Abstract
AKI is a major clinical problem with extremely high mortality and morbidity. Kidney hypoxia or ischemia-reperfusion injury inevitably occurs during surgery involving renal or aortic vascular occlusion and is one of the leading causes of perioperative AKI. Despite the growing incidence and tremendous clinical and financial burden of AKI, there is currently no effective therapy for this condition. The pathophysiology of AKI is orchestrated by renal tubular and endothelial cell necrosis and apoptosis, leukocyte infiltration, and the production and release of proinflammatory cytokines and reactive oxygen species. Effective management strategies require multimodal inhibition of these injury processes. Despite the past theoretical concerns about the nephrotoxic effects of several clinically utilized volatile anesthetics, recent studies suggest that modern halogenated volatile anesthetics induce potent anti-inflammatory, antinecrotic, and antiapoptotic effects that protect against ischemic AKI. Therefore, the renal protective properties of volatile anesthetics may provide clinically useful therapeutic intervention to treat and/or prevent perioperative AKI. In this review, we outline the history of volatile anesthetics and their effect on kidney function, briefly review the studies on volatile anesthetic-induced renal protection, and summarize the basic cellular mechanisms of volatile anesthetic-mediated protection against ischemic AKI.
Keywords: acute renal failure, ischemia-reperfusion, ischemic renal failure
AKI is a major clinical problem with extremely high mortality and morbidity costing >$10 billion annually in the United States.1,2 AKI is a growing clinical concern because the number of patients who develop AKI has nearly doubled over the past 7 years.3 In hospitalized patients, the incidence of AKI approaches 5%–20%, but it is significantly higher (>36%) in patients who require intensive care unit (ICU) admission and exceeds 60% during the ICU stay.4 Of all cases of AKI in the hospital, approximately 30%–40% occur during the perioperative period.5,6 AKI-related mortality remains extremely high, in which 7%–23% patients die after suffering from uncomplicated AKI and 50%–80% die in the ICU setting.7,8
Renal ischemia-reperfusion (IR) injury is a leading cause of perioperative AKI and frequently complicates major vascular, transplant, cardiac, and liver surgeries.9,10 Although hemodialysis and continuous hemofiltration can acutely treat the symptoms of AKI, allowing the kidney function to recover, significant mortality occurs from AKI as a result of the lack of effective clinical therapy to prevent and/or facilitate the repair of renal cell damage from AKI.11 Furthermore, AKI-induced extrarenal organ injury to the lung, liver, intestine, and brain frequently leads to multiple organ dysfunction syndrome, sepsis, and death.12–14 As the disease progresses, multiple organs are affected, which significantly contributes to morbidity and mortality.7
Tissue injury from surgery or ischemic/hypoxic organ damage creates major stress responses, including autonomic, hormonal, and metabolic changes, and stimulates systemic inflammatory reactions, which are associated with increased postoperative morbidity and mortality. By acting on the brain and on the spinal cord, sufficient clinical anesthetic depth achieved with volatile anesthetics reduces these stress responses.15 However, the invisible nonanesthetic effects of volatile anesthetics in modulating the inflammatory responses have for the most part been ignored and hidden under the shadow of detrimental halogenated anesthetic renal toxicity.16 This brief review provides evidence that halogenated volatile anesthetics are not nephrotoxic as previously believed, but, instead, are renal protective due to their powerful anti-ischemic and anti-inflammatory effects; reviews the protective effects of volatile anesthetics against ischemic AKI in preclinical studies; summarizes the cellular mechanisms of volatile anesthetic-induced kidney protection; and discusses their potential use to protect kidney function during the perioperative period.
Historical Volatile Anesthetics and Their Effect on Renal Function
Volatile anesthetics are administered to virtually all patients subjected to general anesthesia and are an integral part of the perioperative period. In addition to their analgesic and anesthetic properties, currently utilized halogenated volatile anesthetics are well known to have powerful nonanesthetic properties. For example, several clinically utilized volatile anesthetics (e.g., isoflurane, sevoflurane, and desflurane) have effects on systemic and pulmonary BP, cardiac inotropy, heart rate, and airway smooth muscle tone.17
Since the first successful administration of the general anesthesia with ethyl ether by Morton in 1846,18 significant efforts have been made to develop stable and nonflammable anesthetics. The first nonflammable halogenated volatile anesthetic gas, methoxyflurane, was first synthesized in 1948 by a team of chemists involved in the Manhattan Project during World War II.19 Unfortunately, the clinical use of fluorinated methoxyflurane led to frequent and significant kidney toxicity.20 Methoxyflurane causes vasopressin-resistant high-output renal insufficiency secondary to biotransformation of methoxyflurane to inorganic fluoride by the hepatic cytochrome P450 system.21,22 The inorganic fluoride formation as the cause of volatile anesthetic–induced nephrotoxicity was subsequently generalized to newer fluorinated anesthetics without any sound scientific evidence. Indeed, subsequent animal and human studies demonstrated that neither the peak value of fluoride nor the duration of systemic fluoride exposure correlated with anesthetic nephrotoxicity.23,24 Decades later, another potential concern was raised for clinical use of a widely popular volatile anesthetic, sevoflurane, due to the degradation of sevoflurane by carbon dioxide absorbers (strong alkali). When sevoflurane comes in contact with soda lime absorbers, it undergoes dehydrofluorination to form haloalkenes (called compound A) that have been shown to be severely nephrotoxic in rats.25 Unlike in vivo rat studies, clinical studies indicate that compound A formation during sevoflurane anesthesia has no clinically significant renal effects at any fresh gas flow rate.26 Therefore, the effects of compound A on renal function are not a contemporary clinical concern.
Contrary to the generalized and scientifically unproven historical perception of halogenated anesthetic–induced nephrotoxicity, recent studies show that volatile anesthetics possess powerful multiorgan protective effects during and after ischemic and inflammatory conditions that frequently occur during the perioperative period. It is becoming increasingly clear that volatile anesthetics powerfully modulate IR injury and inflammation in vivo and in vitro.27 In the heart, pretreatment with halothane or isoflurane improves left ventricular systolic function after 15 minutes of left anterior descending coronary artery occlusion.28 Subsequent studies discovered the protective effects of volatile anesthetic pretreatment before prolonged ischemia in other organs (e.g., liver, brain) and this phenomenon was termed anesthetic preconditioning.29,30 Furthermore, volatile anesthetics also protect several organs, including the kidney and heart, when administered after completion of ischemic insult and this phenomenon was termed anesthetic postconditioning.31 These studies establish volatile anesthetics as new potential therapeutic agents to protect against organ inflammation and ischemia.
