Abstract
Objective
Regulation of angiogenesis is critical for many diseases. Specifically, pathological retinal neovascularization, a major cause of blindness, is suppressed with dietary ω3-long-chain polyunsaturated fatty acids (ω3LCPUFAs) through antiangiogenic metabolites of cyclooxygenase and lipoxygenase. Cytochrome P450 epoxygenases (CYP2C8) also metabolize LCPUFAs, producing bioactive epoxides, which are inactivated by soluble epoxide hydrolase (sEH) to transdihydrodiols. The effect of these enzymes and their metabolites on neovascularization is unknown.
Approach and Results
The mouse model of oxygen-induced retinopathy was used to investigate retinal neovascularization. We found that CYP2C (localized in wild-type monocytes/macrophages) is upregulated in oxygen-induced retinopathy, whereas sEH is suppressed, resulting in an increased retinal epoxide:diol ratio. With a ω3LCPUFA-enriched diet, retinal neovascularization increases in Tie2-driven human-CYP2C8–overexpressing mice (Tie2-CYP2C8-Tg), associated with increased plasma 19,20-epoxydocosapentaenoic acid and retinal epoxide:diol ratio. 19,20-Epoxydocosapentaenoic acids and the epoxide:diol ratio are decreased with overexpression of sEH (Tie2-sEH-Tg). Overexpression of CYP2C8 or sEH in mice does not change normal retinal vascular development compared with their wild-type littermate controls. The proangiogenic role in retina of CYP2C8 with both ω3LCPUFA and ω6LCPUFA and antiangiogenic role of sEH in ω3LCPUFA metabolism were corroborated in aortic ring assays.
Conclusions
Our results suggest that CYP2C ω3LCPUFA metabolites promote retinal pathological angiogenesis. CYP2C8 is part of a novel lipid metabolic pathway influencing retinal neovascularization.
Keywords: angiogenesis factor, cytochrome P450 CYP2C8 (human), pathologic neovascularization
Pathological neovascularization in retinopathy is a major cause of blindness.1,2 ω3 long-chain polyunsaturated fatty acids (ω3LCPUFA), docosahexaenoic acid (DHA), and eicosapentaenoic acid (EPA) suppress retinal neovascularization and improve visual function in animal and clinical retinopathy studies3,4 through metabolites of cyclooxygenase and lipoxygenase.3,5–7 Cytochrome P450s (CYPs) also metabolize both ω3LCPUFAs and ω6LCPUFAs into bioactive epoxides, which are hydrolyzed by soluble epoxide hydrolase (sEH) to form less active transdihydrodiols (diols), hence dampening the biological effects of LCPUFA epoxides (Figure 1A).8,9 It is important to further elucidate enzymes that generate bioactive ω3LCPUFA (and ω6LCPUFA) metabolites (CYP2C) and enzymes that deactivate them (sEH) to identify pro- and antiangiogenic pathways.
