Abstract
The microtubule, which is one of the major targets of anthelmintics, anticancer drugs, and fungicides, is composed mainly of α- and β-tubulins. We focused on a unique characteristic of an Aspergillus nidulans benA33 mutant to screen for microtubule-disrupting antifungal agents. This mutant, which has a β-tubulin with a mutation of a single amino acid, undergoes mitotic arrest due to the formation of hyperstable microtubules at 37°C. The heat sensitivity of the mutant is remedied by some antimicrotubule agents. We found that an agar plate assay with the mutant was able to distinguish three types of microtubule inhibitors. The growth recovery zones of the mutant were formed around paper disks containing microtubule inhibitors, including four benzimidazoles, ansamitocin P-3, griseofulvin, and rhizoxin, on the agar plate at 37°C. Nocodazole, thiabendazole, and griseofulvin reversed the mitotic arrest of the mutant and promoted its hyphal growth. Ansamitocin P-3 and rhizoxin showed growth recovery zones around the growth-inhibitory zones. Benomyl and carbendazim also reversed mitotic arrest but produced weaker growth recovery than the aforementioned drugs. Other microtubule inhibitors, such as colchicine, Colcemid, paclitaxel, podophyllotoxin, TN-16, vinblastine, and vincristine, as well as some cytoskeletal inhibitors tested, did not show such activity. In our screening, we newly identified two mycotoxins, citrinin and patulin, two sesquiterpene dialdehydes, polygodial and warburganal, and four phenylalanine derivatives, arphamenine A, l-2,5-dihydrophenylalanine (DHPA), N-tosyl-l-phenylalanine chloromethylketone, and N-carbobenzoxy-l-phenylalanine chloromethyl ketone. In a wild-type strain of A. nidulans, DHPA caused selective losses of microtubules, as determined by fluorescence microscopy, and of both α- and β-tubulins, as determined by Western blot analysis. This screening method involving the benA33 mutant of A. nidulans is useful, convenient, and highly selective. The phenylalanine derivatives tested are of a novel type of microtubule-disrupting antifungal agents, producing an accompanying loss of tubulins, and are different from well-known tubulin inhibitors affecting the assembly of tubulin dimers into microtubules.
The microtubule, a component of the eukaryotic cytoskeleton, is involved in the separation of chromatids during mitosis and in the maintenance of cell shape during interkinesis (13). This fiber is a complex composed of an assembly of heterodimers of α- and β-tubulins and microtubule-associated proteins (24). Various microtubule inhibitors have been obtained from microbial sources and chemical libraries. Practical uses for some of these microtubule inhibitors are as follows. Vinca alkaloids such as vinblastine and vincristine, which are anticancer drugs isolated from plants, inhibit microtubule assembly (14, 32). Paclitaxel (taxol), which is also an anticancer drug isolated from a plant, binds to the N-terminal region of β-tubulin and promotes the formation of highly stable microtubules that resist depolymerization (33). Ansamitocin P-3 and rhizoxin are macrocyclic antitumor antibiotics, isolated as a cytotoxic metabolite of an actinomycete and as a phytotoxin produced by a plant-pathogenic fungus, respectively (14). Their binding sites partially overlap the vinblastine-binding site (14). Benzimidazoles such as benomyl, carbendazim, and nocodazole, which are used as anthelmintics and fungicides (9, 12, 18, 25), bind to the colchicine-binding site of tubulin.
The benA gene, referred to as the benomyl resistance gene, encodes the β-tubulin of the filamentous fungus Aspergillus nidulans. A number of benA mutant strains have been isolated so far (40). A benA33 mutant, which undergoes mitotic arrest at temperatures as high as 37°C, has a glutamine-to-lysine mutation at the 134th amino acid in the β-tubulin gene (16). The formation of hyperstable microtubules is considered to cause the mitotic arrest of the mutant under high-temperature conditions (6, 30).
Oakley and Morris found that the heat sensitivity of the benA33 mutant was remedied by some antimicrotubule agents (30). We further found that under mitotic-arrest-producing conditions, the growth recovery zones of the mutant formed around paper disks containing microtubule inhibitors, including ansamitocin P-3, four benzimidazoles, griseofulvin, and rhizoxin, in an agar plate assay. Other microtubule inhibitors and some cytoskeletal inhibitors tested did not show such effects. In addition, we newly found that this assay was able to distinguish three types of microtubule inhibitors. Therefore, we applied this characteristic of the mutant in screening for microtubule-disrupting antifungal agents. In our screening, we identified two mycotoxins, citrinin and patulin, two sesquiterpene dialdehydes, polygodial and warburganal, and four phenylalanine derivatives, arphamenine A, l-2,5-dihydrophenylalanine (DHPA), N-tosyl-l-phenylalanine chloromethylketone (TPCK),and N-carbobenzoxy-l-phenylalanine chloromethyl ketone (ZPCK). In this paper, we describe a new screening method for microtubule-disrupting antifungal agents that uses a mitotic-arrest mutant of A. nidulans and demonstrate the convenience and high selectivity of this method. Moreover, we discuss the possibility of isolation for microtubule-disrupting agents of a novel type among the compounds screened by this method.
MATERIALS AND METHODS
Chemicals.
All chemicals were obtained from Sigma Chemical Co. (St. Louis, Mo.) unless otherwise stated. Arphamenine A was a product of the Peptide Institute (Osaka, Japan). Alamar blue, carbendazim, griseofulvin, and TN-16 were purchased from Wako Pure Chemical (Osaka, Japan). Citrinin, DHPA, polygodial, and warburganal were prepared previously (10, 11, 19, 20, 36, 38).
A. nidulans strains and culture.
A wild-type A. nidulans strain (FGSC A4) and its benA33 mutant strain (FGSC A820) were obtained from the Fungal Genetics Stock Center (FGSC; Kansas City, Kans.). These strains were cultured in a complete medium (0.5% yeast extract, 1% glucose, and 0.1% trace elements). The trace element composition was obtained from the A. nidulans Linkage Map website (http://www.gla.ac.uk/Acad/IBLS/molgen/aspergillus/).
Assay of growth recovery of A. nidulans benA33 mutant from mitotic arrest.
The growth recovery of the benA33 mutant from mitotic arrest was assayed by a paper disk method. Paper disks (diameter, 8 mm) with or without each drug were placed on a 1.5% agar plate containing the complete medium seeded with 1 × 104 conidia of the mutant/ml. The plate was incubated at 37°C for 2 to 7 days. After incubation, the formation of growth recovery zones around the paper disks within and on the surface of the agar medium was observed.
In another assay, a 96-well plate method was used. Twenty microliters of the cell suspension containing 1 × 106 conidia was added to 180 μl of the complete medium in each well. After 1 μl of the drug solution had been loaded into each well, the plate was incubated at 37°C for 24 h. After incubation, 20 μl of Alamar blue dye solution was added, and then incubation was continued for another 4 h. Fluorescence was detected with a Millipore Cytofluor 2300 (excitation wavelength, 530 nm; emission wavelength, 590 nm). All assays were performed at least three times on separate occasions.
Antifungal susceptibility test.
The MICs for fungal strains were determined by a slightly modified broth microdilution method outlined by the National Committee for Clinical Laboratory Standards (29). Aspergillus niger ATCC 6275 was obtained from the American Type Culture Collection (ATCC; Manassas, Va.). Aspergillus fumigatus IFO 5840, A. niger IFO 4416, A. niger IFO 9455, Candida albicans IFO 1061, and Yarrowia lipolytica IFO 1746 were obtained from the Institute for Fermentation Osaka (IFO; Osaka, Japan). C. albicans NBRC 1385, Fusarium oxysporum NBRC 5942, Fusarium solani NBRC 5899, and F. solani NBRC 8505 were purchased from the NITE Biological Resource Center (NBRC; Chiba, Japan). All fungal strains tested except A. nidulans FGSC A4 were cultured in 2.5% malt extract medium (Oriental Yeast, Tokyo, Japan). A. nidulans FGSC A4 was cultured in the complete medium described above. In the assay, the 96-well plate method was used. Twenty microliters of the cell suspension containing 1 × 106 conidia or 1 × 106 cells was added to 180 μl of the medium in each well. After 1 μl of the drug solution had been loaded into each well, the plate was incubated at 30°C for 48 h. The MIC was the lowest drug concentration to produce a prominent decrease in turbidity compared with control growth. All assays were performed at least three times on separate occasions.