Volatile Anesthetics and Renal Protection Mechanisms
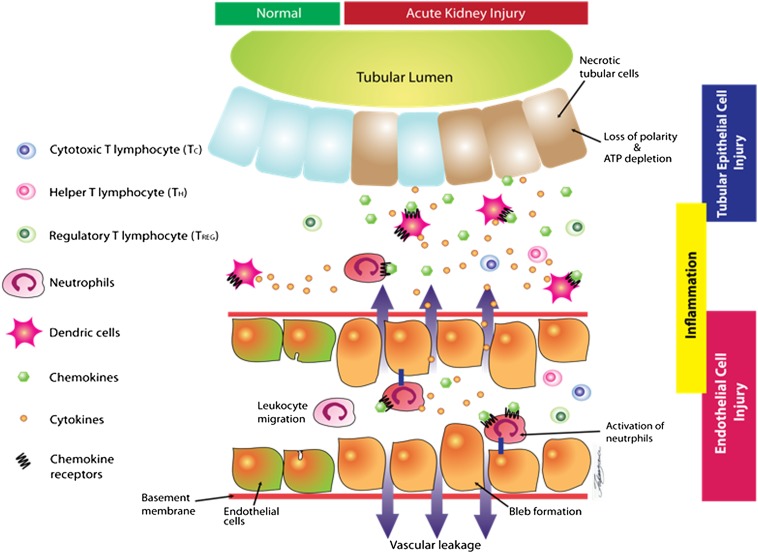
The cellular mechanisms of ischemic AKI are briefly summarized in Figure 1. Renal IR results in upregulation of several proinflammatory cytokines (e.g., TNF-α), chemokines (e.g., monocyte chemoattractant protein-1, macrophage inflammatory protein-2, and IL-8), and adhesion molecules (e.g., intercellular adhesion molecule-1, vascular cell adhesion molecule-1) in several cell types in the kidney.32 Proinflammatory cytokines are produced in dying or injured proximal tubules and endothelial cells, as well as in infiltrating leukocytes, including neutrophils, macrophages, and lymphocytes.16 In addition to cytokine-induced local inflammation, chemokines attract cytotoxic neutrophils and cytotoxic T lymphocytes to the kidney and contribute to local inflammation after IR injury.33,34 On the other hand, a subset of the T-cell population (regulatory T cells) plays an important role in protecting the kidney from ischemic AKI by suppressing inflammation and facilitating recovery. Regulatory T cells produce multiple anti-inflammatory mediators, including IL-10, TGF-β1, and programmed cell death protein-1, which can counteract the effects of IR injury.35,36 Furthermore, regulatory T cells express ectonucleoside triphosphate diphosphohydrolase (CD39) and ecto-5′-nucleotidase (CD73), which convert proinflammatory ATP to cytoprotective adenosine. Enhanced adenosine generation in turn produces powerful anti-inflammatory effects via A2a adenosine receptors.37
Figure 1.
Cellular mechanisms of ischemic AKI. Prolonged renal ischemia causes significant depletion of ATP, leading to various cellular changes (e.g., bleb formation and loss of renal tubular polarity). Adhesion molecules and neutrophil chemoattractants expressed on the endothelial cells cause migration of neutrophils. Cytokines and chemokines produced by renal tubular epithelial cells further cause renal tubular and endothelial inflammation. Breakdown of the endothelial basement membrane causes vascular leakage and neutrophil migration into the interstitial space. Orchestration of dendritic cells, neutrophils, and T lymphocytes further promotes epithelial and endothelial injury by inducing inflammation and cytokine/chemokine generation. On the other hand, a subset of the T-cell population, called regulatory T cells, plays an important role in protecting the kidney from IR injury by suppressing inflammation and by facilitating recovery. Volatile anesthetics via release of multiple cytoprotective and anti-inflammatory molecules can target many of the pathways involved in renal tubular, endothelial, and interstitial inflammation and injury.
Several studies show that volatile anesthetics have profound protective effects on the kidney by attenuating renal tubular necrosis and decreasing the nephrotoxic effects of proinflammatory leukocyte infiltration and cytokine generation after renal IR injury.16,38,39 Isoflurane or sevoflurane treatment during ischemia and 3-hour reperfusion decreased plasma creatinine by half, with marked improvements in kidney histology and markers of inflammation.16 We also showed that volatile anesthetics decrease the nuclear translocation of NF-κB, a key proinflammatory transcription factor.16 To note, volatile anesthetics promote or produce some of the identical anti-inflammatory mediators involved in renal protection provided by regulatory T cells, including TGF-β1 release, CD73 activation, and adenosine generation (see below).40,41
The detailed mechanisms of action and the exact target location for volatile anesthetics to induce general anesthesia (analgesia, amnesia, immobility) remain ambiguous despite decades of extensive research; however, the lipid membrane is considered a primary site of anesthetic action. This hypothesis is rooted from the striking observations made by Meyer and Overton in 1899, in which the authors noted that anesthetic potencies of 17 different volatile anesthetic agents are linearly related to lipid solubility. This phenomenon was called Meyer and Overton’s rule.42,43 Therefore, lipid solubility and its capacity to interact with the lipid membrane bilayer differ among clinically used volatile anesthetics.44 Interestingly, we determined that desflurane, the least lipid-soluble volatile anesthetic, was less potent in providing renal protection compared with other more lipid-soluble volatile anesthetics, such as sevoflurane, isoflurane, or halothane.16 Consistent with these findings in the kidney, liver heme oxygenase-1 expression is differentially regulated by several volatile anesthetics; isoflurane and sevoflurane upregulated heme oxygenase-1 mRNA and protein expression, whereas desflurane failed to induce heme oxygenase-1.45
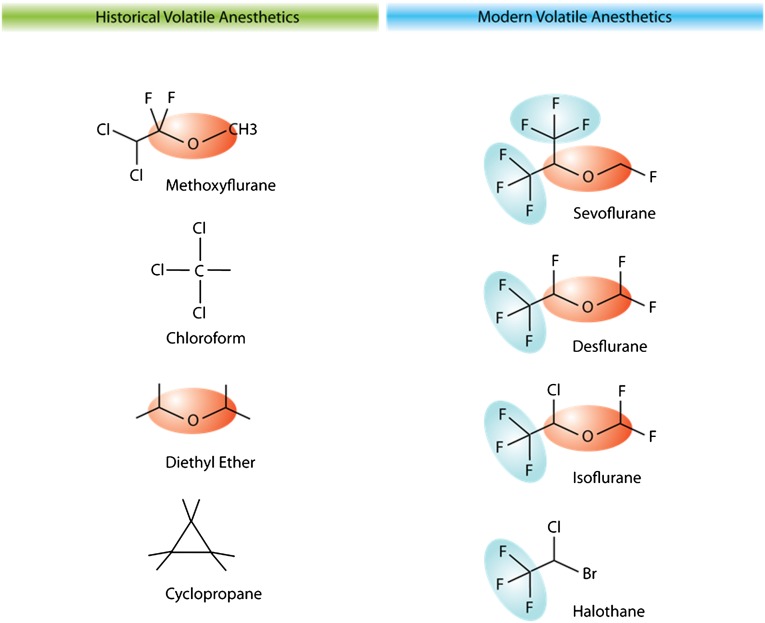
In vitro studies in epithelial and endothelial cells suggest that halogenation (fluorinated carbon groups) is responsible for the immunomodulatory effects of volatile anesthetics.46 The trifluorocarbon (CF3) molecule, which is shared in all newer volatile anesthetics, has been suggested as the specific molecular group of a volatile anesthetic that exerts immunomodulatory effects (Figure 2). In pulmonary epithelial and endothelial cells, modulation of chemical structures of volatile anesthetics showed that the CF3 molecular group is required for decreasing multiple proinflammatory chemokine and cytokine markers after LPS treatment. Diethyl ether or structure-similar nonfluorinated molecules failed to produce anti-inflammatory effects.
