Abstract
To distinguish active from inactive/chronic infection in Toxoplasma gondii-seropositive individuals, we have developed an enzyme-linked immunosorbent assay (ELISA) using specific peptides derived from Toxoplasma matrix antigen MAG1. We used this assay to measure matrix specific antibodies and pilot studies with infected mice established the validity of two peptides. The immune response against MAG1 occurs in about 12 days postinfection and displays a sex difference later on in mouse model, with males producing higher antibody titers than females. Serum samples from 22 patients with clinical toxoplasmosis and from 26 patients with serological evidence of past exposure to Toxoplasma (more than one year infection history) were analyzed. Both MAG1 peptides detected antibodies significant frequently and robustly from active stage than from the chronic stage of toxoplasmosis. The results indicate that both MAG1 peptides may be used as a tool to differentiate active from inactive infection. It also may be considered in the design of potential vaccines in humans.
Keywords: MAG1_4, MAG1_5, Sex-difference, Diagnostic marker, Early immune response
1. Introduction
Due to the generally asymptomatic nature of toxoplasmosis, accurate differentiation between a recently acquired or reactivated infection and a past infection maintained in a quiescent state is difficult. The finding of Toxoplasma-specific IgM antibodies does not necessarily mean an acute infection since IgM antibodies can persist in some patients with past infection [1]. Two-test strategies with IgM-capture assays and direct IgG assays followed by an assay for Toxoplasma-specific IgG-avidity ratio is presently the best option for diagnosing a recent infection [2]. However, a high proportion of infected pregnant women show persistent, low-avidity IgG antibodies to Toxoplasma [3]. As serology remains a key approach to diagnose toxoplasmosis, a large number of recombinant antigens have been produced in Escherichia coli and evaluated for their potential to serve as diagnostic markers of recent Toxoplasma infections in the past [4-6]. While more proteins could be produced and screened this strategy requires cloning, expression and purification of recombinant proteins. More recently, highly purified chemically synthesized peptides can be produced easily and in large quantities, making them potentially useful for diagnostic tests.
The pathogenesis of toxoplasmosis is related to the complex life cycle of the parasite, as well as to parasite genotype in a lower degree [7,8]. There are two stages of asexual reproduction in the intermediate hosts including humans: tachyzoites and bradyzoites. Tachyzoites are thought to be responsible for active infection. They can transform into bradyzoites and vice versa depending on the environmental (i.e. host) conditions. The development of bradyzoites is a stress mediated differentiation response that leads to lifelong persistence in brain, heart and skeletal muscle. Bradyzoites are usually associated with chronic/inactive infection but bradyzoites are formed as early as 3 days postinfection [9]. While they cause little pathology in a healthy host but, they can reconvert into the tachyzoite stage and cause potentially fatal encephalitis, or disseminated toxoplasmosis in immunocompromised individuals [8].
Due to the central role of bradyzoite in the parasite life cycle, we hypothesize that the cyst burden might be different between active and chronic patients. Given that development and rupture of cysts are controlled by the immune system, this work focused on determining whether cyst-specific antibody responses differ in active and chronic toxoplasmosis. Taking into account previous work [4,10], we focused on the bradyzoite antigen BAG1 and the matrix antigen MAG1, which are thought to be the only bradyzoite-specific proteins that are immunogenic in infection [4]. The bradyzoite antigen BAG1 is a 30-kDa cytoplasmic protein with homology to the small heat shock proteins of plants. The matrix antigen MAG1 is a protein of 65-kDa abundantly expressed within the cyst and in the cyst wall surrounding the bradyzoites. Both antigens contribute to the early stimulation of both humoral and cell-mediated immunity against Toxoplasma infection in host including humans and thus appear to play a major role in the host resistance to Toxoplasma infection [4]. In this study, we designed and synthesized several peptides from both antigens (BAG1 and MAG1), developed peptide ELISA assay to detect anti-peptide antibodies and measured antibody responses in experimentally infected mice and naturally infected humans to determine whether these responses could provide a reliable marker for discriminating active from chronic Toxoplasma infection.
2. Materials and methods
2.1. Selection of peptides
We designed 8 peptides (Table 1) from the BAG1 and MAG1 antigens of Toxoplasma based on antigenic sites predicted by the Protean program in the Dnastar Lasergene software package. The peptides were chemically synthesized by GenScript (NJ, USA).
Table 1.
Amino acid sequence of peptides designed from BAG1 and MAG1 antigens. Numbering of amino acids is indicated for each sequence.
| Genetic locus | Peptide sequence | aa position |
|---|---|---|
| BAG1-1 | CYDDLRNRLSHDKNVRPVASQQLD | 48–71 |
| BAG1-2 | DVEFDSKKKEADLPGLQKDDVTIEVDNGA KGEKTSKEAEKVDDGK | 122–173 |
| BAG1-3 | KGEKTSKEAEKVDDGKTKNILTERVSGYFA RRFQLPSNYKPDG | 158–200 |
| MAG1-1 | KAYREATGKLEADELESERGPAVSPRRRLV DLIKDNQRRLR | 222–262 |
| MAG1-2 | SPRRRLVDLIKDNQRRLRAALQKIKIQKK LEEIDD | 245–279 |
| MAG1-3 | SQKAKEIREKAASLSSLLGVDAVEKQLRR VEPEHEDNTR | 319–357 |
| MAG1-4 | RALLEAKTKELVEPTSKEAEEARQIL AEQAA | 422–452 |
| MAG1-5 | DCEEQQEQGDTTLSDHDFHSGGTEQEGL PETEVAHQHETEEQ | 107–148 |
2.2. Analysis of peptide response in Toxoplasma-infected mice
2.2.1. Mouse immune response against peptides over time
Male and female BALB/c mice (9 weeks old, The Jackson Laboratory, Bar Harbor, ME) were infected intraperitoneally with 400 tachyzoites of Toxoplasma Prugniaud strain (PRU, type II). A series of sera were collected at 0, 6, 12, 15, 21 and 28 days following infection. Infection was confirmed by seroconversion using a commercial ELISA assay (VIR-ELISA, Viro-Immun Labor-Diagnostika, Oberursel Germany). A second set of sera were collected at 42 days postinfection (dpi) from male and female mice to confirm sex differences in the level of IgG MAG1 antibody. Serum samples from 24 uninfected male and female mice were used as negative controls.
