Summary
Infants with MLL-rearranged (MLL-R) acute lymphoblastic leukaemia (ALL) have a dismal prognosis. While most patients achieve remission, approximately half of patients recur with a short latency to relapse. This suggests that chemotherapy-resistant leukaemia stem cells (LSCs) survive and can recapitulate the leukaemia. We hypothesized that interactions between LSCs and the bone marrow microenvironment mediate survival and chemotherapy resistance in MLL-R ALL. Using primary samples of infant MLL-R ALL, we studied the influence of bone marrow stroma on apoptosis, proliferation, and cytotoxicity induced by the FLT3 inhibitor lestaurtinib. MLL-R ALL were differentially protected by stroma from spontaneous apoptosis compared to non-MLL-R ALL. Co-culture of bulk MLL-R ALL in direct contact with stroma or with stroma-produced soluble factors promoted proliferation and cell cycle entry. Stroma also protected bulk MLL-R ALL cells and MLL-R ALL LSCs from lestaurtinib-mediated cytotoxicity. Previous studies have demonstrated that CXCR4 mediates bone marrow microenvironment signalling. Using a xenograft model of MLL-R ALL, we demonstrated that CXCR4 inhibition with AMD3100 (plerixafor) led to markedly enhanced efficacy of lestaurtinib. Therefore, the bone marrow microenvironment is a mediator of chemotherapy resistance in MLL-R ALL and targeting leukaemia-stroma interactions with CXCR4 inhibitors may prove useful in this high-risk subtype of paediatric ALL.
Keywords: acute lymphoblastic leukaemia, CXCR4, microenvironment, mixed lineage leukaemia, stroma
Advances in the diagnosis and treatment of paediatric acute lymphoblastic leukaemia (ALL) have led to cure rates approaching 90% (Pui et al, 2011). However, a significant proportion of children with ALL have high-risk features and the outcomes for these children have been relatively poor, especially in infants younger than 12 months of age, with a 5-year overall survival rate of 53% (Hunger et al, 2012). One reason for the poor prognosis in infants with ALL is the high prevalence of mixed lineage leukaemia (MLL) gene rearrangement (80%) in this age group (Pieters et al, 2007). MLL, which is located on chromosome 11q23, encodes for a DNA histone methyltransferase that helps to regulate gene expression through epigenetic modification (Krivtsov & Armstrong, 2007). When rearrangements of MLL occur, most commonly t(4;11)(q21;q23) (MLL-AFF1), t(11;19)(q23;p13·3) (MLL-MLLT1) and t(9;11)(p22;q23) (MLL-MLLT3), the resulting fusion proteins lead to aberrant histone modification and therefore aberrant gene expression, including expression of the homeobox genes HOX A9 and MEIS1 (Milne et al, 2005). The adverse prognostic significance of MLL rearrangements has also been demonstrated in children aged older than 1 year with ALL (Salzer et al, 2010) and in both de novo and therapy-induced acute myeloid leukaemia (AML) (Bernt & Armstrong, 2011).
Increased expression of the tyrosine kinase FLT3 (Armstrong et al, 2002; Yeoh et al, 2002), the expression of genes that mediate glucocorticoid resistance (Kotani et al, 2010; Stam et al, 2010; Spijkers-Hagelstein et al, 2012), and epigenetic influences on gene expression (Schafer et al, 2010; Zuber et al, 2011) have all been implicated as intrinsic mechanisms that may contribute to MLL-R leukaemia cell survival. As a result, strategies to target these mechanisms have emerged, including the use of tyrosine kinase inhibitors (TKIs) (Brown et al, 2005, 2006; Spijkers-Hagelstein et al, 2012), DNA methyltransferase inhibitors (Schafer et al, 2010) and inhibitors to bromodomain-containing proteins (Zuber et al, 2011). Studies have also suggested that infant MLL-R ALL differentially respond to certain chemotherapy agents in vitro (Ramakers-van Woerden et al, 2004) and remission rates of up to 97% have been reported with intensified treatment regimens (Pieters, 2009). However, a recent analysis of Interfant-99 demonstrated that approximately 60% of infants with MLL-R ALL still had minimal residual disease (MRD) as detected by real-time quantitative polymerase chain reaction at the end of consolidation therapy (Van der Velden et al, 2009), underscoring the resistance to chemotherapy in this ALL subtype. In addition, early relapse continues to be problematic with relapse rates of 50% and median time to relapse of just 8 months (Pieters, 2009), suggesting that leukaemia stem cells (LSCs) are able to survive in protective niches and eventually give rise to recurrent disease. Thus, it is possible that the poor outcome associated with MLL-R leukaemias is mediated both by unique survival pathways intrinsic to the leukaemia itself, such as inherent chemotherapy resistance, and by extrinsic factors, including increased protection of LSCs by the bone marrow microenvironment.
The bone marrow microenvironment is clearly important in the survival and quiescence of normal haematopoietic stem cells (HSCs) (Calvi et al, 2003; Yin & Li, 2006; Colmone et al, 2008; Ayala et al, 2009; Méndez-Ferrer et al, 2010). It is also becoming increasingly clear that interactions between leukaemia cells, particularly LSCs, and bone marrow stroma enhance leukaemia survival by promoting quiescence and inhibiting apoptosis (Konopleva et al, 2002; Jin et al, 2008). Perhaps the most studied leukaemia- stroma pathway is the interaction between CXCR4 and CXCL12 (also termed stromal cell-derived factor-1; SDF1), and CXCR4/CXCL12 signalling appears to play an important role in therapeutic resistance in leukaemias (Burger et al, 2000, 2003; Tavor et al, 2004). Several groups have demonstrated that inhibition of CXCR4 can lead to chemosensitivity in preclinical models of ALL (Juarez et al, 2003) and AML (Tavor et al, 2004, 2008; Nervi et al, 2009; Zeng et al, 2009; Jacobi et al, 2010). In addition, overexpression of CXCR4 has been correlated with inferior outcomes in both ALL (Crazzolara et al, 2001; Schneider et al, 2002) and AML (Rombouts et al, 2004; Konoplev et al, 2007; Spoo et al, 2007).
Given the high rates of MRD and relapse, and overall poor prognosis for infants with MLL-R ALL, we speculated that the bone marrow stroma may provide a protective niche for MLL-R ALL bulk cells and LSCs, through CXCR4/CXCL12 signalling. We also postulated that by disrupting the LSC-stroma interaction, MLL-R ALL LSCs may be rendered more vulnerable to targeted therapy with FLT3 TKIs. Our data show that MLL-R ALL cells are better protected from spontaneous apoptosis than non-MLL-R ALL cells when co-cultured with bone marrow stromal cells. We were also able to demonstrate that co-culture with bone marrow stromal cells protects MLL-R ALL cells from the cytotoxic effects of FLT3 inhibition, particularly when leukaemia and stromal cells are in direct contact. Further, using ex vivo culture followed by in vivo xenografting into non-obese diabetic/severe combined immunodeficient (NOD/SCID) mice, we found that co-culture of MLL-R ALL in physical contact with bone marrow stroma cells enhances the survival of LSCs in the presence and absence of FLT3 TKIs. Finally, we also showed that utilizing an antagonist of CXCR4, AMD3100, plus granulocyte colony-stimulating factor (G-CSF) enhances the efficacy of FLT3 TKIs against MLL-R ALL LSCs in vivo.
Materials and methods
Reagents
RPMI 1640 culture medium, AIM V culture medium, 100×penicillin-streptomycin solution (PS) and 200 m mol/l L-glutamine solution (LG) (all purchased from Invitrogen, Carlsbad, CA), fetal bovine serum (FBS, Gemini Bio-Products, West Sacramento, CA, USA), and 50 μmol/l hydrocortisone solution (Sigma-Aldrich, St. Louis, MO, USA) were used to maintain primary ALL and normal human bone marrow stromal cell cultures. The FLT3 inhibitor lestaurtinib was kindly provided by Cephalon, Inc. (Frazer, PA, USA). AMD3100 was purchased from Sigma-Aldrich. Stock solutions were prepared according to the manufacturer’s instructions and were stored at −80°C until ready for use.
Primary patient leukaemia samples
Diagnostic bone marrow and peripheral blood samples were collected under a Johns Hopkins institutional review board-approved protocol from newly diagnosed children with leukaemia. At the time of collection, primary leukaemic cells were enriched by density centrifugation using Ficoll-Paque PLUS (GE Healthcare, Piscataway, NJ, USA). Cells were either used fresh, or resuspended in 90% FBS/10% dimethyl sulfoxide (DMSO) and viably cryopreserved in liquid nitrogen. To perform our experiments on cryopreserved cells, vials were thawed, resuspended in media, and live leukaemic cells were enriched by density centrifugation. Primary ALL cells were maintained in AIM V medium supplemented with 10% FBS, 1% PS, and 1% LG. All leukaemia cell cultures were incubated at 37°C in 5% CO2. None of the MLL-R ALL primary samples harboured a FLT3 mutation.
Normal human bone marrow cultures
Normal human bone marrow was collected from healthy adult bone marrow transplant donors according to a Johns Hopkins institutional review board-approved protocol. Mononuclear cells were isolated via density centrifugation. Normal human bone marrow cultures were maintained in RPMI 1640 media supplemented with 10% FBS, 1% PS, 1% LG, and 2% hydrocortisone. Media was changed every 2 weeks and cultures were maintained for at least 4 weeks to select for adherent cells. To create tissue culture plates, 2 ml of Trypsin-EDTA (Sigma-Aldrich) were added to each tissue culture flask to detach adherent cells. Cells were then plated into tissue culture plates at a density of 50 000 cells/ml. Media was changed every 2 weeks and tissue plate cultures were maintained for at least 4 weeks in order to achieve confluence of stromal cells prior to co-culture with leukaemia cells. All stromal cell cultures were incubated at 33°C in 5% CO2.
