Abstract
Biomineralization is the process by which living organisms deposit mineral in the extracellular matrix. In nature, almost 50% of biominerals are calcium-bearing minerals. In addition to calcium, we also find biominerals formed from silica and magnetite. Calcium containing biominerals could be either calcium phosphate as in apatite found in vertebrates or calcium carbonate as in calcite and aragonite found in many invertebrates. Since all biomineralization is matrix mediated, an understanding of the nature of the proteins involved is essential in elucidating its mechanism. This review will discuss some of the proteins involved in the process of biomineralization involving calcium. Two proteins, dentin matrix protein 1 and dentin phosphoprotein (Phosphophoryn) will serve as models for the vertebrate system, and two others - P16 and phosphodontin will serve as models for the invertebrate system.
Keywords: DMP1, DSPP, sea urchin P16, Phosphodontin
INTRODUCTION
I am pleased to have been asked to contribute to this set of papers published in recognition of the career of Dr. Arthur Veis, with whom I have had the pleasure of working with for the last 16 years. In the 1960’s Veis chose to study why the collagen matrix of bone and dentin became mineralized, while the collagen of skin and tendon did not. That work led to the discovery of a highly phosphorylated dentin protein absent from tendon and skin (1, 2). This protein was later named phosphophoryn, and is now known as dentin sialophosphoprotein (DSPP). DSPP is comprised of two distinct domains, sialylated DSP and phosphorylated DPP. The discovery of DPP led the Veis lab to focus on the specific role of the acidic, phosphorylated proteins in biomineralization of the collagen matrix. Following the studies of Weiner and Hood (3) demonstrating the similar important role of acidic proteins in the development of calcium carbonate containing mineral shells and spicules of invertebrates, Veis hypothesized that there might indeed be commonalities in all biomineralization mechanisms (4). In an attempt to look for commonalities in the proteins involved in tooth mineralization we have started to isolate and clone the mineral occluded proteins in the echinoderm tooth. This review will focus on the acidic phosphoproteins involved in biomineralization, with an emphasis on those proteins isolated in the Veis laboratory.
1. Vertebrate proteins
DSPP belongs to a family of proteins now known as Small Integrin-Binding Ligand N-linked Glycoproteins-SIBLING’s (5). The SIBLING family includes DSPP, osteopontin (OPN), bone sialoprotein (BSP), dentin matrix protein 1 (DMP1), and matrix extracellular phosphoglycoprotein (MEPE); (6). They are characterized by common exon–intron features, the presence of the integrin binding tripeptide Arg-Gly-Asp (RGD), and several conserved phosphorylation and N-glycosylation sites (5). The genes for the SIBLING proteins are all clustered within an approximately 375 kb span of nucleotides on human chromosome 4q21 (mouse 5q21). Although present in both bone and tooth, BSP and OPN are found in greater amounts in the bone, while DSPP and DMP1 are in greater amounts in the tooth. While initially thought to be bone and tooth specific, all the members of the SIBLING family have been shown to be present in other soft tissues, where they conceivable play other roles besides mineralization. (7–13). It has been reported that genes for three major enamel extra cellular matrix proteins [amelogenin (AMEL), ameloblastin (AMBN), and enamelin (ENAM)] and five dentin/bone extra cellular matrix proteins [dentinsialophosphoprotein (DSPP), dentin matrix acidic phosphoprotein 1 (DMP1), integrin-binding sialoprotein (IBSP), matrix extracellular phosphoglycoprotein (MEPE), and osteopontin (OPN)] as well as milk caseins all arose from a common ancestor SPARCL1 (SPARC-like 1), by gene duplication to form the secretory calcium binding phosphoprotein (SCPP) family (14)
1a. Dentin Matrix Protein 1 (DMP1)
A cDNA library prepared from rat incisors was screened with a polyclonal antibody to a mixture of purified phosphophoryns and an antisense “wobble” poly Asp probe. This led to the isolation of the first dentin matrix protein, appropriately named DMP1 (Fig. 1A). (15). DMP1 has a composition of 115 serine-, 64 Asp- and 77 Glu-residues. Based on its amino acid composition, DMP1 has a calculated isoelectric point of 3.72 and a net charge of -105 at pH 7.0. Phosphorylation of the potential serine residues would lower its isoelectric point and increase the net charge at physiologic pH, making it a highly acidic protein. In situ hybridization analysis demonstrated that initial expression of DMP1 coincides with dentin mineralization (16). As the dentin matrix mineralizes, there is a down regulation of DMP1 and it completely disappears when dentin mineralization is complete (17). DMP1 has also been shown to be expressed constitutively in osteoblasts throughout ossification (18). In vivo DMP1 is cleaved generating a 37 kDa N-terminal polypeptide and a 57 kDa C-terminal peptide(19). It has been proposed that the 57 kDa polypeptide is the functional moiety of DMP1 (20). He et al has shown that the C-terminal polypeptide contains two collagen binding domains, 349DSESSEEDR357 and 424SEENRDSDSQDSSR437 (21). As seen from the sequences, these two domains are rich in acidic amino acid residues and have a potential serine phosphorylation site. In addition, the C-polypeptide contains all the functional domains, which include the RGD domain (22), the nuclear localization signal (23) and the peptide functioning as a nucleator (24). In vitro studies show that DMP1 is capable of binding calcium (25), and can nucleate the formation of hydroxyapatite in a multistep process that begins by DMP1 binding calcium ions and initiating mineral deposition. The nucleated amorphous calcium phosphate precipitates ripen and nanocrystals form. Subsequently, these expand and coalesce into microscale crystals elongated in the c-axis direction (26). Tartaix et al showed that the highly phosphorylated C-terminal 57-kDa fragment (containing 42 phosphates/mol), of DMP1, was a hydroxyapatite nucleator (27).