2.2.2. Analysis of the mouse immune response specificity regarding Toxoplasma stages
To determine the bradyzoite-specificity of MAG1 peptides, 8 sera were collected from mouse infected with a Toxoplasma insertional mutant [11] that does not undergo normal differentiation to bradyzoites. These sera were kindly provided by Prof. V. Carruthers, University of Michigan School of Medicine, and were collected at 0, 3, 8, 10, 12, 15, 18 and 21 dpi.
2.2.3. Analysis of the mouse immune response specificity regarding Toxoplasma genotypes
In order to examine whether there is a genotype difference in the MAG1 peptide antibody response, a number of sera obtained approximately 2 months after infection from mice infected intraperitoneally with three canonical genotypes have been analyzed [12]. These sera include 8 samples from mice infected with type I, 2 from mice infected with type II and 7 from mice infected with type III. Due to the small number of mouse sera infected by type II, we added a group of human sera (n = 12) known to be infected with type II to this measurement (12). Information regarding the infective stage for these 12 human sera is not available; therefore, these sera could not be used in the dataset (Table 2B) to calculate the cutoff for active infection.
Table 2B.
MAG1 reactivity, serotype and serology data in patients with chronic Toxoplasma infection.
| Patient no | Sex | Current serology
|
History Serologya
|
Serotype | Positivityb
|
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| ELISA IgG | ELISA IgM | ISAGA IgM | ISAGA IgA | ELISA IgG | ELISA IgM | MAG1_4 (≥0.139) | MAG1_5 (≥0.083) | |||
| 1 | F | 260 | 0.09 | 12 | 12 | 240 | 0.08 | II | Pc (0.205) | Nd (0.040) |
| 2 | F | 150 | 0.01 | /e | / | 220 | 0.02 | II | N (0.045) | N (0.040) |
| 3 | M | 92 | 0.02 | / | / | 82 | 0.01 | II | N (0.046) | N (0.040) |
| 4 | M | 130 | 0.02 | / | / | 120 | 0.02 | Uncer | N (0.046) | P (0.116) |
| 5 | M | 270 | 0.02 | / | / | 150 | 0.02 | II | N (0.044) | N (0.041) |
| 6 | M | 160 | 0.03 | / | / | 200 | 0.02 | I | N (0.045) | N (0.041) |
| 7 | M | 15 | 0.03 | / | / | 11 | 0.03 | II | N (0.051) | N (0.041) |
| 8 | M | 40 | 0.04 | / | / | 97 | 0.03 | Uncer | N (0.044) | N (0.042) |
| 9 | M | 41 | 0.15 | / | / | 40 | 0.18 | II | N (0.044) | N (0.042) |
| 10 | F | 150 | 0.04 | / | / | 99 | 0.06 | II | N (0.049) | N (0.042) |
| 11 | M | 48 | 0.08 | / | / | 77 | 0.04 | Uncer | N (0.050) | N (0.042) |
| 12 | F | 15 | 0.04 | / | / | 12 | 0.05 | II | N (0.045) | N (0.043) |
| 13 | F | 51 | 0.02 | / | / | 22 | 0.03 | Uncer | N (0.071) | N (0.043) |
| 14 | F | 120 | 0.07 | / | / | 160 | 0.08 | II | N (0.050) | N (0.045) |
| 15 | F | 500 | 0.04 | 0 | 12 | 350 | 0.05 | II | N (0.043) | N (0.048) |
| 16 | F | 34 | 0.03 | / | / | 42 | 0.07 | II | N (0.049) | N (0.049) |
| 17 | F | 11 | 0.05 | / | / | 10 | 0.01 | Uncer | N (0.061) | N (0.049) |
| 18 | F | 64 | 0.04 | / | / | 150 | 0.06 | I | N (0.070) | N (0.051) |
| 19 | M | 18 | 0.03 | / | / | 14 | 0.03 | II | N (0.048) | N (0.052) |
| 20 | F | 77 | 0.37 | 12 | 3 | 150 | 1.58 | Uncer | N (0.044) | N (0.053) |
| 21 | F | 28 | 0.02 | / | / | 57 | 0.06 | II | N (0.045) | N (0.054) |
| 22 | M | 34 | 0.1 | / | / | 19 | 0.13 | II | N (0.044) | N (0.060) |
| 23 | M | 11 | 0.01 | / | / | 18 | 0.04 | Uncer | N (0.090) | N (0.061) |
| 24 | M | 120 | 0.05 | / | / | 86 | 0.04 | II | N (0.127) | N (0.066) |
| 25 | F | 61 | 0.02 | / | / | 49 | 0.02 | II | N (0.128) | N (0.067) |
| 26 | F | 45 | 0.03 | / | / | 36 | 0.02 | II | N (0.081) | N (0.069) |
History serology: data obtained 12 month ago.
Positivity: antibody reactivity above the cutoff for active infection.
P: positive.
N: Negative.
/:Not tested. The OD values of IgG antibodies to MAG1 peptides in individuals were indicated (numbers in parentheses).
2.3. Human responses against MAG1 peptides: Patients, samples, and in vitro tests
2.3.1. Patient and sample characterizations
79 human serum samples from 68 individuals were obtained from Lille Hospital, France. These sera were characterized using serological and molecular profiles as previously described [13-17]. Anti-Toxoplasma antibodies were measured by ELISA for IgG and IgM (Enzygnost IgG/IgM; Behring, Marburg, Germany) and by ISAGA for IgM and IgA, as previously described [15]. The cutoff for positivity for the Toxoplasma-specific IgG test corresponds to 4 IU/ml, according to the manufacturer’s instructions. ISAGA results were scored as 0 to 12 arbitrary units as previously described [14]: anti-Toxoplasma IgM and IgA were considered positive when scores were higher than 6 in serum. A score of 6 was considered equivocal in serum. In aqueous humor, anti-Toxoplasma IgM and IgA ocular values of at least 6 and 3 were considered positive and equivocal, respectively [15]. DNA extractions were performed with the QIAamp DNA Mini kit (Qiagen) using the biological fluid protocol; Toxoplasma DNA amplification targeted the 529-bp genomic repetitive element described was performed as previously described [13,14,16,17].
According to the serological and molecular results, patients and samples were classified into 3 groups, as follows: (i) Active toxoplasmosis group composed of 31 serum samples was collected from 22 infected immunocompetent individuals with known clinical outcome (Table 2A). It includes: 7 children (12 sera) with congenital toxoplasmosis and 9 pregnant women (13 sera) who had Toxoplasma seroconversion during pregnancy, 4 sera from individuals who had ocular toxoplasmosis, 1 serum from a patient with a cerebral toxoplasmosis, and 1 serum from an individual with an active toxoplasmosis; (ii) chronic group composed of 28 serum samples collected from 26 individuals with serological evidence of past exposure to Toxoplasma (more than one year infection history, Table 2B), and (iii) control group composed of serum samples from 19 individuals who scored negative for toxoplasmosis by serological testing.