Figure 2.
Structures of historical (left) and modern (right) volatile anesthetics. Trifluorinated carbon molecules are shown in blue shades and diethyl ether molecule groups are shown in orange shades.
The mechanisms of volatile anesthetic–mediated protection against ischemic AKI are most likely different from cardiac IR models despite the similar anti-inflammatory effects produced. Whereas volatile anesthetics protect the heart via activation of ATP-dependent potassium (K+ATP) channels,47,48 kidney protection mechanism involves multiple signaling pathways including TGF-β1 generation, sphingosine kinase (SK) activation, adenosine generation, and IL-11 synthesis independent of the K+ATP channels.16 In addition, volatile anesthetics must be present during renal ischemia to provide protection, whereas pretreatment alone is protective against cardiac IR injury.16,49
Volatile Anesthetics Release Renal Tubular TGF-β1 and Promote Plasma Membrane Caveolae Formation
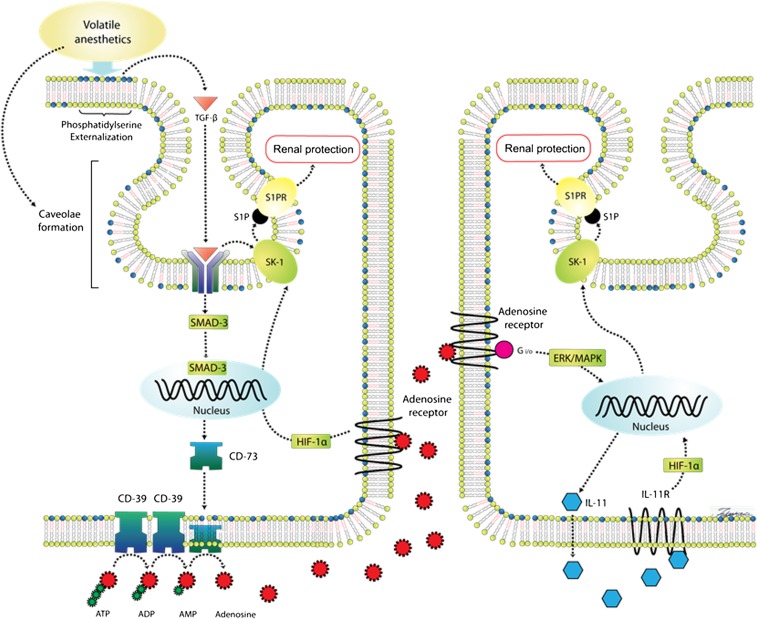
Disruption of the plasma membrane lipid bilayer by volatile anesthetics results in the translocation of phosphatidylserine from the inner leaflet to the outer leaflet of the plasma membrane (Figure 3).50 Phosphatidylserine (PS) externalization in macrophages as well as in renal tubular epithelia leads to the release of a potent anti-inflammatory/antinecrotic molecule TGF-β1 in adjacent cells via ligation of PS receptors.41,51,52 Indeed, TGF-β1 has been recognized as a key signaling molecule in the anti-inflammatory milieu of macrophages ingesting apoptotic cells.51,53 We demonstrated that the release of TGF-β1 is critically involved in generating the anti-inflammatory and antinecrotic effects of volatile anesthetics to protect against ischemic AKI in mice.54 TGF-β1–deficient mice were not protected against renal IR injury with sevoflurane anesthesia. Furthermore, a neutralizing TGF-β1 antibody blocked renal protection with sevoflurane in wild-type mice. In addition, sevoflurane caused nuclear translocation of the mothers against decapentaplegic homolog 3 (SMAD-3) transcription factor in primary cultures of proximal tubules from wild-type mice but not in proximal tubules from TGF-β1–deficient mice. Furthermore, SMAD-3–deficient mice were not protected against renal IR injury with sevoflurane anesthesia. Finally, isoflurane-induced PS externalization also causes an increase in caveolae/caveolin lipid rafts in the buoyant fractions of the plasma membranes, and an increase in caveolae sequestration of several key signaling intermediates, which are critical for volatile anesthetic-mediated renal protection including TGF-β1 receptors, as well as SKs, extracellular signal–regulated kinases, and sphingosine-1-phosphate (S1P) (Figure 3).41
Figure 3.
Proposed renal protection mechanisms of volatile anesthetics. Volatile anesthetics interact with the plasma membrane lipid bilayer in renal tubular cells and induce phosphatidylserine externalization and TGF-β1 generation. Volatile anesthetics also increase the formation of caveolae/caveolin lipid rafts in the buoyant fractions of the renal tubular plasma membranes and facilitate caveolae sequestration of several cytoprotective signaling intermediates (e.g., SK-1, TGF-β1 receptors, and S1P). TGF-β1 generated by volatile anesthetics binds to the TGF-β1 receptor, leading to translocation of SMAD-3 to the nucleus to increase the expression of renal tubular CD73. Increased CD73 expression subsequently increases renal tubular adenosine generation. Activation of renal tubular and perhaps endothelial ARs increases SK-1 protein expression via induction of HIF-1α transcription factor. In addition, activation of A1 ARs increases renal tubular IL-11 synthesis via ERK-MAPK activation. Finally, IL-11 also induces SK-1 generation via the HIF-1α pathway. CD, cluster of differentiation; ERK-MAPK: extracellular signal–regulated kinase mitogen-activated protein kinase; Gi/o, inhibitory regulative G protein; IL-11R, IL-11 receptor; S1PR, S1P receptor.
