Abstract
OBJECTIVE
Groove pancreatitis is a rare form of chronic pancreatitis affecting the “groove” between the pancreatic head, duodenum, and common bile duct. The exact cause is unknown, although there are strong associations with long-term alcohol abuse, functional obstruction of the duct of Santorini, and Brunner gland hyperplasia.
CONCLUSION
Unfortunately, differentiating groove pancreatitis from malignancy on the basis of imaging features, clinical presentation, or laboratory markers can be extraordinarily difficult, and the vast majority of these patients ultimately undergo a pancreaticoduodenectomy (Whipple procedure) because of an inability to completely exclude malignancy. In certain cases, however, the imaging features on CT and MRI can allow the radiologist to prospectively suggest the correct diagnosis.
Keywords: CT, groove pancreatitis, MRI, pancreatic adenocarcinoma, pancreatic cancer
Groove pancreatitis, a rare form of chronic pancreatitis affecting the “groove” between the superior aspect of the pancreatic head, the duodenum, and the common bile duct, was first described by Becker in 1973 [1] and has remained a diagnostic dilemma for radiologists, pathologists, and clinicians since its first description [2, 3]. Groove pancreatitis is an extraordinarily rare form of pancreatitis, and only a few descriptions of it exist in the radiology and pathology literature. Even in the most specialized centers, many radiologists remain unfamiliar with the entity. Unfortunately, even when the possibility of groove pancreatitis is prospectively considered on the basis of the imaging features, a definitive diagnosis can be extraordinarily difficult, and an inability to distinguish groove pancreatitis from a primary duodenal, ampullary, or pancreatic malignancy often ultimately leads to surgery [2]. This review will focus on the underlying pathophysiology of groove pancreatitis, its typical clinical and biochemical manifestations, its radiologic appearance, the differential diagnosis for abnormalities in the pancreaticoduodenal groove, and the correlation between the radiologic and histopathologic features of the process.
Background
The exact underlying cause of groove pancreatitis is unclear, although a number of different theories exist: functional obstruction of the minor papilla or duct of Santorini, increasingly viscous pancreatic secretions as a result of alcohol use or smoking, Brunner gland hyperplasia resulting in stasis of pancreatic secretions in the dorsal pancreas, heterotopic pancreas in the duodenum, and peptic ulcer disease have all been suggested as potential contributing factors [4, 5]. However, a long history of alcohol abuse is thought to be the strongest association [2, 4].
Importantly, there is no known association between groove pancreatitis and autoimmune disease or gallstones. Instead, the demographic features of patients with groove pancreatitis are akin to those of patients with other forms of chronic pancreatitis. Similar to cases of traditional chronic pancreatitis, patients with groove pancreatitis are inevitably middle-aged men with a history of significant alcohol abuse. The incidence of groove pancreatitis in women and younger individuals is considerably lower [4, 6].
The clinical presentation of groove pancreatitis can vary greatly in its acuity, and although some patients can have a presentation similar to that of acute pancreatitis, others can have a more chronic disease course. In the acute setting, patients often present with severe abdominal pain, nausea, vomiting, and, in rare cases, acute gastric outlet obstruction. Alternatively, patients with a chronic presentation often have evidence of jaundice (as a result of distal common bile duct narrowing and strictures) and chronic weight loss, features that are often more suggestive of an underlying malignancy, rather than pancreatitis. The average duration of symptoms is usually 3–6 months, although time courses significantly shorter or longer have been described [4, 7].
Unfortunately, biochemical markers are only of limited use: Pancreatic enzymes are often normal or only minimally elevated, and tumor markers (e.g., carcinoembryonic antigen and CA-19-9) are usually negative [3]. Bilirubin levels can be elevated if the common bile duct is obstructed, and alkaline phosphatase levels can also be elevated even in the absence of ductal obstruction [4].
In cases where an accurate prospective diagnosis of groove pancreatitis is made on the basis of the imaging features, the treatment is usually supportive (similar to cases of conventional acute edematous pancreatitis), typically comprising a combination of fasting, parenteral nutrition, bed rest, and cessation of smoking or alcohol use [5]. Intervention is usually not attempted in the absence of acute complications, such as biliary obstruction or severe gastric outlet obstruction, as a result of duodenal edema, wall thickening, fibrosis, and stricture. In the chronic setting, some patients may require definitive surgery as a result of severe pancreatic insufficiency, weight loss, or intractable pain symptoms, and this subset of patients usually undergoes either a classic or pylorus-sparing pancreaticoduodenectomy (Whipple procedure), although endoscopic drainage of the minor papilla has also been shown to be effective in some patients [4]. The classic Whipple may be preferred over the pylorus-sparing Whipple procedure as a result of chronic inflammation and stricturing of the pylorus [5]. In patients for whom a prospective diagnosis is possible, surgery has been shown to be highly effective in controlling their symptoms, particularly with regard to pain and weight loss [8].
Unfortunately, the prospective diagnosis of this entity remains rare, and a definitive diagnosis can be extraordinarily difficult using any diagnostic modality. Instead, the typical patient course usually involves repeated non-diagnostic fine-needle aspiration (FNA) performed using endoscopic ultrasound guidance, followed by pancreaticoduodenectomy to exclude an underlying pancreatic head, ampullary, or duodenal malignancy [3, 7, 9].