Table 2A.
MAG1 reactivity, serotype and serology data in patients with active Toxoplasmosis.
| No./Sex | Diagnosis | Sampling (interval) | Serology
|
PCR in AHg | Serotype | Positivityj
|
||||
|---|---|---|---|---|---|---|---|---|---|---|
| ELISA IgG | ELISA IgM | ISAGA IgM | ISAGA IgA | MAG1_4 (≥0.139) | MAG1_5 (≥0.083) | |||||
| 1/F | CTa | 1st (0 day) | 320 | 0.01 | 0 | 0 | / | II | Nk (0.118) | N (0.055) |
| 2nd (23 day) | 310 | 0.02 | 12 | 0 | / | II | P (0.232) | P (0.088) | ||
| 2/F | CT | 1st (0 day) | 310 | 0.02 | 12 | 12 | / | II | P (0.146) | N (0.051) |
| – | 2nd (3 day) | 280 | 0.02 | 0 | 0 | / | II | P (0.194) | N (0.071) | |
| 3/M | CT | 1st (1 day) | 2.7 | 0.17 | 12 | 9 | / | II | N (0.044) | N (0.045) |
| 2nd (12 day) | 37 | 0.2 | 12 | 12 | / | II | N (0.044) | N (0.045) | ||
| 4/M | CT | 2 year | 68 | 0.07 | 0 | 0 | / | Unceri | N (0.044) | N (0.052) |
| 5/F | CT | 1st (2 year) | 60 | 0.10 | 0 | 0 | / | Uncer | N (0.046) | N (0.046) |
| 2nd (2.5 year) | 53 | 0.12 | 0 | 0 | / | Uncer | N (0.045) | N (0.051) | ||
| 6/M | CT | 1st (2 year) | 250 | 0.04 | 0 | 0 | / | II | N (0.068) | P (0.111) |
| 2nd (3 year) | 80 | 0.05 | 0 | 0 | / | II | N (0.081) | P (0.119) | ||
| 7/F | CT | Unknown | 489 | /f | / | / | / | II | P (0.153) | P (0.207) |
| 8/F | MSb | 1st (0 day) | <2 | 0.30 | 12 | 6 | / | Uncer | N (0.040) | N (0.041) |
| 2nd (12 day) | 4.1 | 1.35 | 12 | 12 | / | II | N (0.042) | N (0.045) | ||
| 3rd (20 day) | 6.9 | 1.52 | 12 | 12 | / | II | N (0.049) | N (0.044) | ||
| 9/F | MS | 0 day | 79 | 1.06 | 12 | 12 | / | II | N (0.086) | N (0.049) |
| 10/F | MS | 1st (0 day) | 54 | 0.8 | 12 | 12 | / | Uncer | N (0.072) | N (0.062) |
| 2nd (5.5 month) | 16 | 0.21 | 12 | 0 | / | Uncer | N (0.056) | N (0.066) | ||
| 11/F | MS | 0 day | 39 | 0.64 | 12 | 12 | / | Uncer | N (0.048) | N (0.046) |
| 12/F | MS | 1st (0 day) | 2.7 | 1.99 | 12 | 12 | / | Uncer | N (0.062) | P (0.085) |
| 2nd (1 month) | 54 | 1.91 | 12 | 12 | / | II | N (0.059) | P (0.416) | ||
| 13/F | MS | 0 day | 20 | 0.97 | 12 | 3 | / | Uncer | N (0.060) | N (0.064) |
| 14/F | MS | 0 day | 48 | 0.97 | 12 | 12 | / | II | N (0.062) | N (0.042) |
| 15/F | MS | 0 day | 93 | 1.23 | 12 | 12 | / | II | N (0.070) | N (0.047) |
| 16/F | MS | 0 day | 83 | 0.51 | 12 | 12 | / | Uncer | N (0.052) | N (0.054) |
| 17/M | OTc | Unknown | 91 | 0.5 | 12 | 12 | Ph | II | P (0.617) | P (0.087) |
| 18/F | OT | Unknown | 240 | 0.64 | 12 | 3 | P | Atypical | P (0.204) | P (0.109) |
| 19/M | OT | Unknown | 120 | 0.04 | / | / | P | II | P (0.231) | P (0.170) |
| 20/M | OT | Unknown | 330 | 0.03 | 0 | 12 | P | Atypical | N (0.107) | P (0.596) |
| 21/M | CETd | Unknown | 180 | 0.14 | 0 | 12 | / | II | P (0.141) | P (0.115) |
| 22/M | ATe | Unknown | 86 | 0.12 | 12 | 12 | / | Atypical | P (0.286) | P (0.278) |
CT: congenital toxoplasmosis.
MS: maternal seroconversion.
OT: ocular toxoplasmosis.
CET: cerebral toxoplasmosis.
AT: active toxoplasmosis.
/:Not tested.
AH: aqueous humor.
P: positive.
Uncer: uncertain.
Positivity: antibody reactivity above the cutoff for active infection.
N: Negative. The OD values of IgG antibodies to MAG1 peptides in individuals were indicated (numbers in parentheses).
2.3.2. Serotyping of Toxoplasma in human samples
A series of polymorphic peptides (GRA5-II, GRA6-I, GRA6-II, GRA6-III, GRA7-II and GRA7-III) specific to three clonal parasite lineages and derived from three dense granule antigens, GRA5, GRA6 and GRA7 were used for serological typing in human samples as described previously [12]. Briefly, the Toxoplasma serotype was determined by a two-step screening: the first step screening is to distinguish type II from type I/III infection using five peptides (GRA6-I, GRA6-III, GRA5-II, GRA6-II and GRA7-II). The second-step is to distinguish type III from type I infection using GRA7-III peptide.