Fluorescence microscopy.
Fluorescence microscopy of nuclei and microtubules was performed by using a modification of a procedure described previously (31). Samples were fixed in a solution containing 8% formaldehyde, 25 mM EGTA, 5 mM MgSO4, and 5% dimethyl sulfoxide in 50 mM PIPES (pH 6.7) at room temperature for 30 min. The samples were washed three times with 50 mM piperazine-N,N′-bis(2-ethanesulfonic acid) (PIPES; pH 6.7) and then transferred to a digestion solution containing 10 mg of Driselase (ICN Biomedicals Inc., Costa Mesa, Calif.)/ml, 16 mg of β-d-glucanase (Interspex Products Inc., Foster City, Calif.)/ml, and 81.4 U of lyticase (ICN Biomedicals Inc.)/ml in 50 mM sodium citrate (pH 5.8) with 50% (vol/vol) egg white. All washing steps were carried out for 10 min. Prior to use, β-d-glucanase was pretreated to inactivate proteases by the following method. β-d-Glucanase (200 mg/ml) in 100 mM sodium citrate (pH 4.5) was incubated at 55°C for 5 min, kept on ice for 30 min, and stored at −80°C. The samples were incubated in the digestion solution at 25°C for 1 h. After incubation, they were washed three times with PE buffer (50 mM PIPES [pH 6.7] containing 25 mM EGTA) and incubated in −20°C methanol for 10 min. They were further washed three times with PE buffer prior to incubation for 8 h in PE buffer containing 0.5% (vol/vol) NP-40 and an antibody. The antibody was mouse monoclonal anti-α-tubulin antibody DM-1A conjugated to fluorescein isothiocyanate (FITC) diluted at 1:200. After three washes with 50 mM PIPES (pH 6.7), the samples were postfixed with 2 mM 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide hydrochloride in 50 mM PIPES (pH 6.7) for 20 min. They were then rinsed briefly with the same buffer, stained with 10 ng of Hoechst 33258/ml in 50 mM PIPES (pH 6.7) for 30 min, and rinsed with 50 mM PIPES (pH 6.7). Germlings were observed under a standard Olympus microscope. Photographs were obtained with Cool SNAP (Roper Scientific Inc., Tucson, Ariz.) and processed by Adobe Photoshop (Adobe Systems Inc., Mountain View, Calif.).
CMI.
The chromosome mitosis index (CMI) was determined by using a modification of a procedure described previously (31). Conidia of a wild-type A. nidulans strain were incubated at 30°C for 10 to 12 h. They were further incubated with or without each drug. Samples were withdrawn from the cultures at 30-min intervals. The withdrawals were continued over 2 h until the untreated cells had passed through at least one cell cycle (2). After incubation, the cell suspension was fixed in a solution containing 8% formaldehyde, 25 mM EGTA, 5 mM MgSO4, and 5% dimethyl sulfoxide in 50 mM PIPES (pH 6.7) at room temperature for 30 min. The suspension was washed three times with 50 mM PIPES (pH 6.7). The cells were stained with 10 ng of Hoechst 33258/ml in 50 mM PIPES (pH 6.7) for 30 min and rinsed with 50 mM PIPES (pH 6.7). Germlings were observed under a standard Olympus microscope. The percentage of germlings harboring nuclei with condensed chromosomes was calculated. All assays were performed at least three times on separate occasions.
Protein preparation, SDS-PAGE, and Western blot analysis.
Proteins were prepared by using a modification of a procedure described previously (27). For the preparation of total mycelial proteins, the complete medium seeded with 1 × 106 conidia of a wild-type A. nidulans strain/ml was incubated with or without each drug at 30°C for 24 h. The mycelia were harvested by filtration, washed with distilled water, and then frozen with liquid nitrogen. The frozen mycelia were lyophilized, ground to a fine powder by using a mortar and pestle, and then mixed with 4 volumes of protein isolation buffer (see below) and one-half volume of glass beads on ice. To facilitate protein extraction, the mixture was vortexed for 30 s and then allowed to stand for 1 min on ice. This step was repeated three times. Undisrupted mycelia and large mycelial fragments were removed by ultrafiltration with an Ultrafree-MC 0.45-μm filter (Millipore, Bedford, Mass.). The Bradford method was used to determine protein concentrations (3). The relative protein concentrations of different samples were confirmed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and Coomassie blue staining. The protein isolation buffer used contained 300 mM NaCl, 5 mM EDTA, 5 mM EGTA, 0.1% NP-40, 0.2% Triton X-100, 1 mM dithiothreitol, 0.1 mg of N-α-p-tosyl-l-arginine methyl ester/ml, 0.3 mg of benzamidine/ml, 0.01 mg of N-α-p-tosyl-l-lysine chloromethyl ketone/ml, 0.01 mg of soybean trypsin inhibitor/ml, 0.01 mg of N-α-p-tosyl-l-phenylalanine chloromethyl ketone/ml, 0.01 mg of pepstatin/ml, 0.01 mg of leupeptin/ml, 0.01 mg of aprotinin/ml, 5 μg of antipain/ml, 0.01 mg of chymostatin/ml, and 4 mM o-phenanthroline in 50 mM Tris-HCl (pH 7.4). For SDS-PAGE, the samples were added to an equal volume of 125 mM Tris-HCl (pH 6.8) containing 4% SDS, 10% sucrose, 10% 2-mercaptoethanol, and 0.004% bromophenol blue. The mixture was boiled at 95°C for 5 min and cooled quickly on ice.
SDS-PAGE and Western blot analysis were performed by using standard protocols. Each sample was electrophoresed on SDS-15% (wt/vol) PAGE gels. After electrophoresis, proteins were blotted onto polyvinylidene difluoride membranes (Millipore) by using a semidry transblot system (ATTO, Tokyo, Japan). The blots were blocked by incubation for at least 8 h at 4 to 8°C in phosphate-buffered saline (PBS) containing 5% nonfat dry milk and 0.05% Tween 20. Primary and secondary antibodies were diluted with the same buffer. The primary antibodies used were mouse monoclonal anti-α-tubulin DM-1A diluted at 1:1,000, mouse monoclonal anti-β-tubulin TU27B (provided by B. R. Oakley, Ohio State University, Columbus, Ohio) diluted at 1:200, and mouse monoclonal anti-actin C4 (ICN Biomedicals) diluted at 1:100. The blots were incubated with each primary antibody at room temperature for at least 4 h and then washed three times with PBS containing 0.05% Tween 20. All washing steps were carried out for 10 min. The secondary antibody used was goat anti-mouse immunoglobulin G conjugated to horseradish peroxidase (DAKO, Glostrup, Denmark) diluted at 1:1,500. The blots were incubated with the secondary antibody at room temperature for at least 2 h, washed three times with PBS containing 0.05% Tween 20, and then washed once with PBS. The blots were developed with the enhanced chemiluminescence system (Perkin-Elmer, Wellesley, Mass.).
RESULTS
Effects of various cytoskeletal inhibitors on growth recovery of A. nidulans benA33 mutant from mitotic arrest determined by agar plate assay.
The A. nidulans benA33 mutant grows vegetatively at 25°C but undergoes mitotic arrest at 37°C because of the formation of hyperstable microtubules (6, 30). We examined the effects of microtubules and some cytoskeletal inhibitors on the growth recovery of this mutant from mitotic arrest at 37°C by the agar plate assay, as shown in Table 1 and Fig. 1. Microtubule inhibitors, including four benzimidazoles (benomyl, carbendazim, nocodazole, and thiabendazole), induced the recovery of the mutant from mitotic arrest and enhanced hyphal growth around the paper disks (Table 1). Griseofulvin, ansamitocin P-3, and rhizoxin also had such a growth recovery effect. The other microtubule inhibitors, including Colcemid, colchicine, paclitaxel, podophyllotoxin, TN-16, vinblastine, and vincristine, and some cytoskeletal inhibitors, including cytochalasin B, monastrol, and nikkomycin Z, did not prevent mitotic arrest at all (Table 1).
