Abstract
BAL9141, a new antimicrobial agent belonging to the class of parenteral pyrrolidinone-3-ylidenemethyl cephalosporins, is active against most gram-positive microorganisms, including methicillin-resistant variants (methicillin-resistant Staphylococcus aureus [MRSA] and methicillin-resistant Staphylococcus epidermidis [MRSE]), as well as against penicillin-resistant pneumococci (PRP) and many gram-negative microorganisms. BAL9141 is administered as the prodrug BAL5788, which is rapidly converted to BAL9141 by plasma esterases. Pharmacokinetic (PK) data obtained in a previous multiple ascending dose study were used to fit a population PK model to using the NPEM2 program, yielding PK parameter estimates and its covariance matrix for BAL9141. These estimates and matrix were used to perform Monte Carlo simulations (MCSs) and obtain unbiased target attainment rates (TARs) for various time periods during which the concentration remains above the MIC (T>MIC). Assuming a T>MIC of 40%, TARs of 100% were reached with a dose of 500 mg/liter every 12 h for pathogens with MICs of 2 mg/liter and with a dose of 750 mg/liter every 12 h for pathogens with MICs of 4 mg/liter. Because MICs are ≤2 mg/liter for most strains of MRSA, MRSE, and PRP (with some strains showing an MIC of 4 mg/liter), a dosing regimen of 750 mg every 12 h is proposed for clinical studies. The corresponding provisional breakpoint is S (susceptible) ≤ 4 mg/liter.
Antimicrobial resistance among gram-positive cocci is of serious concern (6, 7, 24). The increased prevalence of resistance, particularly in Staphylococcus aureus and pneumococci, has compromised established recommendations for first-line treatment (12). Although β-lactams have traditionally been used to treat infections caused by S. aureus, these antibiotics were not effective against methicillin-resistant S. aureus (MRSA) (5).
BAL9141 (formerly Ro 63-9141), a pyrrolidinone-3-ylidenemethyl broad-spectrum cephalosporin, is administered as the prodrug BAL5788, which is rapidly and quantitatively converted to BAL9141 by plasma esterases. BAL9141 has a unique mechanism of action in that it binds to and inhibits penicillin-binding protein (PBP) PBP2a and PBP2x, the main enzymes involved in conferring resistance to methicillin and penicillin in staphylococci and pneumococci, respectively. BAL9141 also inhibits PBPs of other clinically relevant gram-positive and gram-negative pathogens (11, 13) and is resistant to hydrolysis by many common β-lactamases. BAL9141 is highly active against MRSA and may prove to be a suitable treatment for infections caused by this pathogen.
Phase 1 pharmacokinetic (PK) studies are traditionally followed by phase II dose-finding studies wherein the various dosing regimens are arrived at empirically. However, recently, there has been much progress in understanding the relationship between the dose and therapeutic efficacy of antimicrobial agents. Therapeutic efficacy has been shown to be dependent on particular pharmacodynamic indices (PDIs), i.e., either area under the concentration-time curve (AUC) and/or peak concentration (both in relation to the MIC) or the time period during which the concentration remains above the MIC (T>MIC) (4). The relationship between PDI and therapeutic efficacy has been shown for various antimicrobial agents using in vitro PK and animal models. A significant correlation between T>MIC and therapeutic effect has been shown with β-lactams (26), and a strong correlation between T>MIC and effect was found for BAL9141 in both in vitro and animal studies (D. Andes and W. A. Craig, Abstr. 40th Intersci. Conf. Antimicrob. Agents Chemother., abstr. 1079, 2000).
Based on established dose-effect relationships of a drug, a suitable T>MIC target can be set and a dosing regimen can be chosen with a high probability of clinical success (20, 22). Calculation of a precise therapeutic dosing regimen is confounded, however, by interindividual PK variability. Monte Carlo simulation (MCS) of antimicrobial dosing regimens is a powerful tool for determining the probability of achieving a specific PDI value defined as a target attainment rate (TAR) (9, 10). MCS has been used for AUC-dependent drugs (1, 8, 15, 19, 25), whereby TARs are obtained for selected values of AUC/MIC ratios. An appropriate dosing regimen can be chosen or a breakpoint value (given the dosing regimen) projected from TARs obtained for various simulated dosing regimens, taking into consideration the population distributions of the MICs of a given target pathogen.