Co-culture system
Primary ALL cells were cultured in three conditions: in physical contact with stroma (S); separated from physical contact with stroma by a permeable Transwell membrane (T); and medium only (M). For the T culture condition, leukaemia cells were seeded into Millicell hanging cell culture inserts (0·4 μm membrane pore size, Millipore, Billerica, MA) and placed into stroma-containing wells. The permeable membranes allowed exposure of leukaemia cells to soluble factors produced by stroma, but prevented physical contact with stroma. All leukaemia-stroma co-culture plates were incubated at 37°C in 5% CO2.
Proliferation and apoptosis assays
The Annexin V binding (AVB) assay was used to measure apoptosis in primary ALL cells. After exposure to differing co-culture conditions and/or doses of lestaurtinib, primary ALL cells were harvested and stained with phycoerythrin (PE)-conjugated annexin V and either fluorescein isothicyanate (FITC)-conjugated CD19 for B-lineage samples or FITC-conjugated CD3 for T-lineage samples (BD Pharmingen, San Diego, CA, USA). Flow cytometry was performed on each sample using a FACSCalibur machine (BD Biosciences, San Diego, CA, USA) and results were analysed using FlowJo software (Tree Star, Inc., Ashland, OR, USA). Leukaemic cells were identified by gating on CD19 or CD3 expressing cells. The percent viability for each sample was defined as the percentage of gated cells that were negative for annexin V.
The MTT (3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide) assay was used to measure proliferation according to the manufacturer’s instructions (Cell Proliferation Kit, Roche Applied Science, Indianapolis, IN, USA) as previously described (Brown et al, 2006). Briefly, primary ALL cells were treated with dose ranges of lestaurtinib for 24–48 h in the presence or absence of normal human bone marrow stromal feeder layers in tissue culture plates. Following treatment, MTT labelling reagent and solubilization solution were added to each well, and plates were read on a Model 680 Microplate Reader (Bio-Rad, Hercules, CA, USA).
Western blot: immunoprecipitation and immunoblotting
Primary ALL samples were incubated for 1 h with 50 n mol/l lestaurtinib or DMSO control. Cell lysis, immunoprecipitation with anti-FLT3 antibody (s18, Santa Cruz Biotechnology, Santa Cruz, CA, USA), and sequential blotting with anti-phosphotyrosine antibody (4G10, Millipore, Billerica, MA, USA), followed by stripping and reblotting with anti-FLT3 antibody were performed as previously described (Brown et al, 2005). Protein bands were visualized using enhanced chemiluminescence (ECL; Amersham, Piscataway, NJ, USA) and scanned with an AGFA Arcus 1200 laser scanner (AGFA, Ridgefield Park, NJ, USA).
Cell cycle kinetics assay
To assess the effects of stromal co-culture and lestaurtinib treatment on cell cycle kinetics, primary ALL cells were permeabilized with methanol, then stained with propidium iodide (PI) and analysed by flow cytometry as previously described (Brown et al, 2006).
Animals
NOD/SCID mice were purchased from the Jackson Laboratory (Bar Harbor, ME, USA) and treated according to an approved Johns Hopkins animal care and use protocol. Mice were given water supplemented with trimethoprim-sulfamethoxazole. Adult mice (6–8 weeks old) were sub-lethally irradiated prior to transplantation of primary ALL cells via tail vein injection. For engraftment studies, primary ALL cells were cultured in S, T, and M conditions for 48 h in the presence or absence of 20 nmol/l lestaurtinib prior to transplantation. For in vivo treatment studies, unmanipulated primary ALL cells were transplanted. After 2 weeks to allow engraftment, mice were treated with one of four regimens (n = 5 mice per treatment cohort): untreated control, lestaurtinib, mobilizers (G-CSF plus AMD3100), or mobilizers plus lestaurtinib. In both the engraftment and treatment studies, mice were sacrificed 10 weeks after transplantation. Bone marrow was harvested from femurs and tibias, and cells were stained with anti-human CD45 and anti-murine CD45 fluorescently-labelled antibodies to identify human ALL cells.
Statistical methods
Paired student’s t-tests and independent two-sample t-tests were used to calculate P values, which were considered statistically significant when < 0·05.
Results
Physical interaction with bone marrow stromal cells selectively promote the survival of MLL-rearranged ALL cells
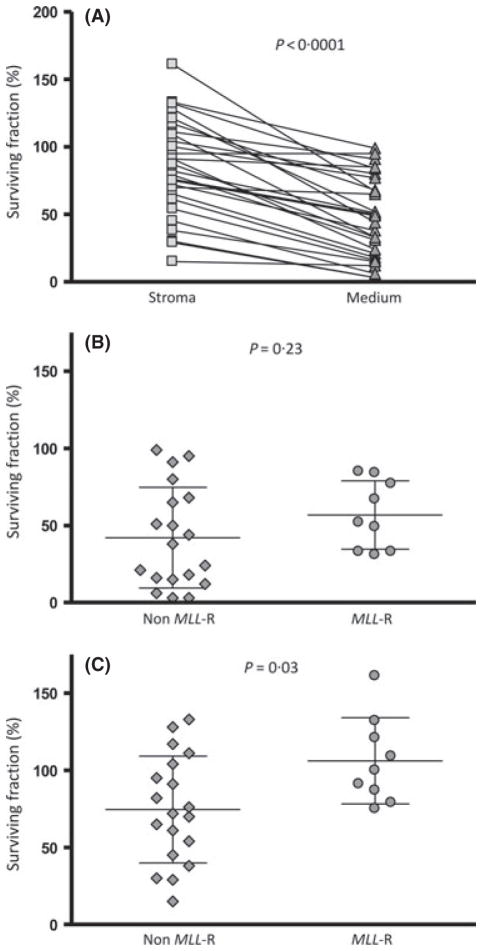
To investigate the role of leukaemia-stromal cell interaction in the survival of ALL cells, 28 primary ALL samples (9 MLL-R, 19 non-MLL-R) were cultured in medium alone and in medium plus normal human bone marrow feeder layers for 48 h. The surviving fraction, as a percentage of the number of viable cells at baseline, was then determined for each sample using the AVB apoptosis assay. Overall, we found that bone marrow stroma co-culture enhanced survival of the leukaemia cells (85% survival on stroma vs. 47% in medium alone, P < 0·0001) (Fig 1A). We next compared those samples with MLL rearrangement to those without and found that in medium alone, there was not a significant difference in the surviving fraction of MLL-R compared to the non-rearranged (57% vs. 42%, respectively, P = 0·23)(Fig 1B). However, on bone marrow stroma, the MLL-R samples had a significantly higher surviving fraction compared to the non-MLL-rearranged (106% vs. 75%, P = 0·03) (Fig 1C). These data suggest that interaction with bone marrow stroma is important in leukaemia cell survival. This also suggests that this interaction provides a greater survival advantage to ALL cells with MLL rearrangements compared to non-MLL rearranged ALL.
Fig. 1.
Human bone marrow stromal co-culture enhances the survival of primary patient ALL cells, particularly those with MLL rearrangement. Primary patient leukaemic blasts were cultured for 48 h in medium alone or on bone marrow stromal feeder layers. The surviving fraction, as a percentage of the number of viable cells at baseline, was determined for each sample using the annexin V binding apoptosis assay. (A) All samples (n = 28). Each line joins the results for one patient sample. P-value derived from paired Student’s t test. (B) Comparison of MLL-R (n = 9) vs. non MLL-R (n = 19) cultured in medium. P-value derived from Student’s t test. (C) Comparison of MLL-R (n = 9) vs non MLL-R (n = 19) co-cultured with normal human marrow stroma. P-value derived from Student’s t test.
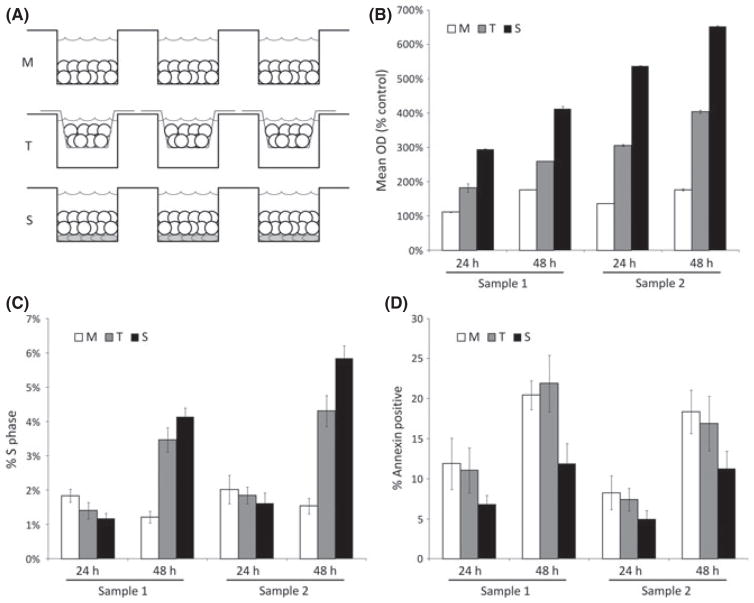
In the bone marrow microenvironment, there are two major aspects of stromal-leukaemia cell interaction: physical interaction between stromal cells and leukaemia cells, and production of soluble cytokines and other factors by stromal cells. To determine the relative contribution of each aspect in enhancing survival of leukaemia cells, we devised an in vitro system in which we could culture leukaemia cells in three different conditions, in medium alone (M), in physical contact with feeder stroma cells (S), and separated from the stromal layer by a transwell that is permeable to soluble factors produced by the stromal cells (T) (Fig 2A). Using this system, we investigated the comparative effects of the three culture conditions on the proliferation, survival, and cell cycle kinetics of two primary leukaemia samples isolated infants with MLL-R ALL (Sample 1: 4-month-old with t(11;19)/MLL-MLLT1 fusion; Sample 2: 3-month-old with t(4;11)/MLL-AFF1 fusion). To assess the pro-proliferative effects of stroma, the cells were cultured in the three culture conditions and proliferation was assessed using the MTT assay at incubation times of 24 and 48 h. The leukaemia cells did not proliferate in the presence of culture medium alone (M), but significant proliferation was noted in both stroma co-culture conditions (S and T) (Fig 2B). The pro-proliferative effect was stronger when the leukaemia cells were in physical contact with the stroma (condition S) than when they were separated from the stroma by a porous membrane (condition T). We next examined the effects of the S, T, and M conditions on cell cycle kinetics of primary ALL cells using flow cytometry analysis of DNA content after PI staining. We cultured primary MLL-R ALL cells in the S, T, and M conditions and performed PI staining at 24 and 48 h. There was a significant increase in the percentage of cells in the S phase of cell cycle between 24 and 48 h of incubation for cells co-cultured with stroma (S and T conditions), compared to cells cultured in media alone (Fig 2C). This effect was more pronounced when leukaemia and stromal cells were in direct contact. Using the AVB assay, we cultured primary MLL-R ALL cells in the three different culture conditions and found that the greatest protection from the induction of apoptosis was in the S condition (Fig 2D). Taken together, these data indicate that for MLL-R ALL cells, co-culture with bone marrow stroma enhances proliferation, accelerates entry into cell cycle, and reduces the induction of spontaneous apoptosis. Further, while soluble factors are able to mediate some effect of stroma on MLL-R ALL cells, physical interaction is required for maximal effect.
