, Rossi M, Conti S, Rottoli D, Longaretti L, Remuzzi A, Remuzzi G, Benigni A: Unlike each drug alone, lisinopril if combined with avosentan promotes regression of renal lesions in experimental diabetes. Am J Physiol Renal Physiol 297: F1448–F1456, 2009 [DOI] [PubMed] [Google Scholar]
- 11.Simonson MS, Ismail-Beigi F: Endothelin-1 increases collagen accumulation in renal mesangial cells by stimulating a chemokine and cytokine autocrine signaling loop. J Biol Chem 286: 11003–11008, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wenzel RR, Littke T, Kuranoff S, Jürgens C, Bruck H, Ritz E, Philipp T, Mitchell A, SPP301 (Avosentan) Endothelin Antagonist Evaluation in Diabetic Nephropathy Study Investigators : Avosentan reduces albumin excretion in diabetics with macroalbuminuria. J Am Soc Nephrol 20: 655–664, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Galiè N, Hoeper MM, Gibbs JS, Simonneau G: Liver toxicity of sitaxentan in pulmonary arterial hypertension. Eur Respir J 37: 475–476, 2011 [DOI] [PubMed] [Google Scholar]
- 14.Fattinger K, Funk C, Pantze M, Weber C, Reichen J, Stieger B, Meier PJ: The endothelin antagonist bosentan inhibits the canalicular bile salt export pump: A potential mechanism for hepatic adverse reactions. Clin Pharmacol Ther 69: 223–231, 2001 [DOI] [PubMed] [Google Scholar]
- 15.Opgenorth TJ, Adler AL, Calzadilla SV, Chiou WJ, Dayton BD, Dixon DB, Gehrke LJ, Hernandez L, Magnuson SR, Marsh KC, Novosad EI, Von Geldern TW, Wessale JL, Winn M, Wu-Wong JR: Pharmacological characterization of A-127722: An orally active and highly potent ETA-selective receptor antagonist. J Pharmacol Exp Ther 276: 473–481, 1996 [PubMed] [Google Scholar]
- 16.Kohan DE, Pritchett Y, Molitch M, Wen S, Garimella T, Audhya P, Andress DL: Addition of atrasentan to renin-angiotensin system blockade reduces albuminuria in diabetic nephropathy. J Am Soc Nephrol 22: 763–772, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.AbbVie: A prospective, double-blind, placebo-controlled, multicenter study to evaluate efficacy and safety of atrasentan, including thoracic bioimpedance, in type 2 diabetic subjects with nephropathy. Available at: http://clinicaltrials.gov/ct2/show/NCT01399580?term=atrasentan+and+bioimpedance&rank=1 Accessed October 10, 2013
- 18.de Zeeuw D, Gansevoort RT, Dullaart RP, de Jong PE: Angiotensin II antagonism improves the lipoprotein profile in patients with nephrotic syndrome. J Hypertens Suppl 13: S53–S58, 1995 [DOI] [PubMed] [Google Scholar]
- 19.Dhaun N, MacIntyre IM, Kerr D, Melville V, Johnston NR, Haughie S, Goddard J, Webb DJ: Selective endothelin-A receptor antagonism reduces proteinuria, blood pressure, and arterial stiffness in chronic proteinuric kidney disease. Hypertension 57: 772–779, 2011 [DOI] [PubMed] [Google Scholar]
- 20.AbbVie: Randomized, multicountry, multicenter, double-blind, parallel, placebo-controlled study of the effects of atrasentan on renal outcomes in subjects with type 2 diabetes and nephropathy SONAR: Study of Diabetic Nephropathy with Atrasentan. Available at: http://clinicaltrials.gov/ct2/show/NCT01858532?term=sonar&rank=1 Accessed October 10, 2013