A MCS approach has seldom been used for antimicrobial agents showing time-dependent (T>MIC) activity (2, 17), mainly because the calculations needed are less straightforward than for AUC-dependent drugs. The objectives of the present study were to propose a dosage regimen for future therapeutic studies with BAL9141, to show that MCS can be used to determine T>MIC activity, and to illustrate the limitations of this approach for β-lactams.
(Part of this study was presented at the 42nd Interscience Conference on Antimicrobial Agents and Chemotherapy, San Diego, Calif., 27 to 30 September 2002.)
MATERIALS AND METHODS
Data.
Plasma drug concentrations used for modeling were taken from a previously performed multiple ascending dose study (Basilea, Inc. [Basel, Switzerland], data on file). Briefly, six subjects received 30-min infusions of BAL9141 as the prodrug BAL5788 (500 mg every 12 h), and six subjects received 750 mg every 12 h for 8 days. Serum samples were collected at days 1 and 8 immediately before start of infusion and at 5, 15, 30 (end of infusion), and 45 min and at 1, 1.25, 1.5, 2, 3, 4, 6, 8, 10, 12, 14, 16, and 24 h after the dose. The degree of protein binding of BAL9141 was 40% as determined by centrifugal ultrafiltration.
Population PK modeling.
Population PK modeling was performed with the NPEM2 program (27) using a two-compartment open model. Population PK parameters were estimated from the multiple ascending dose study using data from days 1 and 8. Parameter ranges were estimated by first running the iterative Bayesian front end. Weighting was performed using the assay error pattern described by the polynomial standard deviation (SD) = 0.6546 + 0.5697D-001 C-0.164D-006 c2, where c is concentration.
Estimation of T>MIC and the MCS.
Various dosing regimens were simulated to obtain T>MIC as a function of MIC (22). MCS was performed using the MICLAB version 2.20 program (Medimatics, Maastricht, The Netherlands) simulating 10,000 subjects for each regimen. The estimated parameters obtained from the population PK analysis were used to obtain TARs for T>MIC, expressed as the percentage of the population reaching or exceeding a specific target. MCS was performed, after correction for protein binding, using two approaches. In the first approach, input values were the estimated parameters and measures of dispersion (SD). In the second approach, the full covariance matrix was used in the simulations.
The TARs at each MIC were then compared with the MIC distributions of several bacterial species, including MRSA.
RESULTS
Population PK analysis.
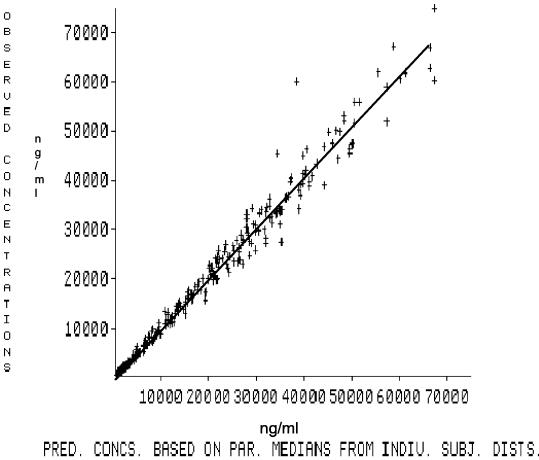
The best model fit to the experimental data using the NPEM2 program was obtained with a two-compartment model and yielded the estimated parameter values shown in Table 1. Table 1 includes the correlation matrix rather than the covariance matrix, although in calculations the covariance matrix was used throughout. Measured versus predicted concentrations of BAL9141 in serum are shown in Fig. 1. The two-compartment model accurately predicted BAL9141 concentration in serum (r2 = 0.98), the line of identity not differing significantly from 1. As further validation, the model was used to simulate the pharmacokinetics of a BAL9141 dose of 750 mg every 12 h. The simulated curve was virtually identical to that obtained experimentally (results not shown).
TABLE 1.