TABLE 1.
Effects of cytoskeletal inhibitors on mitotic arrest of A. nidulans benA33 mutant in a complete agar medium
| Compound | Concn range (μg/disk) | Growth recovery zonea |
|---|---|---|
| Tubulin inhibitors | ||
| Benomyl | 25-200 | +* |
| Carbendazim | 25-200 | +* |
| Nocodazole | 25-200 | + |
| Thiabendazole | 50-200 | + |
| Griseofulvin | 100-200 | + |
| Rhizoxin | 25-200 | +# |
| Ansamitocin P-3 | 25-200 | +# |
| Colchicine | 50-200 | — |
| Colcemid | 50-200 | — |
| Vinblastine | 50-200 | — |
| Vincristine | 50-200 | — |
| Paclitaxel | 50-200 | — |
| Podophyllotoxin | 50-200 | — |
| TN-16 | 50-200 | — |
| Other cytoskeletal inhibitors | ||
| Nikkomycin Z | 50-200 | — |
| Monastrol | 50-200 | — |
| Cytochalasin B | 50-200 | — |
+, a growth recovery zone was observed; —, a growth recovery zone was not observed; *, the density of the hyphae was relatively low; #, the growth-inhibitory zone was observed around a paper disk.
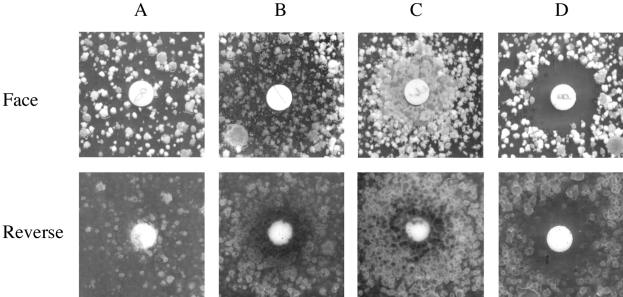
FIG. 1.
Effects of microtubule inhibitors on mitotic arrest of A. nidulans benA33 mutant in a complete agar medium. Paper disks containing no drug (A), 50 μg of benomyl (B), 100 μg of nocodazole (C), and 50 μg of rhizoxin (D) were placed on the complete agar medium seeded with the conidia of the A. nidulans benA33 mutant. The agar plates were incubated at 37°C for 2 to 3 days. Photographs were taken from the top (face) and bottom (reverse) of the agar plate.
For the untreated benA33 mutants, small colonies scattered on the agar medium surface at 37°C (Fig. 1A, face), but the formation of colonies was less extensive within the agar medium (Fig. 1A, reverse). Many colonies were observed around the disks containing benomyl (Fig. 1B, reverse) and carbendazim (data not shown) within the agar medium. However, the density of colonies around the disks on the surface of the agar medium was relatively low (Fig. 1B, face, and C, face). Nocodazole (Fig. 1C), thiabendazole, and griseofulvin (data not shown) caused the mutant to form many colonies around the disks both on the surface of and within the agar medium. Rhizoxin (Fig. 1D) and ansamitocin P-3 (data not shown) produced growth-inhibitory zones around the disks, and colony formation was observed outside the growth-inhibitory zones.
Effects of microtubule inhibitors on growth recovery of A. nidulans benA33 mutant from mitotic arrest determined by multiwell-based Alamar blue assay.
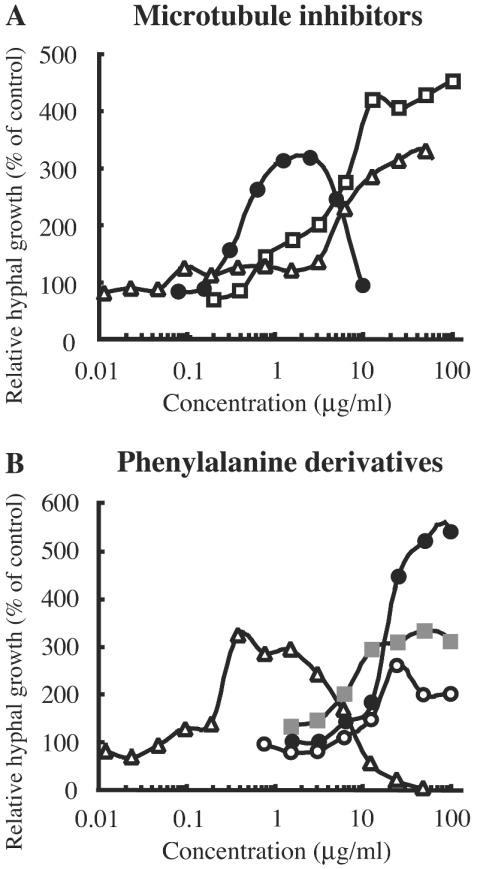
To quantify the potency of the growth recovery effect of microtubule inhibitors on the benA33 mutant, we used the multiwell-based Alamar blue assay (Fig. 2A). Benomyl induced growth recovery from mitotic arrest and enhanced hyphal growth at concentrations from 3 to 50 μg/ml. Griseofulvin exhibited a similar effect at concentrations higher than 1 μg/ml. On the other hand, nocodazole induced growth recovery in a narrower range of concentrations, from 3 to 10 μg/ml, indicating that this microtubule inhibitor is more effective than benomyl and griseofulvin at low concentrations.
FIG. 2.
Effects of known microtubule inhibitors (A) and phenylalanine derivatives (B) on mitotic arrest of A. nidulans benA33 mutant in an aqueous complete medium. (A) Various concentrations of benomyl (triangles), nocodazole (closed circles), and griseofulvin (open squares) were added to an aqueous complete medium in a 96-well plate seeded with conidia of the A. nidulans benA33 mutant. (B) Various concentrations of arphamenine A (open circles), DHPA (closed circles), ZPCK (triangles), and TPCK (gray squares) were added to the medium. The microplates were incubated at 37°C for 24 h. Relative hyphal growth was assessed by the fluorescence derived from Alamar blue dye after cultivation.
Screening for microtubule-disrupting agents by the agar plate assay.
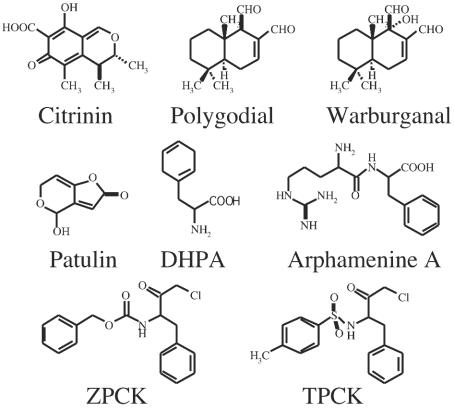
Microtubule-disrupting agents from microbial sources and chemical libraries were screened by the agar plate assay based on the mitotic arrest of the A. nidulans benA33 mutant. Among the culture broths of 400 actinomycetes and 200 compounds, 8 compounds were found to reverse the mitotic block and promote fungal growth. We identified two mycotoxins, citrinin and patulin, two sesquiterpene dialdehydes, polygodial and warburganal, and four phenylalanine derivatives, arphamenine A, DHPA, TPCK, and ZPCK. The chemical structures of these compounds are shown in Fig. 3. Except for arphamenine A, the effects of the phenylalanine derivatives on growth recovery from mitotic arrest were similar to that of nocodazole (Table 2). The effects of arphamenine A and patulin were similar to that of benomyl (Table 2). The effects of citrinin, polygodial, and warburganal were similar to that of rhizoxin (Table 2).
FIG. 3.
Chemical structures of various compounds screened by using a mitotic-arrest benA33 mutant of A. nidulans.
TABLE 2.