2.4. Measurement of MAG1 antibodies in human and animal sera
IgG antibodies in mouse sera and human sera to MAG1 peptides were measured as follows. Peptides were diluted to 8 μg/ml in 0.1 M carbonate buffer, pH 8.5, and 50 μl of each peptide solution was loaded into a well of a polystyrene microtiter plate and incubated overnight at 4 °C. Following removal of unbound peptide, wells were washed and blocked with 300 μl of Starting Block blocking buffer (Pierce, US) for 1–2 min at room temperature. Sera were tested by adding 50 μl of diluted human serum (1:100) to each well and incubating for 3 h at 37 °C. The microtiter plates were washed four times with a PBS/0.1% Tween 20 solution and then reacted with a secondary anti-IgG antibody coupled to horseradish peroxidase (Southern Biotech, USA) for 2 h at 37 °C. After washing in PBS/0.1% Tween 20 solution, color development was with 50 μl of H2O2 ABTS 2,2′-azinobis (3-ethylbenzthiazoline-sulfonic acid) (ABTS) reagent (Kirkegaard and Perry Laboratories, Inc, USA) for 50 min. Absorbance was measured using a microplate (Vmax, USA) colorimeter employing at 405-nm filter.
2.5. Statistical analyses
The positive cutoff values for MAG1 exposure were defined as the mean plus 4 standard deviations (SD) of the negative control samples. To differentiate active from chronic infection, a cutoff point (cutoff level for active infection) was defined using mean + 2SD optical intensity value of the chronic infection samples. We assessed the statistical significance of differences in means (IgG reactivity) or frequencies (MAG1 peptides positivity) between the active and chronic infection group using the student’s t test or Fisher’s test, and a p value of less than 0.05 was considered significant in the two-tailed test.
3. Results
3.1. Peptide analysis and selection in mouse model
Preliminary studies were performed by measurement of antibodies to the 8 peptides derived from the BAG1 and MAG1 proteins. The amino acid composition of the peptides is depicted in Table 1. Reactivity was measured using a series of sera from Toxoplasma infected male and female mice collected at different time points postinfection (0, 6, 12, 15, 21 and 28 dpi). With the sera tested here, remarkably distinct seroconversion in both males and females appeared at 12 dpi measured by commercial ELISA. Criteria for exclusion of unqualified peptides included (i) having less than 50% of reactivity with all seropositive sera; (ii) lacking unique reactivity when compared to other qualified peptides. Of the 8 peptides originally tested, two (MAG1_4 and MAG1_5) yielded efficient reactions and were therefore used for subsequent analyses.
3.2. Immunoreactivity against MAG1 peptides occurs early in infection and displays sex difference in mouse model
Fig. 1 displays the kinetics of humoral response against MAG1_4 and MAG1_5 peptides in mice during Toxoplasma infection. For MAG1_4 peptide (Fig. 1A and B), anti-peptide IgG antibodies first appeared at 15 dpi in 12.5% and 16.7% of female and male mice, respectively. The level of reactivity increased at each time point and by 28 dpi, 83.3% of sera from females and 100% of sera from males were seropositive. For MAG1_5 peptide (Fig. 1C and D), peptide specific antibodies were first detected at 12 dpi, by which time 100% of sera from both females and males were seropositive. These MAG1_4 and MAG1_5 antibody levels remained quite stable when measured at 4 months post-infection from a separate cohort of mice (data not shown). No reactivity was detected when sera from uninfected mice were assayed.
Fig. 1.
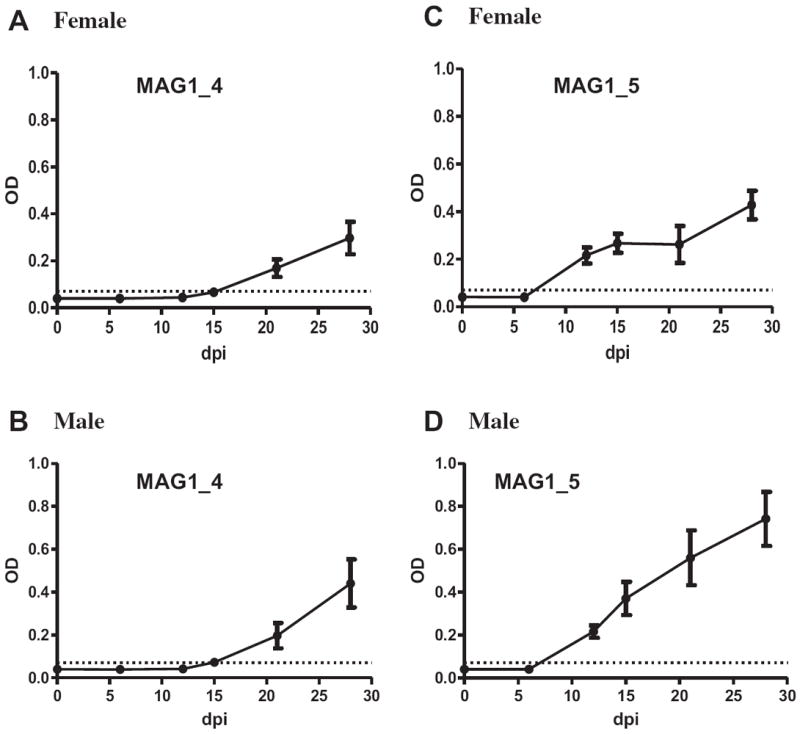
Kinetics of humoral response against MAG1_4 and MAG1_5 peptides in mice during Toxoplasma infection. Female (A and C); male (B and D). Shown are mean ± SEM. …… = cutoff level for exposure to the peptide (mean of negative + 4SD). Infected female: n = 12 at 0 and 6 dpi; n = 10 at 12 dpi; n = 8 at 15 dpi; n = 6 at 21 and 28 dpi. Infected male: n = 12 at 0, 6, 12, and 15 dpi; n = 9 at 21 and 28 dpi.
A significant sex difference in level of antibody reactivity against MAG1_5 peptide was observed at 28 dpi (p = 0.039), with male mice exhibited higher antibody level than female mice. The level of antibody against MAG1_4 showed a trend in the same direction between males and females. The sex difference in MAG1 peptide seroreactivity was confirmed using an independent set of serum samples from infected male and female mice with antibody measurements done on samples collected at 7 weeks postinfection (wpi) (Fig. 2). In the commercial ELISA assay which uses whole tachyzoite as the antigen, such a significant difference in the level of seroreactivity between Toxoplasma infected males and females was not found between early and late time points (data not shown).
Fig. 2.