Volatile Anesthetics Induce Renal Tubular SK-1 and S1P Synthesis
Most volatile anesthetics are lipophilic molecules and can activate sphingomyelin hydrolysis.55 The membrane-bound lysophospholipid receptor for S1P is one of the molecules sequestrated in the plasma membrane caveolae.41 Lysophospholipid S1P is a product of sphingomyelin hydrolysis by SK and functions as both an extracellular ligand for specific G protein–coupled receptors, as well as an intracellular second messenger. S1P promotes cell growth and survival and regulates lymphocyte egress and migration.56–58 S1P binds to five subtypes of specific G protein–coupled receptors and mediates its antiapoptotic effects via pathways involving Akt and extracellular signal–regulated kinase signaling in hepatic myofibroblasts,58 lung epithelium,59 and melanocytes.60 We have shown that SK and S1P signaling play a major role in volatile anesthetic–induced protection against renal IR injury.41,61 Clinically used volatile anesthetic isoflurane-induced S1P synthesis via induction of SK-1 synthesis in renal proximal tubules and cultured endothelial cells.61,62 In addition, S1P receptor blockade by selective S1P receptor antagonists reversed the isoflurane-mediated renal protection.61 The administration of SK-1 inhibitors also reversed the isoflurane-mediated protection against renal IR injury.61 In addition, mice lacking the SK-1 enzyme were not protected against renal IR injury with isoflurane.63
Volatile Anesthetics Induce Renal Tubular Adenosine Synthesis via Activation of CD73
It is becoming increasingly clear that volatile anesthetic–mediated TGF-β1 release activates additional cytoprotective pathways. We recently demonstrated that isoflurane-mediated release of TGF-β1 induces CD73 synthesis in vivo as well as in vitro, leading to increased renal tubular adenosine generation.40 Isoflurane-mediated induction of CD73 and adenosine generation was critical for protection against ischemic AKI in mice. A selective CD73 inhibitor or CD73 deficiency prevented the renal protective effect of isoflurane anesthesia.40 Cell-surface CD73 catalyzes the hydrolysis of AMP to generate adenosine and is considered the rate-limiting step in extracellular adenosine generation.64 Adenosine signaling regulates diverse physiologic effects, including cardiovascular control, tissue injury, and inflammation in many organs by binding to four subtypes of adenosine receptors (ARs).65–68 Indeed, CD73 activation and adenosine generation protect against renal, intestinal, and cardiac IR injury by reducing necrosis and inflammation.69–71 In particular, renal tubular A1AR activation reduces necrosis and apoptosis, whereas A2aAR stimulation leads to tissue protection by attenuating leukocyte-mediated inflammation after renal IR. Renal A1ARs dramatically reduce proximal tubular necrosis and apoptosis via activation of extracellular signal–regulated kinase, Akt, and heat shock protein 27 through a pertussis toxin–sensitive G protein pathway.72,73 Furthermore, A1AR activation may produce anti-inflammatory effects (in addition to modulating necrosis and apoptosis) via direct induction and activation of SK-1 and S1P synthesis.61,74 Finally, isoflurane postconditioning after renal ischemia significantly improved outer medullary renal blood flow via CD73 induction, adenosine generation, and activation of kidney vascular ARs.40 Therefore, isoflurane-mediated adenosine generation likely protects against ischemic AKI by attenuating the post-ischemic no-reflow phenomenon in addition to its antinecrotic, anti-inflammatory, and antiapoptotic effects on renal tubule cells.
Volatile Anesthetics Induce Renal Tubular IL-11 Synthesis via A1AR Activation
Volatile anesthetic therapy for critically ill patients may be limited by its anesthetic and hemodynamic effects. One way to mitigate this is to utilize the distal signaling molecules synthesized with volatile anesthetic treatment devoid of systemic hemodynamic and anesthetic effects. Volatile anesthetic isoflurane-mediated induction of CD73 activation and adenosine generation subsequently induces IL-11 expression in human renal proximal tubular cells and in mouse kidney.75 We recently showed that isoflurane-mediated protection against ischemic AKI is additionally mediated by A1AR-mediated renal tubular IL-11 synthesis and release.75 IL-11 is a 20-kD member of the IL-6–type cytokine family. IL-11 promotes megakaryocyte maturation76 and is already clinically approved to treat severe thrombocytopenia in patients receiving chemotherapy.77 In addition to its hematopoietic effects, IL-11 protects against intestinal, cardiomyocyte, and endothelial cell death.78 We also recently showed that recombinant human IL-11 treatment before or after renal ischemia attenuated ischemic AKI in mice. Specifically, IL-11 administration significantly attenuated necrosis, inflammation, and apoptosis after ischemic AKI closely mimicking the renal protective effects of volatile anesthetics. Furthermore, this IL-11–mediated protection against ischemic AKI also requires the downstream induction of SK-1, another cytoprotective protein.79
Renal IR injury results in intensive tissue hypoxia with subsequent hypoxia-inducible factor (HIF) stabilization, and HIF-1α–dependent transcriptional regulation plays a critical role in modulating tissue hypoxia and inflammation as well as promoting cellular repair.80,81 Interestingly, HIF-1α signaling is critical for IL-11–mediated protection against ischemic AKI.82 Moreover, ischemia or hypoxia promotes HIF-1α–dependent transcriptional regulation of adenosine-generating enzymes (CD39 or CD73)80 that can directly stimulate IL-11 synthesis.79 Previous studies also suggest that a widely used volatile anesthetic, isoflurane, increases HIF-1α expression in a renal carcinoma cell line and in the rabbit myocardium.83,84 Finally, a recent study showed that isoflurane failed to protect mice pretreated with HIF-1α small interfering RNA, suggesting a critical role for HIF-1α signaling in isoflurane-mediated protection against ischemic AKI.85
Clinical Evidence for Volatile Anesthetic–Mediated Organ Protection
Current American Heart Association guidelines recommend the use of volatile anesthetics for maintenance of general anesthesia during noncardiac surgery in high-risk and hemodynamically stable patients at risk for perioperative myocardial ischemia (class IIb evidence).86 In 64 patients subjected to liver resection, sevoflurane preconditioning significantly decreased postoperative elevation of transaminase levels.87 In terms of renal protection, in 72 patients subjected to coronary artery bypass graft surgery, 10 minutes of sevoflurane preconditioning not only significantly decreased the release of brain natriuretic peptide, a biochemical marker of myocardial contractile dysfunction, but also significantly reduced postoperative plasma cystatin C concentrations, suggesting improvements in cardiac and renal function after major heart surgery.88
It is clear that more randomized clinical studies are needed to better define the renal protective role for volatile anesthetics against human AKI. There are currently several prospective surgical trials registered in ClinicalTrials.gov comparing the renal outcome after anesthesia with volatile anesthetics or with intravenous anesthetics. Preliminary findings from one of these studies suggest that eGFR improved during the early postoperative period after living donor kidney transplant surgery when the kidney donors were anesthetized with a volatile anesthetic compared with propofol.89 One of the major challenges in assessing renal protective effects of volatile anesthetics is lack of a sensitive biomarker to detect early efficacy of volatile anesthetic–mediated protection against AKI. Currently, clinically utilized markers (e.g., plasma creatinine, eGFR) are insensitive to truly test the efficacy of renal protection.