Parameter estimates and correlation matrix obtained after population modellinga
| Parameter | Parameter estimate (mean ± SD) | Correlation matrix
|
||
|---|---|---|---|---|
| Volume (liter) | CL (liter/h) | Kcp (h−1) | ||
| Volume (liter) | 10.386 ± 2.013 | 1 | ||
| CL (liter/h) | 5.111 ± 0.518 | 0.07657 | 1 | |
| Kcp (h−1) | 0.542 ± 0.237 | −0.61920 | 0.42430 | 1 |
| Kpc (h−1) | 0.883 ± 0.335 | −0.09290 | 0.25250 | 0.73700 |
Abbreviations: CL, clearance; Kcp and Kpc, equilibrium constants from central to peripheral compartment and from peripheral to central compartment, respectively.
FIG. 1.
Plot of predicted versus observed concentrations (in nanograms per milliliter) of BAL9141 for 12 patients by the population model, based on experimental data. The r2 value was 0.98 (P < 0.0001). The x axis shows the predicted concentrations based on parameter medians for the distribution of individual subjects. The figure shows the individual datum points, the regression line, and the y = x line for the entire population. The regression line superimposes on the y = x line.
Estimation of T>MIC for various dosing regimens.
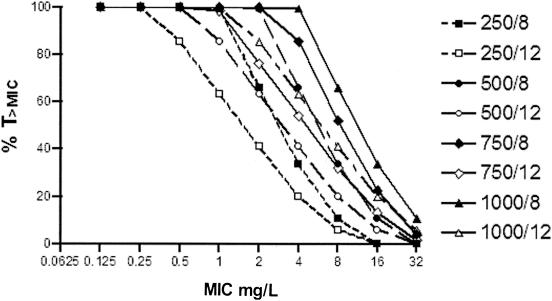
Estimates of mean values of serum BAL9141 concentrations from the NPEM2 analysis were used to simulate various dosing regimens and to obtain graphs of T>MIC versus MIC (Fig. 2). From these results, three dosing regimens were selected for MCSs: 500 mg every 8 h, 500 mg every 12 h, and 750 mg every 12 h.
FIG. 2.
T>MIC as a function of MIC for various dosing regimens of BAL9141. Various dosing regimens of BAL9141 were used: 250, 500, 750, and 1,000 mg/liter every 8 or 12 h.
MCSs.
TARs for four values of T>MIC obtained after MCSs using the full covariance matrix as input are shown in Table 2. A T>MIC of 40% was included, since this value yielded close to maximum effects in PD studies with BAL9141 (Andes and Craig, 40th ICAAC). At a BAL9141 concentration of 4 mg/liter, a T>MIC of ≥40% was achieved for the entire population with a dosing regimen of 750 mg every 12 h.
TABLE 2.
TARs for three dosing regimens and four targets
| BAL9141 concn (mg/liter) | TAR for dosing regimen and targeta
|
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 500 mg every 12 h
|
500 mg every 8 h
|
750 mg every 12 h
|
||||||||||
| 30 | 40 | 50 | 60 | 30 | 40 | 50 | 60 | 30 | 40 | 50 | 60 | |
| 0.5 | ||||||||||||
| 1 | 100 | 100 | ||||||||||
| 2 | 100 | 100 | 72 | 100 | 100 | 100 | 99 | |||||
| 4 | 100 | 59 | 1 | 0 | 100 | 100 | 99 | 79 | 100 | 100 | 78 | 15 |
| 8 | 0 | 0 | 0 | 80 | 13 | 0 | 0 | 69 | 3 | 0 | 0 | |
| 16 | 0 | 0 | 0 | 0 | ||||||||
| 32 | ||||||||||||
| 100% TARb | 4 | 2 | 2 | 1 | 4 | 4 | 2 | 2 | 4 | 4 | 2 | 1 |
TARs for three dosing regimens and four targets, i.e., 30, 40, 50, and 60% T>MIC. TARs are shown as percentages.
BAL9141 concentration (in milligrams per liter) that gives 100% TAR.