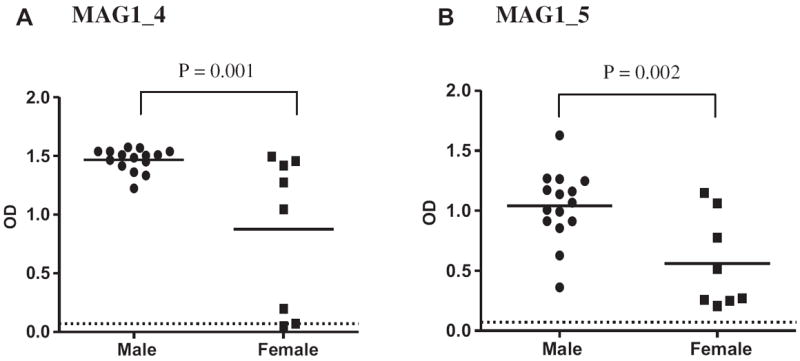
Immunoreactivity against MAG1 displays sex difference in mice. (A) MAG1_4; (B) MAG1_5. —— : denotes mean of reactivity. …… = cutoff level for exposure to the peptide (mean of negative + 4SD). Infected male, n = 15; infected female, n = 8.
3.3. Immunoreactivity against MAG1 peptides displays bradyzoite specificity as well as Toxoplasma type differences in mouse model
3.3.1. Bradyzoite specificity of MAG1_4 and MAG1_5 peptides
8 serum samples collected at different time points from mice infected with mutant strain that is unable to differentiate tachyzoite into bradyzoite, were used to examine the bradyzoite-specificity of the two peptides. IgG antibodies against the whole Toxoplasma organism were first detected at 8 dpi and peaked at 2–3 weeks postinfection. In contrast, none of these sera reacted with the MAG1-4 or MAG1_5 peptides (data not shown). These results are consistent with a bradyzoite-specificity of these two peptides.
3.3.2. Discrepant reaction of Toxoplasma genotypes with MAG1_4 and MAG1_5 peptides
Both MAG1 peptides exhibited similar reaction patterns with sera infected with one of the 3 canonical genotypes of Toxoplasma strains (Fig. 3). For MAG1_4 peptide, the majority of sera infected with type II (1 of 2 mouse sera and 10 of 12 human sera) and type III (6 of 7 mouse sera) displayed positive reaction, while only 2 of 8 sera from mice infected with type I were positive (Fig. 3A). For MAG1_5 peptide, all sera from mice infected with type III (n = 7) displayed positive reactions; the majority of sera infected with type II (2 of 2 mouse sera and 9 of 12 human sera) had reactions. In contrast, only 3 of 8 sera from mice infected with type I exhibited positive reactions (Fig. 3B).
Fig. 3.
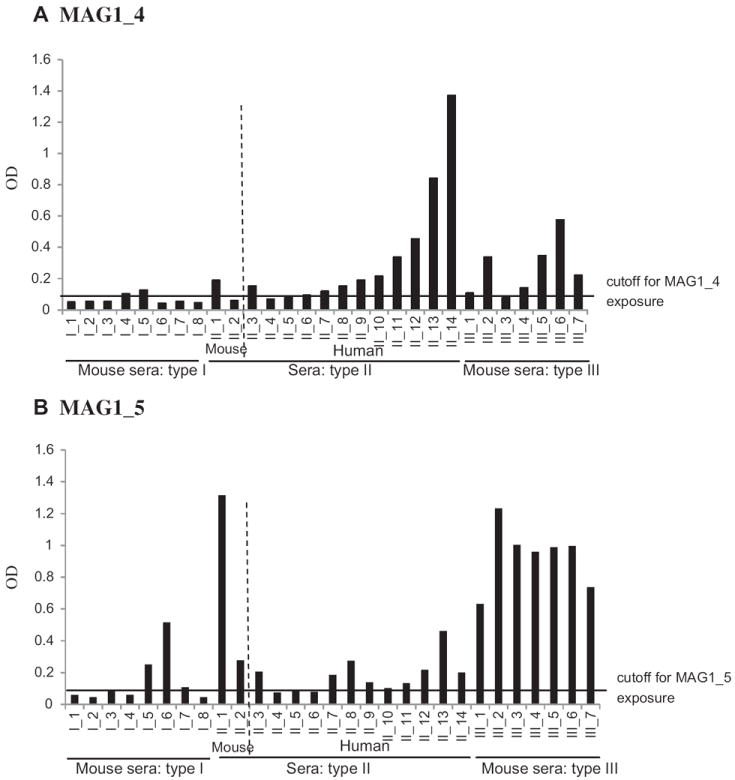
Reactivity of sera from mice and human infected with different Toxoplasma genotypes against MAG1 peptides. Both MAG1_4 (A) and MAG1_5 (B) peptides exhibited similar discrepancies in the reaction patterns with sera infected with different canonical genotypes of Toxoplasma strains. —— : cutoff value for exposure to the peptide (mean of negative + 4SD).
3.4. Human responses against MAG1 peptides
3.4.1. Human active infection reacts more frequently with MAG1_4 and MAG1_5 peptides
We assayed IgG reactivity of sera obtained from 22 patients with active Toxoplasma infections, 26 patients with chronic infections and 19 uninfected controls with MAG1_4 and MAG1_5 peptides in ELISA (Table 2 and Fig. 4). There were no false-positive reactions in the uninfected control group. All individuals who were repeatedly sampled showed similar patterns except 1 individual with congenital toxoplasmosis, whose sample from early time point was negative but the later time point scored positive (Case No. 1 in Table 2A). When cutoff for MAG1_4 exposure was applied (mean of negative + 4SD = 0.060), 72.7% (16 of 22) versus 26.9% (7 of 26) of sera from active and chronic infection, respectively, were MAG1_4 antibody positive (p = 0.003). When cutoff for MAG1_5 exposure was applied (mean of negative + 4SD = 0.067), 45.5% (10 of 22) versus 11.5% (3 of 26) of sera from active and chronic infection, respectively, were MAG1_5 antibody positive (p = 0.011). When the cutoff for MAG1_4 active infection was applied (mean of chronic + 2SD = 0.139), 36.3% (8 of 22) versus 3.85% (1 of 26) of sera from active and chronic group, respectively, were positive for active infection (p = 0.007, Fig. 4A). Similarly, when the cutoff for MAG1_5 active infection was applied (mean of chronic + 2SD = 0.083), 40.9% (9 of 22) versus 3.85% (1 of 26) of sera from active and chronic group, respectively, were positive for active infection (p = 0.003, Fig. 4B). The small sample size and skewed sex ratio (female:male = 14:8) in the active group precluded further analysis of MAG1 antibody level by sex. There was no sex difference in antibody level of MAG1_4 or MAG1_5 reactivity in chronic group (female:male = 14:12).
Fig. 4.