Fig. 2.
Stromal co-culture induces higher proliferation, cell cycle entry, and inhibition of apoptosis of bulk MLL-R ALL cells, especially with direct leukaemia-stroma contact. (A) Schema illustrating in vitro bone marrow stroma co-culture system. Leukaemic samples are cultured in three different conditions: in physical contact with stromal feeder layers (S), with stromal feeder layers but separated by permeable transwell (T) membranes (which expose leukaemia cells to soluble factors produced by stroma but prevent cell-cell interactions), and in culture medium only (M). (B) Proliferation was measured after 24 and 48 h of culture using the MTT assay. Error bars represent standard error of the mean (SEM) of triplicate wells. (C) Flow cytometry analysis of DNA content after PI staining was used to measure cell cycle kinetics after 24 and 48 h of culture. Error bars represent SEM of duplicate fluorescence-activated cell sorting (FACS) tubes. (D) Apoptosis was measured using the AVB assay. Error bars represent SEM of duplicate FACS tubes.
Interaction with bone marrow stromal cells protects MLL-R bulk leukaemia cells from the cytotoxic effects of targeted therapy
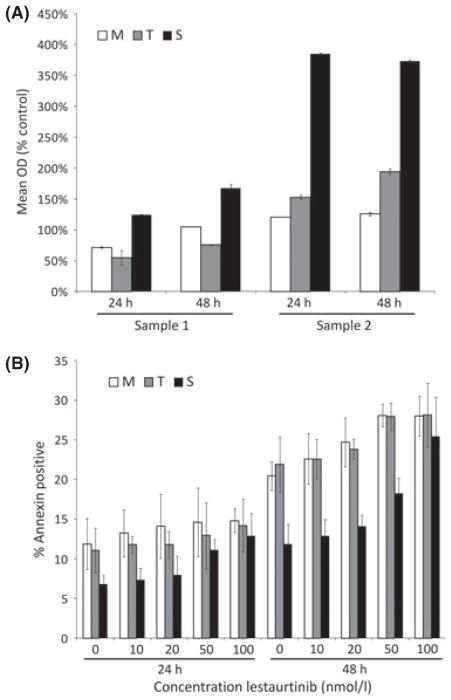
MLL-R ALL cells have high expression of and constitutive activation of the tyrosine kinase, FLT3. Previous work in our laboratory has demonstrated that inhibition of FLT3 activation is cytotoxic to MLL-R ALL cells (Brown et al, 2005). Therefore, we next investigated the effect of the presence or absence of bone marrow stroma co-culture on FLT3 inhibitor (lestaurtinib)-mediated cytotoxicity. Three MLL-R patient samples (Samples 3, 4 and 5) and three non-MLL-rearranged patient samples were cultured in medium alone and in medium plus bone marrow stroma at 6 dose levels (0–100 n mol/l) of lestaurtinib. After 48 h in culture, the samples were analysed using the MTT assay. To control for the MTT metabolized by the bone marrow stroma, and for any potential cytotoxic effect of lestaurtinib on the stroma itself, the optical density (OD) of control wells containing only bone marrow stroma was subtracted from the OD of the wells with both stroma and leukaemic blasts after treatment with the same concentration of lestaurtinib for the 48-h assay period. No cytotoxicity was observed in the stroma-only control wells at concentrations up to 100 n mol/l lestaurtinib (data not shown). The MLL-R samples were protected from the cytotoxic effects of lestaurtinib at all dose levels when co-cultured with bone marrow stromal cells (Fig 3A). At 50 n mol/l, for example, the mean OD for the 3 samples treated in medium alone was 25% untreated control vs. 47% for the same samples treated with stromal co-culture (P = 0·03). This effect was not seen in samples without MLL rearrangement (data not shown; mean OD in medium 93% vs. 103% with stromal co-culture, P = 0·27). In addition, Western blots revealed that treatment with 50 n mol/l lestaurtinib, which caused significant cytotoxicity in MLL-R cells, resulted in significant inhibition of FLT3 phosphorylation, suggesting that the cytotoxic effects are mediated through FLT3 (Fig 3B).
Fig. 3.
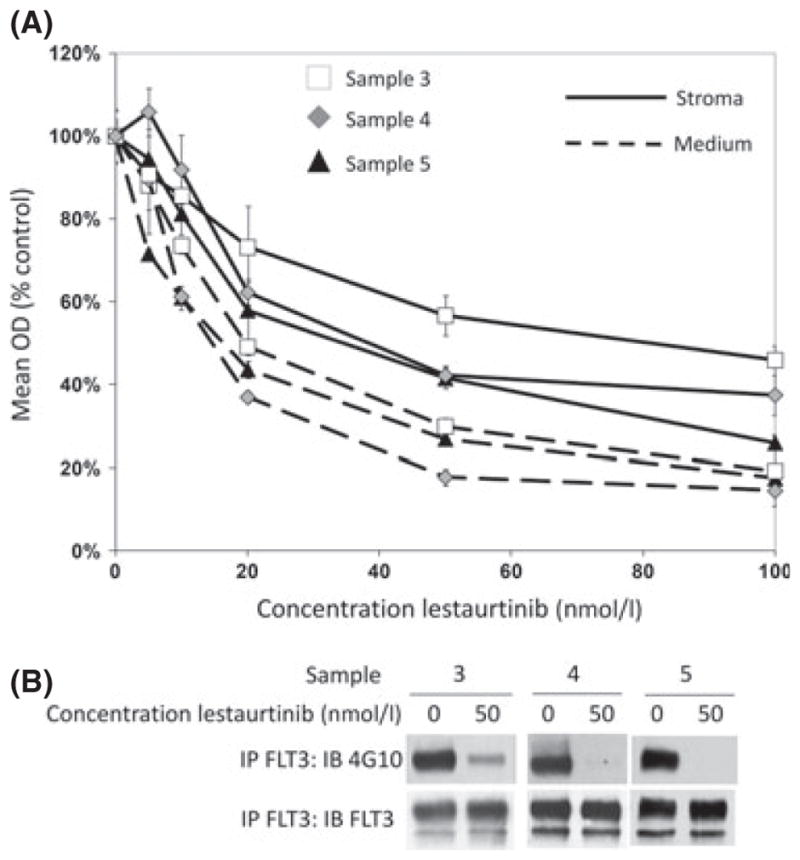
Human bone marrow stromal co-culture protects primary patient MLL-rearranged ALL cells from the cytotoxic effects of FLT3 inhibition. (A) MTT dose-response curves showing cytotoxic response to lestaurtinib (normalized to untreated controls) for three primary MLL-rearranged ALL patient samples cultured either in medium alone (dashed lines), or on bone marrow stromal feeder layers (solid lines) for 48 h. Error bars represent standard error of the mean (SEM) of triplicate wells. (B) Western blot for phosphorylated and total FLT3 after treatment with or without 50 n mol/l lestaurtinib.
To determine the relative importance of soluble factors produced by the bone marrow stroma and direct physical contact with the bone marrow stromal cells to the resistance to lestaurtinib-induced cytotoxicity, we cultured MLL-R ALL cells (Samples 1 and 2, as above) in the three different culture conditions in the presence of increasing concentrations of lestaurtinib (0–100 n mol/l). Despite the presence of lestaurtinib, cells cultured in the S condition demonstrated significant proliferation, compared to cells cultured in M or T (Fig 4A, showing results with 50 n mol/l lestaurtinib). We also measured the effect of stromal co-culture on protection from lestaurtinib-induced apoptosis. Using the AVB assay (for Sample 1 only - there were insufficient cells available for Sample 2 for this assay), we found that the S condition protected cells from lestaurtinib-induced apoptosis at doses of up to 50 n mol/l (Fig 4B). These data indicate that for MLL-R ALL cells, physical interaction with bone marrow stroma abrogates FLT3 TKI-mediated cytotoxicity.
Fig. 4.
Direct stromal contact results in higher pro-proliferative effects and inhibition of apoptosis, particularly in the presence of FLT3 inhibition. Primary MLL-R ALL cells were cultured in S, T, M conditions and treated with dose ranges of 0–100 n mol/l lestaurtinib for 24 and 48 h. (A) Proliferation was measured using the MTT assay. Data for 50 n mol/l lestaurtinib are shown. Error bars represent standard error of the mean (SEM) of triplicate wells. (B) Apoptosis was measured using the AVB assay. Error bars represent SEM of duplicate FACS tubes.
In summary, these data suggest that inhibition of FLT3 activation is cytotoxic to MLL-R ALL cells and that the bone marrow microenvironment may contribute to therapeutic resistance of MLL-R ALL cells to FLT3 inhibition.