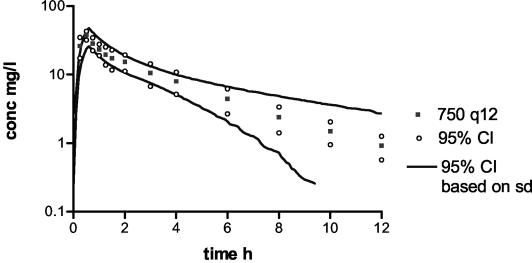
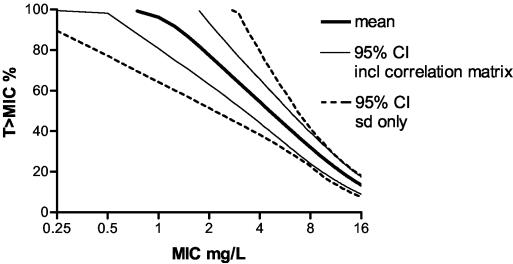
To demonstrate the importance of the covariance matrix as input in the MCSs, simulations were performed using only means and SDs as input. Figure 3 shows the simulated curve of the dosing regimen of 750 mg every 12 h with 95% confidence intervals (95% CI) and the 95% CI measured for subject samples. The 95% CI obtained without covariance matrix input is larger than that obtained with covariance matrix input, particularly at lower serum BAL9141 concentrations. TARs and 95% CI for the two approaches (Fig. 4) show that the 95% CI is wider when the covariance matrix is not considered, though at lower concentrations of BAL9141 in serum, a widening of the interval occurs with both approaches.
FIG. 3.
Simulation of a dosing regimen of 750 mg every 12 h (750 q12), with 95% CI based on SDs without the full covariance matrix and the 95% CI as measured from samples from subjects.
FIG. 4.
TARs and 95% CI (thus, attainment rates at 2.5 and 97.5%, respectively) as obtained with simulations using means and SDs with or without the full covariance matrix.
DISCUSSION
T>MIC is the PDI most likely to be associated with the efficacy of BAL9141, since β-lactams show time-dependent antimicrobial activity (3, 4, 18, 23). This relationship has been specifically confirmed for BAL9141 in animal model studies in which T>MIC correlated with efficacy better than either the peak drug concentration in serum or AUC from 0 to 24 h (Andes and Craig, 40th ICAAC). Accordingly, in order to select a rational dosing regimen for use in therapeutic studies with BAL9141, simulations of various dosing regimens with estimated PK parameter values were employed to obtain T>MICs. T>MIC plotted as a function of MIC for various dosing regimens (Fig. 2) indicated several possible optional dosing regimens. In vitro assays have indicated that the highest MIC for MRSA is 4 mg/liter (13, 14), so 4 mg/liter was used as the target MIC for which T>MIC should be optimized when selecting the dosing regimen.
In this study, MCS was used to simulate 10,000 subjects per dosing regimen. T>MIC was calculated for each subject for a range of MICs, and the proportion of the population attaining a certain T>MIC was determined. Ideally, the optimal dosing regimen should show 100% TAR (Table 2) at concentrations equal to or surpassing the highest MIC for the microorganism(s) targeted for therapy. A T>MIC of 40% was considered a reasonable target on the basis of animal data (Andes and Craig, 40th ICAAC). This is probably a conservative estimate, because in a study of a neutropenic mouse model of acute pneumococcal pneumonia, T>MICs lower than 40% afforded optimal effects (A. Azoulay-Dupuis, J. Mohler, J. Bedos, A. Schmitt-Hoffmann, and M. A. Shapiro, Abstr. 42nd Intersci. Conf. Antimicrob. Agents Chemother., abstr. F-335, 2002).
Historically, breakpoints have usually been set once dosing regimens were established, and consequently, debates have ensued as to where to set the breakpoints based on PK and PD information and the MIC distribution of strains (16, 21). Recently, MCS was used to reevaluate breakpoints for pneumococci for ceftriaxone, cefotaxime, and cefepime (M. N. Dudley and P. G. Ambrose, Abstr. 42nd Intersci. Conf. Antimicrob. Agents Chemother., abstr. A-635, 2002) and penicillin G (P. G. Ambrose, W. A. Craig, S. M. Bhavnani, and M. N. Dudley, Abstr. 42nd Intersci. Conf. Antimicrob. Agents Chemother., abstr. A-1263, 2002). The conclusions from these analyses were that the breakpoints for the three cephalosporins should be adjusted, while the susceptibility breakpoint for penicillin should not. MCS in the preclinical phases of drug development has led to the realization that breakpoints could, and should, be set on the basis of MIC distribution data before initiating clinical trials, using PK and PD information to identify dosing regimens affording acceptable TARs (9). In this study, the possible dosing regimens to pursue for BAL9141 in further studies were based on MIC distributions, in particular for MRSA.