Figure 1. The amino acid sequence of rat DMP1 and DPP.
A is the sequence of DMP1. The signal peptide sequence is highlighted in green, the casein kinase consensus sequence is highlighted in red and the collagen binding sites are underlined. B is the amino acid composition of Dentin Phosphoprotein (Phosphophoryn). The DSS repeats are highlighted in red.
Feng and colleagues developed a DMP1 knock out mouse (DMP1 KO). The DMP1 KO mice postnatally develop a profound tooth phenotype characterized by a partial failure of maturation of predentin into dentin, enlarged pulp chambers, increased width of predentin zone with reduced dentin wall, and hypomineralization. The tooth phenotype of these mice is strikingly similar to that in dentin sialophosphoprotein (Dspp) null mice and shares some features of the human disease dentinogenesis imperfecta III (28). When Dmp-1 was expressed using the Col1a promoter on the DMP1 KO mouse background, the defects in mineralization and dentinal tubules, were completely rescued (29). There was also rescue of the phenotype, when only the C-terminal polypeptide of DMP1 was expressed as a transgene in a DMP1 KO background (20). In addition to dentin, there were also abnormalities in the bone of DMP1 KO mice. The mineral-to-matrix ratios were significantly lower in DMP1 KO mice tibias compared with wild type (WT) or heterozygous (HET) mice. The mineral crystallinity (crystal size/perfection) was significantly increased in DMP1 KO and HET mice relative to WT. Histological analysis suggested that DMP1 KO mice had osteomalacia. Finally, the DMP1 KO mice had significantly lower ionic calcium and phosphate concentrations relative to wild type (30).
Like a majority of other proteins in biological systems, the members of the SIBLING family of proteins also play multiple roles. In addition to the mineralized tissues, studies have shown that DMP1 is also expressed in the non-mineralized tissues such as kidney, brain, pancreas and salivary glands (31, 32). Studies have shown that DMP1 can translocate to the nucleus suggesting it may act as a transcription factor (33). In keeping with this, George and colleagues have shown that DMP1 regulates dentin sialophosphoprotein gene transcription during early odontoblast differentiation (34). DMP1 interacts with αvβ3 integrin and stimulates phosphorylation of focal adhesion kinase and activation of downstream effectors of the MAPK pathways, namely extracellular signal-related kinases (ERK) and Jun N-terminal kinases (JNK1/2). Extracellular treatment of MC3T3-E1 cells with DMP1 stimulates the translocation of phosphorylated JNK to the nucleus and a concomitant up-regulation of transcriptional activation by phosphorylated c-Jun (35). Besides binding to the αvβ3 integrin, DMP1 has also been shown to bind to the endoplasmic reticulum chaperone protein GRP78 and get internalized (36). Endocytosis of DMP1 mediates elevation of intracellular free calcium. This calcium flux leads to activation of stress response and cellular mediators of the stress kinase pathway resulting in downstream induction of transcription factors, such as Runx-2, which is a key modulator of osteoblast differentiation (37).
1.b. Dentin Phosphoprotein (Phosphophoryn; DPP)
DPP is synthesized as a part of a larger precursor protein, dentin sialophosphoprotein (DSPP), (38) that in vivo is rapidly processed by scission of a central sequence freeing the amino terminal domain, dentin sialoprotein (DSP), and the carboxyl-terminal domain DPP (39,40), plus a central connecting peptide sequence (41). DPP from various species contain 35–45 residue % aspartic acid and 40–55 residue % serine, of which as many as 90% of the serine residues are phosphorylated (42,43). Figure 1B shows the amino acid sequence of DPP. The DSS repeats are highlighted in red. Based on its amino acid sequence, DPP has an isoeletric point of 2.67 and a charge of −144 at pH 7.0. Once again as in DMP1, the protein will be much more acidic after phosphorylation of the serine residues. A significant fraction of DPP is cross-linked to dentin collagen in vivo. In vitro studies also show DPP has the capability to bind to collagen. At neutral pH and low PP/collagen mixing ratios, a single interaction site was evident, centered at approximately 210 nm from the N-terminus. The binding interaction induced a local conformational change in the collagen, bending the molecule and reducing its effective length. At high PP/collagen ration, the DPP bound all along the collagen fibril (44). In vitro nucleation studies have shown that immobilized native DPP could nucleate apatite crystals with similar morphology to that found in mineralized dentin. However, recombinant non-phosphorylated DPP mediated crystal deposits were shown to be amorphous calcium phosphate by electron diffraction patterns (45). Thus, even though both native and recombinant DPP could sequester calcium ions, recombinant non-phosphorylated DPP failed in transforming the amorphous calcium phosphate into apatite crystals suggesting that the ordered arrays of phosphate groups in DPP are necessary for facilitating localized apatite nucleation. Recently it has been shown that expression of DPP in NIH3T3 cells (which do not normally mineralize) is sufficient for the induction of matrix mineralization. Expression of DPP in these cells led to the formation of mineral nodules. Analysis of the mineral phase by X-ray diffraction showed the presence of hydroxyapatite (46).