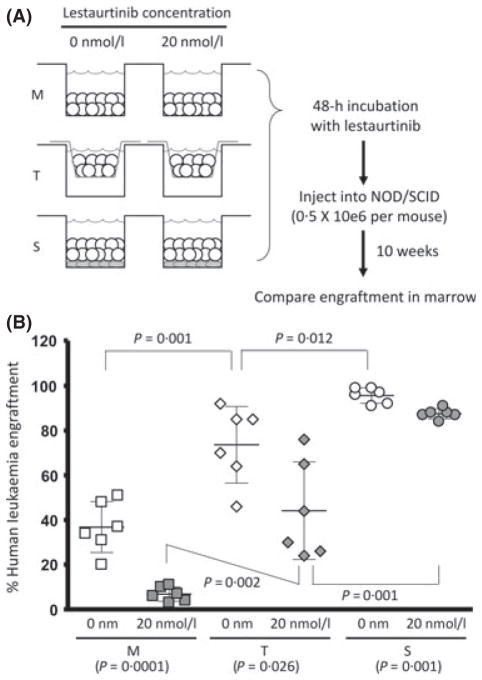
Co-culture with bone marrow stroma promotes the survival of LSCs ex vivo and protects LSCs from the cytotoxic effects of ex vivo exposure to a FLT3 TKI
We next sought to evaluate the role of the interaction between normal human bone marrow feeder layers and LSCs using ex vivo culture followed by in vivo xenografting (Fig 5A). Leukaemia cells from a primary MLL-R infant ALL sample (Sample 1, as above) were incubated for 48 h in S, T and M conditions, either in the presence or absence of 20 n mol/l lestaurtinib. We then injected 0·5 × 106 cells from each culture condition into cohorts of 6- to 8-week-old sub-lethally irradiated NOD/SCID mice. We hypothesized that stromal co-culture conditions (T and S) would preferentially protect LSCs from spontaneous and lestaurtinib-induced apoptosis. Bone marrow engraftment was examined after the mice were sacrificed 10 weeks later, reflecting LSC activity. Engraftment was defined as the percentage of CD45 + cells in the bone marrow that were of human origin. The S condition was clearly superior to either the T or M condition in promoting the survival of the LSC ex vivo in the absence of lestaurtinib (Fig 5B, open data points). After ex vivo exposure to 20 n mol/l lestaurtinib, cells cultured in the S condition led to even higher differences in engraftment compared to the T and M conditions, suggesting that physical interaction with stroma leads to protection from the cytotoxic effects of FLT3 inhibition (Fig 5B, filled data points). These data suggest that the physical interaction between LSCs and the bone marrow stromal cells play an important role in LSC survival and resistance to anti-leukaemic therapy.
Fig. 5.
Ex vivo stromal co-culture enhances LSC survival and protects LSC from cytotoxic effects of FLT3 inhibition. (A) Schema of experiment where primary MLL-R ALL cells were cultured in S, T and M condition in the absence or presence of 20 n mol/l lestaurtinib, then injected into sub-lethally irradiated NOD/SCID mice. Ten weeks after injection, mice were sacrificed and assessed for engraftment of human leukaemia cells. (B) S and T conditions significantly enhanced LSC survival ex vivo, both in absence (open data points) and presence (filled data points) of 20 n mol/l lestaurtinib. Engraftment is expressed as percentage of marrow CD45 + cells that were of human origin as determined by flow cytometric analysis. Bars show mean and standard deviation for each cohort.
CXCR4 antagonist AMD3100 in combination with G-CSF potently enhances the efficacy of FLT3 TKIs against MLL-R ALL LSCs
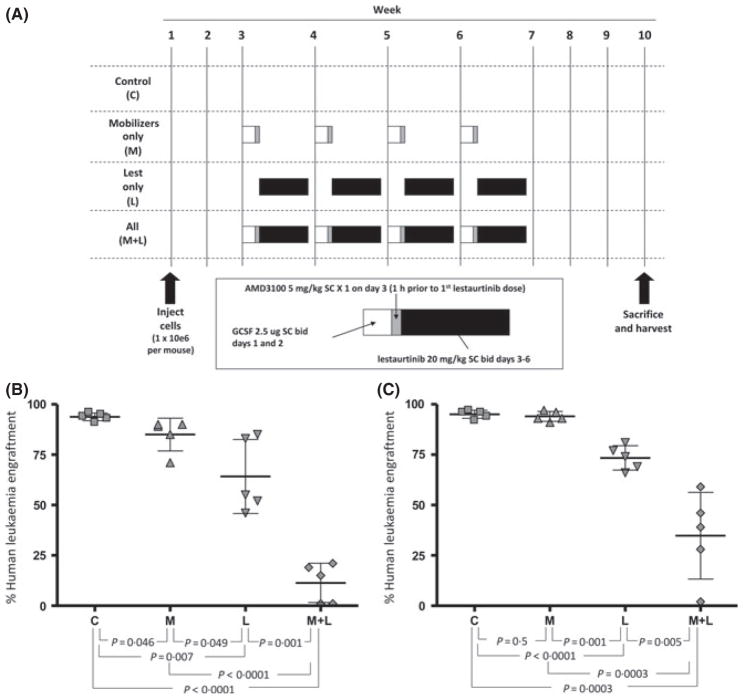
Finally, we investigated if targeting the interaction between the stromal microenvironment and leukaemia cells could enhance the efficacy of anti-leukaemic therapy. We used the CXCR4 antagonist, AMD3100 (plerixafor), plus G-CSF as a strategy to disrupt the leukaemia-stroma interaction and mobilize MLL-R ALL LSCs from their protective stromal environment. We hypothesized that mobilization of MLL-R ALL LSCs would increase susceptibility to FLT3 TKIs. We performed these experiments using samples from two infants with MLL-R ALL (Samples 1 and 2, as above). For each of the two samples, we transplanted four cohorts (n = 5 per cohort) of sub-lethally irradiated 6- to 8-week-old NOD/SCID mice with 1 × 106 primary leukaemia cells via tail vein injection. The mice were allowed to engraft for 2 weeks with no treatment, then were treated with one of four regimens (Fig 6A): (i) a control group which received no treatment (group C); (ii) a marrow mobilization regimen consisting of G-CSF 2·5 μg subcutaneously (SC) twice daily for 2 d (day 1–2) followed by AMD3100 5 mg/kg SC × 1 on day 3 (group M); (iii) lestaurtinib 20 mg/kg (SC) twice daily for 4 consecutive days per week (days 3–6) weekly for 4 weeks (group L); or (iv) a combination of mobilization and lestaurtinib, with lestaurtinib dosing beginning 1 h after the AMD3100 dose on day 3 (group M+L). The mice were then sacrificed and harvested after a total of 10 weeks from leukaemia cell injection. Human leukaemic engraftment was assessed by flow cytometric analysis of anti-murine CD45 and anti-human CD45 stained bone marrow. The bone marrow of the control mice was nearly fully replaced by human leukaemia cells, with greater than 90% engraftment by both patient samples (Fig 6B for Sample 1, Fig 6C for Sample 2). Both mobilization alone and lestaurtinib treatment alone had a modest inhibitory effect on LSC engraftment. The combination of mobilization and lestaurtinib, however, markedly decreased LSC engraftment at 10 weeks indicating that the G-CSF/AMD3100 combination potently enhanced the efficacy of FLT3 inhibitors against MLL-R ALL LSCs. These findings indicate that interruption of leukaemia-stromal cell signalling is critical for the treatment of MLL-R ALL.
Fig. 6.
In vivo treatment of NOD/SCID mice with G-CSF and AMD3100 sensitizes LSC to FLT3 inhibition. (A) Schema illustrating in vivo treatment of NOD/SCID mice injected with primary MLL-R ALL cells. Cohorts of sub-lethally irradiated NOD/SCID mice were injected with primary leukaemia cells (n = 2 primary samples), then treated weekly from week 3 to week 6 with one of four regimens: vehicle-treated control (C); mobilizers (M), consisting of G-CSF 2·5 μg SC twice daily on days 1 and 2 plus AMD3100 (plerixafor) 5 mg/kg SC once on day 3; lestaurtinib 20 mg/kg SC twice daily on days 3–6 alone (L); or mobilizers plus lestaurtinib (M+L). The mice were sacrificed and assessed for engraftment of human leukaemia cells 10 weeks after injection (expressed as the percentage of bone marrow CD45 + cells that were of human origin, as determined by flow cytometry analysis). (B) Results for Sample 1, (C) results for Sample 2.
Discussion
In spite of improvements in the treatment of paediatric ALL, children with high-risk disease continue to have suboptimal cure rates. High-risk paediatric ALL is commonly defined by the clinical criteria of age (<1 or ≥ 10 years) and elevated white blood cell count (>50 × 109/l) (Smith et al, 1996). Current Children’s Oncology Group protocols then further stratify risk according to the presence of MRD after induction therapy and cytogenetic abnormalities, and the presence of an MLL rearrangement increases risk in both standard-and high-risk groups (Schultz et al, 2007). We chose to study infant MLL-R ALL as this ALL subtype has historically had the lowest event-free and overall survival rates, apart from BCR-ABL1 + ALL, which now has significantly improved outcomes since the introduction of targeted BCR-ABL1 TKIs (Schultz et al, 2009). We also investigated the bone marrow microenvironment as a mediator of therapy resistance, as infants with MLL-R ALL have both high rates of relapse and short time to relapse, suggesting that MLL-R LSCs are differentially protected from chemotherapy. In our experiments, we first demonstrated that co-culture with normal human bone marrow stromal cells promotes the survival of ALL cells, particularly those with rearrangements of the MLL gene. We also showed that bone marrow stroma provides a microenvironment for MLL-R ALL cells that is protective against the cytotoxic effects of the FLT-3 inhibitor, lestaurtinib. Maximal growth and survival benefits were observed when there was direct contact between stromal cells and leukaemia cells both in the absence and presence of lestaurtinib. These findings are consistent with a study of B-lineage leukaemia cell lines, which demonstrated that direct contact with stromal cells protected leukaemia cells from cytotoxic chemotherapy, whereas soluble factors had negligible effects (Mudry et al, 2000). We also demonstrated that stromal co-culture promoted the survival of LSCs ex vivo and their ability to propagate leukaemia in vivo. Finally, we demonstrated that in vivo CXCR4 inhibition led to the enhanced efficacy of lestaurtinib against MLL-R LSCs.
