Abstract
The sodium pump Na+/K+-ATPase, expressed in virtually all cells of higher organisms, is involved in establishing a resting membrane potential and in creating a sodium gradient to facilitate a number of membrane-associated transport activities. Na+/K+-ATPase is an oligomer of α, β, and γ subunits. Four unique genes encode each of the α and β subunits. In dental enamel cells, the spatiotemporal expression of Na+/K+-ATPase is poorly characterized. Using the rat incisor as a model, this study provides a comprehensive expression profile of all four α and all four β Na+/K+-ATPase subunits throughout all stages of amelogenesis. Real-time PCR, western blot analysis, and immunolocalization revealed that α1, β1, and β3 are expressed in the enamel organ and that all three are most highly expressed during late-maturation-stage amelogenesis. Expression of β3 was significantly higher than expression of β1, suggesting that the dominant Na+/K+-ATPase consists of an α1β3 dimer. Localization of α1, β1, and β3 subunits in ameloblasts was primarily to the cytoplasm and occasionally along the basolateral membranes. Weaker expression was also noted in papillary layer cells during early maturation. Our data support that Na+/K+-ATPase is functional in maturation-stage ameloblasts.
Keywords: ameloblast, biomineralization, calcium transport, enamel, sodium pump
Na+/K+-ATPase is a P-type ATPase composed of three subunits: α, β, and γ (1). The catalytic α subunit contains 10 transmembrane domains; the glycosylated β subunit is a type II membrane protein, responsible for protein folding and membrane integration; and the auxillary γ subunit (the FXYD protein) is a type I membrane protein, which modulates substrate affinity but is not required for enzymatic activity (2). Na+/K+-ATPase is widely expressed in many tissues and is responsible for the active transport of Na+ and K+ across plasma membranes. For each unit of ATP consumed, Na+/K+-ATPase exports three Na+ and imports two K+, creating an electrical gradient and a concentration gradient. The electrical gradient is the basis for excitability in nerve and muscle cells, whilst the chemical gradient provides the driving force for several facilitated transporters and creates an osmotic gradient for water absorption (3, 4). Different molecular variants have evolved to fulfill the functional versatility. So far, four α isoforms (α1–4) and four β isoforms (β1–4) have been identified, each produced respectively from a different gene, Atp1a1–4 and Atp1b1–4 (1, 5). In addition, seven isoforms of the γ subunit have been identified, which regulate Na+/K+-ATPase in a tissue- and isoform-specific manner (6).
Dental enamel formation can be divided into secretory and maturation stages (7, 8). The secretory-stage enamel organ is composed of four cell layers: outer enamel epithelium; stellate reticulum; stratum intermedium; and the inner ameloblast layer. At this stage, ameloblasts secrete enamel matrix proteins and orchestrate the formation of the final enamel prismatic architecture composed primarily of hydroxyapatite (HA) crystallites. The maturation-stage enamel organ is composed of papillary layer cells and ameloblasts. Maturation-stage ameloblasts are involved in a number of activities, including ion transport, pH regulation, and endocytosis (7–9).
Based on chemically mediated Na+/K+-ATPase inhibition studies, which resulted in delayed enamel maturation in developing teeth, it has been proposed that the enamel organ Na+/K+-ATPase may participate in the net flow (i.e. removal) of the organic matrix components, including water, from the enamel matrix environment during enamel maturation (10). Our earlier array data also indicated that expression of the Na+/K+-ATPase α1 subunit significantly increased in enamel organ cells during enamel maturation (8). These data prompted a more thorough investigation of the Na+/K+-ATPase in amelogenesis. The purpose of our investigation was to complete a systematic study and to obtain a comprehensive profile of the expression of Na+/K+-ATPase in enamel organ cells throughout the stages of amelogenesis.
Material and methods
Sample collection from rat mandibular enamel organ
All vertebrate animal manipulation complied with Institutional and Federal guidelines. This study has been independently reviewed and approved by the Institutional Animal Care and Use Committee of the University of Southern California.
The dissection was performed as previously described (11). Briefly, mandibles were dissected from 100-g (4 wk of age) male Wistar Hannover rats from Taconic (Hudson, NY, USA). The mandibles were then frozen in liquid nitrogen overnight and were subsequently lyophilized for 24 h. Enamel organ cells at three stages of development (secretory, early maturation, and late maturation) were collected (11).
Real-time PCR
Secretory, early-maturation, and late-maturation enamel organ cells from two male rats were pooled, and RNA extraction was performed using a QIAshredder, an RNeasy Protect Mini Kit, and DNase I solution from Qiagen (Valencia, CA, USA). Reverse transcription and real-time PCR were performed using the iScript cDNA Synthesis kit and SYBR Green Supermix from BioRad, respectively. Real-time PCR was performed on the CFX96 system (BioRad Laboratories, Hercules, CA, USA) in 20-µl volumes with a final primer concentration of 100 nm, for 40 cycles at 95°C for 10 s and 58°C for 45 s. Three independent real-time PCR analyses were conducted (a total of six rats) for each gene of interest (the primers are listed in Table 1), and for each of the three stages of amelogenesis. Beta-actin (Actb or β-actin) and enamelin (Enam) served as control transcripts – Actb served as a normalizing control and Enam as a gene transcript that was significantly down-regulated during maturation-stage amelogenesis. The Student’s t-test was used to compare the expression of each gene between the secretory and early-maturation stages, and between the secretory and late-maturation stages.