Various mutations in the DSPP gene results in phenotypes of dentinogenesis imperfecta types II or/and III or dentine dysplasia. The Dspp-null mice develop tooth defects similar to human dentinogenesis imperfecta III with enlarged pulp chambers, increased width of predentin zone, hypomineralization and pulp exposure. Electron microscopy revealed an irregular mineralization front and a lack of calcospherites coalescence in the dentin (47). Recently the generation of DPP conditional knockout (DPPcKO) mice, in which only DSP is expressed on a Dspp null background utilizing the DSP transgene under the control of the Dspp promoter has been reported (48). SEM and micro-CT data showed that DSP expression in these mice mainly reversed the deficiency in the dentin volume but not in the dentin mineral density observed in Dspp null mice, suggesting that the process of mineral maturation, such as the assembly of hierarchically ordered crystal structures, requires DPP.
In addition to playing a major role in dentin mineralization, DPP has also been shown to modulate cell differentiation through integrin signaling and activation of p38, ERK1/2, and JNK, three components of the MAPK pathway (10) DPP has been shown to activate the Smad pathway. DPP caused an increase in the phosphorylation of Smad1, which was then translocated to the nucleus, resulting in the upregulation of the Smad1 target genes, Smad6, Dlx5, and Runx2 (49). We have also shown that the developing kidney metanephroi exposed in culture to anti-DMP2 or antisense oligonucleotides have elongated ureteric bud branches but with reduced branching arborization (50). There was also a reduction in the number of glomeruli. Analysis of a Dspp null mouse at embryonic day 13.5 showed a delayed maturation of the nephron elements and fewer ureteric buds as compared with wild type. Delay in the maturation of the nephrons was reflected by overall smaller size of the metanephros and less polarized epithelial layers in the nephrons (50). More recently we have shown that in the rat ureteric bud cell line, DPP is localized on the cell membrane where it is associated with annexin 2 (51). When these cells were treated with anti-DPP antibodies the influx of calcium into the cells was reduced by 40%, implying that in non-mineralizing tissues, DPP may play a role in calcium transport (51).
2. Invertebrate Proteins
Weiner and colleagues have shown the presence of aspartic acid-rich proteins, in the prismatic shell matrix of the bivalve Atrina rigida (52). These proteins were highly acidic in nature. Acidic proteins rich in Asp-residues have also been identified in pearl oyster shells (53). The aspartic acid rich residues of these acidic proteins are reminiscent of the aspartic acid rich residues present in DPP. The Veis lab has chosen to analyze the proteins present in the tooth of the eichinoderm Lytechinus variegatus the common green sea urchin. The echinoderms branched from the mainstream of invertebrate evolution onto the path from which the vertebrates also evolved. Thus echinoderms are invertebrate deuterostomes and are more closely related to the deuterostome vertebrates (and other chordates) than are other invertebrates (54). In their mature form the echinoderms, like the vertebrates, have a mineralized endoskeletal structure, the stereom, formed from mesenchymal cells. In the early 1980’s, Veis had come to the hypothesis that the same kinds of mineral-related proteins might exist in the mineral-related skeletal elements of invertebrates (4) even though these mainly presented carbonate-based mineral. In 1986 (55) the Veis lab demonstrated that antibodies prepared to and specific to the phosphoproteins of bovine dentin, were cross-reactive with similar acidic proteins from the teeth of Lytechinus variegatus the common green urchin. (55). In the past few years the Veis lab has been concentrating on the isolation and characterization of the mineral occluded proteins of the sea urchin tooth. Sea urchin teeth are first ground in liquid nitrogen and extracted with 6M Guanidine Hydrochloride. The residual mineralized powder pellet which contains the mineral occluded proteins is then extracted with 0.6N HCl. Gel electrophoresis of the proteins present in the 0.6 N HCl extract showed strong polychromatic staining with Stains-All (Fig 2A) typical of strongly acidic proteins. Many of the proteins were identified as phosphoproteins, when the gel was stained with ProQ Diamond Phophoprotein staining (Fig 2B), and had the capability of binding calcium (Fig 2C). 2D gel analysis confirmed that the majority of the phosphoproteins had Isoelectric point values less than 4 (56). We have isolated and cloned three of the proteins present in the 0.6N HCL extract of the L variegatus teeth. The first protein was mortalin which may be involved in the formation of the cell syncytia (57). The next two proteins isolated from the L. variegatus tooth were P16 and P19 (56)
Figure 2. SDS-PAGE of proteins present in the 0.6 N HCl extract of Lytechinus variegatus tooth.