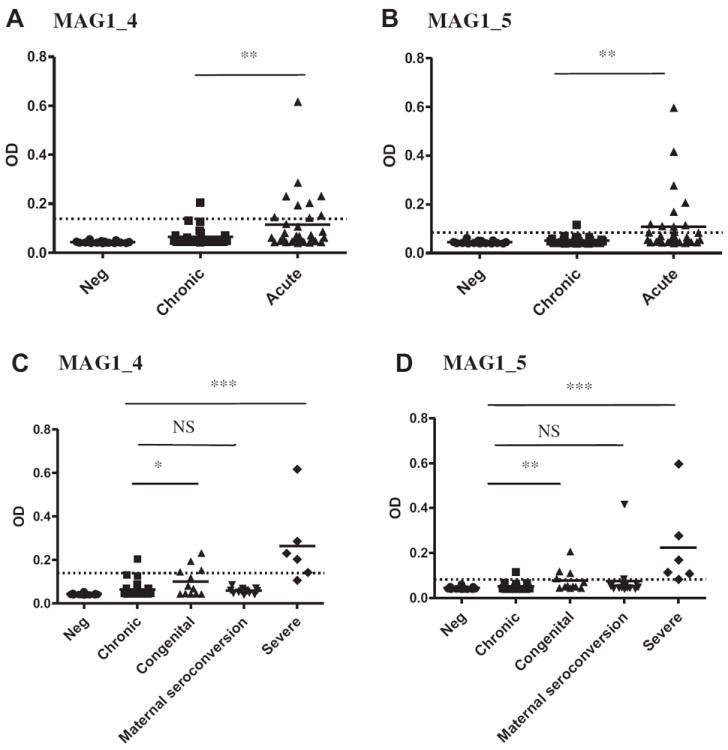
Human serological response against MAG1_4 (A and C) and MAG1_5 (B and D) peptides with sera from patients characterized as negative (n = 19), having a chronic (n = 26), and active (n = 22) Toxoplasma infection. …… = cutoff level for active infection (mean of chronic + 2SD); *p < 0.05; **p < 0.001; ***p < 0.000; NS: not significant.
Regarding the different toxoplasmosis histories, among the 7 patients with congenital toxoplasmosis, 9 women with seroconversion during pregnancy, and 6 patients with other severe toxoplasmosis (Table 2A), 42.9%, 0%, and 83.3% patients were seropositive to MAG1_4 using the cutoff for active infection, respectively (p = 0.028, p = 0.394, and p < 0.000, respectively, Fig. 4C). Similarly, there were 42.9%, 11.1%, and 100% patients were seropositive to MAG1_5, respectively (p = 0.005, p = 0.062, and p < 0.000, respectively, Fig. 4D).
3.4.2. Toxoplasma typing and human response against MAG1_4 and MAG1_5 peptides
Since sera from mice infected with type I strain showed less reactivity to MAG1 peptides, we conducted serological typing for human samples in order to examine whether lack of reactivity with MAG1 peptides was associated with Toxoplasma serotype (Table 2A). Serological typing results showed that type II (positive reaction with one or more type II peptides designated GRA5_II, GRA6_II, and GRA7_II) is highly prevalent in both active (59.1%) and chronic (65.4%) infections, consistent with the European origin of toxoplasmosis. The second most common category for the active and chronic groups (27.3% versus 26.9%), was uncertain (lack of reactivity to all the peptides). An atypical category (reacted with any combination of three types of peptides) was found frequently in patients with ocular toxoplasmosis (50%). Since none of the human Toxoplasma was identified as type I, we were not able to document the link between a weak response against MAG1 and an infection with type I parasites we identified in our mouse model.
4. Discussion
In this study we have evaluated the usefulness of peptides originated from MAG1 and BAG1 antigen for discriminating active from chronic Toxoplasma infection. We targeted these two antigens because they are thought to be the most immunogenic antigens in bradyzoite and they are known to play a role in the early stimulation of humoral and cell-mediated immunity against Toxoplasma infection in humans [4]. Among the 8 peptides studied here, we identified two peptides MAG1_4 and MAG1_5 within MAG1 antigen that were recognized by antibodies from experimentally infected mice. The humoral response against MAG1 occurs as early as 12 dpi and displays a sex difference later in the magnitude of the response among mice. Results from human samples revealed that sera from patients with active Toxoplasma infection reacted more frequently and more strongly against MAG1 peptides than sera from patients with chronic infection. This result agrees with previous reports that MAG1 antigen should be considered a marker specific for active toxoplasmosis [5].
A previous study [4] suggested that IgG antibodies against MAG1 antigen occur early (1 month) after infection. Our results confirmed and extended this finding in a mouse model, showing that an antibody response against MAG1_4 and MAG1_5 peptides occurs at as early as 12 dpi (Fig. 1). Since bradyzoites form within a few days after infection [9], this very early host immune response against MAG1 could originate from this transformation. MAG1 was originally identified in the cyst matrix [18], but further experiments revealed that MAG1 is also expressed in tachyzoites and secreted into the parasitophorous vacuole, albeit less abundantly than in bradyzoites [19]. However, the MAG1 antigen expressed in tachyzoite, does not appear to induce an immune response since no reactivity to this antigen was detected in sera of mice experimentally infected with a mutant Toxoplasma strain which does not undergo normal differentiation into bradyzoites.
The MAG1 peptides exhibited discrepancies in the reaction patterns with sera from mice and human infected with the 3 canonical genotypes of Toxoplasma strains. The majority of sera infected with type II and III displayed positive reaction with both peptides, while sera from mice infected with a type I strain responded less frequently. This is consistent with previous results indicating that type II and III strains are more capable of bradyzoite differentiation than type I strains.
We observed a sex-dependent MAG1 antibody response in mice, with males producing higher antibody titers than females. Although Toxoplasma infection is more lethal in female mice than male mice [20], the sex difference in the magnitude of the antibody response to MAG1 was observed among mice that survived for 7 weeks. One potential explanation for the sex difference in antibody response is a higher cyst burden in male mice compared to female mice. Indeed, recent data from our laboratory, obtained by counting the number of cyst from mouse brain, show males have higher number of tissue cysts than females (G Kannan and MV Pletnikov, unpublished data). However, another study reported more cysts in brains of infected female mice compared to male mice [21]. Male mice reportedly have a rapid response to infection associated with production of high levels of TNF-α and IFN-r. In addition to accounting for the better controls of parasite multiplication and improved survival in male mice compared to female mice, this robust innate immune response may also support induction of a stronger humoral immune response. Interestingly, the sex-dependent MAG1 antibody response in mice was not observed in either active or chronic human infection in the current study. The sex difference in mice may be uniquely related to Toxoplasma infection of BALB/c mice. However, the skewed sex ratio (female:male = 14:8) in the active group and small sample size in the chronic group (female:male = 14:12) may have preclude the possibility of observing a gender difference in humans. Further studies with larger samples size are needed to address whether there is a difference in human infection.