Pathologic Profile
Groove pancreatitis has traditionally been divided into two forms. The pure form affects only the pancreaticoduodenal groove (i.e., between the pancreatic head and duodenum), whereas the segmental form is centered in the pancreaticoduodenal groove but also extends medially into the pancreatic head. The demarcation between these two forms of groove pancreatitis is not always completely clear. Some cases of pure groove pancreatitis can result in progressive narrowing of the pancreatic duct and, subsequently, lead to diffuse changes of chronic pancreatitis in the entirety of the pancreatic parenchyma [2].
On gross examination (Figs. 1–4), the duodenal mucosa between the major and minor papillae is often severely thickened. On sectioning, the epicenter of the process is typically in the spaces between the pancreas and duodenum and between the major and minor papillae. The involved areas can be relatively solid, almost gelatinous, areas of edema and fibrosis or can contain cysts. The cysts may contain small calculi and generally have a smooth white surface [7–9].
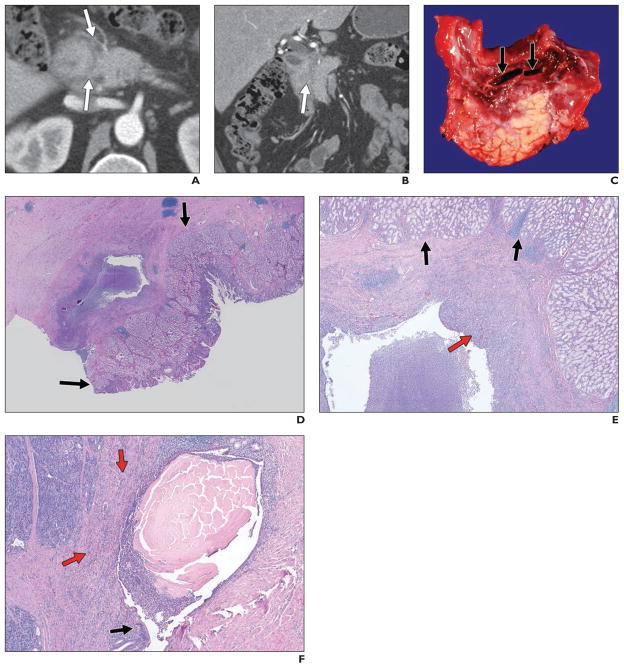
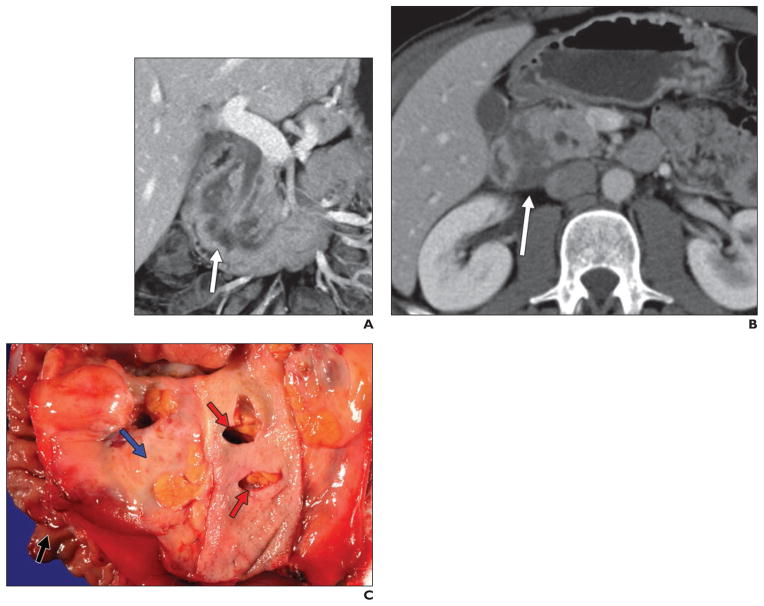
Fig. 1. 43-year-old man who initially presented with epigastric pain.
A and B, Contrast-enhanced CT revealed subtle infiltrating soft tissue (arrows) in pancreaticoduodenal groove. He underwent ERCP and endoscopic ultrasound, both of which suggested duodenal wall thickening and discrete mass, although biopsy results were negative. On basis of these findings, patient was thought to have primary duodenal malignancy. However, this process was found to represent groove pancreatitis after pancreaticoduodenectomy.
C, Gross specimen shows paraampullary duodenal wall cysts (arrows) with surrounding erythema and edema. Duodenal wall (top) and underlying pancreas (lower half) are thickened and fibrotic.
D, Histologic specimen, low-power view (H and E, ×1), shows Brunner gland hyperplasia (arrows) in duodenum, overlying inflamed cyst in groove region.
E, Histologic specimen (H and E, ×10) shows duodenal Brunner gland hyperplasia (black arrows) overlying inflamed cyst. Red arrow shows inflamed fibrous tissue in cyst wall. Cyst wall is composed of markedly inflamed fibrous tissue.
F, Histologic specimen (H and E, ×20) shows partially denuded duct with luminal concretions. Cyst is partially lined by ductal epithelium (black arrow) and contains thick proteinaceous secretions. Background pancreatic parenchyma (red arrows) is inflamed and fibrotic.