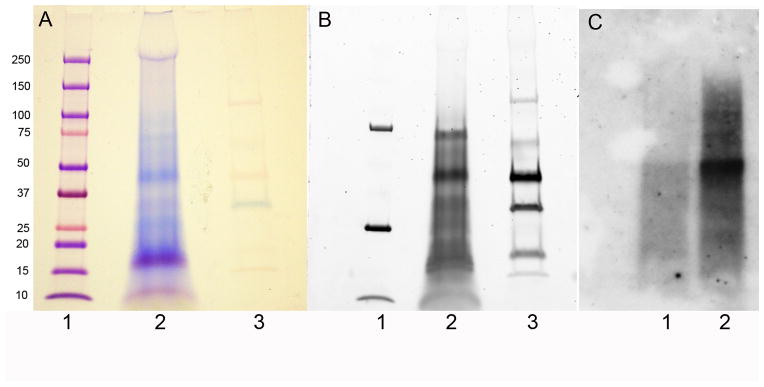
In A the gel was stained with Stains All. In B the same gel was stained with ProQ Diamond phosphoprotein staining Kit prior to staining with Stains all. In C the gel was transferred to nitrocellulose and over laid with Ca45. Lanes A1 and B1 are molecular weight standards. Lanes A2 and B2 are 0.6 N HCl extracts and lanes A3 and B3 are phosphorylated standards. In C lane 1 is the 6M Guanidine Hydrochloride extract and lane 2 is 0.6 N HCl extract
2.a: Lytechinus variegatus P16 and P19
P19 does not have a signal peptide and most likely is not secreted from the cell syncytia. However p19 has a nuclear import signal -RKKK-, at residues 64–67, suggesting that it may have a regulatory role within the cell nuclei. This is consistent with the prediction that the P19 may have one or more threonine or serine phosphorylation sites, most prominently at the 61TST63 site adjacent to the nuclear localization signal. Thus, P19 may be one of those proteins not directly involved in the extracellular mineralization process, but is trapped when the mineral forms around the syncytial cells and so is inaccessible to extraction prior to demineralization.
P16 on the other hand, has a signal peptide a membrane spanning region and a number of predicted phosphorylations sites. (Fig 3A). In addition, P16 has an acidic domain rich in Asp- and Ser-residues. Based on its amino acid composition, P16 has an isoeletric point of 3.6 and a charge of −11 at physiological pH. P16 and P19, have previously been shown to be present in the spicules of the sea urchin S. purpuratus (58). Thus, proteins present in the embryonic spicules, mineralized tissues arising transiently during embryogenesis, are also present in the adult mineralized tooth. The P16 amino acid sequence is highly conserved in both of the species studied; S. purpuratus and L. variegatus. Cheers and Ettensohn (59) microinjected morpholino antisense oligonucleotides (MOs) for P16 into fertilized eggs of S. purpuratus and L. variegatus, blocking the synthesis of the P16 mRNA. They showed that P16 plays an essential role in skeletogenesis. P16 is not required for primary mesenchyme cell (PMC) specification, ingression, migration, or fusion, but is essential for skeletal rod elongation. Using the GFP-tagged form of P16, they also show that the protein is enriched in the plasma membrane and in the perinuclear region that may be the golgi apparatus, suggesting that it may function to receive signals required for skeletogenesis or may play a more direct role in the deposition of biomineral. The presence of a membrane spanning region and the localization of the GFP-tagged protein at the plasma membrane, suggest that P16 is a membrane anchored protein. With this in mind, an antibody was prepared to recombinant L. variegatus P16 and used to locate P16 in the growing urchin tooth. We have previously shown that at ~10 mm from the origin of the plumula, the mineralized primary plates, and the developing secondary plates, are labeled, The P16 fluorescence concentrates brightly at the cell membrane boundaries of the calcite plates (55). Figure 4 shows that even at the very early stage of tooth development in the plumula region where syncytial formation is beginning, P16 is associated with the syncytial membranes.
Figure 3. The amino acid composition of Lytechinus variegatus P16 and Phosphodontin.
A is the sequence of P16. The signal peptide is highlighted in green. The potential phosphorylation sites are highlighted in red and the acidic amino acids are highlighted in blue. The transmembrane domain is underlined. B is the sequence of Phosphodontin: The signal peptide is highlighted in green. The Ser-Ser pairs which are casein kinase 2 substrates are highlighted in red, while the acidic amino acids are highlighted in blue.
Figure 4. Immunofluorescence of the plumula stained with anti-P16 antibodies.
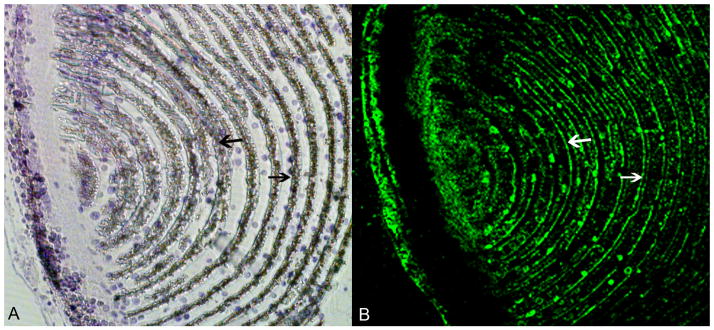
In A the section was stained with toluidine blue. In B the adjacent section was stained with anti-P16 antibody. The arrows point to the developing syncytial membranes
2.b Phosphodontin
Mann et al (60) using MS-based phosphoproteomics have shown the presence of a number of phosphorylated proteins in the S. purpuratus sea urchin tooth and test. One of the proteins not previously described was an acidic and highly phosphorylated protein that was specific to the tooth proteome and absent in the test, hence named phosphodontin (Fig 3B). Phosphodontin has a isolectric point of 3.77 and due to its high content of Glu- and Asp-residues has a net charge of −109 at pH 7.0. In addition phosphodontin also has a number of Ser-Ser pairs scattered all along the sequence. These Ser-Ser pairs are potential casein kinase 2 phosphorylation substrates for at least one Ser-residue. The sequence of phosphodontin is very reminiscent of DPP present in rat tooth. Recently von Marschall et al have reported that the IPV motif at the amino terminal of DSPP is responsible for trafficking of the protein out of the ER (61). Many secreted acidic calcium binding proteins have an IPV-like hydrophobic-proline-hydrophobic tripeptide suggesting a common pathway for trafficking such proteins out of the ER. Interestingly phosphodontin starts with an IPV-like (APV) motif. As yet, neither the cDNA nor the full length protein for phosphodontin has been isolated, thus preventing the study of its role in mineralization or its location within the teeth using specific antibodies.