Future Implications and Challenges
Despite the growing incidence of AKI, there is currently no effective clinical therapy for AKI.11 Harnessing the anti-inflammatory and anti-ischemic effects of clinically utilized volatile anesthetics would be an excellent therapy to mitigate the detrimental effects of AKI. Volatile anesthetics activate multiple pathways to synthesize several key cytoprotective and anti-inflammatory signaling molecules to attenuate ischemic AKI, including TGF-β1 release, caveolae organization of SK and S1P signaling, CD73 activation, adenosine synthesis, and IL-11 generation (Figure 3). The optimal therapeutic window, method of delivery, dose, and exposure time of volatile anesthetics for renal protection need to be further elucidated. In addition, the molecular mechanisms of volatile anesthetics including lipid interactions and protein kinases involved in the downstream effects of anesthetic-induced renal protection need to be further studied. Finally, clinical introduction of a sensitive biomarker to detect AKI is critically needed to better assess the early efficacy of volatile anesthetic–mediated protection against ischemic AKI. New therapeutic use of volatile anesthetics will facilitate their off-label use as adjuncts for kidney protection during the perioperative period. In summary, harnessing the nonanesthetic properties of volatile anesthetics may have important clinical implications for critically ill patients anesthetized in the operating room and sedated in the ICU.
Disclosures
None.
Acknowledgments
This work was supported by the Department of Anesthesiology at Columbia University College of Physicians and Surgeons and by the National Institutes of Health (Grants R01-GM067081 and T32-GM00846).
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
References
- 1.Chertow GM, Burdick E, Honour M, Bonventre JV, Bates DW: Acute kidney injury, mortality, length of stay, and costs in hospitalized patients. J Am Soc Nephrol 16: 3365–3370, 2005 [DOI] [PubMed] [Google Scholar]
- 2.Jones DR, Lee HT: Perioperative renal protection. Best Pract Res Clin Anaesthesiol 22: 193–208, 2008 [DOI] [PubMed] [Google Scholar]
- 3.Hsu CY, McCulloch CE, Fan D, Ordoñez JD, Chertow GM, Go AS: Community-based incidence of acute renal failure. Kidney Int 72: 208–212, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bagshaw SM, George C, Bellomo R, ANZICS Database Management Committee : Early acute kidney injury and sepsis: A multicentre evaluation. Crit Care 12: R47, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thakar CV, Christianson A, Freyberg R, Almenoff P, Render ML: Incidence and outcomes of acute kidney injury in intensive care units: A Veterans Administration study. Crit Care Med 37: 2552–2558, 2009 [DOI] [PubMed] [Google Scholar]
- 6.Uchino S, Kellum JA, Bellomo R, Doig GS, Morimatsu H, Morgera S, Schetz M, Tan I, Bouman C, Macedo E, Gibney N, Tolwani A, Ronco C, Beginning and Ending Supportive Therapy for the Kidney (BEST Kidney) Investigators : Acute renal failure in critically ill patients: A multinational, multicenter study. JAMA 294: 813–818, 2005 [DOI] [PubMed] [Google Scholar]
- 7.Liaño F, Junco E, Pascual J, Madero R, Verde E, The Madrid Acute Renal Failure Study Group : The spectrum of acute renal failure in the intensive care unit compared with that seen in other settings. Kidney Int Suppl 66: S16–S24, 1998 [PubMed] [Google Scholar]
- 8.Lafrance JP, Miller DR: Acute kidney injury associates with increased long-term mortality. J Am Soc Nephrol 21: 345–352, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Abelha FJ, Botelho M, Fernandes V, Barros H: Determinants of postoperative acute kidney injury. Crit Care 13: R79, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Koyner JL, Garg AX, Coca SG, Sint K, Thiessen-Philbrook H, Patel UD, Shlipak MG, Parikh CR, TRIBE-AKI Consortium : Biomarkers predict progression of acute kidney injury after cardiac surgery. J Am Soc Nephrol 23: 905–914, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ratliff BB, Rabadi MM, Vasko R, Yasuda K, Goligorsky MS: Messengers without borders: Mediators of systemic inflammatory response in AKI. J Am Soc Nephrol 24: 529–536, 2013 [DOI] [PubMed] [Google Scholar]
- 12.Bellomo R, Kellum JA, Ronco C: Defining and classifying acute renal failure: From advocacy to consensus and validation of the RIFLE criteria. Intensive Care Med 33: 409–413, 2007 [DOI] [PubMed] [Google Scholar]
- 13.Faubel S: Pulmonary complications after acute kidney injury. Adv Chronic Kidney Dis 15: 284–296, 2008 [DOI] [PubMed] [Google Scholar]
- 14.Liu M, Liang Y, Chigurupati S, Lathia JD, Pletnikov M, Sun Z, Crow M, Ross CA, Mattson MP, Rabb H: Acute kidney injury leads to inflammation and functional changes in the brain. J Am Soc Nephrol 19: 1360–1370, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Desborough JP: The stress response to trauma and surgery. Br J Anaesth 85: 109–117, 2000 [DOI] [PubMed] [Google Scholar]
- 16.Lee HT, Ota-Setlik A, Fu Y, Nasr SH, Emala CW: Differential protective effects of volatile anesthetics against renal ischemia-reperfusion injury in vivo. Anesthesiology 101: 1313–1324, 2004 [DOI] [PubMed] [Google Scholar]
- 17.Pagel PS, Kampine JP, Schmeling WT, Warltier DC: Comparison of the systemic and coronary hemodynamic actions of desflurane, isoflurane, halothane, and enflurane in the chronically instrumented dog. Anesthesiology 74: 539–551, 1991 [DOI] [PubMed] [Google Scholar]
- 18.Miller RD: Miller's Anesthesia, Philadelphia, Elsevier, 2005 [Google Scholar]
- 19.Smith I, Nathanson M, White PF: Sevoflurane—a long-awaited volatile anaesthetic. Br J Anaesth 76: 435–445, 1996 [DOI] [PubMed] [Google Scholar]