Effects of phenylalanine derivatives and other antifungals on mitotic arrest of A. nidulans benA33 mutant in a complete agar medium
| Compound | Concn range (μg/Disk) | Growth recovery zonea |
|---|---|---|
| Phenylalanine derivatives | ||
| Arphamenine A | 100-300 | +* |
| DHPA | 100-300 | + |
| TPCK | 100-300 | + |
| ZPCK | 100-300 | + |
| Other antifungals | ||
| Citrinin | 25-100 | +*# |
| Patulin | 100-200 | + |
| Polygodial | 25-100 | +# |
| Warburganal | 25-100 | +# |
+, a growth recovery zone was observed; *, the density of the hyphae was relatively low; #, the growth-inhibitory zone was observed around a paper disk.
Effects of phenylalanine derivatives on mitotic arrest of A. nidulans benA33 mutant determined by multiwell-based Alamar blue assay.
The effects of arphamenine A, DHPA, ZPCK, and TPCK were also quantified by the multiwell-based Alamar blue assay (Fig. 2B). Arphamenine A, DHPA, ZPCK, and TPCK showed the same growth recovery effects as those obtained by the agar plate assay. The growth recovery effects of arphamenine A, DHPA, and TPCK were similar to those of benomyl and griseofulvin. The expression pattern of the growth recovery effect of ZPCK was similar to that of nocodazole.
Antifungal susceptibility tests of phenylalanine derivatives against other fungal strains.
An antifungal susceptibility test was conducted with four phenylalanine derivatives against various fungal strains after 48 h of incubation (Table 3). DHPA, TPCK, and ZPCK showed antifungal activities against a wild-type strain, A. nidulans FGSC A4. Arphamenine A had little antifungal susceptibility except to the dimorphic fungi Y. lipolytica IFO 1746 and A. nidulans FGSC A4. DHPA, TPCK, and ZPCK had similar antifungal spectra except for Fusarium spp. and C. albicans IFO 1061. DHPA especially showed fungicidal effects against Y. lipolytica IFO 1746 and three strains of A. niger tested (data not shown). The MICs of DHPA for these strains remained the same even after 96 h of incubation, but the antifungal activities of TPCK and ZPCK disappeared after 72 h of incubation.
TABLE 3.
MICs of four phenylalanine derivatives for other fungal strains
| Strain | MIC (μg/ml) ofa:
|
|||
|---|---|---|---|---|
| Arphamenine A | DHPA | TPCK | ZPCK | |
| C. albicans IFO 1061 | >100 | 12.5 | >100 | >100 |
| C. albicans NBRC 1385 | >100 | 100 | 100 | 100 |
| Y. lipolytica IFO 1746 | 12.5 | 12.5 | 25 | 1.56 |
| A. fumigatus IFO 5840 | >100 | 25 | 50 | 25 |
| A. nidulans FGSC A4 | 100 | 62.5 | 25 | 6.25 |
| A. niger ATCC 6275 | >100 | 25 | 50 | 12.5 |
| A. niger IFO 4416 | ND | 12.5 | ND | ND |
| A. niger IFO 9455 | ND | 6.25 | ND | ND |
| F. oxysporum NBRC 5942 | >100 | >100 | 50 | 50 |
| F. solani NBRC 5899 | >100 | >100 | 50 | 100 |
| F. solani NBRC 8505 | >100 | >100 | 25 | 6.25 |
ND, not determined.
CMIs of a wild-type A. nidulans strain treated with DHPA and nocodazole.
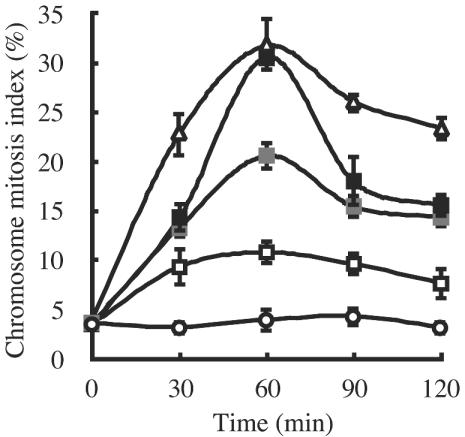
CMIs for a wild-type A. nidulans strain, FGSC A4, treated with DHPA and nocodazole were determined (Fig. 4). Under the control conditions, the CMIs were kept at less than 5%. DHPA at 62.5 μg/ml (he MIC) gradually elevated the CMI. The maximum index was 11% after 60 min of incubation. However, the CMIs under treatment conditions were significantly higher than those obtained under the control conditions. CMIs reached 20 and 30% at DHPA concentrations of 250 μg/ml (4 times the MIC) and 1,000 μg/ml (16 times the MIC), respectively. These results indicate that CMIs increased with the elevation of the concentration of DHPA. Nocodazole as a positive control elevated the CMI to 30% at 0.1 μg/ml (the MIC) after 60 min of incubation. The CMIs for DHPA and nocodazole gradually increased within 60 min and thereafter gradually dropped.
FIG. 4.
CMIs of the germlings in a wild-type A. nidulans strain treated with DHPA and nocodazole. Conidia of a wild-type A. nidulans strain were incubated at 30°C for 10 to 12 h. They were further incubated with or without each drug. As a control, the sample was treated with the solvent in which DHPA was dissolved (open circles). Concentrations of DHPA were 62.5 μg/ml (the MIC; open squares), 250 μg/ml (4 times the MIC; gray squares), and 1,000 μg/ml (16 times the MIC; closed squares). Concentrations of nocodazole were 0.1 μg/ml (the MIC; triangles). The percentages of germlings in which nuclei had condensed chromosomes are presented. Results are expressed as means ± standard deviations for three independent observations.
Fluorescence microscopy of microtubules and nuclei in the germlings of a wild-type A. nidulans strain.
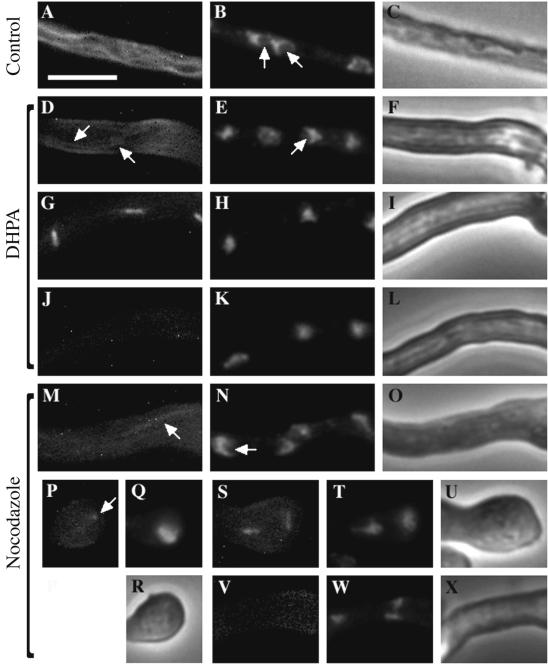
The effects of DHPA on the microtubules and nuclei in the germlings of a wild-type A. nidulans strain, FGSC A4, were observed with fluorescence microscopy by using Hoechst 33258 and anti-α-tubulin antibody DM-1A conjugated to FITC, respectively (Fig. 5 and 6). Germlings and conidia were incubated for 60 min and 18 h, respectively. In the interphase germlings, nuclei with uncondensed chromatin and detectable nucleoli were observed (Fig. 5B and 6B). Generally, the nucleoli are not stained but are detectable as dark regions. The cytoplasmic microtubules formed filamentous and mesh networks in parallel to their growth axes in the germlings of the untreated control (Fig. 5A and 6A). An anaphase germling showed the nuclei (arrows) moving to the poles of spindles (Fig. 6E) and normal mitotic microtubules (Fig. 6D). In the DHPA-treated germlings, nuclei with condensed chromatin and a few detectable nucleoli were observed (Fig. 5E, H, and K and 6H). A network of cytoplasmic microtubules was observed (Fig. 5D and 6G), and normal mitotic microtubules were detected (Fig. 5G). Some germlings did not have any detectable microtubules (Fig. 5J). Nocodazole as a positive control showed an effect similar to that of DHPA against microtubules and nuclei. Nuclei with condensed chromatin and a few detectable nucleoli were observed (Fig. 5N, Q, T, and W). The network of cytoplasmic microtubules was observed (Fig. 5M), and normal mitotic microtubules were detected (Fig. 5S). Most of the germlings did not have any detectable microtubules (Fig. 5V). Few germlings showed dotted microtubules (Fig. 5P).