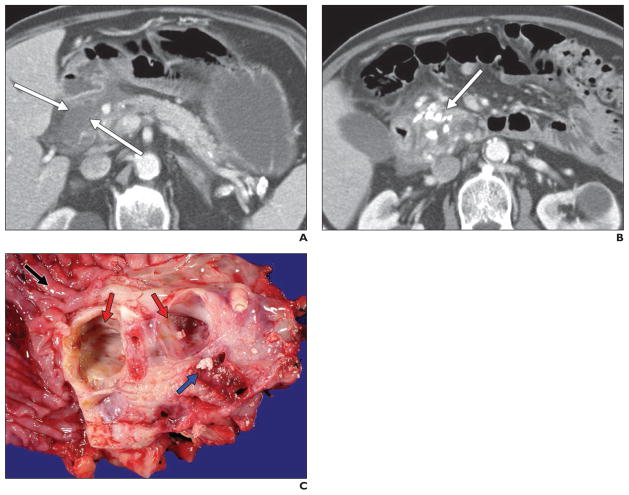
Fig. 4. 72-year-old man who presented with abdominal pain and weight loss.
A and B, Axial CT images with contrast agent show low-density soft tissue or fluid in pancreaticoduodenal groove (arrows, A), with multiple calcifications in pancreatic parenchyma (arrow, B). Given calcifications, it was thought that this pancreaticoduodenal groove low-attenuation material could represent sequelae of pancreatitis, but patient underwent surgery because of inability to completely exclude malignancy. Postsurgical pathology confirmed diagnosis of groove pancreatitis, with findings of chronic pancreatitis in remainder of gland.
C, Gross pathologic specimen (duodenum, black arrow) shows cysts (red arrows) in duodenal wall and subjacent pancreas, which are unilocular and contain multiple calculi (blue arrow).
Microscopic evaluation (Figs. 1, 3, and 5) typically reveals marked thickening of the duodenal wall, with Brunner gland and smooth muscle hyperplasia, edema, and inflammation. In some cases, heterotopic pancreatic tissue can be visualized, a feature thought to predispose patients to groove pancreatitis [7–9].
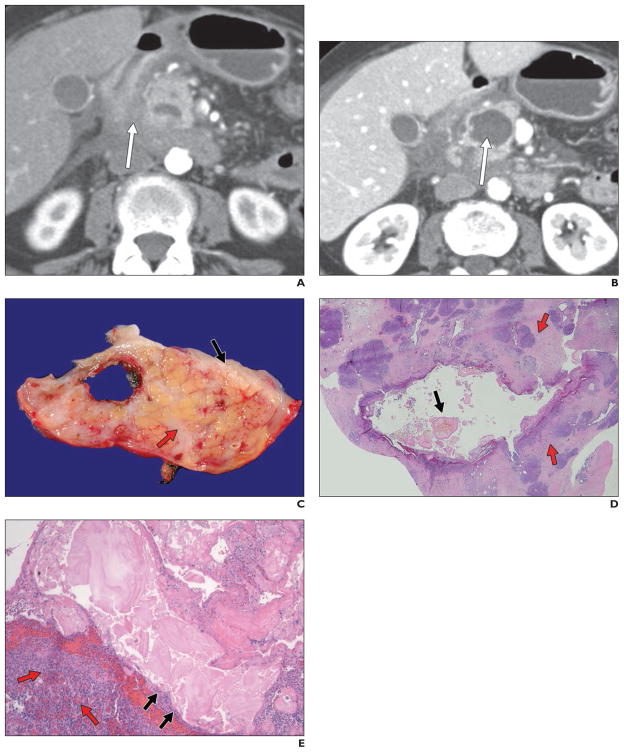
Fig. 3. 61-year-old woman who presented with 4-month history of abdominal pain, nausea, and vomiting.
A and B, Axial contrast-enhanced CT images show induration between duodenum and pancreatic head (arrow, A), some fluid in retroperitoneum or pararenal spaces, and cystic lesion in pancreatic head (arrow, B). Endoscopic ultrasound–guided fine-needle aspiration was negative for malignancy, although appearance was concerning for infiltrating neoplasm. Diagnosis of groove pancreatitis was confirmed on postsurgical pathology.
C, Gross specimen shows pancreatic cyst located subjacent to duodenal wall (black arrow). Pancreatic parenchyma (red arrow) is fibrotic.
D, Histologic specimen (H and E, ×1) shows dilated pancreatic duct. Duct lumen contains characteristic thick eosinophilic inspissated secretions (black arrow). Background pancreas is expanded by dense fibrosis (red arrows) and other stigmata of obstructive chronic pancreatitis.
E, Histologic specimen (H and E, ×40) shows dilated pancreatic duct. At this higher magnification, luminal secretions are associated with denudation (black arrows) of ductal epithelium. Spindle cells and mixed fibroinflammatory infiltrate (red arrows) of plasma cells, eosinophils, neutrophils, and lymphocytes are present in cyst wall.
Fig. 5. 62-year-old man who presented with painless jaundice and underwent placement of biliary drainage catheter.
A and B, Coronal (A) and axial (B) contrast-enhanced CT images show masslike enlargement of pancreatic head with central cystic focus (arrow, A), which is relatively isodense to surrounding pancreas, as well as diffuse pancreatic ductal dilatation. There is subtle soft tissue in pancreaticoduodenal groove (arrow, B), which is best seen on axial image. Endoscopic ultrasound suggested presence of discrete mass in this location (although biopsy results were negative), and patient underwent Whipple procedure under assumption that mass represented pancreatic adenocarcinoma. However, postsurgical pathology revealed it to be segmental form of groove pancreatitis with involvement of pancreatic head.