The bone marrow microenvironment is composed of stromal cells, extracellular matrix and soluble factors, such as cytokines and growth factors (Li & Dalton, 2006; Yin & Li, 2006). Interactions between ITGA4 (VLA-4)/fibronectin (Mudry et al, 2000; Matsunaga et al, 2003; DiVietro et al, 2007; Petty et al, 2009), TIE2 (TEK)/Angiopoietin 1 (ANG-PT1) (Arai et al, 2004), and integrin-linked kinase (ILK)/Akt (AKT1) (Tabe et al, 2007) all appear to be important in leukaemic cell survival. Multiple groups have demonstrated that a key player in leukaemic cell protection by bone marrow stroma is the interaction between CXCR4 and CXCL12. The CXCR4/CXCL12 axis is functionally important in the homing and adhesion of normal HSCs and leukaemia cells to bone marrow niches (Bleul et al, 1998; Mohle et al, 1998; Peled et al, 1999, 2000; Bradstock et al, 2000; Shen et al, 2001; Burger et al, 2003; Tavor et al, 2004). CXCR4 is a chemokine receptor expressed by HSCs and malignant haematopoietic cells (Mohle et al, 1998; Peled et al, 1999; Crazzolara et al, 2001; Konoplev et al, 2007). CXCL12 is the ligand for CXCR4 and is constitutively secreted by stromal cells in the bone marrow microenvironment (Nagasawa et al, 1994, 1996; Bleul et al, 1996, 1998; Ma et al, 1998). Phosphorylation and activation of CXCR4 through the binding of CXCL12 leads to activation of a number of downstream signalling pathways, including the Janus kinase (JAK)/signal transducer and activator of transcription (STAT), phosphoinositol 3-kinase (PI3K)/Akt, and extracellular signalling-related kinase (ERK) pathways, which promote migration, adhesion, and survival of leukaemia cells (Busillo & Benovic, 2007). Ultimately, activation of CXCR4 by microenvironment- produced CXCL12 leads to stromal-mediated protection from spontaneous and drug-induced cell death (Burger et al, 2000; Juarez et al, 2003).
Extrinsic signals from bone marrow stroma promote quiescence of both haematopoietic stem cells (Pietras et al, 2011) and AML LSCs (Ishikawa et al, 2007). Induction of quiescence is one mechanism by which stroma protects leukaemia cells from chemotherapy. However, we found that co-culture of MLL-R ALL cells with stroma promoted entry into the cell cycle. These effects probably reflected the behaviour of bulk leukaemia cells, as the LSC population is probably very small. Because the phenotype of LSCs in ALL has not been definitively identified, we were unable to perform our in vitro experiments using a pure LSC population. We instead used a functional assay to demonstrate their presence. When we cultured bulk MLL-R ALL cells ex vivo prior to transplantation into immunodeficient mice, we demonstrated functionally that stroma promotes the survival of LSCs both in the absence and presence of lestaurtinib, as cells cultured with stroma led to significantly higher engraftment compared to those cultured with soluble factors or medium alone. We suspect that stroma induced quiescence in the LSC population, allowing the LSCs to survive suboptimal conditions ex vivo and ultimately engraft and proliferate in vivo.
Clinically, high expression of CXCR4 is associated with both extramedullary disease (Crazzolara et al, 2001) and poor overall survival in childhood ALL (Schneider et al, 2002). In addition, high expression of phosphorylated CXCR4 was recently associated with poor overall survival in adults with ALL (Konoplev et al, 2011). In AML, high expression of CXCR4 is associated with a poor outcome (Spoo et al, 2007) and patients harbouring a FLT3 internal tandem duplication mutation appear to have higher expression of CXCR4 compared to those with wild-type FLT3 (Rombouts et al, 2004). Given these data, it is conceivable that resistance to chemotherapy is mediated in part by CXCR4 expression. A recent study also suggested an interaction between MLL targets and the CXCR4/CXCL12 axis. Knockdown of MEIS1 and HOXA family genes in an MLL-R ALL cell line led to decreased migration toward an CXCL12 gradient as well as impaired engraftment in immunodeficient mice, suggesting that MLL targets may influence CXCR4-mediated signalling (Orlovsky et al, 2011). Thus, we postulated that the CXCR4/CXCL12 axis probably plays an important role in MLL-R leukaemia-stromal cell interaction. Interestingly, review of the publicly-available genomic databases did not demonstrate differences in CXCR4 mRNA expression in MLL-R versus non-MLL-R ALL (Yeoh et al, 2002; Ross et al, 2003; Tsutsumi et al, 2003; Bhojwani et al, 2008; Kang et al, 2010). However, the studies that associated high CXCR4 expression with poor outcome measured CXCR4 expression by flow cytometric measurement of cell surface CXCR4. Therefore, it is possible that surface CXCR4 expression is more important than total CXCR4 expression, and we plan to investigate this hypothesis further in future experiments.
Our findings have clear therapeutic implications. We targeted the CXCR4/CXCL12 axis in our xenograft model using the CXCR4 inhibitor AMD3100 (now known as plerixafor) in combination with G-CSF. In preclinical and clinical trials, plerixafor resulted in rapid mobilization of HSCs into the peripheral blood, particularly when combined with G-CSF (Devine et al, 2004; Broxmeyer et al, 2005; Liles et al, 2005; Cashen et al, 2008; DiPersio et al, 2009a,b). Given this data, plerixafor was approved by the US Food and Drug Administration in December 2008 for stem cell mobilization into the peripheral blood for collection for autologous stem cell transplant. Preclinical data also indicate that inhibition of CXCR4 can mobilize leukaemia cells from the protective, growth-promoting bone marrow microenvironment and make them more susceptible to anticancer drugs by disrupting the stromal-leukaemia cell interaction (Juarez et al, 2007; Nervi et al, 2009). There are several ongoing clinical trials in adults and children using plerixafor in combination with chemotherapy to mobilize leukaemia cells from the protective bone marrow microenvironment. A general concern of this strategy is that normal HSCs will be mobilized out of their protective marrow niches and exposed to the effects of cytotoxic drugs, resulting in prolonged cytopenias. However, preliminary results from phase I trials of plerixafor and chemotherapy in adults with AML suggest that the use of a CXCR4 inhibitor as a blast mobilizing and chemosensitizing agent is safe and does not cause untoward haematological toxicity (Uy et al, 2011, 2012). In addition, using targeted therapies, such as FLT3 inhibitors, would diminish the effects on normal HSCs. Recent studies have also demonstrated that combining a CXCR4 inhibitor with the FLT3 TKI sorafenib (Zeng et al, 2009) or the BCR-ABL1 TKI nilotinib (Parameswaran et al, 2011) led to increased sensitivity of leukaemia cells harbouring FLT3 activating mutations and BCR-ABL1, respectively. Our results demonstrate that CXCR4 inhibition enhances the anti-leukaemic effects of a FLT3 TKI in a high-risk subtype of paediatric ALL. Therefore, combining plerixafor with additional targeted therapies or chemotherapy may prove useful in other high-risk paediatric leukaemias.
Acknowledgments
This work was supported by Damon Runyon-Lilly Clinical Investigator Award (P.B.), Leukemia and Lymphoma Society (LLS) Translational Research Program Grant (P.B.), LLS Scholar in Clinical Research Award (P.B.) and St. Baldrick’s Foundation Fellow Award (R.E.R. and E.A.S.).
Footnotes
Authorship
E.M., L.L., and P.B. performed the research; P.B. designed the research study; D.S. contributed the mice for the study; R.E.R., E.A.S., and P.B. analysed the data; and R.E.R., E.A.S. and P.B. wrote the paper.