FIG. 5.
Effects on microtubules and nuclei of a wild-type A. nidulans strain treated with DHPA and nocodazole for 60 min under phase-contrast (C, F, I, L, O, R, U, and X) and fluorescence (A, B, D, E, G, H, J, K, M, N, P, Q, S, T, V, and W) microscopic observation. The germlings of a wild-type A. nidulans FGSC A4 strain were treated with DHPA (D to L), with nocodazole (M to X), or without a drug (A to C). Concentrations of DHPA and nocodazole were 62.5 μg/ml (the MIC) (D to L) and 0.1 μg/ml (the MIC) (M to X), respectively. After 60 min of incubation, nuclei and microtubules were observed with fluorescence microscopy by using Hoechst 33258 (B, E, H, K, N, Q, T, and W) and anti-α-tubulin antibody DM-1A conjugated to FITC (A, D, G, J, M, P, S, and V), respectively. An interphase germling shows nuclei with uncondensed chromatin (B). The nucleoli (indicated by arrows) are not stained but are detectable as dark regions (B). A normal network of cytoplasmic microtubules is present (A). Interphase germling after 60 min of treatment with 62.5 μg of DHPA/ml had a network of microtubules (indicated by arrows) (D), normal mitotic microtubules (G), and no visible microtubules (J). Nuclei with condensed chromatin and no detectable nucleoli were observed (H and K). Some germlings had nuclei with detectable nucleoli (indicated by an arrow) (E). Germlings after 60 min of treatment with 0.1 μg of nocodazole/ml had a network of microtubules (indicated by an arrow) (M), a few dotted microtubules (P), normal mitotic microtubules (S), and no visible microtubules (V). Nuclei with condensed chromatin and no detectable nucleoli were observed (Q, T, and W). Some germlings had nuclei with detectable nucleoli (indicated by an arrow) (N). The magnification is the same for all micrographs. Bar, 15 μm.
FIG. 6.
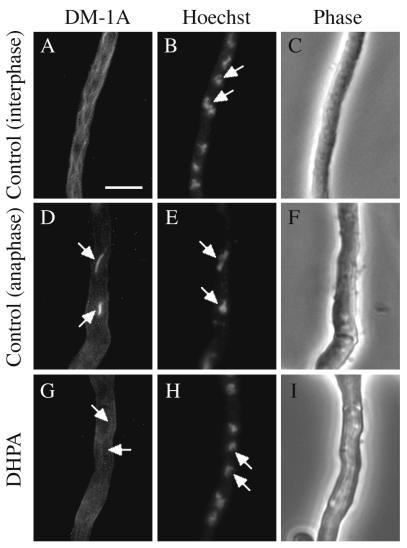
Effects on microtubules and nuclei of a wild-type A. nidulans strain treated with or without DHPA for 18 h observed with phase-contrast microscopy (C, F, and I) and fluorescence microscopy (A, B, D, E, G, and H). The conidia of a wild-type A. nidulans strain, FGSC A4, were treated with (G to I) or without (A to F) 62.5 μg of DHPA (the MIC). After 18 h of incubation, nuclei and microtubules were observed with fluorescence microscopy by using Hoechst 33258 (B, E, and H) and anti-α-tubulin antibody DM-1A conjugated to FITC (A, D, and G), respectively. An interphase germling shows nuclei with uncondensed chromatin (B). The nucleoli (indicated by arrows) are not stained but are detectable as dark regions (B). A normal network of cytoplasmic microtubules is present (A). Anaphase germlings show nuclei (indicated by arrows) moving to the poles of spindles (E) and normal mitotic microtubules (D). Conidia after 18 h of treatment with DHPA at 62.5 μg/ml show visible networks of microtubules (indicated by arrows) (G). Nuclei (indicated by arrows) with uncondensed chromatin and detectable nucleoli were observed (H). The magnification was the same for all micrographs. Bar, 15 μm.
Western blot analysis of α- and β-tubulins and actin in the germlings of a wild-type A. nidulans strain.
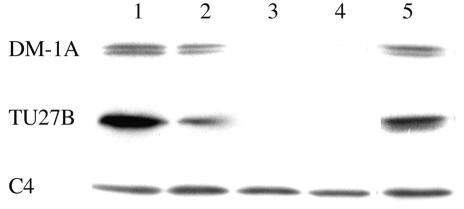
The effects of DHPA and nocodazole on α- and β-tubulins and actin in the germlings of a wild-type A. nidulans strain, FGSC A4, were demonstrated by Western blot analysis with specific antibodies (Fig. 7). Samples were obtained after 24 h of incubation with or without each drug. Actin proteins were consistently found in all samples. In the untreated control, both α- and β-tubulins were detected (lane 1). However, in the DHPA-treated mycelia, α- and β-tubulins disappeared with an increase in drug concentration (lanes 2 to 4). In the nocodazole-treated mycelia, α- and β-tubulins were detected (lane 5).
FIG. 7.
Effects of DHPA and nocodazole on cytoskeletal proteins of a wild-type A. nidulans strain as determined by Western blot analysis. Samples were obtained after 24 h of incubation with DHPA (lanes 2 to 4), with nocodazole (lane 5), or without any drug (lane 1). Concentrations of DHPA were 62.5 μg/ml (the MIC; lane 2), 250 μg/ml (4 times the MIC; lane 3), and 1,000 μg/ml (16 times the MIC; lane 4). The concentration of nocodazole was 0.1 μg/ml (the MIC; lane 5). The samples were analyzed by SDS-PAGE followed by Western blot analysis with specific antibodies against α-tubulin, β-tubulin, and actin (DM-1A, TU27B, and C4, respectively).
DISCUSSION
A. nidulans benA33 mutants undergo mitotic arrest under high-temperature conditions because hyperstable microtubules are formed (6, 30). The heat sensitivity of the mutant is remedied by some antimicrotubule agents (30). We applied this characteristic in screening for microtubule-disrupting agents. First, the agar plate assay was used to evaluate microtubule inhibitors and other target-associated drugs (Table 1 and Fig. 1). Oakley and Morris inoculated the benA33 mutant onto agar plates containing some antimicrotubule agents (30). Conversely, we put paper disks containing the agents onto the agar plates inoculated with the conidia of the benA33 mutant. The benzimidazoles benomyl, carbendazim, nocodazole, and thiabendazole are well-known microtubule inhibitors (18). They prevented the mitotic arrest of the mutant and enhanced hyphal growth, as previously reported (30). Although both benomyl and carbendazim enhanced hyphal growth at relatively low densities on the surface and at high densities within the agar medium, nocodazole and griseofulvin enhanced it at high densities both on the surface and within the agar medium. The reason for the difference between growth recovery on the surface and that within the agar media is still unclear. This difference could result partly from the different affinities to the tubulins of A. nidulans. Nocodazole has a higher affinity to tubulin than do benomyl and carbendazim (5). In fact, nocodazole induced the recovery of the mutant from mitotic arrest at lower concentrations than the two drugs used in the Alamar blue assay (Fig. 2). Ansamitocin P-3 and rhizoxin also had growth recovery effects, but their effects were different from those of benzimidazoles and griseofulvin. Ansamitocin P-3 and rhizoxin induced growth recovery zones outside the growth-inhibitory zones around paper disks. These drugs have the same binding site for tubulin (14). Their binding site is distinct from those of benzimidazoles and griseofulvin (14). This difference could explain the unique effect. Paclitaxel did not prevent mitotic arrest. Paclitaxel alone can tightly bind to the microtubules of A. nidulans and promote the formation of highly stable microtubules, thereby preventing cell division in mitosis (33, 44). Treatment with paclitaxel was able to cause the formation of extremely stable microtubules in accordance with the characteristics of the benA33 mutant. Colchicine, vinblastine, podophyllotoxin, and TN-16 had no effect on the mitotic arrest of the mutant either, because the microtubules of A. nidulans are insensitive to these drugs (34). Some cytoskeletal inhibitors, such as cytochalasin B, monastrol, and nikkomycin Z, which inhibit actin, kinesin, and cell wall synthesis, respectively, also did not prevent mitotic arrest. Other inhibitors, including actinomycin D, amphotericin B, cycloheximide, and miconazole, also had no effect (data not shown).