C, Histologic specimen (H and E, ×40) shows dilated pancreatic duct with thick proteinaceous secretions. Multiple smaller dilated ducts are present. Ducts are lined by low cuboidal-to-columnar mucinous ductal epithelium.
Characteristically, the process itself often arises around a partially denuded small duct leading into the minor papilla. This partially denuded duct may contain proteinaceous concretions and is surrounded by a proliferation of myofibroblasts that can extend outward to involve the duodenum, pancreas, and even bile duct. Inflammatory cells can be abundant or sparse [2, 8]. The stromal tissue surrounding these cysts can potentially show some degree of reactive cellular atypia. In the setting of a mass lesion, the atypia can potentially be confused for malignancy [10].
Unfortunately, cytology results of endoscopic ultrasound–guided FNA can be very variable, with the most common finding being spindled stromal cells. Most often, cytology results of FNA are interpreted as negative for malignancy, although sampling of an area with many spindle cells can be mistakenly interpreted as a spindle cell neoplasm [10]. Unfortunately, the specific diagnosis of groove pancreatitis cannot be made solely on the basis of FNA and cytology, and it can be very difficult to confidently exclude an underlying malignancy purely on the basis of a negative cytology result.
Imaging Findings
The MDCT findings of groove pancreatitis vary between the segmental and pure forms of the process. In the pure form, the appearance can range from ill-defined fat stranding and inflammatory change in the groove between the pancreatic head and duodenum, to frank soft tissue in the groove (Figs. 1, 3, 4, and 6). Notably, this soft tissue often has a “sheetlike” curvilinear crescentic shape that is best appreciated on coronal multiplanar reformatted images [11] (Figs. 2 and 7). If multiphase imaging is performed, this soft tissue tends to show increasing delayed enhancement as a result of a significant fibrotic component. It is not rare to appreciate thickening of the medial duodenal wall (particularly on the coronal images), and small cysts are a common feature either within the thickened duodenal wall or the pancreaticoduodenal groove itself [12] (Figs. 2, 7, and 8).
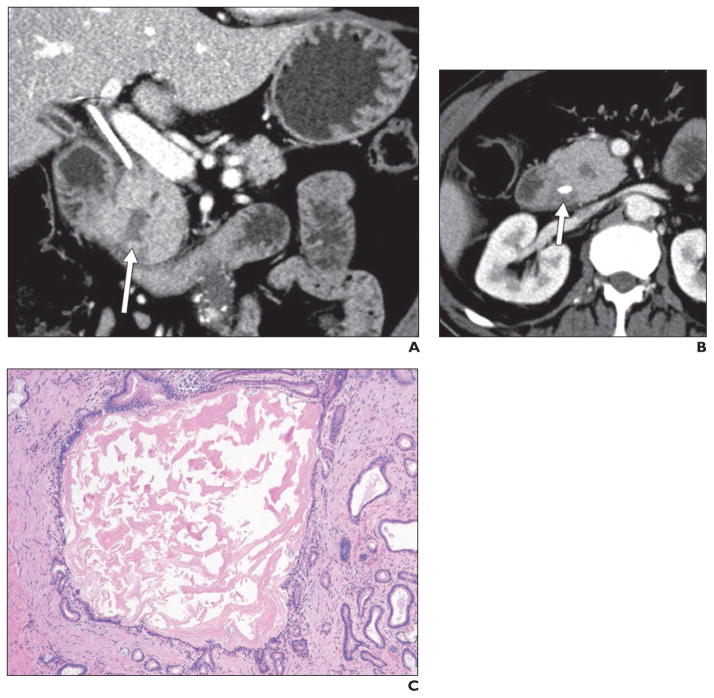
Fig. 6. 45-year-old man with history of alcoholism who presented with acute renal failure and abdominal pain.
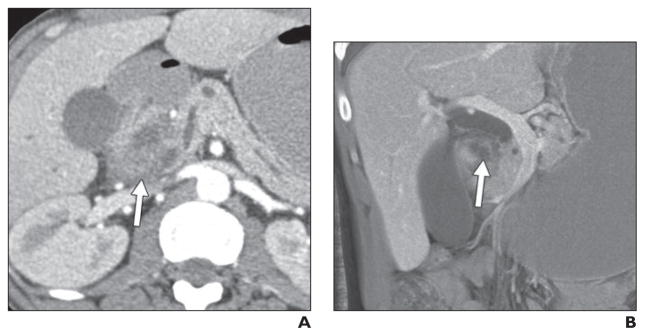
A and B, Axial (A) and coronal maximum-intensity-projection (B) contrast-enhanced CT images with contrast agent show hypodense soft tissue (arrow, A) in pancreaticoduodenal groove, with some areas of lower density (arrow, B), which are possibly cystic changes. Possibility of groove pancreatitis was raised on basis of imaging findings. Endoscopic ultrasound was performed and raised possibility of distinct mass in this location (although biopsy results were negative for tumor). Given inability to completely exclude tumor, patient underwent Whipple procedure, where diagnosis of groove pancreatitis was confirmed.
Fig. 2. 39-year-old man who presented with abdominal pain.