References
- Arai F, Hirao A, Ohmura M, Sato H, Matsuoka S, Takubo K, Ito K, Koh GY, Suda T. Tie2/angiopoietin-1 signaling regulates hematopoietic stem cell quiescence in the bone marrow niche. Cell. 2004;118:149–161. doi: 10.1016/j.cell.2004.07.004. [DOI] [PubMed] [Google Scholar]
- Armstrong SA, Staunton JE, Silverman LB, Pieters R, den Boer ML, Minden MD, Sallan SE, Lander ES, Golub TR, Korsmeyer SJ. MLL translocations specify a distinct gene expression profile that distinguishes a unique leukemia. Nature Genetics. 2002;30:41–47. doi: 10.1038/ng765. [DOI] [PubMed] [Google Scholar]
- Ayala F, Dewar R, Kieran M, Kalluri R. Contribution of bone microenvironment to leukemogenesis and leukemia progression. Leukemia: Official Journal of the Leukemia Society of America, Leukemia Research Fund, UK. 2009;23:2233–2241. doi: 10.1038/leu.2009.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernt KM, Armstrong SA. Targeting epigenetic programs in MLL-rearranged leukemias. Hematology/the Education Program of the American Society of Hematology. 2011;2011:354–360. doi: 10.1182/asheducation-2011.1.354. [DOI] [PubMed] [Google Scholar]
- Bhojwani D, Kang H, Menezes RX, Yang W, Sather H, Moskowitz NP, Min DJ, Potter JW, Harvey R, Hunger SP, Seibel N, Raetz EA, Pieters R, Horstmann MA, Relling MV, den Boer ML, Willman CL, Carroll WL Children’s Oncology Group Study, Dutch Childhood Oncology Group & German Cooperative Study Group for Childhood Acute Lymphoblastic Leukemia. Gene expression signatures predictive of early response and outcome in high-risk childhood acute lymphoblastic leukemia: a Children’s Oncology Group study [corrected] Journal of Clinical Oncology: Official Journal of the American Society of Clinical Oncology. 2008;26:4376–4384. doi: 10.1200/JCO.2007.14.4519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bleul CC, Fuhlbrigge RC, Casasnovas JM, Aiuti A, Springer TA. A highly efficacious lymphocyte chemoattractant, stromal cellderived factor 1 (SDF-1) The Journal of Experimental Medicine. 1996;184:1101–1109. doi: 10.1084/jem.184.3.1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bleul CC, Schultze JL, Springer TA. B lymphocyte chemotaxis regulated in association with microanatomic localization, differentiation state, and B cell receptor engagement. The Journal of Experimental Medicine. 1998;187:753–762. doi: 10.1084/jem.187.5.753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradstock KF, Makrynikola V, Bianchi A, Shen W, Hewson J, Gottlieb DJ. Effects of the chemokine stromal cell-derived factor-1 on the migration and localization of precursor-B acute lymphoblastic leukemia cells within bone marrow stromal layers. Leukemia: Official Journal of the Leukemia Society of America, Leukemia Research Fund, UK. 2000;14:882–888. doi: 10.1038/sj.leu.2401729. [DOI] [PubMed] [Google Scholar]
- Brown P, Levis M, Shurtleff S, Campana D, Downing J, Small D. FLT3 inhibition selectively kills childhood acute lymphoblastic leukemia cells with high levels of FLT3 expression. Blood. 2005;105:812–820. doi: 10.1182/blood-2004-06-2498. [DOI] [PubMed] [Google Scholar]
- Brown P, Levis M, McIntyre E, Griesemer M, Small D. Combinations of the FLT3 inhibitor CEP-701 and chemotherapy synergistically kill infant and childhood MLL-rearranged ALL cells in a sequence-dependent manner. Leukemia: Official Journal of the Leukemia Society of America, Leukemia Research Fund, UK. 2006;20:1368–1376. doi: 10.1038/sj.leu.2404277. [DOI] [PubMed] [Google Scholar]
- Broxmeyer HE, Orschell CM, Clapp DW, Hangoc G, Cooper S, Plett PA, Liles WC, Li X, Graham-Evans B, Campbell TB, Calandra G, Bridger G, Dale DC, Srour EF. Rapid mobilization of murine and human hematopoietic stem and progenitor cells with AMD3100, a CXCR4 antagonist. The Journal of Experimental Medicine. 2005;201:1307–1318. doi: 10.1084/jem.20041385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burger JA, Tsukada N, Burger M, Zvaifler NJ, Dell’Aquila M, Kipps TJ. Blood-derived nurse-like cells protect chronic lymphocytic leukemia B cells from spontaneous apoptosis through stromal cell-derived factor-1. Blood. 2000;96:2655–2663. [PubMed] [Google Scholar]
- Burger JA, Spoo A, Dwenger A, Burger M, Behringer D. CXCR4 chemokine receptors (CD184) and alpha4beta1 integrins mediate spontaneous migration of human CD34 + progenitors and acute myeloid leukaemia cells beneath marrow stromal cells (pseudoemperipolesis) British Journal of Haematology. 2003;122:579–589. doi: 10.1046/j.1365-2141.2003.04466.x. [DOI] [PubMed] [Google Scholar]
- Busillo JM, Benovic JL. Regulation of CXCR4 signaling. Biochimica Et Biophysica Acta. 2007;1768:952–963. doi: 10.1016/j.bbamem.2006.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calvi LM, Adams GB, Weibrecht KW, Weber JM, Olson DP, Knight MC, Martin RP, Schipani E, Divieti P, Bringhurst FR, Milner LA, Kronenberg HM, Scadden DT. Osteoblastic cells regulate the haematopoietic stem cell niche. Nature. 2003;425:841–846. doi: 10.1038/nature02040. [DOI] [PubMed] [Google Scholar]
- Cashen A, Lopez S, Gao F, Calandra G, Mac-Farland R, Badel K, DiPersio J. A phase II study of plerixafor (AMD3100) plus G-CSF for autologous hematopoietic progenitor cell mobilization in patients with Hodgkin lymphoma. Biology of Blood and Marrow Transplantation: Journal of the American Society for Blood and Marrow Transplantation. 2008;14:1253–1261. doi: 10.1016/j.bbmt.2008.08.011. [DOI] [PubMed] [Google Scholar]
- Colmone A, Amorim M, Pontier AL, Wang S, Jablonski E, Sipkins DA. Leukemic cells create bone marrow niches that disrupt the behavior of normal hematopoietic progenitor cells. Science. 2008;322:1861–1865. doi: 10.1126/science.1164390. [DOI] [PubMed] [Google Scholar]
- Crazzolara R, Kreczy A, Mann G, Heitger A, Eibl G, Fink FM, Möhle R, Meister B. High expression of the chemokine receptor CXCR4 predicts extramedullary organ infiltration in childhood acute lymphoblastic leukaemia. British Journal of Haematology. 2001;115:545–553. doi: 10.1046/j.1365-2141.2001.03164.x. [DOI] [PubMed] [Google Scholar]
- Devine SM, Flomenberg N, Vesole DH, Liesveld J, Weisdorf D, Badel K, Calandra G, DiPersio JF. Rapid mobilization of CD34 + cells following administration of the CXCR4 antagonist AMD3100 to patients with multiple myeloma and non-Hodgkin’s lymphoma. Journal of Clinical Oncology: Official Journal of the American Society of Clinical Oncology. 2004;22:1095–1102. doi: 10.1200/JCO.2004.07.131. [DOI] [PubMed] [Google Scholar]
- DiPersio JF, Micallef IN, Stiff PJ, Bolwell BJ, Maziarz RT, Jacobsen E, Nademanee A, McCarty J, Bridger G, Calandra G. Phase III prospective randomized double-blind placebo-controlled trial of plerixafor plus granulocyte colony-stimulating factor compared with placebo plus granulocyte colony-stimulating factor for autologous stem-cell mobilization and transplantation for patients with non-Hodgkin’s lymphoma. Journal of Clinical Oncology: Official Journal of the American Society of Clinical Oncology. 2009a;27:4767–4773. doi: 10.1200/JCO.2008.20.7209. [DOI] [PubMed] [Google Scholar]
- DiPersio JF, Stadtmauer EA, Nademanee A, Micallef IN, Stiff PJ, Kaufman JL, Maziarz RT, Hosing C, Früehauf S, Horwitz M, Cooper D, Bridger G, Calandra G. Plerixafor and G-CSF versus placebo and G-CSF to mobilize hematopoietic stem cells for autologous stem cell transplantation in patients with multiple myeloma. Blood. 2009b;113:5720–5726. doi: 10.1182/blood-2008-08-174946. [DOI] [PubMed] [Google Scholar]
- DiVietro JA, Brown DC, Sklar LA, Larson RS, Lawrence MB. Immobilized stromal cell-derived factor-1α triggers rapid VLA-4 affinity increases to stabilize lymphocyte tethers on VCAM-1 and subsequently initiate firm adhesion. The Journal of Immunology: Official Journal of the American Association of Immunologists. 2007;178:3903–3911. doi: 10.4049/jimmunol.178.6.3903. [DOI] [PubMed] [Google Scholar]
- Hunger SP, Lu X, Devidas M, Camitta BM, Gaynon PS, Winick NJ, Reaman GH, Carroll WL. Improved survival for children and adolescents with acute lymphoblastic leukemia between 1990 and 2005: a report from the Children’s Oncology Group. Journal of Clinical Oncology: Official Journal of the American Society of Clinical Oncology. 2012;30:1663–1669. doi: 10.1200/JCO.2011.37.8018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa F, Yoshida S, Saito Y, Hijikata A, Kitamura H, Tanaka S, Nakamura R, Tanaka T, Tomiyama H, Saito N, Fukata M, Miyamoto T, Lyons B, Ohshima K, Uchida N, Taniguchi S, Ohara O, Akashi K, Harada M, Shultz LD. Chemotherapy- resistant human AML stem cells home to and engraft within the bone-marrow endosteal region. Nature Biotechnology. 2007;25:1315–1321. doi: 10.1038/nbt1350. [DOI] [PubMed] [Google Scholar]
- Jacobi A, Thieme S, Lehmann R, Ugarte F, Malech HL, Koch S, Thiede C, Müller K, Bornhäuser M, Ryser M, Brenner S. Impact of CXCR4 inhibition on Flt3-ITD-positive human AML blasts. Experimental Hematology. 2010;38:180–190. doi: 10.1016/j.exphem.2009.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin L, Tabe Y, Konoplev S, Xu Y, Leysath CE, Lu H, Kimura S, Ohsaka A, Rios MB, Calvert L, Kantarjian H, Andreeff M, Konopleva M. CXCR4 up-regulation by imatinib induces chronic myelogenous leukemia (CML) cell migration to bone marrow stroma and promotes survival of quiescent CML cells. Molecular Cancer Therapeutics. 2008;7:48–58. doi: 10.1158/1535-7163.MCT-07-0042. [DOI] [PubMed] [Google Scholar]