Moreover, this agar plate assay was able to distinguish the following three types of microtubule inhibitors. Benzimidazoles such as benomyl and carbendazim, which have low affinity to tubulin, induced growth zones in the hyphae at relatively low densities. Nocodazole and griseofulvin, which have high affinities to tubulin, induced growth zones in the hyphae at high densities. Ansamitocin P-3 and rhizoxin, which have the same binding site for tubulin, which is different from those of benzimidazoles and griseofulvin, induced growth zones around the growth-inhibitory zones.
Alamar blue dye, a noncytotoxic reagent that yields a fluorescent product after reduction, is used as an indicator of the redox state of cells (19). Since it is often difficult to obtain reproducibility in the turbidity measurement of filamentous fungi, this dye is also used to evaluate the cell proliferation and antifungal susceptibility of filamentous fungi (4, 19, 22). Therefore, we used this dye to quantify the concentration required for the growth recovery of the benA33 mutant from mitotic arrest. It was observed that there is are optimum concentrations of benomyl and nocodazole for the growth recovery of the mutant from mitotic arrest. Benomyl (Fig. 2) and carbendazim (data not shown) induced growth recovery at high concentrations. On the other hand, nocodazole induced growth recovery at lower concentrations than the other microtubule inhibitors tested. Such a growth recovery effect at low concentrations would result only from a high affinity of nocodazole to microtubules in A. nidulans (5). Conversely, the growth inhibition at high concentrations of nocodazole probably resulted from the mitotic arrest induced by extensive microtubule depolymerization. Rhizoxin showed an effect similar to that of nocodazole (data not shown). When the agar plate assay was used, we clearly distinguished the effects of nocodazole and rhizoxin. Griseofulvin and benomyl induced growth recovery at relatively high concentrations. We therefore confirmed that the multiwell-based Alamar blue assay, as well as the agar plate assay, is able to screen for microtubule inhibitors. Although this assay cannot distinguish the types of microtubule inhibitors, it is useful in determining the effective concentrations of the drugs. This method is also suitable for the high-throughput screening of microtubule inhibitors.
We screened for antifungal microtubule-disrupting agents among the culture broths of 400 actinomycetes and 200 compounds by using the agar plate assay. Two mycotoxins, citrinin and patulin, two sesquiterpene dialdehydes, polygodial and warburganal, and four phenylalanine derivatives, arphamenine A, DHPA, TPCK, and ZPCK, were identified (Table 2 and Fig. 3). Citrinin and patulin have been reported to show antifungal activities (1, 10, 11, 43). Practically, citrinin and patulin had antifungal activities, and their MICs for a wild-type strain of A. nidulans were 50 and 100 μg/ml, respectively. These mycotoxins inhibit microtubule assembly in vitro (35). Patulin interacts with the thiol groups of microtubules (35). Polygodial and warburganal also have been reported to show antifungal activities (20, 21, 23), and practically, their MICs for the wild-type strain were 6.25 and 3.13 μg/ml, respectively. The two sesquiterpene dialdehydes and citrinin react with thiol groups (23, 38). Therefore, polygodial, warburganal, and citrinin could also bind to the thiol groups of microtubules to inhibit microtubule assembly. However, their effects on the benA33 mutant were different from those of patulin on agar plates (Table 2). Polygodial, warburganal, and citrinin, as well as ansamitocin P-3 and rhizoxin, induced growth recovery zones around inhibitory zones. Thus, these three drugs could inhibit microtubules in a manner similar to that of ansamitocin P-3 and rhizoxin. On the other hand, the effect of patulin was similar to those of benomyl and carbendazim. Patulin could prevent microtubule polymerization by interacting with the benomyl (or carbendazim)-binding site. As stated above, citrinin and patulin are mycotoxins and are toxic to animals and plants (1). Polygodial and warburganal might have effects similar to those of the mycotoxins. Thus, we did not further investigate the mycotoxins and the sesquiterpene dialdehydes.
In this report, we focused on phenylalanine derivatives. These derivatives showed growth recovery effects on the benA33 mutant that were similar to those of well-known microtubule inhibitors. Among the fungi tested, arphamenine A showed antifungal activities against only the dimorphic fungi Y. lipolytica IFO 1746 and A. nidulans FGSC A4 (Table 3). The antifungal spectrum of TPCK was similar to that of ZPCK (Table 3). The antifungal activities of both TPCK and ZPCK disappeared after 72-h incubation. In addition, their effects were not fungicidal (data not shown). Among the four phenylalanine derivatives screened, three compounds, arphamenine A, TPCK, and ZPCK, are well-known protease inhibitors. Arphamenine A has previously been reported to be an aminopeptidase B inhibitor in microbial culture broth (39). TPCK and ZPCK are specific inhibitors of chymotrypsin-type serine proteases (15, 37). On the other hand, DHPA, a phenylalanine analogue, is known as an antimicrobial agent with a broad spectrum (7). In fact, DHPA showed antifungal activities against several strains of fungi (Table 3). The mechanism of the antifungal action has not been explained. The MICs of DHPA for the strains, including those of A. nidulans, were unchanged even after 96 h of incubation. Likewise, another phenylalanine analogue, p-fluoro-phenylalanine (FPA), induces the growth recovery of the benA33 mutant (30). Almost all of the benA mutants are resistant to FPA (28). Morris and Oakley supposed that FPA should inhibit growth and cause nondisjunction by a direct effect on the polymerization of tubulin (28). However, the effects and action mechanisms of FPA and DHPA on microtubules have not yet been elucidated. Thus, we examined the effect of DHPA on microtubules in detail.
The microtubule is also the major component of the mitotic apparatus and is involved in the separation of chromatids during mitosis (13). Therefore, the mitotic apparatus fails to assemble and then to function when treatment with microtubule inhibitors is applied. Consequently, cells are caused to arrest in mitosis and the CMI values increase compared with those for untreated cells (31). DHPA at the MIC (62.5 μg/ml) slightly elevated the CMI value (Fig. 4), which was still significantly higher than that of the control. In addition, the increase in the CMI values depends on the dose of DHPA. DHPA at a concentration of 16 times the MIC was needed for the elevation of the CMI to 30%, which could be obtained by treatment with nocodazole at the MIC. These results indicate that DHPA's antifungal effect on a wild-type strain and its growth recovery effect on the benA33 mutant could not be explained only by the assembly and disassembly of the mitotic apparatus.