In a recent in vitro evaluation (14), the activity of BAL9141 was tested against a large number of recent isolates from various international surveillance programs. For many gram-positive bacterial pathogens, including both oxacillin-susceptible (n = 50) and -resistant (n = 96) S. aureus and penicillin-resistant pneumococci (n = 114), the MICs of BAL9141 were ≤2 mg/liter; with a TAR of 2 mg/liter, 500 mg every 12 h is the dosing regimen corresponding to a 100% TAR at a T>MIC of 40%. However, the MIC was 4 mg/liter for some coagulase-negative staphylococcus strains, and 13% of a collection of MRSA strains (unpublished data) also showed MICs of 4 mg/liter, for which a dosage regimen of, for instance, 750 mg every 12 h would be required to achieve a 100% TAR at a T>MIC of 40%. Since BAL9141 is indicated for treatment of infections due to MRSA, a dosing regimen of 750 mg every 12 h is a conservative recommendation for treatment of infections caused by MRSA. The susceptibility-resistance breakpoint for BAL9141 is thus ≥ 8 mg/liter (National Committee for Clinical Laboratory Standards [NCCLS] notation) or >4 mg/liter (The European Committee on Antimicrobial Susceptibility Testing [EUCAST] notation) for gram-positive microorganisms. This breakpoint may not be applicable to gram-negative microorganisms, both because the T>MIC correlating to efficacy is more than 40% of the dosing interval (Andes and Craig, 40th ICAAC) and because the MIC distributions of gram-negative microorganisms are different.
A good indication of the T>MICs achievable using various dosing regimens (Fig. 2) is obtained using values for the population mean. However, it is important that every patient receiving therapy achieve the specified T>MIC. Calculations must therefore take interpatient variability into account, to ensure that even patients with relatively rapid elimination of BAL9141 are exposed to adequate drug concentrations. MCSs using estimated values of PK parameters require incorporation of a measure of dispersion, usually the SD. When models are fitted to PK data, the PK parameter estimates and their measures of dispersion are not independent, in which case population modeling must be performed in order to obtain the covariance matrix for estimated PK parameters. Simulations failing to take the covariance matrix into account lead to overestimates of the dispersion of the simulated concentration-time profiles (Fig. 3 and 4). For β-lactam antibiotics, this overestimation is greater at lower concentrations, resulting in large variations in T>MIC, primarily because at the peak concentrations, variance is determined by the volume of distribution, whereas over time, variance in intercompartmental rates and clearance play increasing important roles. In addition, variation in T>MIC increases as drug levels fall below the MICs near the end of the dosing interval. In the present study, the 95% CI are not very different between the results obtained with and without application of the covariance matrix for time points up to approximately 5 h after administration of the drug. However, as time progresses and concentrations decrease further, the differences become more prominent. Thus, increasing the dosing interval using higher doses will lead to a higher variability at clinically relevant concentrations.
The PK parameter values used to perform MCSs in this study were estimated from time-concentration data from 12 human volunteers. These 12 persons do not necessarily represent the whole patient population the drug is to be administered to; consequently, the parameter values and the measure of dispersion may be overestimated or underestimated. Importantly, however, the approach taken does provide a method to obtain a reasonable estimate of the attainment rates in the absence of anything else. Also, clearance in human volunteers in general is higher than in patients, and the breakpoints suggested are on the conservative side. In that respect, it is also important to note that the number of subjects simulated should be sufficient to include the extremes of the population distribution. We have used 10,000 patient simulations in this study, a large enough number to include the tails of the population distribution. However, it is important to evaluate the PK data from phase 2 trials and use these data to validate the simulations in this study.
We conclude that a dosing regimen for BAL9141 of 750 mg every 12 h has a high probability of therapeutic efficacy as shown by MCSs.