CONCLUSIONS
Biomineralization, the capacity of living systems to sequester mineral deposits, is a widespread phenomenon, and carbonate, silicates and phosphates comprise the principle skeletal elements. In the vertebrate system the primary calcium containing biomineral is apatite, while the invertebrates primary deposit carbonates. It is believed that all biomineralization is matrix controlled and the proteins present in the extracellular matrix are responsible for nucleation and crystal growth. In order for this to occur the proteins need to be anchored either to collagen as in the vertebrate system or to a lipid bilayer in invertebrates. Although the two systems calcite and apatite appear to be vastly different, there seems to be commonalities in the proteins involved. The proteins involved in the two systems although not having the same amino acid sequences are all highly acidic and phosphorylated. There are motifs like the Asp repeats and Ser-Ser repeats present in the proteins in both systems. Recently Dorvee and Veis have considered the role of water as a participant in biogenic mineralization (62). This raises the possibility that the role of acidic phosphoproteins goes beyond just calcium sequestration and its potential for epitaxial mineral deposition. The highly acidic and negatively charged nature of these proteins may be conveying on them the ability to regulate the kinetics of water-ion exchange.
Acknowledgments
I would like to thank Dr. Arthur Veis for his support throughout these years. This work was supported by National Institutes of Health Grant DE001374 (to A.V.)
Footnotes
Declaration of Interest;
The author declares that there is no conflict of interest. The author alone is responsible for the content and writing of the paper
References
- 1.Veis A, Schlueter RJ. Presence of phosphate-mediated cross-linkages in hard tissue collagens Nature. 1963;197:1204. doi: 10.1038/1971204a0. [DOI] [PubMed] [Google Scholar]
- 2.Veis A, Perry A. The phosphoprotein of the dentin Matrix. Biochemistry. 1967;6(8):2409–2416. doi: 10.1021/bi00860a017. [DOI] [PubMed] [Google Scholar]
- 3.Weiner S, Hood L. Soluble-protein of organic matrix of mollusk shells potential template for shell formation. Science. 1975;190:987–988. doi: 10.1126/science.1188379. [DOI] [PubMed] [Google Scholar]
- 4.Veis A, Sabsay B. Bone and Tooth Formation. Insights into Mineralization Strategies. In: Westbroek P, Jong EW, editors. Biomineralization and Biological Metal Accumulation. Springer; Netherlands: 1982. pp. 273–284. [Google Scholar]
- 5.Fisher LW, Torchia DA, Fohr B, Young MF, Fedarko NS. Flexible structures of SIBLING proteins, bone sialoprotein, and osteopontin. Biochem Biophys Res Commun. 2001;280:460–465. doi: 10.1006/bbrc.2000.4146. [DOI] [PubMed] [Google Scholar]
- 6.Fisher LW, Fedarko NS. Six genes expressed in bones and teeth encode the current members of the SIBLING family of proteins. Connective Tiss Res. 2003;(Suppl 1):33–40. [PubMed] [Google Scholar]
- 7.Hudkins KL, Giachelli CM, Cui Y, Couser WG, Johnson RJ, Alpers CE. Osteopontin expression in fetal and mature human kidney. J Am Soc Nephrol. 1999;10:444– 457. doi: 10.1681/ASN.V103444. [DOI] [PubMed] [Google Scholar]
- 8.Jain A, Karadag A, Foh B, Fisher LW, Fedarko NS. Three SIBLINGs (small integrin-binding ligand, N-linked glycoproteins) activity enabling MCP-like cellular evasion of complement-mediated attack. J Biol Chem. 2002;277:13700–13708. doi: 10.1074/jbc.M110757200. [DOI] [PubMed] [Google Scholar]
- 9.Riminucci M, Corsi A, Peris K, Fisher LW, Chimenti S, Bianco P. Coexpression of bone sialoprotein (BSP) and the pivotal transcriptional regulator of osteogenesis, Cbfa1/Runx2, in malignant melanoma. Calcif Tissue Int. 2003;73(3):281–9. doi: 10.1007/s00223-002-2134-y. [DOI] [PubMed] [Google Scholar]
- 10.Jadlowiec J, Koch H, Zhang X, Campbell PG, Seyedain M, Sfei C. Phosphophoryn regulates the gene expression and differentiation of NIH3T3, MC3T3–E1, and human mesenchymal stem cells via the Integrin/MAPK signaling pathway. J Biol Chem. 2004;279:53323–53330. doi: 10.1074/jbc.M404934200. [DOI] [PubMed] [Google Scholar]
- 11.Saito T, Kobayashi F, Fujii T, Bessho K. Effect of phosphophoryn on rhBMP-2-induced bone formation. Arch Oral Biol. 2004;49:239–243. doi: 10.1016/j.archoralbio.2003.08.003. [DOI] [PubMed] [Google Scholar]
- 12.Chaplet M, Waltregny D, Detry C, Fisher LW, Castronovo V, Bellahcene A. Expression of dentin sialophosphoprotein in human prostate cancer and its correlation with tumor aggressiveness. Int J Cancer. 2006;118:850–856. doi: 10.1002/ijc.21442. [DOI] [PubMed] [Google Scholar]