In humans, active infection exhibited higher level of MAG1 reactivity than chronic infection. We propose several potential explanations here: (i) severe infection (e.g. ocular, cerebral, and active toxoplasmosis) in patients occurs mainly through reactivation of latent cysts into the invasive tachyzoites. MAG1, located within the cyst and in the cyst wall, would be released and exposed to the immune system during the rupture of cysts. This process may account for the more frequent and stronger response to MAG1 found in sera from active, severe infection. (ii) During primary infection, the transformation of tachyzoites into bradyzoites would be expected to induce an immune response against bradyzoites. The appearance of MAG1 antibodies during the course of primary infection and then the absence of detectable MAG1 antibody in the chronic phase of infection implies that the response must wane over time. Although we could not observe any decline in MAG1 antibody levels in mice 4 months after experimental infection, the mouse may not be a good model for human infections.
One limitation of our peptide ELISA is that sera from patients with maternal seroconversion during pregnancy have less reactivity with the MAG1 peptides compared to sera from other type of active infection. This might be due to specific population characteristics, such as early stage of Toxoplasma infection when women were sampled or the use of anti-parasitic drugs. The majority of sera (7 of 13) in our study had low levels of IgG reactivity in routine serology (e.g. <40 IU in standard IgG assay), an indication of a very early immune response. Pfrepper et al. [6] measured antibodies against MAG1 over time in Toxoplasma-infected pregnant women using an ELISA assay with a recombinant antigen (30–452 amino acid residues). In their study, the sensitivity for the detection of IgG MAG1 antibodies was lower (31.8%) in sera collected initially at the time of infection in contrast to sera collected at 3 months (56.9%) and between 3 and 6 months (70.0%) postinfection. Most of our samples were collected at the time of initial infection and thus are consistent with Pfrepper’s results. Another possible reason for the lower level of MAG1 reactivity may be related to the effect of anti-Toxoplasma medications. In France, when seroconversion is detected in the mother, Spiramycin (9 MUI/day) treatment is generally started. Notably, this medicine has been mentioned to modify antibodies production [22].
Not surprisingly, serological typing indicated that type II was over-represented in both active (59.1%) and chronic (65.4%) infection compared with other types as our human samples were originated from France. It is worth noting that atypical strain was identified in samples from two of four patients (50%) one with ocular toxoplasmosis and one with active toxoplasmosis, whereas it was absent in patients with chronic infection. Our result is in agreement with Grigg et al.’s finding [23] that unusual abundance of atypical strains associated with human ocular toxoplasmosis. Moreover, this data also indicated a possible correlation between severe toxoplasmosis and atypical serotype, as it has been recently proposed [8,24].
Although the reason(s) why MAG1 antigens were recognized more robustly by active than chronic infection is unknown in human immune response, the IgG reactivity to MAG1_4 and MAG1_5 peptides described here has the potential to distinguish active from chronic Toxoplasma infection. Such differentiation will help in identifying patients at high risk of developing a severe or an invasive disease, especially when they exhibit an immunocompromised status. Certainly, further and more extensive studies are required and will be performed to strengthen and specify the data presented in this study. Additionally, these peptides should be considered in the design of potential vaccines in humans given the broad recognition of them by the immune system, supporting the hypothesis that a combination of bradyzoite and tachyzoite antigens must be used for vaccine development [4].
Acknowledgments
We thank Prof. V. Carruthers for providing mouse sera infected with mutant strain that is unable to differentiate into bradyzoite. This work was supported by Stanley Medical Research Institute. The Parasitology-Mycology Department of Lille Hospital (Dr L Delhaes) is member of the French National Reference Centre for Toxoplasmosis (Centre National de Référence de la Toxoplasmose).
References
- 1.Liesenfeld O, Montoya JG, Tathineni NJ, Davis M, Brown BW, Jr, Cobb KL, Parsonnet J, Remington JS. Confirmatory serologic testing for acute toxoplasmosis and rate of induced abortions among women reported to have positive Toxoplasma immunoglobulin M antibody titers. Am J Obstet Gynecol. 2001;184:140–145. doi: 10.1067/mob.2001.108341. [DOI] [PubMed] [Google Scholar]
- 2.Roberts A, Hedman K, Luyasu V, Zufferey J, Bessières MH, Blatz RM, Candolfi E, Decoster A, Enders G, Gross U, Guy E, Hayde M, Ho-Yen D, Johnson J, Lécolier B, Naessens A, Pelloux H, Thulliez P, Petersen E. Multicenter evaluation of strategies for serodiagnosis of primary infection with Toxoplasma gondii. Eur J Clin Microbiol Infect Dis. 2001;20:467–474. doi: 10.1007/pl00011289. [DOI] [PubMed] [Google Scholar]
- 3.Petersen E, Borobio MV, Guy E, Liesenfeld O, Meroni V, Naessens A, Spranzi E, Thulliez P. European multicenter study of the LIAISON automated diagnostic system for determination of Toxoplasma gondii-specific immunoglobulin G (IgG) and IgM and the IgG avidity index. J Clin Microbiol. 2005;43:1570–1574. doi: 10.1128/JCM.43.4.1570-1574.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Di Cristina M, Del Porto P, Buffolano W, Beghetto E, Spadoni A, Guglietta S, Piccolella E, Felici F, Gargano N. The Toxoplasma gondii bradyzoite antigens BAG1 and MAG1 induce early humoral and cell-mediated immune responses upon human infection. Microbes Infect. 2004;6:164–171. doi: 10.1016/j.micinf.2003.11.009. [DOI] [PubMed] [Google Scholar]