A and B, Contrast-enhanced coronal (A) and axial (B) images show infiltrating soft tissue (arrows) in pancreaticoduodenal groove. This “sheetlike” soft tissue is crescentic in appearance and is associated with thickening of medial duodenal wall and several cysts in wall of duodenum. Although possibility of groove pancreatitis was entertained on basis of CT appearance and negative endoscopic ultrasound biopsy results, patient underwent Whipple procedure because of inability to completely exclude duodenal malignancy. Postsurgical pathology results confirmed diagnosis of groove pancreatitis.
C, Gross specimen shows paraampullary duodenal wall cyst. Cyst is present between duodenal wall (black arrow) and adjacent pancreas (blue arrow), which show marked edema. Common bile duct has been opened and has few extraneous knife marks from dissection (red arrows); it does not show luminal narrowing or stricture formation.
Fig. 7. 45-year-old woman with long history of multiple bouts of abdominal pain refractory to conservative management.
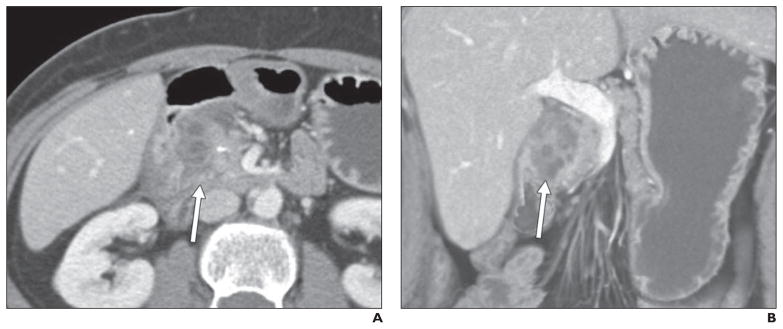
A and B, Axial (A) and coronal (B) contrast-enhanced CT images show infiltrating crescentic soft tissue (arrows) in pancreaticoduodenal groove with multiple cystic spaces. Given her long history, this was prospectively thought to represent groove or chronic pancreatitis, and patient underwent Whipple procedure because of continued inability to control her pain symptoms. Diagnosis of groove pancreatitis was confirmed on postsurgical pathology.
Fig. 8. 46-year-old man who presented with pruritus. He was found to have biliary and pancreatic ductal dilatation and underwent biliary stent placement.
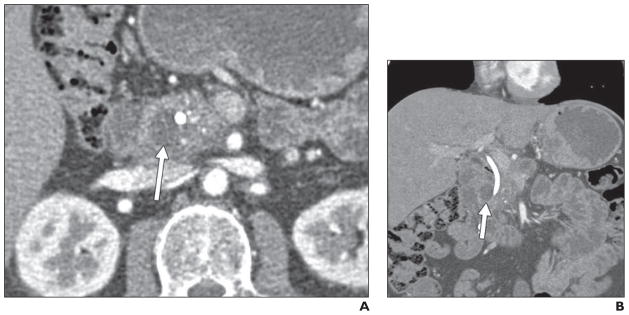
A and B, Axial (A) and coronal (B) contrast-enhanced CT images show subtle thickening between duodenum and pancreas, with cystic focus (arrows) in pancreaticoduodenal groove. ERCP showed irregular stricture of distal common bile duct, which was thought to be concerning for malignancy (although brush biopsy results were negative). Diagnosis of groove pancreatitis was confirmed on postsurgical pathology.
The segmental form can be much more difficult to appreciate, because involvement of the groove is often obscured by masslike enlargement of the pancreatic head. The segmental form of groove pancreatitis is very commonly confused for a pancreatic head mass, and differentiating the two entities can be nearly impossible on the basis of imaging (Fig. 5).
Regardless of the specific form of groove pancreatitis, the diffuse retroperitoneal inflammatory change seen in acute edematous pancreatitis is usually absent with groove pancreatitis (Fig. 3). It is rare to visualize fluid in the pararenal spaces or surrounding the pancreas, and diffuse inflammatory change is usually minimal [11–14]. Notably, in both forms, the common bile duct can appear attenuated and narrowed, a feature often best appreciated on the coronal multiplanar reformats. In most cases, this narrowing is relatively smooth, tapered, and regular, without evidence of “shouldering,” irregularity, or abrupt margins. The pancreatic duct can also be narrowed toward the downstream pancreatic head, typically in a smooth gradual fashion. In a more chronic setting, changes in the pancreatic parenchyma resembling those of traditional chronic pancreatitis can develop secondary to this progressive narrowing and fibrosis of the downstream pancreatic duct, including pancreatic calcifications, ductal dilatation, and ductal beading or irregularity [11–14] (Fig. 4).
Findings on MRI largely mirror those seen on CT. The sheetlike crescentic soft tissue found in the pancreaticoduodenal groove is typically mildly hypointense on T1-weighted images and variable in signal intensity on T2-weighted images and shows progressive enhancement on delayed images as a result of fibrotic tissue. In particular, the T2 intensity of this soft tissue can vary widely depending on the acuity of the process. In the acute phase, the tissue tends to be more T2 hyperintense because of edema and fluid and becomes progressively more hypointense over time because of the accumulation of a fibrotic component [2, 15] (Fig. 9).
Fig. 9.