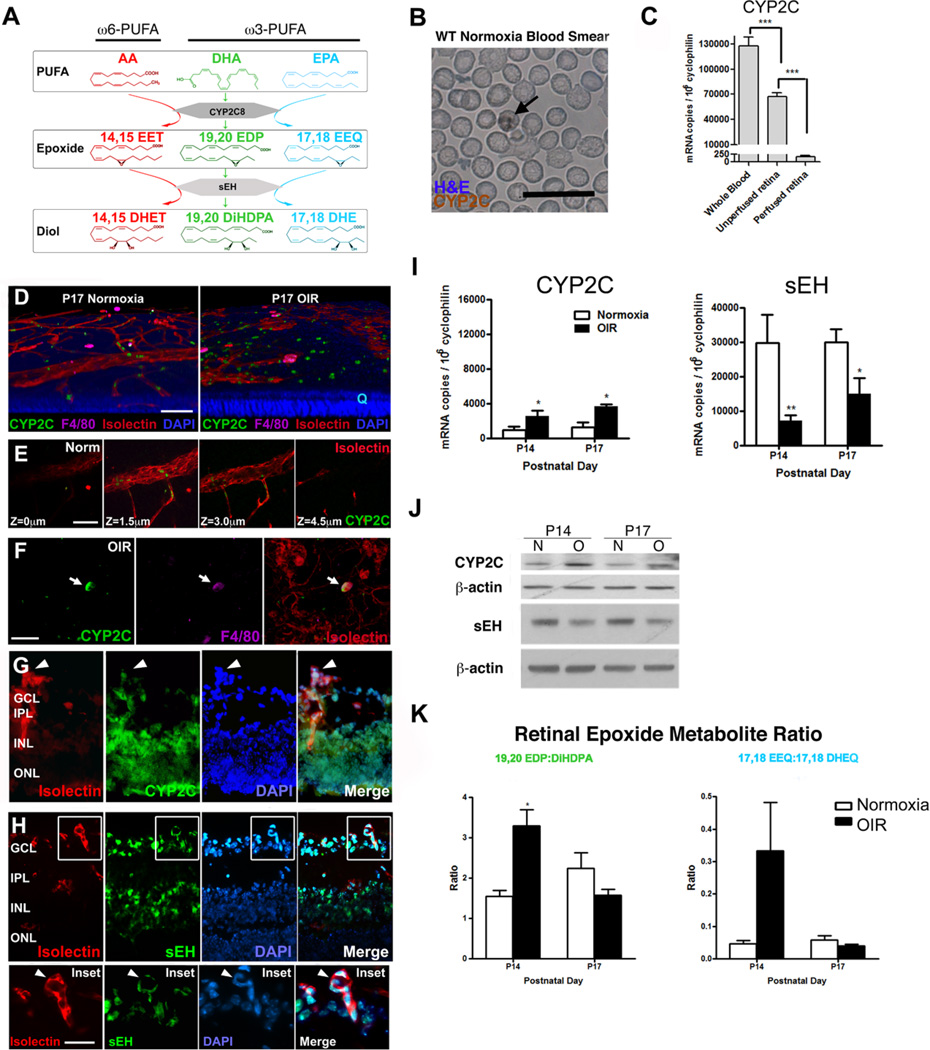
Figure 1.
Retinal expression of cytochrome P450 epoxygenases (CYP2C8) homologue, soluble epoxide hydrolase (sEH), and their products ratio in normoxia vs oxygen-induced retinopathy oxygen-induced retinopathy (OIR). A, Schematic diagram of CYP2C8 and sEH metabolism of arachidonic acid (AA), docosahexaenoic acid (DHA), and eicosapentaenoic acid (EPA). B, Wild-type (WT) blood smear indicates CYP2C-positive leukocytes (arrows). Scale bar, 20 µm. C, mRNA level of CYP2C in blood and retina with or without perfusion. D, Three-dimensional (3D) reconstruction of confocal images of postnatal day (P) 17 WT normoxia and oxygen-induced retinopathy (OIR) retinal flat-mount stained with CYP2C (green), F4/80 (purple), isolectin (red), and 4',6-diamidino-2-phenylindole (DAPI; blue). Scale bar, 100 µm. E, Layer-by-layer confocal image across a vein of normoxia retina. F, Colocalization of CYP2C and F4/80 (arrow) in OIR retinal flat-mount. G, Retinal cross-sectional staining with isolectin (red), CYP2C (green), and DAPI (blue) shows CYP2C is expression in neovascular tufts (arrowhead), as well as in the neurons of the ganglion cell layer (GCL), inner nuclear layers (INL), and outer nuclear layer (ONL). H, Retinal cross-sectional staining with isolectin (red), sEH (green), and DAPI (blue) shows sEH is expressed in neovascular tufts (arrow-head), as well as in neurons of GCL and INL. Scale bar, 10 µm. I, CYP2C and sEH mRNA expression in retina during OIR (n=6). J, CYP2C and sEH protein expression in normoxia (N) versus OIR (O) retina. K, The ratio of corresponding DHA and EPA epoxides to diols by liquid chromatography/mass spectrometry/mass spectrometry oxylipid analysis (n=4–6 per group; 2-way ANOVA with Bonferroni post test; *P<0.05). Three-dimensional reconstruction was made using Volocity 3D Image Analysis Software. All confocal images were taken by Leica SP2 confocal microscope with ×40 objective lens. Blood smear pictures was taken by Zeiss AxioObserver microscope under ×20 objective lens. DHET indicates dihydroxyeicosatrienoic acid; DiHDPA, dihydroxy-docosapentaenoic acid; EDP, epoxydocosapentaenoic acid; EET, epoxyeicosatrienoic acid; EEQ, epoxyeicosatetraenoic acid; and PUFA, polyunsaturated fatty acid.