- 5.Holec L, Hiszczyńska-Sawicka E, Gasior A, Brillowska-Dabrowska A, Kur J. Use of MAG1 recombinant antigen for diagnosis of Toxoplasma gondii infection in humans. Clin Vaccine Immunol. 2007;14:220–225. doi: 10.1128/CVI.00419-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pfrepper KI, Enders G, Gohl M, Krczal D, Hlobil H, Wassenberg D, Soutschek E. Seroreactivity to and avidity for recombinant antigens in toxoplasmosis. Clin Diagn Lab Immunol. 2005;12:977–982. doi: 10.1128/CDLI.12.8.977-982.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ajzenberg D, Yera H, Marty P, Paris L, Dalle F, Menotti J, Aubert D, Franck J, Bessières MH, Quinio D, Pelloux H, Delhaes L, Desbois N, Thulliez P, Robert-Gangneux F, Kauffmann-Lacroix C, Pujol S, Rabodonirina M, Bougnoux ME, Cuisenier B, Duhamel C, Duong TH, Filisetti D, Flori P, Gay-Andrieu F, Pratlong F, Nevez G, Totet A, Carme B, Bonnabau H, Dardé ML, Villena I. Genotype of 88 Toxoplasma gondii isolates associated with toxoplasmosis in immunocompromised patients and correlation with clinical findings. J Infect Dis. 2009;199:1155–1167. doi: 10.1086/597477. [DOI] [PubMed] [Google Scholar]
- 8.Delhaes L, Mraz JC, Fréalle E, Durand-Joly I, Magro L, Ajzenberg D, Dardé ML, Dei-Cas E, Yakoub-Agha I. Severe pulmonary toxoplasmosis after allo-SCT in two patients: from Toxoplasma genotyping to clinical management. Bone Marrow Transplant. 2010;45:580–583. doi: 10.1038/bmt.2009.167. [DOI] [PubMed] [Google Scholar]
- 9.Dubey JP, Lindsay DS, Speer CA. Structures of Toxoplasma gondii tachyzoites, bradyzoites, and sporozoites and biology and development of tissue cysts. Clin Microbiol Rev. 1998;11:267–299. doi: 10.1128/cmr.11.2.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim SK, Boothroyd JC. Stage-specific expression of surface antigens by Toxoplasma gondii as a mechanism to facilitate parasite persistence. J Immunol. 2005;174:8038–8048. doi: 10.4049/jimmunol.174.12.8038. [DOI] [PubMed] [Google Scholar]
- 11.Huynh MH, Carruthers VB. Toxoplasma MIC2 is a major determinant of invasion and virulence. PLoS Pathog. 2006;2:e84. doi: 10.1371/journal.ppat.0020084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xiao J, Buka SL, Cannon TD, Suzuki Y, Viscidi RP, Torrey EF, Yolken RH. Serological pattern consistent with infection with type I Toxoplasma gondii in mothers and risk of psychosis among adult offspring. Microbes Infect. 2009;11:1011–1018. doi: 10.1016/j.micinf.2009.07.007. [DOI] [PubMed] [Google Scholar]
- 13.Homan WL, Vercammen M, De Braekeleer J, Verschueren H. Identification of a 200- to 300-fold repetitive 529 bp DNA fragment in Toxoplasma gondii, and its use for diagnostic and quantitative PCR. Int J Parasitol. 2000;30:69–75. doi: 10.1016/s0020-7519(99)00170-8. [DOI] [PubMed] [Google Scholar]
- 14.Reischl U, Bretagne S, Krüger D, Ernault P, Costa JM. Comparison of two DNA targets for the diagnosis of Toxoplasmosis by real-time PCR using fluorescence resonance energy transfer hybridization probes. BMC Infect Dis. 2003;3:7. doi: 10.1186/1471-2334-3-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Simon A, Labalette P, Ordinaire I, Frealle E, Dei-Cas E, Camus D, Delhaes L. Use of fluorescence resonance energy transfer hybridization probes to evaluate quantitative real-time PCR for diagnosis of ocular toxoplasmosis. J Clin Microbiol. 2004;42:3681–3685. doi: 10.1128/JCM.42.8.3681-3685.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sterkers Y, Varlet-Marie E, Cassaing S, Brenier-Pinchart MP, Brun S, Dalle F, Delhaes L, Filisetti D, Pelloux H, Yera H, Bastien P. Multicentric comparative analytical performance study for molecular detection of low amounts of Toxoplasma gondii from simulated specimens. J Clin Microbiol. 2010;48:3216–3222. doi: 10.1128/JCM.02500-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yera H, Filisetti D, Bastien P, Ancelle T, Thulliez P, Delhaes L. Multicenter comparative evaluation of five commercial methods for toxoplasma DNA extraction from amniotic fluid. J Clin Microbiol. 2009;47:3881–3886. doi: 10.1128/JCM.01164-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Parmley SF, Yang S, Harth G, Sibley LD, Sucharczuk A, Remington JS. Molecular characterization of a 65-kilodalton Toxoplasma gondii antigen expressed abundantly in the matrix of tissue cysts. Mol Biochem Parasitol. 1994;66:283–296. doi: 10.1016/0166-6851(94)90155-4. [DOI] [PubMed] [Google Scholar]
- 19.Ferguson DJ, Parmley SF. Toxoplasma gondii MAG1 protein expression. Trends Parasitol. 2002;18:482. doi: 10.1016/s1471-4922(02)02349-8. [DOI] [PubMed] [Google Scholar]
- 20.Liesenfeld O, Nguyen TA, Pharke C, Suzuki Y. Importance of gender and sex hormones in regulation of susceptibility of the small intestine to peroral infection with Toxoplasma gondii tissue cysts. J Parasitol. 2001;87:1491–1493. doi: 10.1645/0022-3395(2001)087[1491:IOGASH]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 21.Roberts CW, Cruickshank SM, Alexander J. Sex-determined resistance to Toxoplasma gondii is associated with temporal differences in cytokine production. Infect Immun. 1995;63:2549–2555. doi: 10.1128/iai.63.7.2549-2555.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Meroni V, Genco F, Tinelli C, Lanzarini P, Bollani L, Stronati M, Petersen E. Spiramycin treatment of Toxoplasma gondii infection in pregnant women impairs the production and the avidity maturation of T. gondii-specific immunoglobulin G antibodies. Clin Vaccine Immunol. 2009;16:1517–1520. doi: 10.1128/CVI.00253-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Grigg ME, Ganatra J, Boothroyd JC, Margolis TP. Unusual abundance of atypical strains associated with human ocular toxoplasmosis. J Infect Dis. 2001;184:633–639. doi: 10.1086/322800. [DOI] [PubMed] [Google Scholar]
- 24.Delhaes L, Ajzenberg D, Sicot B, Bourgeot P, Darde ML, Dei-Cas E, Houfflin-Debarge V. Severe congenital toxoplasmosis due to a Toxoplasma gondii strain with an atypical genotype: case report and review. Prenatal Diagn. 2010;30:902–905. doi: 10.1002/pd.2563. [DOI] [PubMed] [Google Scholar]