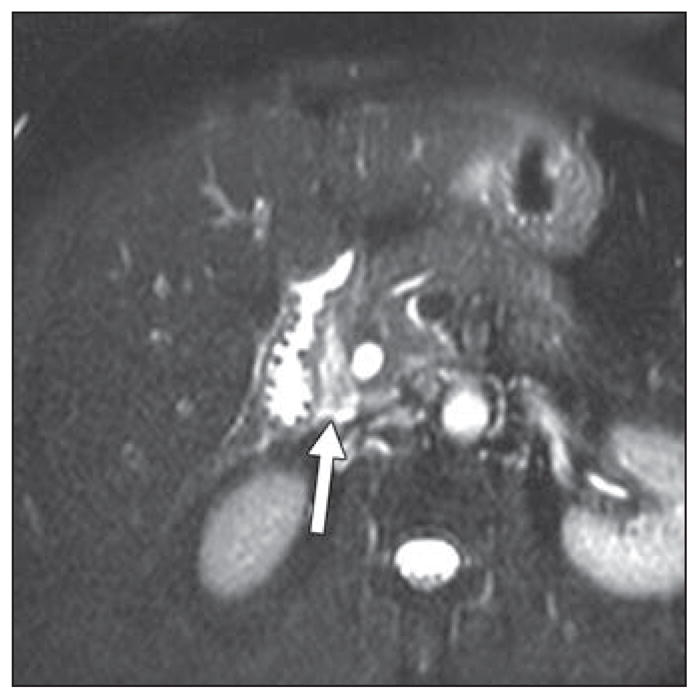
66-year-old woman with history of recurrent abdominal pain and presumptive groove pancreatitis. MRI shows T2-hyperintense sheetlike soft tissue in pancreaticoduodenal groove (arrow). She underwent endoscopic ultrasound, which revealed soft tissue in pancreaticoduodenal groove. However, because of imaging features and negative endoscopic ultrasound biopsy results, “lesion” was followed with surveillance scans and remained stable over time.
Involvement of the pancreas is well visualized on MRI in the segmental form, with progressive loss of T1 signal intensity in the pancreatic head as a result of parenchymal atrophy and fibrosis. The medial duodenal wall is involved in both the pure and segmental forms of groove pancreatitis, with duodenal wall thickening and multiple T2 hyperintense cysts in both the duodenal wall and pancreaticoduodenal groove. The medial duodenal wall can appear focally as T2 hyperintense and hyperenhancing at the site of abnormality [2, 15].
MRCP can nicely reveal abnormalities of the distal common bile duct and downstream pancreatic duct, both of which tend to be narrowed near the ampulla. The presence of an abnormality in the groove can be surmised by evaluating the distance between the ampulla and the duodenal lumen, which is typically widened in cases of groove pancreatitis (as a result of soft tissue in the groove and thickening of the duodenal wall). Finally, as a result of narrowing at the ampulla and strictures of the distal common bile duct, a dilated “banana-shaped” gallbladder has been described as an ancillary finding [2, 15].
The appearance of groove pancreatitis with both conventional abdominal ultrasound and endoscopic ultrasound is not well described in the literature, despite the growing use of endoscopic ultrasound in the evaluation and biopsy of pancreatic abnormalities. The appearance with both modalities is similar and varies depending on the course of the patient’s symptoms. In the early stages of the process, when there is more of an inflammatory component (rather than fibrosis), one can expect to visualize hypoechoic bandlike thickening of the pancreaticoduodenal groove, as well as thickening of the adjacent duodenum and a hypoechoic heterogeneous pancreatic head (in the segmental form of the process). In the chronic stages of the process, however, fibrosis dominates over inflammation, and the hypoechoic bandlike thickening is replaced by a hyperechoic band in the pancreaticoduodenal groove, contiguous with hyperechoic thickening of the duodenum and an increasingly hyperechoic pancreatic head [16]. On endoscopic ultrasound, it is common to visualize smooth narrowing of the common bile duct, and the Santorini duct, which is typically well visualized in normal examinations, often becomes undetectable [5]. Evaluation with ERCP is limited to visualization of a tapered lower bile duct, which can sometimes be difficult to differentiate from the irregular narrowing of the common duct seen with malignancies [5, 9].
Anatomy of the Pancreaticoduodenal Groove and Differential Diagnosis
The pancreaticoduodenal groove is a small theoretic space bordered by the pancreatic head (medial), second portion of the duodenum (lateral), third portion of the duodenum and inferior vena cava (posterior), and duodenal bulb (superior). The distal common bile duct, main pancreatic duct, accessory pancreatic duct, major papilla, and minor papilla are all found within this space, within either the pancreatic head or duodenum. A number of small arteries and veins lie within this space, the most important of which is the superior pancreaticoduodenal artery, as well as a number of small lymph nodes [17]. A number of other disorders centered in this space can mimic groove pancreatitis and should be considered in the differential diagnosis, as discussed in the following subsections.
Pancreatic Adenocarcinoma
The differentiation of pancreatic adenocarcinoma from groove pancreatitis can be extremely difficult, and many cases ultimately proceed to surgery because of an inability to reliably make this distinction [6] (Fig. 10). This is particularly the case with malignancies, which arise immediately adjacent to the groove itself and do not show the typical pancreatic ductal cutoff, ductal obstruction, and upstream atrophy present with most adenocarcinomas. Notably, however, unlike groove pancreatitis, most pancreatic adenocarcinomas do not show internal cystic change and are much more likely to infiltrate posteriorly into the retroperitoneum and encase the vasculature (including the gastroduodenal artery). Moreover, thickening of the medial duodenal wall, a common finding with groove pancreatitis, is quite uncommon with pancreatic adenocarcinoma. Finally, some researchers have suggested that the enhancement pattern for groove pancreatitis tends to be more patchy and heterogeneous compared with pancreatic adenocarcinoma, which is usually more homogeneously hypodense [18].