CYP2C8, a dominant human epoxygenase, is induced by hypoxia,10 which in turn is critical in retinal neovascular development. sEH is implicated in cardiovascular diseases11 and expressed in endothelial cells (ECs)12 and may regulate angiogenesis. ω6LCPUFA-derived epoxyeicosatrienoic acids (EETs), synthesized by CYP2C8 from arachidonic acid (AA), promote angiogenesis and EC migration.13–17 However, the angiogenic effect of ω3LCPUFA-derived epoxy metabolites from CYP2C8: DHA-derived epoxydocosapentaenoic acids (EDPs) and EPA-derived epoxyeicosatetraenoic acids (EEQs; Figure 1A) is unknown.18 Both EDPs and EEQs exhibit potent vasodilatory and cardioprotective effects,19 with EDPs suggested to suppress EC migration and angiogenesis in tumors.20
We investigated whether CYP2C8 and its ω3LCPUFA metabolites promoted or suppressed retinal neovascularization using blood vessel system–specific CYP2C8 and sEH-overexpressing mice (Tie2-CYP2C8-Tg, Tie2-sEH-Tg)21 and germ-line knockout of sEH (sEH−/−) with wild-type (WT) littermate controls with a ω3LCPUFA-enriched diet in a mouse model of oxygen-induced retinopathy (OIR). CYP2C8 and sEH metabolites from a ω6LCPUFA-enriched diet were examined similarly in OIR.
Materials and Methods
Methods and Materials are available in the online-only Supplement.
Results
CYP2C-positive leukocytes are detected in circulating cells from WT normoxic mice (Figure 1B). The CYP2C mRNA level is highest in blood cells and much higher in nonperfused versus perfused retina (Figure 1C). We then identified the source of the mouse CYP2C8 homologue, CYP2C. CYP2C-positive cells are found within blood vessel lumens in normoxic retina (Figure1D and 1E) and outside vessels in P17 OIR retina (Figure 1D), when the maximal neovascularization is observed in mouse OIR.22,23 Some F4/80-positive macrophages outside vessels express CYP2C in OIR, indicating macrophages are one of the sources of CYP2C in the OIR retina. Other sources are not excluded (Figure 1F). Pathological neovessels and neural tissue express CYP2C and sEH in OIR (Figure1G and 1H). We next examined whether OIR affects levels of CYP2C (producing active metabolites) or sEH (reducing active metabolite levels).
OIR Induces Increased CYP2C8 and Decreased sEH Retinal Levels, Resulting in an Increased Retinal DHA-Derived Epoxide:Diol Ratio
CYP2C (mRNA and protein) is induced in OIR retinas, whereas sEH is suppressed (P<0.05; Figure1I and 1J). In OIR versus normoxia at P14 (on normal chow) when the neovessel formation starts,23 the DHA-derived retinal epoxide:diol ratio (19,20-EDP:19,20-dihydroxy-docosapentaenoic acid [DiHDPA]) is increased >2-fold (P=0.017; Figure 1K). We next determined how this increase in active metabolites influences retinal neovessel formation.
In Tie2-CYP2C8-Tg OIR Mice, ω3LCPUFA Feed Increases Neovascularization and Increases VEGF-A Expression
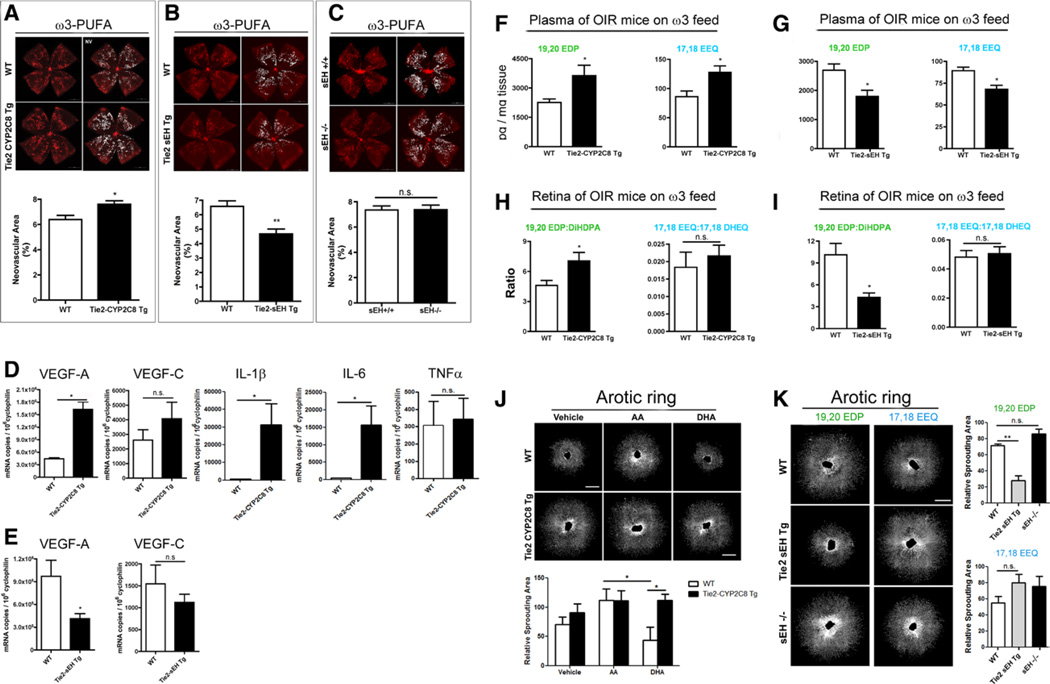