- Juarez J, Bradstock KF, Gottlieb DJ, Bendall LJ. Effects of inhibitors of the chemokine receptor CXCR4 on acute lymphoblastic leukemia cells in vitro. Leukemia: Official Journal of the Leukemia Society of America, Leukemia Research Fund, UK. 2003;17:1294–1300. doi: 10.1038/sj.leu.2402998. [DOI] [PubMed] [Google Scholar]
- Juarez J, Dela Pena A, Baraz R, Hewson J, Khoo M, Cisterne A, Fricker S, Fujii N, Bradstock KF, Bendall LJ. CXCR4 antagonists mobilize childhood acute lymphoblastic leukemia cells into the peripheral blood and inhibit engraftment. Leukemia: Official Journal of the Leukemia Society of America, Leukemia Research Fund, UK. 2007;21:1249–1257. doi: 10.1038/sj.leu.2404684. [DOI] [PubMed] [Google Scholar]
- Kang H, Chen IM, Wilson CS, Bedrick EJ, Harvey RC, Atlas SR, Devidas M, Mullighan CG, Wang X, Murphy M, Ar K, Wharton W, Borowitz MJ, Bowman WP, Bhojwani D, Carroll WL, Camitta BM, Reaman GH, Smith MA, Downing JR, Hunger SP, Willman CL. Gene expression classifiers for relapse-free survival and minimal residual disease improve risk classification and outcome prediction in pediatric B-precursor acute lymphoblastic leukemia. Blood. 2010;115:1394–1405. doi: 10.1182/blood-2009-05-218560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konoplev S, Rassidakis GZ, Estey E, Kantarjian H, Liakou CI, Huang X, Xiao L, Andreeff M, Konopleva M, Medeiros LJ. Overexpression of CXCR4 predicts adverse overall and event-free survival in patients with unmutated FLT3 acute myeloid leukemia with normal karyotype. Cancer. 2007;109:1152–1156. doi: 10.1002/cncr.22510. [DOI] [PubMed] [Google Scholar]
- Konoplev S, Jorgensen JL, Thomas DA, Lin E, Burger J, Kantarjian HM, Andreeff M, Medeiros LJ, Konopleva M. Phosphorylated CXCR4 is associated with poor survival in adults with B-acute lymphoblastic leukemia. Cancer. 2011;117:4689–4695. doi: 10.1002/cncr.26113. [DOI] [PubMed] [Google Scholar]
- Konopleva M, Konoplev S, Hu W, Zaritskey AY, Afanasiev BV, Andreeff M. Stromal cells prevent apoptosis of AML cells by up-regulation of anti-apoptotic proteins. Leukemia: Official Journal of the Leukemia Society of America, Leukemia Research Fund, UK. 2002;16:1713–1724. doi: 10.1038/sj.leu.2402608. [DOI] [PubMed] [Google Scholar]
- Kotani A, Ha D, Schotte D, den Boer ML, Armstrong SA, Lodish HF. A novel mutation in the miR-128b gene reduces miRNA processing and leads to glucocorticoid resistance of MLL-AF4 acute lymphocytic leukemia cells. Cell Cycle. 2010;9:1037–1042. doi: 10.4161/cc.9.6.11011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krivtsov AV, Armstrong SA. MLL translocations, histone modifications and leukaemia stem-cell development. Nature Reviews Cancer. 2007;7:823–833. doi: 10.1038/nrc2253. [DOI] [PubMed] [Google Scholar]
- Li ZW, Dalton WS. Tumor microenvironment and drug resistance in hematologic malignancies. Blood Reviews. 2006;20:333–342. doi: 10.1016/j.blre.2005.08.003. [DOI] [PubMed] [Google Scholar]
- Liles WC, Rodger E, Broxmeyer HE, Dehner C, Badel K, Calandra G, Christensen J, Wood B, Price TH, Dale DC. Augmented mobilization and collection of CD34 + hematopoietic cells from normal human volunteers stimulated with granulocytecolony- stimulating factor by single-dose administration of AMD3100, a CXCR4 antagonist. Transfusion. 2005;45:295–300. doi: 10.1111/j.1537-2995.2005.04222.x. [DOI] [PubMed] [Google Scholar]
- Ma Q, Jones D, Borghesani PR, Segal RA, Nagasawa T, Kishimoto T, Bronson RT, Springer TA. Impaired B-lymphopoiesis, myelopoiesis, and derailed cerebellar neuron migration in CXCR4- and SDF-1-deficient mice. Proceedings of the National Academy of Sciences of the United States of America. 1998;95:9448–9453. doi: 10.1073/pnas.95.16.9448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsunaga T, Takemoto N, Sato T, Takimoto R, Tanaka I, Fujimi A, Akiyama T, Kuroda H, Kawano Y, Kobune M, Kato J, Hirayama Y, Sakamaki S, Kohda K, Miyake K, Niitsu Y. Interaction between leukemic-cell VLA-4 and stromal fibronectin is a decisive factor for minimal residual disease of acute myelogenous leukemia. Nature Medicine. 2003;9:1158–1165. doi: 10.1038/nm909. [DOI] [PubMed] [Google Scholar]
- Méndez-Ferrer S, Michurina TV, Ferraro F, Mazloom AR, Macarthur BD, Lira SA, Scadden DT, Ma’ayan A, Enikolopov GN, Frenette PS. Mesenchymal and haematopoietic stem cells form a unique bone marrow niche. Nature. 2010;466:829–834. doi: 10.1038/nature09262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milne TA, Martin ME, Brock HW, Slany RK, Hess JL. Leukemogenic MLL fusion proteins bind across a broad region of the Hox a9 locus, promoting transcription and multiple histone modifications. Cancer Research. 2005;65:11367–11374. doi: 10.1158/0008-5472.CAN-05-1041. [DOI] [PubMed] [Google Scholar]
- Mohle R, Bautz F, Rafii S, Moore MA, Brugger W, Kanz L. The chemokine receptor CXCR-4 is expressed on CD34 + hematopoietic progenitors and leukemic cells and mediates transendothelial migration induced by stromal cell-derived factor-1. Blood. 1998;91:4523–4530. [PubMed] [Google Scholar]
- Mudry RE, Fortney JE, York T, Hall BM, Gibson LF. Stromal cells regulate survival of B-lineage leukemic cells during chemotherapy. Blood. 2000;96:1926–1932. [PubMed] [Google Scholar]
- Nagasawa T, Kikutani H, Kishimoto T. Molecular cloning and structure of a pre-B-cell growth-stimulating factor. Proceedings of the National Academy of Sciences of the United States of America. 1994;91:2305–2309. doi: 10.1073/pnas.91.6.2305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagasawa T, Hirota S, Tachibana K, Takakura N, Nishikawa S, Kitamura Y, Yoshida N, Kikutani H, Kishimoto T. Defects of B-cell lymphopoiesis and bone-marrow myelopoiesis in mice lacking the CXC chemokine PBSF/SDF-1. Nature. 1996;382:635–638. doi: 10.1038/382635a0. [DOI] [PubMed] [Google Scholar]
- Nervi B, Ramirez P, Rettig MP, Uy GL, Holt MS, Ritchey JK, Prior JL, Piwnica-Worms D, Bridger G, Ley TJ, Dipersio JF. Chemosensitization of acute myeloid leukemia (AML) following mobilization by the CXCR4 antagonist AMD3100. Blood. 2009;113:6206–6214. doi: 10.1182/blood-2008-06-162123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orlovsky K, Kalinkovich A, Rozovskaia T, Shezen E, Itkin T, Alder H, Ozer HG, Carramusa L, Avigdor A, Volinia S, Buchberg A, Mazo A, Kollet O, Largman C, Croce CM, Nakamura T, Lapidot T, Canaani E. Down-regulation of homeobox genes MEIS1 and HOXA in MLL-rearranged acute leukemia impairs engraftment and reduces proliferation. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:7956–7961. doi: 10.1073/pnas.1103154108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parameswaran R, Yu M, Lim M, Groffen J, Heisterkamp N. Combination of drug therapy in acute lymphoblastic leukemia with a CXCR4 antagonist. Leukemia: Official Journal of the Leukemia Society of America, Leukemia Research Fund, UK. 2011;25:1314–1323. doi: 10.1038/leu.2011.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peled A, Petit I, Kollet O, Magid M, Ponomaryov T, Byk T, Nagler A, Ben-Hur H, Many A, Shultz L, Lider O, Alon R, Zipori D, Lapidot T. Dependence of human stem cell engraftment and repopulation of NOD/SCID mice on CXCR4. Science. 1999;283:845– 848. doi: 10.1126/science.283.5403.845. [DOI] [PubMed] [Google Scholar]
- Peled A, Kollet O, Ponomaryov T, Petit I, Franitza S, Grabovsky V, Slav MM, Nagler A, Lider O, Alon R, Zipori D, Lapidot T. The chemokine SDF-1 activates the integrins LFA-1, VLA-4, and VLA-5 on immature human CD34 + cells: role in transendothelial/stromal migration and engraftment of NOD/SCID mice. Blood. 2000;95:3289–3296. [PubMed] [Google Scholar]
- Petty JM, Lenox CC, Weiss DJ, Poynter ME, Suratt BT. Crosstalk between CXCR4/stromal derived factor-1 and VLA-4/VCAM-1 pathways regulate neutrophil retention in the bone marrow. The Journal of Immunology: Official Journal of the American Association of Immunologists. 2009;182:604–612. doi: 10.4049/jimmunol.182.1.604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pieters R. Infant acute lymphoblastic leukemia: lessons learned and future directions. Current Hematologic Malignancy Reports. 2009;4:167– 174. doi: 10.1007/s11899-009-0023-4. [DOI] [PubMed] [Google Scholar]
- Pieters R, Schrappe M, De Lorenzo P, Hann I, De Rossi G, Felice M, Hovi L, LeBlanc T, Szczepanski T, Ferster A, Janka G, Rubnitz J, Silverman L, Stary J, Campbell M, Li CK, Mann G, Suppiah R, Biondi A, Vora A, Valsecchi MG. A treatment protocol for infants younger than 1 year with acute lymphoblastic leukaemia (Interfant-99): an observational study and a multicentre randomised trial. Lancet. 2007;370:240–250. doi: 10.1016/S0140-6736(07)61126-X. [DOI] [PubMed] [Google Scholar]