Fig. 10.
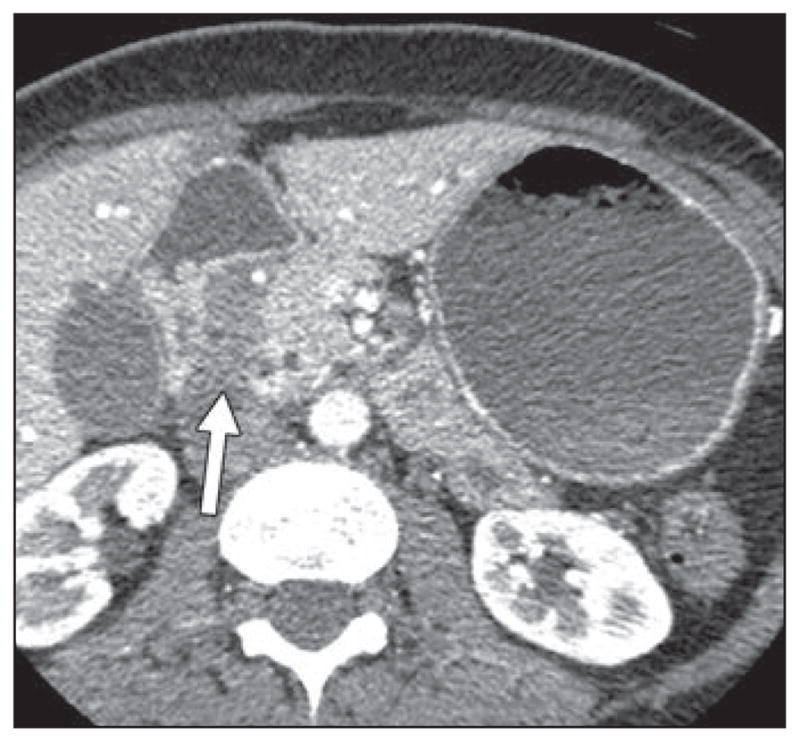
49-year-old woman with 1-month history of nausea and vomiting. CT image shows homogeneous hypodense thickening in pancreaticoduodenal groove, without evidence of vascular encasement, as well as smooth tapering of distal common bile duct and pancreatic duct (not shown). This was thought preoperatively to represent groove pancreatitis but was found on postoperative pathology to represent groove pancreatic adenocarcinoma.
Duodenal Adenocarcinoma
These tumors can be quite difficult to differentiate from groove pancreatitis, especially when they present as focal thickening of the medial duodenal wall. Most small-bowel adenocarcinomas arise from the duodenum or proximal small bowel, and close attention to the coronal multiplanar reformats may allow the accurate distinction of a mass arising in the duodenal wall from a process truly centered in the pancreaticoduodenal groove.
Ampullary Carcinomas
The focality of these malignant lesions at the ampulla should be distinguished from the more ill-defined crescentic soft tissue seen with groove pancreatitis. However, especially when ampullary carcinomas grow larger, the distinction may not be so simple.
Duodenal Gastrointestinal Stromal Tumor and Carcinoid
Gastrointestinal stromal tumors, in particular, can be quite variable in their appearance, and the more hypodense lesions arising from the submucosal layer of the medial duodenal wall could potentially mimic groove pancreatitis. However, some gastrointestinal stromal tumors and most carcinoid or neuroendocrine tumors are avidly hypervascular and will not typically be confused with groove pancreatitis.
Paraduodenal Pancreatitis
Several different terms have been coined for chronic inflammatory processes centered in the pancreaticoduodenal groove, including groove pancreatitis, cystic dystrophy of the heterotopic pancreas, periampullary duodenal wall cyst, pancreatic hamartoma of the duodenal wall, myoadenomatosis, and cystic dystrophy of the duodenal wall [5, 19]. This group of disorders, all of which are considered to be relatively similar from a histopathologic perspective, have clinically been grouped together and termed “paraduodenal pancreatitis.” Practically speaking, the different entities in this group do not substantially differ in terms of their clinical presentations and radiologic appearances and should all be considered as being within the spectrum of groove pancreatitis.
Conventional Edematous Pancreatitis With Involvement of the Groove
In some instances, an acute presentation of groove pancreatitis can be difficult to distinguish from acute edematous pancreatitis that secondarily involves the pancreaticoduodenal groove. Groove pancreatitis typically shows little retroperitoneal inflammation or fluid, and even in the segmental form, involvement of the pancreas is usually limited to the pancreatic head. This should be contrasted with typical acute edematous pancreatitis, which usually involves a substantial portion of the pancreatic parenchyma, does not appear centered in the groove, and is typically characterized by peripancreatic fluid and inflammation tracking into the pararenal spaces. Moreover, elevated lipase levels are not characteristic of groove pancreatitis and are an important differentiating feature. Finally, typical edematous pancreatitis should usually resolve on follow-up studies, whereas the imaging findings associated with groove pancreatitis often persist.
Conclusion
The prospective diagnosis of groove pancreatitis can be quite difficult regardless of the radiologic modality (CT or MRI), and, despite the presence of several suggestive imaging features, differentiating this entity from malignancy (particularly pancreatic ductal adenocarcinoma and duodenal adenocarcinoma) may not always be possible. In most cases, given the inability to reliably exclude an underlying malignancy, patients ultimately undergo pancreaticoduodenectomy. However, in those cases where the imaging features are highly characteristic and the radiologist is able to strongly suggest the diagnosis on presentation, major surgery can potentially be avoided.