With ω3LCPUFA feed, Tie2-human-CYP2C8-Tg mice develop more OIR neovascularization than WT (Tie2-CYP2C8-Tg: 7.60±0.29% versus WT: 6.40±0.33% of total retinal area; P=0.014) at P17 (Figure 2A). Moreover, Tie2-sEH-Tg retinas develop less neovascularization in OIR (Tie2-sEH-Tg: 4.67±0.34% versus WT: 6.59±0.38%; P=0.0027; Figure 2B). Germ-line deletion of sEH (sEH−/−) had no effect on neovascularization (sEH−/−:7.39±0.34% versus WT: 7.35±0.32%; P=0.95; Figure 2C). With ω3LCPUFA feed, Tie2-CYP2C8-Tg OIR mice had 2.6-fold greater vascular endothelial growth factor (VEGF)-A expression than WT (P=0.011), whereas Tie2-sEH-Tg had 57% less VEGF-A expression (P=0.030). No significant difference in VEGF-C levels was detected (Figure2D and 2E). Significantly higher retinal interleukin-1β and interleukin-6 levels were found in Tie2-CYP2C8-Tg versus WT OIR mice (Figure 2D). We next examined the active and inactive metabolite levels or ratios in plasma and retina.
Figure 2.
With ω3-long-chain polyunsaturated fatty acid (ω3LCPUFA) feed, oxygen-induced retinopathy (OIR) neovascularization and corresponding epoxides level are modified in Tie2-CYP2C8-Tg and Tie2-sEH-Tg mice; the alternation of angiogenesis was also shown in aortic ring sprouting using Tie2-CYP2C8-Tg and Tie2-sEH-Tg treated with docosahexaenoic acid (DHA) and eicosapentaenoic acid (AA) or epoxide metabolites. A, Neovascular area of Tie2-CYP2C8-Tg mice exposed to OIR comparing with wild-type (WT) littermate control (n=11–13 per group). Scale bar, 500 µm. B, Neovascular area in OIR of Tie2-sEH-Tg comparing with WT (n=14–19 per group). C, Neovascular area in OIR of systemic soluble epoxide hydrolase (sEH) knockout (sEH−/−; n=8–15 per group). D and E, Reverse transcription polymerase chain reaction of vascular endothelial growth factor (VEGF)-A, VEGF-C, interleukin (IL)-1β, IL-6, and tumor necrosis factor (TNF)-α in OIR Tie2-CYP2C8-Tg (D) and VEGF-A, VEGF-C in OIR Tie2-sEH-Tg (E) compared with WT (t test; *P<0.05 and **P<0.01). Retinal whole mount pictures were taken by Zeiss AxioObserver microscope under ×5 objective lens. F, Plasma levels of 19,20-epoxydocosapentaenoic acid (EDP) and 17,18-epoxyeicosatetraenoic acid (EEQ) in Tie2-CYP2C8-Tg mice (n=4–6 per group). G, Plasma levels of 19,20-EDP and 17,18-EEQ in Tie2-sEH-Tg mice (n=4–6 per group). H, Retinal 19,20-EDP:dihydroxy-docosapentaenoic acid (DiHDPA) and 17,18-EEQ:17,18-dihydroxy-eicosatetraenoic acid (DHEQ) ratio of Tie2-CYP2C8-Tg vs WT (n=4–6 per group). I, Retinal 19,20-EDP:DiHDPA and 17,18-EEQ:17,18-DHEQ ratio of Tie2-sEH-Tg vs WT (n=4–6 per group; t test; *P<0.05 and **P<0.01). J, Vehicle control, AA (30 µmol/L) or DHA (30 µmol/L) induced aortic ring sprouting of WT and Tie2-CYP2C8-Tg mice (n=3–7 per group). K, Aortic sprouting from Tie2-sEH-Tg and sEH−/− treated with 17,18-EDP and 19,20-EEQ (n=4–8 per group). Scale bars, 50 µm (t test; *P<0.05 and **P<0.01). Aortic ring pictures were taken by Zeiss AxioObserver microscope under ×10 objective lens. n.s. indicates not significant.