- Pietras EM, Warr MR, Passegué E. Cell cycle regulation in hematopoietic stem cells. The Journal of Cell Biology. 2011;195:709–720. doi: 10.1083/jcb.201102131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pui CH, Carroll WL, Meshinchi S, Arceci RJ. Biology, risk stratification, and therapy of pediatric acute leukemias: an update. Journal of Clinical Oncology: Official Journal of the American Society of Clinical Oncology. 2011;29:551–565. doi: 10.1200/JCO.2010.30.7405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramakers-van Woerden NL, Beverloo HB, Veerman AJ, Camitta BM, Loonen AH, van Wering ER, Slater RM, Harbott J, den Boer ML, Ludwig WD, Haas OA, Janka- Schaub GE, Pieters R. In vitro drugresistance profile in infant acute lymphoblastic leukemia in relation to age, MLL rearrangements and immunophenotype. Leukemia: Official Journal of the Leukemia Society of America, Leukemia Research Fund, UK. 2004;18:521–529. doi: 10.1038/sj.leu.2403253. [DOI] [PubMed] [Google Scholar]
- Rombouts EJ, Pavic B, Löwenberg B, Ploemacher RE. Relation between CXCR-4 expression, Flt3 mutations, and unfavorable prognosis of adult acute myeloid leukemia. Blood. 2004;104:550–557. doi: 10.1182/blood-2004-02-0566. [DOI] [PubMed] [Google Scholar]
- Ross ME, Zhou X, Song G, Shurtleff SA, Girtman K, Williams WK, Liu HC, Mahfouz R, Raimondi SC, Lenny N, Patel A, Downing JR. Classification of pediatric acute lymphoblastic leukemia by gene expression profiling. Blood. 2003;102:2951–2959. doi: 10.1182/blood-2003-01-0338. [DOI] [PubMed] [Google Scholar]
- Salzer WL, Devidas M, Carroll WL, Winick N, Pullen J, Hunger SP, Camitta BA. Long-term results of the Pediatric Oncology Group studies for childhood acute lymphoblastic leukemia 1984–2001: a report from the Children’s Oncology Group. Leukemia: Official Journal of the Leukemia Society of America, Leukemia Research Fund, UK. 2010;24:355–370. doi: 10.1038/leu.2009.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafer E, Irizarry R, Negi S, McIntyre E, Small D, Figueroa ME, Melnick A, Brown P. Promoter hypermethylation in MLL-r infant acute lymphoblastic leukemia: biology and therapeutic targeting. Blood. 2010;115:4798–4809. doi: 10.1182/blood-2009-09-243634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider P, Vasse M, Al Bayati A, Lenormand B, Vannier JP. Is high expression of the chemokine receptor CXCR-4 of predictive value for early relapse in childhood acute lymphoblastic leukaemia? British Journal of Haematology. 2002;119:579–580. doi: 10.1046/j.1365-2141.2002.03835_6.x. [DOI] [PubMed] [Google Scholar]
- Schultz KR, Pullen DJ, Sather HN, Shuster JJ, Devidas M, Borowitz MJ, Carroll AJ, Heerema NA, Rubnitz JE, Loh ML, Raetz EA, Winick NJ, Hunger SP, Carroll WL, Gaynon PS, Camitta BM. Risk- and responsebased classification of childhood B-precursor acute lymphoblastic leukemia: a combined analysis of prognostic markers from the Pediatric Oncology Group (POG) and Children’s Cancer Group (CCG) Blood. 2007;109:926–935. doi: 10.1182/blood-2006-01-024729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz KR, Bowman WP, Aledo A, Slayton WB, Sather H, Devidas M, Wang C, Davies SM, Gaynon PS, Trigg M, Rutledge R, Burden L, Jorstad D, Carroll A, Heerema NA, Winick N, Borowitz MJ, Hunger SP, Carroll WL, Camitta B. Improved early event-free survival with imatinib in Philadelphia chromosome-positive acute lymphoblastic leukemia: a Children’s Oncology Group study. Journal of Clinical Oncology: Official Journal of the American Society of Clinical Oncology. 2009;27:5175– 5181. doi: 10.1200/JCO.2008.21.2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen W, Bendall LJ, Gottlieb DJ, Bradstock KF. The chemokine receptor CXCR4 enhances integrin-mediated in vitro adhesion and facilitates engraftment of leukemic precursor- B cells in the bone marrow. Experimental Hematology. 2001;29:1439–1447. doi: 10.1016/s0301-472x(01)00741-x. [DOI] [PubMed] [Google Scholar]
- Smith M, Arthur D, Camitta B, Carroll AJ, Crist W, Gaynon P, Gelber R, Heerema N, Korn EL, Link M, Murphy S, Pui CH, Pullen J, Reamon G, Sallan SE, Sather H, Shuster J, Simon R, Trigg M, Tubergen D, Uckun F, Ungerleider R. Uniform approach to risk classification and treatment assignment for children with acute lymphoblastic leukemia. Journal of Clinical Oncology: Official Journal of the American Society of Clinical Oncology. 1996;14:18–24. doi: 10.1200/JCO.1996.14.1.18. [DOI] [PubMed] [Google Scholar]
- Spijkers-Hagelstein JA, Schneider P, Hulleman E, de Boer J, Williams O, Pieters R, Stam RW. Elevated S100A8/S100A9 expression causes glucocorticoid resistance in MLL-rearranged infant acute lymphoblastic leukemia. Leukemia: Official Journal of the Leukemia Society of America, Leukemia Research Fund, UK. 2012;26:1255–1265. doi: 10.1038/leu.2011.388. [DOI] [PubMed] [Google Scholar]
- Spoo AC, Lübbert M, Wierda WG, Burger JA. CXCR4 is a prognostic marker in acute myelogenous leukemia. Blood. 2007;109:786–791. doi: 10.1182/blood-2006-05-024844. [DOI] [PubMed] [Google Scholar]
- Stam RW, Den Boer ML, Schneider P, de Boer J, Hagelstein J, Valsecchi MG, de Lorenzo P, Sallan SE, Brady HJ, Armstrong SA, Pieters R. Association of high-level MCL-1 expression with in vitro and in vivo prednisone resistance in MLL-rearranged infant acute lymphoblastic leukemia. Blood. 2010;115:1018–1025. doi: 10.1182/blood-2009-02-205963. [DOI] [PubMed] [Google Scholar]
- Tabe Y, Jin L, Tsutsumi-Ishii Y, Xu Y, McQueen T, Priebe W, Mills GB, Ohsaka A, Nagaoka I, Andreeff M, Konopleva M. Activation of integrin-linked kinase is a critical prosurvival pathway induced in leukemic cells by bone marrow-derived stromal cells. Cancer Research. 2007;67:684–694. doi: 10.1158/0008-5472.CAN-06-3166. [DOI] [PubMed] [Google Scholar]
- Tavor S, Petit I, Porozov S, Avigdor A, Dar A, Leider-Trejo L, Shemtov N, Deutsch V, Naparstek E, Nagler A, Lapidot T. CXCR4 regulates migration and development of human acute myelogenous leukemia stem cells in transplanted NOD/SCID mice. Cancer Research. 2004;64:2817–2824. doi: 10.1158/0008-5472.can-03-3693. [DOI] [PubMed] [Google Scholar]
- Tavor S, Eisenbach M, Jacob-Hirsch J, Golan T, Petit I, Benzion K, Kay S, Baron S, Amariglio N, Deutsch V, Naparstek E, Rechavi G. The CXCR4 antagonist AMD3100 impairs survival of human AML cells and induces their differentiation. Leukemia: Official Journal of the Leukemia Society of America, Leukemia Research Fund, UK. 2008;22:2151–5158. doi: 10.1038/leu.2008.238. [DOI] [PubMed] [Google Scholar]
- Tsutsumi S, Taketani T, Nishimura K, Ge X, Taki T, Sugita K, Ishii E, Hanada R, Ohki M, Aburatani H, Hayashi Y. Two distinct gene expression signatures in pediatric acute lymphoblastic leukemia with MLL rearrangements. Cancer Research. 2003;63:4882–4887. [PubMed] [Google Scholar]
- Uy GL, Avigan D, Cortes JE, Becker PS, Chen RW, Liesveld JL, Hewes B, Johns D, Erba HP. Safety and tolerability of plerixafor in combination with cytarabine and daunorubicin in patients with newly diagnosed acute myeloid leukemia- preliminary results from a phase I study. Blood (ASH Annual Meeting Abstracts) 2011;118:82. [Google Scholar]
- Uy GL, Rettig MP, Motabi IH, McFarland K, Trinkaus KM, Hladnik LM, Kulkarni S, Abboud CN, Cashen AF, Stockerl-Goldstein KE, Vij R, Westervelt P, Dipersio JF. A phase 1/2 study of chemosensitization with the CXCR4 antagonist plerixafor in relapsed or refractory acute myeloid leukemia. Blood. 2012;119:3917–3924. doi: 10.1182/blood-2011-10-383406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Velden VH, Corral L, Valsecchi MG, Jansen MW, De Lorenzo P, Cazzaniga G, Panzer-Grümayer ER, Schrappe M, Schrauder A, Meyer C, Marschalek R, Nigro LL, Metzler M, Basso G, Mann G, Den Boer ML, Biondi A, Pieters R, Van Dongen JJ. Prognostic significance of minimal residual disease in infants with acute lymphoblastic leukemia treated within the Interfant-99 protocol. Leukemia: Official Journal of the Leukemia Society of America, Leukemia Research Fund, UK. 2009;23:1073–1079. doi: 10.1038/leu.2009.17. [DOI] [PubMed] [Google Scholar]
- Yeoh EJ, Ross ME, Shurtleff SA, Williams WK, Patel D, Mahfouz R, Behm FG, Raimondi SC, Relling MV, Patel A, Cheng C, Campana D, Wilkins D, Zhou X, Li J, Liu H, Pui CH, Evans WE, Naeve C, Wong L, Downing JR. Classification, subtype discovery, and prediction of outcome in pediatric acute lymphoblastic leukemia by gene expression profiling. Cancer Cell. 2002;1:133–143. doi: 10.1016/s1535-6108(02)00032-6. [DOI] [PubMed] [Google Scholar]
- Yin T, Li L. The stem cell niches in bone. The Journal of Clinical Investigation. 2006;116:1195–1201. doi: 10.1172/JCI28568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng Z, Shi YX, Samudio IJ, Wang RY, Ling X, Frolova O, Levis M, Rubin JB, Negrin RR, Estey EH, Konoplev S, Andreeff M, Konopleva M. Targeting the leukemia microenvironment by CXCR4 inhibition overcomes resistance to kinase inhibitors and chemotherapy in AML. Blood. 2009;113:6215–6224. doi: 10.1182/blood-2008-05-158311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuber J, Shi J, Wang E, Rappaport AR, Herrmann H, Sison EA, Magoon D, Qi J, Blatt K, Wunderlich M, Taylor M, Johns C, Chicas A, Mulloy JC, Kogan SC, Brown P, Valent P, Bradner JE, Lowe SW, Vakoc CR. RNAi screen identifies Brd4 as a therapeutic target in acute myeloid leukaemia. Nature. 2011;478:524–528. doi: 10.1038/nature10334. [DOI] [PMC free article] [PubMed] [Google Scholar]