- 13.Ogbureke KU, Fisher LW. Renal expression of SIBLING proteins and their partner matrix metalloproteinases (MMPs) Kidney Int. 2005;68:155–166. doi: 10.1111/j.1523-1755.2005.00389.x. [DOI] [PubMed] [Google Scholar]
- 14.Kawasaki K, Suzuki T, Weiss KM. Genetic basis for the evolution of vertebrate mineralized tissue. Proc Natl Acad Sci USA. 2004;101:11356–11361. doi: 10.1073/pnas.0404279101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.George A, Sabsay B, Simonian PA, Veis A. Characterization of a novel dentin matrix acidic phosphoprotein. Implications for induction of biomineralization. J Biol Chem. 1993;268:12624–12630. [PubMed] [Google Scholar]
- 16.George A, Silberstein R, Veis A. In situ hybridization shows Dmp1 (AG1) to be a developmentally regulated dentin-specific protein produced by mature odontoblasts. Connect Tissue Res. 1995;33:67–72. doi: 10.3109/03008209509016984. [DOI] [PubMed] [Google Scholar]
- 17.Hao J, Zou B, Narayanan K, George A. Differential expression patterns of the dentin matrix proteins during mineralized tissue formation. Bone. 2004;34:921–932. doi: 10.1016/j.bone.2004.01.020. [DOI] [PubMed] [Google Scholar]
- 18.D’Souza RN, Cavender A, Sunavala G, Alvarez J, Ohshima T, Kulkarni AB, MacDougall M. Gene expression patterns of murine dentin matrix protein 1 (Dmp1) and dentin sialophosphoprotein (DSPP) suggest distinct developmental functions in vivo. J Bone Miner Res. 1997;12:2040–2049. doi: 10.1359/jbmr.1997.12.12.2040. [DOI] [PubMed] [Google Scholar]
- 19.Qin C, Brunn JC, Cook RG, Orkiszewski RS, Malone JP, Vei A, Butler WT. Evidence for the proteolytic processing of dentin matrix protein 1. Identification and characterization of processed fragments and cleavage sites. J Biol Chem. 2003;278:34700–34708. doi: 10.1074/jbc.M305315200. [DOI] [PubMed] [Google Scholar]
- 20.Lu Y, Qin C, Xie Y, Bonewald LF, Feng JQ. Studies of the DMP1 57-kDa functional domain both in vivo and in vitro. Cells Tissues Organs. 2009;189(1–4):175–185. doi: 10.1159/000151727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.He G, George A. Dentin matrix protein 1 immobilized on type I collagen fibrils facilitates apatite deposition in vitro. J Biol Chem. 2004;279:11649–11656. doi: 10.1074/jbc.M309296200. [DOI] [PubMed] [Google Scholar]
- 22.Kulkarni GV, Chen B, Malone JP, Narayanan AS, George A. Promotion of selective cell attachment by the RGD sequence in dentine matrix protein 1. Arch Oral Biol. 2000;45:475–484. doi: 10.1016/s0003-9969(00)00010-8. [DOI] [PubMed] [Google Scholar]
- 23.Narayanan K, Ramachandran A, Hao J, He G, Park KW, Cho M, George A. Dual functional roles of dentin matrix protein 1: implications in biomineralization and gene transcription by activation of intracellular Ca 2+store. J Biol Chem. 2003;278:17500–17508. doi: 10.1074/jbc.M212700200. [DOI] [PubMed] [Google Scholar]
- 24.Gajjeraman S, Narayanan K, Hao J, Qin C, George A. Matrix macromolecules in hard tissues control the nucleation and hierarchical assembly of hydroxyapatite. J Biol Chem. 2007;282:1193–1204. doi: 10.1074/jbc.M604732200. [DOI] [PubMed] [Google Scholar]
- 25.He G, Dahl T, Veis A, George A. Dentin matrix protein 1 initiates hydroxyapatite formation in vitro. Connect Tissue Res. 2003;44 (Suppl 1):240–245. [PubMed] [Google Scholar]
- 26.He G, Dahl T, Veis A, George A. Nucleation of apatite crystals in vitro by self-assembled dentin matrix protein 1. Nature Materials. 2003;2:552– 558. doi: 10.1038/nmat945. [DOI] [PubMed] [Google Scholar]
- 27.Tartaix PH, Doulaverakis M, George A, Fisher LW, Butler WT, Qin C, Salih E, Tan M, Fujimoto Y, Spevak L, Boskey AL. In Vitro Effects of Dentin Matrix Protein-1 on Hydroxyapatite Formation Provide Insights into in Vivo Functions. J Biol Chem. 2004;279:18115–18120. doi: 10.1074/jbc.M314114200. [DOI] [PubMed] [Google Scholar]
- 28.Ye L, MacDougall M, Zhang S, Xie Y, Zhang J, Li Z, Lu Y, Mishina Y, Feng JQ. Deletion of Dentin Matrix Protein-1 Leads to a Partial Failure of Maturation of Predentin into Dentin, Hypomineralization, and Expanded Cavities of Pulp and Root Canal during Postnatal Tooth Development. J Biol Chem. 2004;279:19141–19148. doi: 10.1074/jbc.M400490200. [DOI] [PubMed] [Google Scholar]