References
- 1.Becker V. Bauchspeicheldruse (Inselapperat ausgenommen) In: Doerr W, editor. Spezielle pathologische anatomie. Berlin, Germany: Springer-Verlag; 1973. [Google Scholar]
- 2.Blasbalg R, Baroni RH, Costa DN, et al. MRI features of groove pancreatitis. AJR. 2007;189:73–80. doi: 10.2214/AJR.06.1244. [DOI] [PubMed] [Google Scholar]
- 3.Balakrishnan V, Chatni S, Radhakirshnan L, et al. Groove pancreatitis: a case report and review of the literature. JOP. 2007;8:592–597. [PubMed] [Google Scholar]
- 4.Triantopoulou C, Dervenis C, Giannakou N, et al. Groove pancreatitis: a diagnostic challenge. Eur Radiol. 2009;19:1736–1743. doi: 10.1007/s00330-009-1332-7. [DOI] [PubMed] [Google Scholar]
- 5.Tezuka K, Makino TY, Hirai I, Kimura W. Groove pancreatitis. Dig Surg. 2010;27:149–152. doi: 10.1159/000289099. [DOI] [PubMed] [Google Scholar]
- 6.Manzelli A, Petrou A, Lazzaro A, et al. Groove pancreatitis: a mini-series report and review of the literature. JOP. 2011;12:230–233. [PubMed] [Google Scholar]
- 7.Kim JD, Han YS, Choi DL. Characteristic clinical and pathologic features for preoperative diagnosed groove pancreatitis. J Korean Surg Soc. 2011;80:342–347. doi: 10.4174/jkss.2011.80.5.342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Levenick JM, Gordon SR, Sutton JE, et al. A comprehensive, case-based review of groove pancreatitis. Pancreas. 2009;38:e169–e175. doi: 10.1097/MPA.0b013e3181ac73f1. [DOI] [PubMed] [Google Scholar]
- 9.Maide DJ, Oliveira-Cunha M, Smith AM. Pancreatic carcinoma masquerading as groove pancreatitis: case report and review of the literature. JOP. 2011;12:598–602. [PubMed] [Google Scholar]
- 10.Chute DJ, Stelow EB. Fine-needle aspiration features of paraduodenal pancreatitis (groove pancreatitis): a report of three cases. Diagn Cytopathol. 2012;40:1116–1121. doi: 10.1002/dc.21722. [DOI] [PubMed] [Google Scholar]
- 11.Wook KS, Suk K, Woo LJ, et al. Evaluation of unusual causes of pancreatitis: role of cross-sectional imaging. Eur J Radiol. 2009;71:296–312. doi: 10.1016/j.ejrad.2008.04.006. [DOI] [PubMed] [Google Scholar]
- 12.Itoh S, Yamakawa K, Shimamoto K, et al. CT findings in groove pancreatitis: correlation with histopathological findings. J Comput Assist Tomogr. 1994;18:911–915. doi: 10.1097/00004728-199411000-00011. [DOI] [PubMed] [Google Scholar]
- 13.Perez-Johnston R, Sainani NI, Sahani DV. Imaging of chronic pancreatitis (including groove and autoimmune pancreatitis) Radiol Clin North Am. 2012;50:447–466. doi: 10.1016/j.rcl.2012.03.005. [DOI] [PubMed] [Google Scholar]
- 14.Shanbhogue AKP, Fasih N, Surabhi VR, et al. A clinical and radiologic review of uncommon types and causes of pancreatitis. RadioGraphics. 2009;29:1003–1026. doi: 10.1148/rg.294085748. [DOI] [PubMed] [Google Scholar]
- 15.Castell-Monsalve FJ, Sousa-Martin JM, Carranza-Carranza A. Groove pancreatitis: MRI and pathologic findings. Abdom Imaging. 2008;33:342–348. doi: 10.1007/s00261-007-9245-x. [DOI] [PubMed] [Google Scholar]
- 16.Wronski M, Karkocha D, Slodkowski M, et al. Sonographic findings in groove pancreatitis. J Ultrasound Med. 2011;30:111–115. doi: 10.7863/jum.2011.30.1.111. [DOI] [PubMed] [Google Scholar]
- 17.Hernandez-Jover D, Pernas JC, Gonzalez-Ceballos S, et al. Pancreatoduodenal junction: review of anatomy and pathologic conditions. J Gastrointest Surg. 2011;15:1269–1281. doi: 10.1007/s11605-011-1443-8. [DOI] [PubMed] [Google Scholar]
- 18.Ishigami K, Tajima T, Nishie A, et al. Differential diagnosis of grove pancreatic carcinoma vs. groove pancreatitis: usefulness of the portal venous phase. Eur J Radiol. 2010;74:e95–e100. doi: 10.1016/j.ejrad.2009.04.026. [DOI] [PubMed] [Google Scholar]
- 19.Pezzilli R, Santini D, Calculli L, et al. Cystic dystrophy of the duodenal wall is not always associated with chronic pancreatitis. World J Gastroenterol. 2011;17:4349–4364. doi: 10.3748/wjg.v17.i39.4349. [DOI] [PMC free article] [PubMed] [Google Scholar]