In OIR With ω3LCPUFA Feed, Tie2-CYP2C8-Tg Increase and Tie2-sEH-Tg Decrease Plasma Epoxide Levels and Retinal Epoxide:Diol Ratios at P17
In OIR, ω3LCPUFA-fed Tie2-CYP2C8-Tg mouse plasma has more 19,20-EDP (≈1.6-fold; P=0.029) and more 17,18-EEQ (≈1.5-fold; P=0.030) than WT. The DHA-derived 19,20-EDP level is 30-fold higher than EPA-derived 17,18-EEQ in both mutant and WT (Figure 2F), indicating that DHA contributes more than EPA to the effect. No significant change was found in the plasma EETs levels (Figure IIB in the online-only Data Supplement). In ω3LCPUFA-fed Tie2-sEH-Tg mice, 19,20-EDP and 17,18-EEQ levels were reduced by 34% (P=0.034) and 24% (P=0.016; Figure 2G). In OIR, ω3LCPUFA-fed Tie2-CYP2C8-Tg retinas had a 52% higher 19,20-EDP:DiHDPA ratio than WT (P=0.045; Figure 2H); the 17,18-EEQ:17,18-dihydroxy-eicosatetraenoic acid (DHEQ) ratio was unchanged. In ω3LCPUFA-fed Tie2-sEH-Tg retinas, the 19,20-EDP:DiHDPA ratio decreased by 58% (P=0.028; Figure 2I); the 17,18-EEQ:17,18-DHEQ ratio was unchanged. We next examined the effect of sEH and CYP2C in ex vivo transgenic mouse aortic ring sprouting to confirm our findings.
ω3LCPUFA Metabolite Promotes Aortic Vascular Sprouting From Tie2-CYP2C8-Tg Mice, Which Is Lost in Tie2-sEH-Tg Mice
The proangiogenic effect of CYP2C8 and suppressive effect of sEH with ω3LCPUFA substrate were further confirmed with aortic ring-sprouting assays. A total of 30 µmol/L DHA (versus 30 µmol/L AA as a LCPUFA DHA control) attenuated aortic sprouting in WT (P=0.01), which was abolished in Tie2-CYP2C8-Tg (P<0.05; Figure 2J). Tie2-sEH-Tg suppressed aortic sprouting versus WT with 19,20-EDP treatment (≈50%; P<0.01; Figure 2K), whereas there was no effect with 17,18-EEQ, confirming the relative importance of DHA versus EDP with ω3LCPUFA effects.
Discussion
Although the effects of ω3LCPUFA on human health have been reported since the beginning of the last century,24 the knowledge of the primary mechanisms of action of these lipids is still relatively limited.3,5,6,8 We have shown previously that the 5-lipoxygenase ω3LCPUFA metabolite 4-hydroxy-docosahexaenoic acid suppresses retinal neovascularization.25 Here, we investigated whether CYP2C8, one of the dominant human CYP epoxygenases, also mediates angiogenic effects of ω3LCPUFA in pathological retinal neovascularization.
We found much higher levels of mouse CYP2C homologue in nonperfused retina than perfused retina, indicating that CYP2C in normal retina comes from circulating blood cells. In OIR, CYP2C is localized in some macrophages outside vessels, consistent with migration of circulating cells from increased vascular leakage in OIR.26 Increased retinal DHA-derived epoxide:diol ratio in OIR is consistent with increased CYP2C and decreased sEH levels. Recruited CYP2C-expressing macrophages may contribute to the increased CYP2C in OIR retinas. The increased levels of active metabolites and decreased level of less active breakdown products in OIR, in turn, lead to an increased DHA-derived epoxide:diol ratio.
No significant difference in normal vascular development is observed in Tie2-CYP2C8-Tg and Tie2-sEH-Tg mice compared with WT littermate controls at P7 (Figure I in the online-only Data Supplement). There is no global suppression in cyclooxygenase/lipoxygenase activity in Tie2-CYP2C8-Tg versus WT mice on both feeds (Figure III in the online-only Data Supplement). In OIR, neovascularization is increased in Tie2-CYP2C8-Tg and reduced in Tie2-sEH-Tg with ω3LCPUFA feed, suggesting a proretinopathy role of CYP2C8 ω3LCPUFA metabolites in OIR. However, unchanged neovascularization is observed in ω3LCPUFA-fed sEH−/− mice, possibly because of a faster normal vascular development at P7, which is more resistant to hyperoxia (Figure I in the online-only Data Supplement), or likely reflecting already low sEH expression in OIR. In OIR with ω3LCPUFA feed, Tie2-CYP2C8-Tg increases VEGF-A expression, whereas Tie2-sEH-Tg decreases VEGF-A expression, consistent with the neovascular phenotypes. We have shown that 5-lipoxygenase ω3LCPUFA metabolite 4-hydroxydocosahexaenoic acid suppresses retinal neovascularization without changing VEGF-A expression.25 VEGF-A has been identified to be positively involved in retinal pathological angiogenesis.27 These studies suggest an opposite effect on VEGF-A production of CYP2C8 ω3LCPUFA metabolites in retinal angiogenesis, possibly contributing to the increased neovessel formation.