To clarify the above-mentioned differences, we examined the effect of DHPA on the microtubules of a wild-type A. nidulans strain by using fluorescence microscopy (Fig. 5 and 6). Since it takes approximately 2 h for a wild-type strain of A. nidulans to pass through at least one cell cycle, almost all of the microtubule inhibitors start affecting microtubules and nuclei within 2 h after treatment with the agents (31). Therefore, we observed the germlings after 60 min, at which time the CMIs of DHPA and nocodazole reached their maxima (Fig. 4). In the germlings of the control, normal nuclei and microtubules were virtually present (Fig. 5A and B). The DHPA-treated germlings, however, contained nuclei with condensed chromatin and a few detectable nucleoli (Fig. 5E, H, and K). Most of the germlings did not have any detectable microtubules (Fig. 5J). They had a few microtubules, which were classified into two types: the network of cytoplasmic microtubules (Fig. 5D) and normal mitotic microtubules (Fig. 5G). In the nocodazole-treated germlings, microtubules and nuclei were very similar to those of DHPA-treated germlings (Fig. 5M, N, S, T, V, and W). However, few germlings had dotted microtubules (Fig. 5P). At a subgrowth inhibitory concentration of nococazole, the average numbers of cytoplasmic microtubules in wild-type and tub4 mutant strains of Saccharomyces cerevisiae are reduced (42). This fact is consistent with our results concerning the decrease in cytoplasmic microtubules. It also seems to be consistent with previously reported results obtained with benomyl (17, 31). Jung et al. demonstrated that the effect of benomyl against microtubules depends on the drug concentration (17). A relatively high concentration of benomyl (2.4 μg/ml) causes rapid and complete elimination of the cytoplasmic and mitotic microtubules in A. nidulans (31). On the other hand, low concentrations of benomyl partially translocate the equilibrium away from microtubule polymer (17), thereby inducing a decrease in cytoplasmic microtubules. Moreover, nanomolar concentrations of nocodazole reduce microtubule turnover rates in animal cells and in vitro, while catastrophe and rescue frequencies may increase or decrease depending on the cell type or the experimental system (26, 41). Thus, in our experiments, nocodazole caused a decrease in cytoplasmic microtubules. Since DHPA showed an effect similar to that of nocodazole, it might affect microtubule turnover.
Decreases in the amounts of microtubules were also confirmed by Western blot analysis (Fig. 7). All samples were collected after 24 h of incubation because there was no difference between the conditions of microtubules and nuclei after 60 min of incubation with DHPA (Fig. 5) and those after 24 h of incubation (Fig. 6) with DHPA under fluorescence microscopic observation. In the DHPA-treated germlings, both α- and β-tubulins disappeared with an increase in drug concentration, indicating a blockade of tubulin biosynthesis including inhibition of transcription, reduction of tubulin mRNA stability, inhibition of translation, or enhanced proteolysis of α- and β-tubulins. DHPA indirectly inhibits protein biosynthesis by the nonspecific incorporation of DHPA into proteins in Escherichia coli and sarcoma 180 (36). However, DHPA restored the growth arrest of the benA33 mutant, suggesting that its action must be tubulin-specific. Thus, the inhibition of tubulin synthesis may be the most likely mechanism. On the other hand, conventional protein synthesis inhibitors, including cycloheximide, did not show the same effect as DHPA (data not shown). Another cytoskeletal protein, actin, was consistently detectable even at high DHPA concentrations (Fig. 7). Therefore, the effect of DHPA on microtubules may be dependent on the proteolysis of tubulins. We also tested the effects of two other phenylalanine derivatives, TPCK and ZPCK. Both of these derivatives also had effects very similar to those of DHPA when analyzed by fluorescence microscopy and Western blot analysis with anti-α-tubulin antibody DM-1A (data not shown). As mentioned above, TPCK and ZPCK are the specific inhibitors of chymotrypsin-type proteases (15, 37). The effect of TPCK on fungi has been reported (8). Several mitotic mutants of Schizosaccharomyces pombe are sensitive to this inhibitor. One of the mutants, tsm1-512, has a mutation in the presumptive component of the anaphase-promoting complex (APC) (8). The APC is required for the ubiquitin-dependent proteolysis of certain proteins during exit from mitosis. The tsm1-512 gene is also important for microtubule integrity (8). The growth recovery effects of the phenylalanine derivatives could depend on certain proteases, such as chymotrypsin-type proteases, or on the presumptive component of the APC. In fact, we are currently acquiring some preliminary data about the relationship between recovery from the mitotic arrest caused by phenylalanine derivatives and ubiquitin-dependent proteolysis (data not shown). In contrast to DHPA, nocodazole did not show the remarkable loss of tubulins in the Western blot analysis. This result might explain why DHPA made the CMI increase to a lesser extent than nocodazole did. While microtubule inhibitors such as nocodazole cause very rapid disassembly of microtubules, DHPA presumably affects microtubule turnover via the inhibition of tubulin synthesis or ubiquitin-dependent proteolysis related to the presumptive component of the APC, thereby causing a gradual loss of tubulins. We are currently investigating the mechanism in detail.
Our screening method involving a mitotic-arrest mutant of A. nidulans was convenient and selective for the screening of microtubule-disrupting antifungal agents. The agar plate assay was suitable both for rough screening and for discriminating types of microtubule inhibitors. The multiwell-based assay was suitable for high-throughput screening. The phenylalanine derivatives screened, DHPA, TPCK, and ZPCK, are of a novel type of microtubule-disrupting antifungal agents with high potency. DHPA induced the selective loss of both α- and β-tubulins in the germlings of a wild-type A. nidulans strain. The effects of phenylalanine derivatives, including DHPA, on microtubules presumably depends on the disruption of microtubule turnover via the inhibition of tubulin synthesis or proteolysis related to the presumptive component of the APC. We hope that this study will be useful in elucidating the regulatory mechanisms of microtubule polymerization and depolymerization and, consequently, in developing antifungal agents.
Acknowledgments
This work was partly supported by the Sasakawa Scientific Research grant from the Japan Science Society.
We thank B. R. Oakley (Ohio State University, Columbus, Ohio) for providing us with mouse monoclonal anti-β-tubulin TU27B. We also thank T. Nakamura and Y. Nakase (Osaka City University, Osaka, Japan) for teaching us the general technique of SDS-PAGE and Western blot analysis and J. Clutterbuck (University of Glasgow, Glasgow, Scotland, United Kingdom) for teaching us general techniques concerning A. nidulans.
REFERENCES
- 1.Bennett, J. W., and M. Klich. 2003. Mycotoxins. Clin. Microbiol. Rev. 16:497-516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bergen, L. G., and N. R. Morris. 1983. Kinetics of the nuclear division cycle of Aspergillus nidulans. J. Bacteriol. 156:155-160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bradford, M. M. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72:248-254. [DOI] [PubMed] [Google Scholar]
- 4.Espinel-Ingroff, A., M. Bartlett, R. Bowden, N. X. Chin, Jr., C. Cooper, A. Fothergill, M. R. McGinnis, P. Menezes, S. A. Messer, P. W. Nelson, F. C. Odds, L. Pasarell, J. Peter, M. A. Pfaller, J. H. Rex, M. G. Rinaldi, G. S. Shankland, T. J. Walsh, and I. Weitzman. 1997. Multicenter evaluation of proposed standardized procedure for antifungal susceptibility testing of filamentous fungi. J. Clin. Microbiol. 35:139-143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Friedman, P. A., and E. G. Platzer. 1978. Interaction of anthelmintic benzimidazoles and benzimidazole derivatives with bovine brain tubulin. Biochim. Biophys. Acta 544:605-614. [DOI] [PubMed] [Google Scholar]
- 6.Gambino, J., L. G. Bergen, and N. R. Morris. 1984. Effects of mitotic and tubulin mutations on microtubule architecture in actively growing protoplasts of Aspergillus nidulans. J. Cell Biol. 99:830-838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Genghof, D. S. 1970. 2,5-Dihydrophenylalanine as an inhibitor of microbial growth. Can. J. Microbiol. 16:545-547. [DOI] [PubMed] [Google Scholar]
- 8.Grishchuk, E. L., J. L. Howe, and J. R. McIntosh. 1998. A screen for genes involved in the anaphase proteolytic pathway identifies tsm1(+), a novel Schizosaccharomyces pombe gene important for microtubule integrity. Genetics 149:1251-1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grover, J. K., V. Vats, G. Uppal, and S. Yadav. 2001. Anthelmintics: a review. Trop. Gastroenterol. 22:180-189. [PubMed] [Google Scholar]
- 10.Haraguchi, H., K. Hashimoto, K. Shibata, M. Taniguchi, T. Tanaka, and S. Oi. 1987. Mechanism of antifungal action of citrinin. Agric. Biol. Chem. 51:1373-1378. [Google Scholar]
- 11.Haraguchi, H., M. Taniguchi, T. Tanaka, S. Oi, and K. Hashimoto. 1989. Citrinin, an electron-acceptor having antifungal activity. Agric. Biol. Chem. 53:1741-1742. [Google Scholar]
- 12.Hollomon, D. W., J. A. Butters, and H. Barker. 1997. Tubulins: a target for anti-fungal agents, p. 152-156. In P. H. Bentley and P. J. O'Hanlon (ed.), Anti-infectives: recent advances in chemistry and structure activity relationships. Royal Society of Chemistry, London, United Kingdom.