REFERENCES
- 1.Ambrose, P. G., and D. M. Grasela. 2000. The use of Monte Carlo simulation to examine pharmacodynamic variance of drugs: fluoroquinolone pharmacodynamics against Streptococcus pneumoniae. Diagn. Microbiol. Infect. Dis. 38:151-157. [DOI] [PubMed] [Google Scholar]
- 2.Ambrose, P. G., R. C. Owens, Jr., M. J. Garvey, and R. N. Jones. 2002. Pharmacodynamic considerations in the treatment of moderate to severe pseudomonal infections with cefepime. J. Antimicrob. Chemother. 49:445-453. [DOI] [PubMed] [Google Scholar]
- 3.Craig, W. A. 1998. Choosing an antibiotic on the basis of pharmacodynamics. Ear Nose Throat J. 77:7-11. [PubMed] [Google Scholar]
- 4.Craig, W. A. 1998. Pharmacokinetic/pharmacodynamic parameters: rationale for antibacterial dosing of mice and men. Clin. Infect. Dis. 26:1-10. [DOI] [PubMed] [Google Scholar]
- 5.Daum, R. S., and J. B. Seal. 2001. Evolving antimicrobial chemotherapy for Staphylococcus aureus infections: our backs to the wall. Crit. Care Med. 29:N92-N96. [DOI] [PubMed] [Google Scholar]
- 6.Diekema, D. J., M. A. Pfaller, R. N. Jones, G. V. Doern, K. C. Kugler, M. L. Beach, H. S. Sader, and the SENTRY Participants Group. 2000. Trends in antimicrobial susceptibility of bacterial pathogens isolated from patients with bloodstream infections in the USA, Canada and Latin America. Int. J. Antimicrob. Agents 13:257-271. [DOI] [PubMed] [Google Scholar]
- 7.Diekema, D. J., M. A. Pfaller, F. J. Schmitz, J. Smayevsky, J. Bell, R. N. Jones, and M. Beach. 2001. Survey of infections due to Staphylococcus species: frequency of occurrence and antimicrobial susceptibility of isolates collected in the United States, Canada, Latin America, Europe, and the Western Pacific region for the SENTRY Antimicrobial Surveillance Program, 1997-1999. Clin. Infect. Dis. 32(Suppl. 2):S114-S132. [DOI] [PubMed] [Google Scholar]
- 8.Drusano, G. L., S. L. Preston, M. H. Gotfried, L. H. Danziger, and K. A. Rodvold. 2002. Levofloxacin penetration into epithelial lining fluid as determined by population pharmacokinetic modeling and Monte Carlo simulation. Antimicrob. Agents Chemother. 46:586-589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Drusano, G. L., S. L. Preston, C. Hardalo, R. Hare, C. Banfield, D. Andes, O. Vesga, and W. A. Craig. 2001. Use of preclinical data for selection of a phase II/III dose for evernimicin and identification of a preclinical MIC breakpoint. Antimicrob. Agents Chemother. 45:13-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dudley, M. N., and P. G. Ambrose. 2000. Pharmacodynamics in the study of drug resistance and establishing in vitro susceptibility breakpoints: ready for prime time. Curr. Opin. Microbiol. 3:515-521. [DOI] [PubMed] [Google Scholar]
- 11.Entenza, J. M., P. Hohl, I. Heinze-Krauss, M. P. Glauser, and P. Moreillon. 2002. BAL9141, a novel extended-spectrum cephalosporin active against methicillin-resistant Staphylococcus aureus in treatment of experimental endocarditis. Antimicrob. Agents Chemother. 46:171-177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fridkin, S. K. 2001. Vancomycin-intermediate and -resistant Staphylococcus aureus: what the infectious disease specialist needs to know. Clin. Infect. Dis. 32:108-115. [DOI] [PubMed] [Google Scholar]
- 13.Hebeisen, P., I. Heinze-Krauss, P. Angehrn, P. Hohl, M. G. Page, and R. L. Then. 2001. In vitro and in vivo properties of Ro 63-9141, a novel broad-spectrum cephalosporin with activity against methicillin-resistant staphylococci. Antimicrob. Agents Chemother. 45:825-836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jones, R. N., L. M. Deshpande, A. H. Mutnick, and D. J. Biedenbach. 2002. In vitro evaluation of BAL9141, a novel parenteral cephalosporin active against oxacillin-resistant staphylococci. J. Antimicrob. Chemother. 50:915-932. [DOI] [PubMed] [Google Scholar]