In OIR with ω3LCPUFA feed, Tie2-CYP2C8-Tg increase and Tie2-sEH-Tg decrease plasma epoxide levels and retinal epoxide:diol ratios at P17. Higher levels of DHA-derived 19,20-EDP in both mutant and WT suggest 19,20-EDP as the main active CYP2C ω3LCPUFA metabolite versus EPA-derived 17,18-EEQ mediating a proretinopathy effect in OIR. These results suggest that with ω3LCPUFA feed, CYP2C8 may potentiate neovascularization primarily by increasing plasma 19,20-EDP and the retinal 19,20-EDP:DiHDPA ratio, which reflects both the activity of CYP2C8, which produces bioactive 19,20-EDP and the activity of sEH, which attenuates bioactivity through conversion to less active diol. The effects of CYP2C and sEH in transgenic mouse aortic ring sprouting confirm that Tie2-CYP2C8-Tg promotes angiogenesis with ω3LCPUFA (primarily DHA) and suggests that decreased neovascularization in Tie2-sEH-Tg may be directly attributable to accelerated degradation of 19,20-EDP by overexpressed sEH.
Finding new approaches to prevent or treat neovascularization are important. Here, we find a novel role of CYP2C8 (and sEH) ω3LCPUFA metabolites in retinopathy. CYP2C8 overexpression potentiates retinal neovascularization with ω3LCPUFA feed by increasing plasma DHA-derived 19,20-EDP (and increasing the retinal 19,20-EDP:DiHDPA ratio), reflecting both production and elimination of 19,20- EDP. A recent study shows that EDPs inhibit ECs migration in tumor angiogenesis by suppressing VEGF-C, but not VEGF-A, in human umbilical vein endothelial cells in vitro.20 VEGF-C is a critical mediator of lymphangiogenesis (as well as angiogenesis)28 and considered as an important therapeutic target for cancer. In current studies, no change in VEGF-C expression is observed in Tie2-CYP2C8-Tg and Tie2-sEH-Tg retina. The different expression pattern of VEGF-A and VEGF-C may contribute to the opposite angiogenic function of CYP2C8 metabolite 19,20-EDP observed in the retina and in tumor, indicating a tissue-specific role of CYP2C8. Previously, cardiomyocytes expressing CYP2C8 were shown to increase recovery after cardiac ischemia/reperfusion, whereas ECs expressing CYP2C8 reduced the recovery.12
OIR neovascularization is potentiated by the CYP2C ω6LCPUFA metabolite (14,15-EET; Figures IV and V in the online-only Data Supplement) and the CYP2C ω3LCPUFA metabolite 19,20-EDP, suggesting that inhibition of CYP2C8 might reduce retinal neovascularization by suppressing ω3LCPUFA and ω6LCPUFA diet-induced proretinopathy lipid metabolites.
Supplementary Material
Significance.
Cytochrome P450 epoxygenase induces retinal neovascularization; inhibition of cytochrome P450 epoxygenases presents a new target for retinopathy treatment. Retinal CYP2C from circulating blood cells increase levels of the proangiogenic CYP ω3-long-chain polyunsaturated fatty acid metabolite 19,20-epoxydocosapentaenoic acid (and ω6-long-chain polyunsaturated fatty acid metabolite 14,15-epoxyeicosatrienoic acid).
Acknowledgments
Sources of Funding
This work was supported by the National Institutes of Health (NIH)/National Eye Institute (EY022275, EY017017, and P01HD18655), RPB Senior Investigator Award, Lowy Medical Foundation, European Commission FP7 project 305485 PREVENT- ROP (LEHS), Canadian Institute of Health Research (Z. Shao), BrightFocus Foundation and BCH Faculty Career Development Award (J. Chen), Canada Research Chair tier II. (P. Sapieha), Deutsche Ophthalmologische Gesellschaft and Freifrau von Nauendorff Foundation (A. Stahl), NCI RO1CA148633-01A4 (D. Panigrahy) and supported, in part, by the Intramural Research Program of the NIH, National Institute of Environmental Health Sciences (Z01025034; D.C. Zeldin).