- 13.Hyams, J. S., and C. W. Lloyd (ed.). 1993. Microtubules. Wiley-Liss, New York, N.Y.
- 14.Iwasaki, S. 1998. Natural organic compounds that affect to microtubule functions. Yakugaku Zasshi 118:112-126. [PubMed] [Google Scholar]
- 15.Johnson, L. A., K. E. Moon, and M. Eisenberg. 1988. Inactivation of chymotrypsin and human skin chymase: kinetics of time-dependent inhibition in the presence of substrate. Biochim. Biophys. Acta 953:269-279. [DOI] [PubMed] [Google Scholar]
- 16.Jung, M. K., G. S. May, and B. R. Oakley. 1998. Mitosis in wild-type and β-tubulin mutant strains of Aspergillus nidulans. Fungal Genet. Biol. 24:146-160. [DOI] [PubMed] [Google Scholar]
- 17.Jung, M. K., N. Prigozhina, C. E. Oakley, E. Nogales, and B. R. Oakley. 2001. Alanine-scanning mutagenesis of Aspergillus γ-tubulin yields diverse and novel phenotypes. Mol. Biol. Cell 12:2119-2136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Katiyar, S. K., V. R. Gordon, G. L. McLaughlin, and T. D. Edlind. 1994. Antiprotozoal activities of benzimidazoles and correlations with β-tubulin sequence. Antimicrob. Agents Chemother. 38:2086-2090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kiso, T., Y. Usuki, X. Ping, K. Fujita, and M. Taniguchi. 2001. l-2,5-Dihydrophenylalanine, an inducer of cathepsin-dependent apoptosis in human promyelocytic leukemia cells (HL-60). J. Antibiot. (Tokyo) 54:810-817. [DOI] [PubMed] [Google Scholar]
- 20.Kubo, I., and M. Taniguchi. 1988. Polygodial, an antifungal potentiator. J. Nat. Prod. 51:22-29. [DOI] [PubMed] [Google Scholar]
- 21.Kubo, I., and M. Himejima. 1992. Potentiation of antifungal activity of sesquiterpene dialdehydes against Candida albicans and 2 other fungi. Experientia 48:1162-1164. [DOI] [PubMed] [Google Scholar]
- 22.Llop, C., I. Pujol, C. Aguilar, J. Sala, D. Riba, and J. Guarro. 2000. Comparison of three methods of determining MICs for filamentous fungi using different end point criteria and incubation periods. Antimicrob. Agents Chemother. 44:239-242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Machida, K., T. Tanaka, and M. Taniguchi. 1999. Depletion of glutathione as a cause of the promotive effects of polygodial, a sesquiterpene on the production of reactive oxygen species in Saccharomyces cerevisiae. J. Biosci. Bioeng. 88:526-530. [DOI] [PubMed] [Google Scholar]
- 24.MacRae, T. H. 1992. Towards an understanding of microtubule function and cell organization: an overview. Biochem. Cell Biol. 70:835-841. [DOI] [PubMed] [Google Scholar]
- 25.Maxwell, W. A., and G. Brody. 1971. Antifungal activity of selected benzimidazole compounds. Appl. Microbiol. 21:944-945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mikhailov, A., and G. G. Gundersen. 1998. Relationship between microtubule dynamics and lamellipodium formation revealed by direct imaging of microtubules in cells treated with nocodazole or taxol. Cell Motil. Cytoskelet. 41:325-340. [DOI] [PubMed] [Google Scholar]
- 27.Mirabito, P. M., and N. R. Morris. 1993. BIMA, a TPR-containing protein required for mitosis, localizes to the spindle pole body in Aspergillus nidulans. J. Cell Biol. 120:959-968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Morris, N. R., and C. E. Oakley. 1979. Evidence that p-fluorophenylalanine has a direct effect on tubulin in Aspergillus nidulans. J. Gen. Microbiol. 114:449-454. [DOI] [PubMed] [Google Scholar]
- 29.National Committee for Clinical Laboratory Standards. 1995. Reference method for broth dilution antifungal susceptibility testing of yeasts. Tentative standard M27-T. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 30.Oakley, B. R., and N. R. Morris. 1981. A β-tubulin mutation in Aspergillus nidulans that blocks microtubule function without blocking assembly. Cell 24:837-845. [DOI] [PubMed] [Google Scholar]
- 31.Ovechkina, Y. Y., R. K. Pettit, Z. A. Cichacz, G. R. Pettit, and B. R. Oakley. 1999. Unusual antimicrotubule activity of the antifungal agent spongistatin 1. Antimicrob. Agents Chemother. 43:1993-1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Owellen, R. J., Jr., A. H. Owens, and D. W. Donigian. 1972. The binding of vincristine, vinblastine and colchicine to tubulin. Biochem. Biophys. Res. Commun. 47:685-691. [DOI] [PubMed] [Google Scholar]
- 33.Parekh, H., and H. Simpkins. 1997. The transport and binding of taxol. Gen. Pharmacol. 29:167-172. [DOI] [PubMed] [Google Scholar]
- 34.Parry, J. M. 1993. An evaluation of the use of in vitro tubulin polymerisation, fungal and wheat assays to detect the activity of potential chemical aneugens. Mutat. Res. 287:23-28. [DOI] [PubMed] [Google Scholar]
- 35.Pfeiffer, E., K. Gross, and M. Metzler. 1998. Aneuploidogenic and clastogenic potential of the mycotoxins citrinin and patulin. Carcinogenesis 19:1313-1318. [DOI] [PubMed] [Google Scholar]
- 36.Pine, M. J. 1975. Incorporation of l-2,5-dihydrophenylalanine into cell proteins of Escherichia coli and sarcoma 180. Antimicrob. Agents Chemother. 7:601-605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schoellmann, G., and E. Shaw. 1963. Direct evidence for the presence of histidine in the active center of chymotrypsin. Biochemistry 2:252-255. [DOI] [PubMed] [Google Scholar]
- 38.Taniguchi, M., T. Adachi, H. Haraguchi, S. Oi, and I. Kubo. 1983. Physiological activity of warburganal and its reactivity with sulfhydryl-groups. J. Biochem. 94:149-154. [DOI] [PubMed] [Google Scholar]
- 39.Umezawa, H., T. Aoyagi, S. Ohuchi, A. Okuyama, H. Suda, T. Takita, M. Hamada, and T. Takeuchi. 1983. Arphamenines A and B, new inhibitors of aminopeptidase B, produced by bacteria. J. Antibiot. (Tokyo) 36:1572-1575. [DOI] [PubMed] [Google Scholar]
- 40.Van Tuyl, J. M. 1977. Genetics of fungal resistance to systemic fungicides. Meded. Landbouwhogesch. Wagening. 2:1-137. [Google Scholar]
- 41.Vasquez, R. J., B. Howell, A.-M. C. Yvon, P. Wadsworth, and L. Cassimeris. 1997. Nanomolar concentrations of nocodazole alter microtubule dynamic instability in vivo and in vitro. Mol. Biol. Cell 8:973-985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vogel, J., and M. Snyder. 2000. The carboxy terminus of Tub4p is required for γ-tubulin function in budding yeast. J. Cell Sci. 113:3871-3882. [DOI] [PubMed] [Google Scholar]
- 43.Yamaji, K., Y. Fukushi, Y. Hashidoko, T. Yoshida, and S. Tahara. 1999. Characterization of antifungal metabolites produced by Penicillium species isolated from seeds of Picea glehnii. J. Chem. Ecol. 25:1643-1653. [Google Scholar]
- 44.Yoon, Y., and B. R. Oakley. 1995. Purification and characterization of assembly-competent tubulin from Aspergillus nidulans. Biochemistry 34:6373-6381. [DOI] [PubMed] [Google Scholar]