- 15.Jones, R. N., C. M. Rubino, S. M. Bhavnani, and P. G. Ambrose. 2003. Worldwide antimicrobial susceptibility patterns and pharmacodynamic comparisons of gatifloxacin and levofloxacin against Streptococcus pneumoniae: report from the Antimicrobial Resistance Rate Epidemiology Study Team. Antimicrob. Agents Chemother. 47:292-296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kahlmeter, G., D. F. J. Brown, F. W. Goldstein, A. P. MacGowan, J. W. Mouton, A. Österlund, A. Rodloff, M. Steinbakk, P. Urbaskova, and A. C. Vatopoulos. 2003. European harmonization of MIC breakpoints for antimicrobial susceptibility testing of bacteria. J. Antimicrob. Chemother. 52:145-158. [DOI] [PubMed] [Google Scholar]
- 17.Kuti, J. L., C. H. Nightingale, R. Quintiliani, and D. P. Nicolau. 2002. Pharmacodynamic profiling of continuously infused piperacillin/tazobactam against Pseudomonas aeruginosa using Monte Carlo analysis. Diagn. Microbiol. Infect. Dis. 44:51-57. [DOI] [PubMed] [Google Scholar]
- 18.Leggett, J. E., S. Ebert, B. Fantin, and W. A. Craig. 1990. Comparative dose-effect relations at several dosing intervals for beta-lactam, aminoglycoside and quinolone antibiotics against gram-negative bacilli in murine thigh-infection and pneumonitis models. Scand. J. Infect. Dis. Suppl. 74:179-184. [PubMed] [Google Scholar]
- 19.Montgomery, M. J., P. M. Beringer, A. Aminimanizani, S. G. Louie, B. J. Shapiro, R. Jelliffe, and M. A. Gill. 2001. Population pharmacokinetics and use of Monte Carlo simulation to evaluate currently recommended dosing regimens of ciprofloxacin in adult patients with cystic fibrosis. Antimicrob. Agents Chemother. 45:3468-3473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mouton, J. W. 2002. Breakpoints: current practice and future perspectives. Int. J. Antimicrob. Agents 19:323-331. [DOI] [PubMed] [Google Scholar]
- 21.Mouton, J. W. 2003. Impact of pharmacodynamics on breakpoint selection for susceptibility testing. Infect. Dis. Clin. N. Am. 17:579-598. [DOI] [PubMed] [Google Scholar]
- 22.Mouton, J. W., and N. Punt. 2001. Use of the t > MIC to choose between different dosing regimens of beta-lactam antibiotics. J. Antimicrob. Chemother. 47:500-501. [DOI] [PubMed] [Google Scholar]
- 23.Mouton, J. W., M. L. van Ogtrop, D. Andes, and W. A. Craig. 1999. Use of pharmacodynamic indices to predict efficacy of combination therapy in vivo. Antimicrob. Agents Chemother. 43:2473-2478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.National Nosocomial Infections Surveillance System. 2001. National Nosocomial Infections Surveillance (NNIS) system report, data summary from January 1992-June 2001, issued August 2001. Am. J. Infect. Control 29:404-421. [DOI] [PubMed] [Google Scholar]
- 25.Nicolau, D. P., and P. G. Ambrose. 2001. Pharmacodynamic profiling of levofloxacin and gatifloxacin using Monte Carlo simulation for community-acquired isolates of Streptococcus pneumoniae. Am. J. Med. 111(Suppl. 9A):13S-18S, 36S-38S. [DOI] [PubMed] [Google Scholar]
- 26.Sanchez-Navarro, A., C. I. Colino, and M. M. Sanchez Recio. 2001. A retrospective analysis of pharmacokinetic-pharmacodynamic parameters as indicators of the clinical efficacy of ceftizoxime. Clin. Pharmacokinet. 40:125-134. [DOI] [PubMed] [Google Scholar]
- 27.Schumitzky, A. 1991. Nonparametric EM algorithms for estimating prior distribution. Appl. Math. Comput. 45:141-157. [Google Scholar]