- 20.Crandell WB, Pappas SG, Macdonald A: Nephrotoxicity associated with methoxyflurane anesthesia. Anesthesiology 27: 591–607, 1966 [DOI] [PubMed] [Google Scholar]
- 21.Kharasch ED: Adverse drug reactions with halogenated anesthetics. Clin Pharmacol Ther 84: 158–162, 2008 [DOI] [PubMed] [Google Scholar]
- 22.Mazze RI: Fluorinated anaesthetic nephrotoxicity: An update. Can Anaesth Soc J 31: S16–S22, 1984 [DOI] [PubMed] [Google Scholar]
- 23.Spencer EM, Willatts SM, Prys-Roberts C: Plasma inorganic fluoride concentrations during and after prolonged (greater than 24 h) isoflurane sedation: Effect on renal function. Anesth Analg 73: 731–737, 1991 [DOI] [PubMed] [Google Scholar]
- 24.Cousins MJ, Mazze RI: Methoxyflurane nephrotoxicity. A study of dose response in man. JAMA 225: 1611–1616, 1973 [DOI] [PubMed] [Google Scholar]
- 25.Anders MW: Formation and toxicity of anesthetic degradation products. Annu Rev Pharmacol Toxicol 45: 147–176, 2005 [DOI] [PubMed] [Google Scholar]
- 26.Bito H, Ikeuchi Y, Ikeda K: Effects of low-flow sevoflurane anesthesia on renal function: Comparison with high-flow sevoflurane anesthesia and low-flow isoflurane anesthesia. Anesthesiology 86: 1231–1237, 1997 [DOI] [PubMed] [Google Scholar]
- 27.Yang H, Liang G, Hawkins BJ, Madesh M, Pierwola A, Wei H: Inhalational anesthetics induce cell damage by disruption of intracellular calcium homeostasis with different potencies. Anesthesiology 109: 243–250, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Warltier DC, al-Wathiqui MH, Kampine JP, Schmeling WT: Recovery of contractile function of stunned myocardium in chronically instrumented dogs is enhanced by halothane or isoflurane. Anesthesiology 69: 552–565, 1988 [DOI] [PubMed] [Google Scholar]
- 29.Xiong L, Zheng Y, Wu M, Hou L, Zhu Z, Zhang X, Lu Z: Preconditioning with isoflurane produces dose-dependent neuroprotection via activation of adenosine triphosphate-regulated potassium channels after focal cerebral ischemia in rats. Anesth Analg 96: 233–237, table of contents, 2003 [DOI] [PubMed] [Google Scholar]
- 30.Lv X, Yang L, Tao K, Liu Y, Yang T, Chen G, Yu W, Lv H, Wu F: Isoflurane preconditioning at clinically relevant doses induce protective effects of heme oxygenase-1 on hepatic ischemia reperfusion in rats. BMC Gastroenterol 11: 31, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pagel PS: Postconditioning by volatile anesthetics: Salvaging ischemic myocardium at reperfusion by activation of prosurvival signaling. J Cardiothorac Vasc Anesth 22: 753–765, 2008 [DOI] [PubMed] [Google Scholar]
- 32.Munshi R, Johnson A, Siew ED, Ikizler TA, Ware LB, Wurfel MM, Himmelfarb J, Zager RA: MCP-1 gene activation marks acute kidney injury. J Am Soc Nephrol 22: 165–175, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Heinzelmann M, Mercer-Jones MA, Passmore JC: Neutrophils and renal failure. Am J Kidney Dis 34: 384–399, 1999 [DOI] [PubMed] [Google Scholar]
- 34.Devarajan P: Update on mechanisms of ischemic acute kidney injury. J Am Soc Nephrol 17: 1503–1520, 2006 [DOI] [PubMed] [Google Scholar]
- 35.Kinsey GR, Sharma R, Huang L, Li L, Vergis AL, Ye H, Ju ST, Okusa MD: Regulatory T cells suppress innate immunity in kidney ischemia-reperfusion injury. J Am Soc Nephrol 20: 1744–1753, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kinsey GR, Sharma R, Okusa MD: Regulatory T cells in AKI. J Am Soc Nephrol 24: 1720–1726, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kinsey GR, Huang L, Jaworska K, Khutsishvili K, Becker DA, Ye H, Lobo PI, Okusa MD: Autocrine adenosine signaling promotes regulatory T cell-mediated renal protection. J Am Soc Nephrol 23: 1528–1537, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Obal D, Rascher K, Favoccia C, Dettwiler S, Schlack W: Post-conditioning by a short administration of desflurane reduced renal reperfusion injury after differing of ischaemia times in rats. Br J Anaesth 97: 783–791, 2006 [DOI] [PubMed] [Google Scholar]
- 39.Hashiguchi H, Morooka H, Miyoshi H, Matsumoto M, Koji T, Sumikawa K: Isoflurane protects renal function against ischemia and reperfusion through inhibition of protein kinases, JNK and ERK. Anesth Analg 101: 1584–1589, 2005 [DOI] [PubMed] [Google Scholar]
- 40.Kim M, Ham A, Kim JY, Brown KM, D’Agati VD, Lee HT: The volatile anesthetic isoflurane induces ecto-5′-nucleotidase (CD73) to protect against renal ischemia and reperfusion injury. Kidney Int 84: 90–103, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Song JH, Kim M, Park SW, Chen SW, Pitson SM, Lee HT: Isoflurane via TGF-beta1 release increases caveolae formation and organizes sphingosine kinase signaling in renal proximal tubules. Am J Physiol Renal Physiol 298: F1041–F1050, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Meyer H: Zur Theorie der Alkoholnarkose. Arch Exp Pathol Pharmacol 42: 109–118, 1899 [Google Scholar]
- 43.Overton C: Studien über die Narkose zugleich ein Beitrag zur allgemeinen Pharmakologie. Trans Faraday Soc 33: 1062–1068, 1901 [Google Scholar]
- 44.Franks NP: General anaesthesia: From molecular targets to neuronal pathways of sleep and arousal. Nat Rev Neurosci 9: 370–386, 2008 [DOI] [PubMed] [Google Scholar]
- 45.Hoetzel A, Geiger S, Loop T, Welle A, Schmidt R, Humar M, Pahl HL, Geiger KK, Pannen BH: Differential effects of volatile anesthetics on hepatic heme oxygenase-1 expression in the rat. Anesthesiology 97: 1318–1321, 2002 [DOI] [PubMed] [Google Scholar]
- 46.Urner M, Limbach LK, Herrmann IK, Müller-Edenborn B, Roth-Z’Graggen B, Schlicker A, Reyes L, Booy C, Hasler M, Stark WJ, Beck-Schimmer B: Fluorinated groups mediate the immunomodulatory effects of volatile anesthetics in acute cell injury. Am J Respir Cell Mol Biol 45: 617–624, 2011 [DOI] [PubMed] [Google Scholar]