- 29.Lu Y, Ye L, Yu S, Zhang S, Xie Y, McKee MD, Li YC, Kong J, Eick JD, Dallas SL, Feng JQ. Rescue of odontogenesis in Dmp1-deficient mice by targeted reexpression of DMP1 reveals roles for DMP1 in early odontogenesis and dentin apposition in vivo. Developmental Biology. 2007;303(1):191–201. doi: 10.1016/j.ydbio.2006.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ling Y, Rios HF, Myers ER, Lu Y, Feng JQ, Boskey AL. DMP1 depletion decreases bone mineralization in vivo: an FTIR imaging analysis. Journal of Bone and Mineral Research. 2005;20(12):2169–77. doi: 10.1359/JBMR.050815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ogbureke KU, Fisher LW. Expression of SIBLINGs and their partner MMPs in salivary glands. J Dent Res. 2004;83:664–670. doi: 10.1177/154405910408300902. [DOI] [PubMed] [Google Scholar]
- 32.Terasawa M, Shimokawa R, Terashima T, Ohya K, Takagi Y, Shimokawa H. Expression of dentin matrix protein 1 (DMP1) in nonmineralized tissues. J Bone Miner Metab. 2004;22:430–438. doi: 10.1007/s00774-004-0504-4. [DOI] [PubMed] [Google Scholar]
- 33.Siyam A, Wang S, Qin C, Mues G, Stevens R, D’Souza RN, Lu Y. Nuclear localization of DMP1 proteins suggests a role in intracellular signaling. Biochem and Biophys Research Commun. 2012;424:641–646. doi: 10.1016/j.bbrc.2012.07.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Narayanan K, Gajjeraman S, Ramachandran A, Hao J, George A. Dentin matrix protein 1 regulates dentin sialophosphoprotein gene transcription during early odontoblast differentiation. J Biol Chem. 2006;281(28):19064–19071. doi: 10.1074/jbc.M600714200. [DOI] [PubMed] [Google Scholar]
- 35.Wu H, Teng P-N, Jayaraman T, Onishi S, Li J, Bannon L, Huang H, Close J, Sfeir C. Dentin Matrix Protein 1 (DMP1) Signals via Cell Surface Integrin. J Biol Chem. 2011;286:29462–29469. doi: 10.1074/jbc.M110.194746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ravindran S, Narayanan K, Eapen AS, Hao J, Amsaveni JA, Blond S, George A. Endoplasmic Reticulum Chaperone Protein GRP-78 Mediates Endocytosis of Dentin Matrix Protein 1. J Biol Chem. 2008;283:29658–29670. doi: 10.1074/jbc.M800786200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Eapen A, Sundivakkam P, Song Y, Ravindran S, Ramachandran A, Tiruppathi C, George A. Calcium-mediated Stress Kinase Activation by DMP1 Promotes Osteoblast Differentiation. J Biol Chem. 2010;285:36339–36351. doi: 10.1074/jbc.M110.145607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Feng JQ, Luan X, Wallace J, Jing D, Ohshima T, Kulkarni AB, D’Souza RN, Kozak CA, MacDougall M. Genomic organization, chromosomal mapping, and promoter analysis of the mouse dentin sialophosphoprotein (Dspp) gene, which codes for both dentin sialoprotein and dentin phosphoprotein. J Biol Chem. 1998;273:9457–9464. doi: 10.1074/jbc.273.16.9457. [DOI] [PubMed] [Google Scholar]
- 39.George A, Srinivasan R, Thotakura SR, Liu K, Veis A. Rat dentin matrix protein 3 is a compound protein of rat dentin sialoprotein and phosphophoryn. Connect Tissue Res. 1999;40:49–57. doi: 10.3109/03008209909005277. [DOI] [PubMed] [Google Scholar]
- 40.Gu K, Chang S, Ritchie HH, Clarkson BH, Rutherford RB. Molecular cloning of a human dentin sialophosphoprotein gene. Eur J Oral Sci. 2000;108:35–42. doi: 10.1034/j.1600-0722.2000.00765.x. [DOI] [PubMed] [Google Scholar]
- 41.Butler WT, Brunn JC, Qin C, McKee MD. Extracellular matrix proteins and the dynamics of dentin formation. Connective Tis Res. 2002;43:301–307. doi: 10.1080/03008200290000682. [DOI] [PubMed] [Google Scholar]
- 42.Stetler-Stevenson WG, Veis A. Bovine dentin phosphophoryn. Composition and molecular weight. Biochemistry. 1983;22:4326–4335. doi: 10.1021/bi00287a025. [DOI] [PubMed] [Google Scholar]
- 43.Rahima M, Veis A. Two classes of dentin phosphophoryns, from a wide range of species, contain immunologically cross-reactive epitope regions. Calcif Tissue Res. 1988;42:104–112. doi: 10.1007/BF02556342. [DOI] [PubMed] [Google Scholar]
- 44.Dahl T, Sabsay B, Veis A. Type I collagen-phosphophoryn interactions: specificity of the monomer-monomer binding. J Struct Biol. 1998;123(2):162–8. doi: 10.1006/jsbi.1998.4025. [DOI] [PubMed] [Google Scholar]
- 45.He G, Ramachandran A, Dahl T, George S, Schultz D, Cookson D, Veis A, George A. Phosphorylation of phosphophoryn is crucial for its function as a mediator of biomineralization. J Biol Chem. 2005;280:33109–33114. doi: 10.1074/jbc.M500159200. [DOI] [PubMed] [Google Scholar]