Nonstandard Abbreviations and Acronyms
- AA
arachidonic acid
- CYP
cytochrome P450 epoxygenase
- DHA
docosahexaenoic acid
- DHEQ
dihydroxy-eicosatetraenoic acid
- DiHDPA
dihydroxy-docosapentaenoic acid
- EC
endothelial cell
- EDP
epoxydocosapentaenoic acid
- EEQ
epoxyeicosatetraenoic acid
- EET
epoxyeicosatrienoic acid
- EPA
eicosapentaenoic acid
- LCPUFAs
long-chain polyunsaturated fatty acids
- OIR
oxygen-induced retinopathy
- sEH
soluble epoxide hydrolase
- VEGF
vascular endothelial growth factor
- WT
wild-type
Footnotes
The online-only Data Supplement is available with this article at http://atvb.ahajournals.org/lookup/suppl/doi:10.1161/ATVBAHA.113.302927/-/DC1.
Disclosures
None.
References
- 1.Friedman DS, O’Colmain BJ, Muñoz B, Tomany SC, McCarty C, de Jong PT, Nemesure B, Mitchell P, Kempen J. Eye Diseases Prevalence Research Group. Prevalence of age-related macular degeneration in the United States. Arch Ophthalmol. 2004;122:564–572. doi: 10.1001/archopht.122.4.564. [DOI] [PubMed] [Google Scholar]
- 2.Kempen JH, O’Colmain BJ, Leske MC, Haffner SM, Klein R, Moss SE, Taylor HR, Hamman RF. Eye Diseases Prevalence Research Group. The prevalence of diabetic retinopathy among adults in the United States. Arch Ophthalmol. 2004;122:552–563. doi: 10.1001/archopht.122.4.552. [DOI] [PubMed] [Google Scholar]
- 3.Connor KM, SanGiovanni JP, Lofqvist C, et al. Increased dietary intake of omega-3-polyunsaturated fatty acids reduces pathological retinal angiogenesis. Nat Med. 2007;13:868–873. doi: 10.1038/nm1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hellström A, Smith LE, Dammann O. Retinopathy of prematurity. Lancet. 2013;382:1445–1457. doi: 10.1016/S0140-6736(13)60178-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sapieha P, Stahl A, Chen J, et al. 5-Lipoxygenase metabolite 4-HDHA is a mediator of the antiangiogenic effect of ω-3 polyunsaturated fatty acids. Sci Transl Med. 2011;3:69ra12. doi: 10.1126/scitranslmed.3001571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stahl A, Sapieha P, Connor KM, Sangiovanni JP, Chen J, Aderman CM, Willett KL, Krah NM, Dennison RJ, Seaward MR, Guerin KI, Hua J, Smith LE. Short communication: PPAR gamma mediates a direct antiangiogenic effect of omega 3-PUFAs in proliferative retinopathy. Circ Res. 2010;107:495–500. doi: 10.1161/CIRCRESAHA.110.221317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sapieha P, Chen J, Stahl A, Seaward MR, Favazza TL, Juan AM, Hatton CJ, Joyal JS, Krah NM, Dennison RJ, Tang J, Kern TS, Akula JD, Smith LE. Omega-3 polyunsaturated fatty acids preserve retinal function in type 2 diabetic mice. Nutr Diabetes. 2012;2:e36. doi: 10.1038/nutd.2012.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Arnold C, Konkel A, Fischer R, Schunck WH. Cytochrome P450-dependent metabolism of omega-6 and omega-3 long-chain polyunsaturated fatty acids. Pharmacol Rep. 2010;62:536–547. doi: 10.1016/s1734-1140(10)70311-x. [DOI] [PubMed] [Google Scholar]
- 9.Panigrahy D, Greene ER, Pozzi A, Wang DW, Zeldin DC. EET signaling in cancer. Cancer Metastasis Rev. 2011;30:525–540. doi: 10.1007/s10555-011-9315-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Michaelis UR, Fisslthaler B, Barbosa-Sicard E, Falck JR, Fleming I, Busse R. Cytochrome P450 epoxygenases 2C8 and 2C9 are implicated in hypoxia-induced endothelial cell migration and angiogenesis. J Cell Sci. 2005;118(Pt 23):5489–5498. doi: 10.1242/jcs.02674. [DOI] [PubMed] [Google Scholar]
- 11.Imig JD, Hammock BD. Soluble epoxide hydrolase as a therapeutic target for cardiovascular diseases. Nat Rev Drug Discov. 2009;8:794–805. doi: 10.1038/nrd2875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Edin ML, Wang Z, Bradbury JA, Graves JP, Lih FB, DeGraff LM, Foley JF, Torphy R, Ronnekleiv OK, Tomer KB, Lee CR, Zeldin DC. Endothelial expression of human cytochrome P450 epoxygenase CYP2C8 increases susceptibility to ischemia-reperfusion injury in isolated mouse heart. FASEB J. 2011;25:3436–3447. doi: 10.1096/fj.11-188300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Imig JD. Epoxides and soluble epoxide hydrolase in cardiovascular physiology. Physiol Rev. 2012;92:101–130. doi: 10.1152/physrev.00021.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang B, Cao H, Rao GN. Fibroblast growth factor-2 is a downstream mediator of phosphatidylinositol 3-kinase-Akt signaling in 14,15-epoxyeicosatrienoic acid-induced angiogenesis. J Biol Chem. 2006;281:905–914. doi: 10.1074/jbc.M503945200. [DOI] [PubMed] [Google Scholar]