- 47.O’Rourke B: Myocardial K(ATP) channels in preconditioning. Circ Res 87: 845–855, 2000 [DOI] [PubMed] [Google Scholar]
- 48.Kersten JR, Gross GJ, Pagel PS, Warltier DC: Activation of adenosine triphosphate-regulated potassium channels: Mediation of cellular and organ protection. Anesthesiology 88: 495–513, 1998 [DOI] [PubMed] [Google Scholar]
- 49.Cope DK, Impastato WK, Cohen MV, Downey JM: Volatile anesthetics protect the ischemic rabbit myocardium from infarction. Anesthesiology 86: 699–709, 1997 [DOI] [PubMed] [Google Scholar]
- 50.Lee HT, Kim M, Kim J, Kim N, Emala CW: TGF-beta1 release by volatile anesthetics mediates protection against renal proximal tubule cell necrosis. Am J Nephrol 27: 416–424, 2007 [DOI] [PubMed] [Google Scholar]
- 51.Huynh ML, Fadok VA, Henson PM: Phosphatidylserine-dependent ingestion of apoptotic cells promotes TGF-beta1 secretion and the resolution of inflammation. J Clin Invest 109: 41–50, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Xiao YQ, Malcolm K, Worthen GS, Gardai S, Schiemann WP, Fadok VA, Bratton DL, Henson PM: Cross-talk between ERK and p38 MAPK mediates selective suppression of pro-inflammatory cytokines by transforming growth factor-beta. J Biol Chem 277: 14884–14893, 2002 [DOI] [PubMed] [Google Scholar]
- 53.Fadok VA, Bratton DL, Konowal A, Freed PW, Westcott JY, Henson PM: Macrophages that have ingested apoptotic cells in vitro inhibit proinflammatory cytokine production through autocrine/paracrine mechanisms involving TGF-beta, PGE2, and PAF. J Clin Invest 101: 890–898, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lee HT, Chen SW, Doetschman TC, Deng C, D’Agati VD, Kim M: Sevoflurane protects against renal ischemia and reperfusion injury in mice via the transforming growth factor-beta1 pathway. Am J Physiol Renal Physiol 295: F128–F136, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhou Y, Lekic T, Fathali N, Ostrowski RP, Martin RD, Tang J, Zhang JH: Isoflurane posttreatment reduces neonatal hypoxic-ischemic brain injury in rats by the sphingosine-1-phosphate/phosphatidylinositol-3-kinase/Akt pathway. Stroke 41: 1521–1527, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Olivera A, Kohama T, Edsall L, Nava V, Cuvillier O, Poulton S, Spiegel S: Sphingosine kinase expression increases intracellular sphingosine-1-phosphate and promotes cell growth and survival. J Cell Biol 147: 545–558, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Spiegel S, Milstien S: Sphingosine-1-phosphate: An enigmatic signalling lipid. Nat Rev Mol Cell Biol 4: 397–407, 2003 [DOI] [PubMed] [Google Scholar]
- 58.Davaille J, Li L, Mallat A, Lotersztajn S: Sphingosine 1-phosphate triggers both apoptotic and survival signals for human hepatic myofibroblasts. J Biol Chem 277: 37323–37330, 2002 [DOI] [PubMed] [Google Scholar]
- 59.Monick MM, Mallampalli RK, Bradford M, McCoy D, Gross TJ, Flaherty DM, Powers LS, Cameron K, Kelly S, Merrill AH, Jr, Hunninghake GW: Cooperative prosurvival activity by ERK and Akt in human alveolar macrophages is dependent on high levels of acid ceramidase activity. J Immunol 173: 123–135, 2004 [DOI] [PubMed] [Google Scholar]
- 60.Kim DS, Hwang ES, Lee JE, Kim SY, Park KC: Sphingosine-1-phosphate promotes mouse melanocyte survival via ERK and Akt activation. Cell Signal 15: 919–926, 2003 [DOI] [PubMed] [Google Scholar]
- 61.Kim M, Kim M, Park SW, Pitson SM, Lee HT: Isoflurane protects human kidney proximal tubule cells against necrosis via sphingosine kinase and sphingosine-1-phosphate generation. Am J Nephrol 31: 353–362, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bakar AM, Park SW, Kim M, Lee HT: Isoflurane protects against human endothelial cell apoptosis by inducing sphingosine kinase-1 via ERK MAPK. Int J Mol Sci 13: 977–993, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kim M, Park SW, Kim M, D’Agati VD, Lee HT: Isoflurane activates intestinal sphingosine kinase to protect against bilateral nephrectomy-induced liver and intestine dysfunction. Am J Physiol Renal Physiol 300: F167–F176, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Colgan SP, Eltzschig HK, Eckle T, Thompson LF: Physiological roles for ecto-5′-nucleotidase (CD73). Purinergic Signal 2: 351–360, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kim J, Kim M, Song JH, Lee HT: Endogenous A1 adenosine receptors protect against hepatic ischemia reperfusion injury in mice. Liver Transpl 14: 845–854, 2008 [DOI] [PubMed] [Google Scholar]
- 66.Csóka B, Németh ZH, Rosenberger P, Eltzschig HK, Spolarics Z, Pacher P, Selmeczy Z, Koscsó B, Himer L, Vizi ES, Blackburn MR, Deitch EA, Haskó G: A2B adenosine receptors protect against sepsis-induced mortality by dampening excessive inflammation. J Immunol 185: 542–550, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Park SW, Chen SW, Kim M, Brown KM, D’Agati VD, Lee HT: Protection against acute kidney injury via A(1) adenosine receptor-mediated Akt activation reduces liver injury after liver ischemia and reperfusion in mice. J Pharmacol Exp Ther 333: 736–747, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lappas CM, Day YJ, Marshall MA, Engelhard VH, Linden J: Adenosine A2A receptor activation reduces hepatic ischemia reperfusion injury by inhibiting CD1d-dependent NKT cell activation. J Exp Med 203: 2639–2648, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hart ML, Jacobi B, Schittenhelm J, Henn M, Eltzschig HK: Cutting edge: A2B adenosine receptor signaling provides potent protection during intestinal ischemia/reperfusion injury. J Immunol 182: 3965–3968, 2009 [DOI] [PubMed] [Google Scholar]