- 46.Sfeir C, Lee D, Li J, Zhang X, Boskey AL, Kumta P. Expression of Phosphophoryn Is Sufficient for the Induction of Matrix Mineralization by Mammalian Cells. J Biol Chem. 2011;286:20228–20238. doi: 10.1074/jbc.M110.209528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sreenath T, Thyagarajan T, Hall B, Longenecker G, D’Souza R, Hong S, Wright JT, MacDougall M, Sauk J, Kulkarni AB. Dentin sialophosphoprotein knockout mouse teeth display widened predentin zone and develop defective dentin mineralization similar to human dentinogenesis imperfecta type III. J Biol Chem. 2003;278:24874–80. doi: 10.1074/jbc.M303908200. [DOI] [PubMed] [Google Scholar]
- 48.Suzuki S, Sreenath T, Haruyama N, Honeycutt C, Terse A, Cho A, Kohler T, Müller R, Goldberg M, Kulkarni AB. Dentin sialoprotein and dentin phosphoprotein have distinct roles in dentin mineralization. Matrix Biology. 2009;28(4):221–229. doi: 10.1016/j.matbio.2009.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jadlowiec JA, Zhang X, Li J, Campbell PG, Sfeir C. ECM-mediated signaling by dentin phosphophoryn involves activation of the Smad pathway independent of BMP. J Biol Chem. 2006;281:5341–5347. doi: 10.1074/jbc.M506158200. [DOI] [PubMed] [Google Scholar]
- 50.Alvares K, Kanwar YS, Veis A. Expression and Potential Role of Dentin Phosphophoryn (DPP) in Mouse Embryonic Tissues Involved in Epithelial- Mesenchymal Interactions and Branching Morphogenesis. Dev Dynam. 2006;235:2980–2990. doi: 10.1002/dvdy.20935. [DOI] [PubMed] [Google Scholar]
- 51.Alvares K, Stern PH, Veis A. Dentin phosphoprotein binds annexin 2 and is involved in calcium transport in rat kidney ureteric bud cells. J Biol Chem. 2013;88(18):13036–13045. doi: 10.1074/jbc.M112.389627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gotliv B-A, Kessle rN, Sumerel JL, Morse DE, Tuross N, Addadi L, Weiner S. Asprich: A Novel Aspartic Acid-Rich Protein Family from the Prismatic Shell Matrix of the Bivalve Atrina rigida. Chem BioChem. 2005;6:304–314. doi: 10.1002/cbic.200400221. [DOI] [PubMed] [Google Scholar]
- 53.Tsukamoto D, Sarashina I, Endo K. Structure and expression of an unusually acidic matrix protein of pearl oyster shells. Biochem Biophys Res Commun. 2004;320:1175–1180. doi: 10.1016/j.bbrc.2004.06.072. [DOI] [PubMed] [Google Scholar]
- 54.Adoutte A, Balavoine G, Lartillot O, Prud’homme B, de Rosa R. The new animal phylogeny: Reliability and implications. Proc Natl Acad Sci. 2000;97:4453–4456. doi: 10.1073/pnas.97.9.4453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Veis DJ, Albinger TM, Clohisy J, Rahima M, Sabsay B, Veis A. Matrix Proteins of the Teeth of the Sea Urchin Lytechinus variegatus. J Exptl Zool. 1986;240:35–46. doi: 10.1002/jez.1402400106. [DOI] [PubMed] [Google Scholar]
- 56.Alvares K, Dixit SN, Lux E, Veis A. Echinoderm Phosphorylated Matrix Proteins UTMP16 and UTMP19 Have Different Functions in Sea Urchin Tooth Mineralization. J Biol Chem. 2009;284:26149–16160. doi: 10.1074/jbc.M109.024018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Alvares K, Dixit SN, Lux E, Barss J, Veis A. The Proteome of the Developing Tooth of the Sea Urchin, Lytechinus Variegatus: Mortalin is a Constituent of the Developing Cell Syncytium. J Exp Zoolog (Mol Dev Evol) 2007;308B:357–370. doi: 10.1002/jez.b.21159. [DOI] [PubMed] [Google Scholar]
- 58.Illies MR, Peeler MT, Dechtiaruk AM, Ettensohn CA. Identification and developmental expression of new biomineralization proteins in the sea urchin Strongylocentrotus purpuratus. Dev Genes Evol. 2002;212:419–431. doi: 10.1007/s00427-002-0261-0. [DOI] [PubMed] [Google Scholar]
- 59.Cheers MS, Ettensohn CA. P16 is an essential regulator of skeletogenesis in the sea urchin embryo. Dev Biol. 2005;283:384–396. doi: 10.1016/j.ydbio.2005.02.037. [DOI] [PubMed] [Google Scholar]
- 60.Mann K, Poustka AJ, Mann M. Phosphoproteomes of Strongylocentrotus purpuratus shell and tooth matrix: identification of a major acidic sea urchin tooth phosphoprotein, phosphodontin. Proteome Sci. 2010;8:6–19. doi: 10.1186/1477-5956-8-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.von Marschall Z, Mok S, Phillips MD, McKnight DA, Fisher LW. Rough endoplasmic reticulum trafficking errors by different classes of mutant dentin sialophosphoprotein (DSPP) cause dominant negative effects in both dentinogenesis imperfecta and dentin dysplasia by entrapping normal DSPP. J Bone Miner Res. 2012;6:1309–1312. doi: 10.1002/jbmr.1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Dorvee JR, Veis A. Water in the formation of biogenic minerals: Peeling away the hydration layers. J Structural Biol. 2013;183:278–303. doi: 10.1016/j.jsb.2013.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]