- 15.Cheranov SY, Karpurapu M, Wang D, Zhang B, Venema RC, Rao GN. An essential role for SRC-activated STAT-3 in 14,15-EET-induced VEGF expression and angiogenesis. Blood. 2008;111:5581–5591. doi: 10.1182/blood-2007-11-126680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Panigrahy D, Edin ML, Lee CR, et al. Epoxyeicosanoids stimulate multiorgan metastasis and tumor dormancy escape in mice. J Clin Invest. 2012;122:178–191. doi: 10.1172/JCI58128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Panigrahy D, Kalish BT, Huang S, et al. Epoxyeicosanoids promote organ and tissue regeneration. Proc Natl Acad Sci USA. 2013;110:13528–13533. doi: 10.1073/pnas.1311565110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Spector AA. Arachidonic acid cytochrome P450 epoxygenase pathway. J Lipid Res. 2009;50(Suppl):S52–S56. doi: 10.1194/jlr.R800038-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Konkel A, Schunck WH. Role of cytochrome P450 enzymes in the bioactivation of polyunsaturated fatty acids. Biochim Biophys Acta. 2011;1814:210–222. doi: 10.1016/j.bbapap.2010.09.009. [DOI] [PubMed] [Google Scholar]
- 20.Zhang G, Panigrahy D, Mahakian LM, et al. Epoxy metabolites of docosahexaenoic acid (DHA) inhibit angiogenesis, tumor growth, and metastasis. Proc Natl Acad Sci USA. 2013;110:6530–6535. doi: 10.1073/pnas.1304321110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee CR, Imig JD, Edin ML, et al. Endothelial expression of human cytochrome P450 epoxygenases lowers blood pressure and attenuates hypertension-induced renal injury in mice. FASEB J. 2010;24:3770–3781. doi: 10.1096/fj.10-160119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Smith LE, Wesolowski E, McLellan A, Kostyk SK, D’Amato R, Sullivan R, D’Amore PA. Oxygen-induced retinopathy in the mouse. Invest Ophthalmol Vis Sci. 1994;35:101–111. [PubMed] [Google Scholar]
- 23.Connor KM, Krah NM, Dennison RJ, Aderman CM, Chen J, Guerin KI, Sapieha P, Stahl A, Willett KL, Smith LE. Quantification of oxygen-induced retinopathy in the mouse: a model of vessel loss, vessel regrowth and pathological angiogenesis. Nat Protoc. 2009;4:1565–1573. doi: 10.1038/nprot.2009.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Holman RT. The slow discovery of the importance of omega 3 essential fatty acids in human health. J Nutr. 1998;128(2 Suppl):427S–433S. doi: 10.1093/jn/128.2.427S. [DOI] [PubMed] [Google Scholar]
- 25.Revermann M, Mieth A, Popescu L, Paulke A, Wurglics M, Pellowska M, Fischer AS, Steri R, Maier TJ, Schermuly RT, Geisslinger G, Schubert-Zsilavecz M, Brandes RP, Steinhilber D. A pirinixic acid derivative (LP105) inhibits murine 5-lipoxygenase activity and attenuates vascular remodelling in a murine model of aortic aneurysm. Br J Pharmacol. 2011;163:1721–1732. doi: 10.1111/j.1476-5381.2011.01321.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fu ZJ, Li SY, Kociok N, Wong D, Chung SK, Lo AC. Aldose reductase deficiency reduced vascular changes in neonatal mouse retina in oxygen-induced retinopathy. Invest Ophthalmol Vis Sci. 2012;53:5698–5712. doi: 10.1167/iovs.12-10122. [DOI] [PubMed] [Google Scholar]
- 27.Pierce EA, Avery RL, Foley ED, Aiello LP, Smith LE. Vascular endothelial growth factor/vascular permeability factor expression in a mouse model of retinal neovascularization. Proc Natl Acad Sci USA. 1995;92:905–909. doi: 10.1073/pnas.92.3.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Skobe M, Hawighorst T, Jackson DG, Prevo R, Janes L, Velasco P, Riccardi L, Alitalo K, Claffey K, Detmar M. Induction of tumor lymphangiogenesis by VEGF-C promotes breast cancer metastasis. Nat Med. 2001;7:192–198. doi: 10.1038/84643. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.