- 70.Grenz A, Zhang H, Eckle T, Mittelbronn M, Wehrmann M, Köhle C, Kloor D, Thompson LF, Osswald H, Eltzschig HK: Protective role of ecto-5′-nucleotidase (CD73) in renal ischemia. J Am Soc Nephrol 18: 833–845, 2007 [DOI] [PubMed] [Google Scholar]
- 71.Eckle T, Krahn T, Grenz A, Köhler D, Mittelbronn M, Ledent C, Jacobson MA, Osswald H, Thompson LF, Unertl K, Eltzschig HK: Cardioprotection by ecto-5′-nucleotidase (CD73) and A2B adenosine receptors. Circulation 115: 1581–1590, 2007 [DOI] [PubMed] [Google Scholar]
- 72.Lee HT, Gallos G, Nasr SH, Emala CW: A1 adenosine receptor activation inhibits inflammation, necrosis, and apoptosis after renal ischemia-reperfusion injury in mice. J Am Soc Nephrol 15: 102–111, 2004 [DOI] [PubMed] [Google Scholar]
- 73.Lee HT, Emala CW: Protective effects of renal ischemic preconditioning and adenosine pretreatment: Role of A(1) and A(3) receptors. Am J Physiol Renal Physiol 278: F380–F387, 2000 [DOI] [PubMed] [Google Scholar]
- 74.Park SW, Kim M, Kim JY, Brown KM, Haase VH, D’Agati VD, Lee HT: Proximal tubule sphingosine kinase-1 has a critical role in A1 adenosine receptor-mediated renal protection from ischemia. Kidney Int 82: 878–891, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ham A, Kim M, Kim JY, Brown KM, Yeh J, D’Agati VD, Lee HT: Critical role of interleukin-11 in isoflurane-mediated protection against ischemic acute kidney injury in mice. Anesthesiology 119: 1389–1401, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Burstein SA, Mei RL, Henthorn J, Friese P, Turner K: Leukemia inhibitory factor and interleukin-11 promote maturation of murine and human megakaryocytes in vitro. J Cell Physiol 153: 305–312, 1992 [DOI] [PubMed] [Google Scholar]
- 77.Isaacs C, Robert NJ, Bailey FA, Schuster MW, Overmoyer B, Graham M, Cai B, Beach KJ, Loewy JW, Kaye JA: Randomized placebo-controlled study of recombinant human interleukin-11 to prevent chemotherapy-induced thrombocytopenia in patients with breast cancer receiving dose-intensive cyclophosphamide and doxorubicin. J Clin Oncol 15: 3368–3377, 1997 [DOI] [PubMed] [Google Scholar]
- 78.Du X, Williams DA: Interleukin-11: Review of molecular, cell biology, and clinical use. Blood 89: 3897–3908, 1997 [PubMed] [Google Scholar]
- 79.Kim JY, Kim M, Ham A, Brown KM, Greene RW, D’Agati VD, Lee HT: IL-11 is required for A1 adenosine receptor-mediated protection against ischemic AKI. J Am Soc Nephrol 24: 1558–1570, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Eltzschig HK, Carmeliet P: Hypoxia and inflammation. N Engl J Med 364: 656–665, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Eltzschig HK, Sitkovsky MV, Robson SC: Purinergic signaling during inflammation. N Engl J Med 367: 2322–2333, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lee HT, Park SW, Kim M, Ham A, Anderson LJ, Brown KM, D’Agati VD, Cox GN: Interleukin-11 protects against renal ischemia and reperfusion injury. Am J Physiol Renal Physiol 303: F1216–F1224, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Benzonana LL, Perry NJ, Watts HR, Yang B, Perry IA, Coombes C, Takata M, Ma D: Isoflurane, a commonly used volatile anesthetic, enhances renal cancer growth and malignant potential via the hypoxia-inducible factor cellular signaling pathway in vitro. Anesthesiology 119: 593–605, 2013 [DOI] [PubMed] [Google Scholar]
- 84.Raphael J, Zuo Z, Abedat S, Beeri R, Gozal Y: Isoflurane preconditioning decreases myocardial infarction in rabbits via up-regulation of hypoxia inducible factor 1 that is mediated by mammalian target of rapamycin. Anesthesiology 108: 415–425, 2008 [DOI] [PubMed] [Google Scholar]
- 85.Zhang L, Huang H, Cheng J, Liu J, Zhao H, Vizcaychipi MP, Ma D: Pre-treatment with isoflurane ameliorates renal ischemic-reperfusion injury in mice. Life Sci 88: 1102–1107, 2011 [DOI] [PubMed] [Google Scholar]
- 86.Fleisher LA, Beckman JA, Brown KA, Calkins H, Chaikof E, Fleischmann KE, Freeman WK, Froehlich JB, Kasper EK, Kersten JR, Riegel B, Robb JF, Smith SC, Jr, Jacobs AK, Adams CD, Anderson JL, Antman EM, Buller CE, Creager MA, Ettinger SM, Faxon DP, Fuster V, Halperin JL, Hiratzka LF, Hunt SA, Lytle BW, Nishimura R, Ornato JP, Page RL, Riegel B, Tarkington LG, Yancy CW, ACC/AHA Task Force Members : ACC/AHA 2007 Guidelines on Perioperative Cardiovascular Evaluation and Care for Noncardiac Surgery: Executive Summary: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the 2002 Guidelines on Perioperative Cardiovascular Evaluation for Noncardiac Surgery): Developed in collaboration with the American Society of Echocardiography, American Society of Nuclear Cardiology, Heart Rhythm Society, Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, Society for Vascular Medicine and Biology, and Society for Vascular Surgery. Circulation 116: 1971–1996, 2007 [DOI] [PubMed] [Google Scholar]
- 87.Beck-Schimmer B, Breitenstein S, Bonvini JM, Lesurtel M, Ganter M, Weber A, Puhan MA, Clavien PA: Protection of pharmacological postconditioning in liver surgery: Results of a prospective randomized controlled trial. Ann Surg 256: 837–844; discussion 844–835, 2012 [DOI] [PubMed] [Google Scholar]
- 88.Julier K, da Silva R, Garcia C, Bestmann L, Frascarolo P, Zollinger A, Chassot PG, Schmid ER, Turina MI, von Segesser LK, Pasch T, Spahn DR, Zaugg M: Preconditioning by sevoflurane decreases biochemical markers for myocardial and renal dysfunction in coronary artery bypass graft surgery: A double-blinded, placebo-controlled, multicenter study. Anesthesiology 98: 1315–1327, 2003 [DOI] [PubMed] [Google Scholar]
- 89.Lee JH, Joo DJ, Kim JM, Park JH, Kim YS, Koo BN: Preconditioning effects of the anesthetic administered to the donor on grafted kidney function in living donor kidney transplantation recipients. Minerva Anestesiol 79: 504–514, 2013 [PubMed] [Google Scholar]