Table 1.
Rat primers used in this study
| Gene symbol | Accession no. | Primer sequences (forward) | Primers sequences (reverse) |
|---|---|---|---|
| Atp1a1 | NM_012504 | AGAAGGAAAGGGACATGGAC | ATGGCTCCAATCCACAGTAA |
| Atp1a2 | NM_012505 | ATGTGCTGGTGATGAAAGGT | TCTGTGGGAAAGTTCAGCTC |
| Atp1a3 | NM_012506 | GCTTTGCCTTTGACTGTGAT | GATGATGCCTACACCTTTGG |
| Atp1a4 | NM_022848 | GCGCAAGGATGTAAGGTAGA | GCCCATTACTGTGTGGTCTC |
| Atp1b1 | NM_013113 | TGGAGACTTACCCTCTGACG | GGATTTCAGTGTCCAAGGTG |
| Atp1b2 | NM_012507 | CAGCAGTTCTGTCCTCACCT | GGAGTTGTTTGGATGACAGG |
| Atp1b3 | NM_012913 | AAAATGGTACCTTGCCAACA | AGAGCATACGACAGGCACTC |
| Atp1b4 | NM_053381 | GCCAAGTTGCTTTGGTTTTA | AAGTTTCCCCATCAGTCCTC |
| Enam | NM_001106001 | ATGCTGGGAACAATCCTACA | GTGGTTTGCCATTGTCTTTC |
| Actb | NM_031144 | CACACTGTGCCCATCTATGA | CCGATAGTGATGACCTGACC |
Western blot analyses
Enamel organ cells from secretory, early-maturation, and late-maturation stages were collected from two 100-g rats, pooled, lysed with RIPA buffer (1% Nonidet P-40, 0.1% SDS, 0.5% deoxycholic acid, 150 mm NaCl, 50 mm Tris, pH 8.0) and protease inhibitor cocktail, complete mini (Roche Applied Sciences, Indianapolis, IN, USA). Samples were homogenized manually with a pestle six times, then sonicated with a BRANSON digital sonifier Model 450 (All-Spec Industries, Wilmington, NC, USA) (10% intensity, 10 s on and 10 s off). Samples were then cleared by centrifugation (15,000 g, 15 min, 4°C). Proteins were quantified using the bicinchoninic acid (BCA) assay (Pierce, Rockford, IL, USA) and equal quantities were loaded (15 µg per lane) onto 4–12% SDS–PAGE resolving gels. Antibodies against α1 (Thermo Scientific, Waltham, MA, USA; catalogue # MA1-16731), β1 (AbCam, Cambridge, MA, USA; catalogue # AB8344), and β3 (Epitomics, Burlingame, CA, USA; catalogue # 6713-1) subunits, and Actb (Sigma-Aldrich, St Louis, MO, USA; catalogue # A5441), were used at dilutions of 1:3,000; 1:1,000; 1:1,000, and 1:10,000, respectively. Secondary antibodies from Cell Signaling (Danvers, MA, USA; catalogue #7074 and #7076) were applied at a dilution of 1:50,000. Pierce ECL Plus Western Blotting Substrate (Thermo Scientific, Rockford, IL, USA; catalogue #32132) was used as the detection system.
Immunohistochemistry
Rats of 100 g in weight were fixed by perfusion (4% paraformaldehyde, 0.1% glutaraldehyde, 0.08 m sodium cacodylate, 0.05% calcium chloride, pH 7.2–7.4), then the maxillae were dissected and decalcified in 4.13% disodium ethylenediaminetetraacetic acid (EDTA) for 2 months at 4°C. The samples were embedded in paraffin and 4-µm sections were cut using a microtome. The paraffin sections were deparaffinized and rehydrated. Endogenous peroxidase activity was quenched by 3% H2O2 in methanol for 10 min. The primary antibodies listed above for α1 and β3 were used for western blotting, whereas an additional antibody for β1 (GeneTex, Irvine, CA, USA; catalogue # GTX113390) was used for immunohistochemical (IHC) analyses. Antibodies were diluted in PBS containing 1% BSA and 0.5% Triton X-100 (anti-α1, 1:100 dilution; anti-β1, 1:250 dilution; and anti-β3, 1:100 dilution). ImmPRESS reagent anti-rabbit Ig peroxidase (MP-7401) and ImmPACT 3,3′-diaminobenzidine (DAB) peroxidase substrate (SK-4105) were purchased from Vector Laboratories (Burlingame, CA, USA) and the recommended protocol methodologies were followed. The wash buffer used throughout staining was PBS containing 0.1% Triton X-100.
Results
Na+/K+-ATPase α and β subunit gene transcripts in enamel organ cells
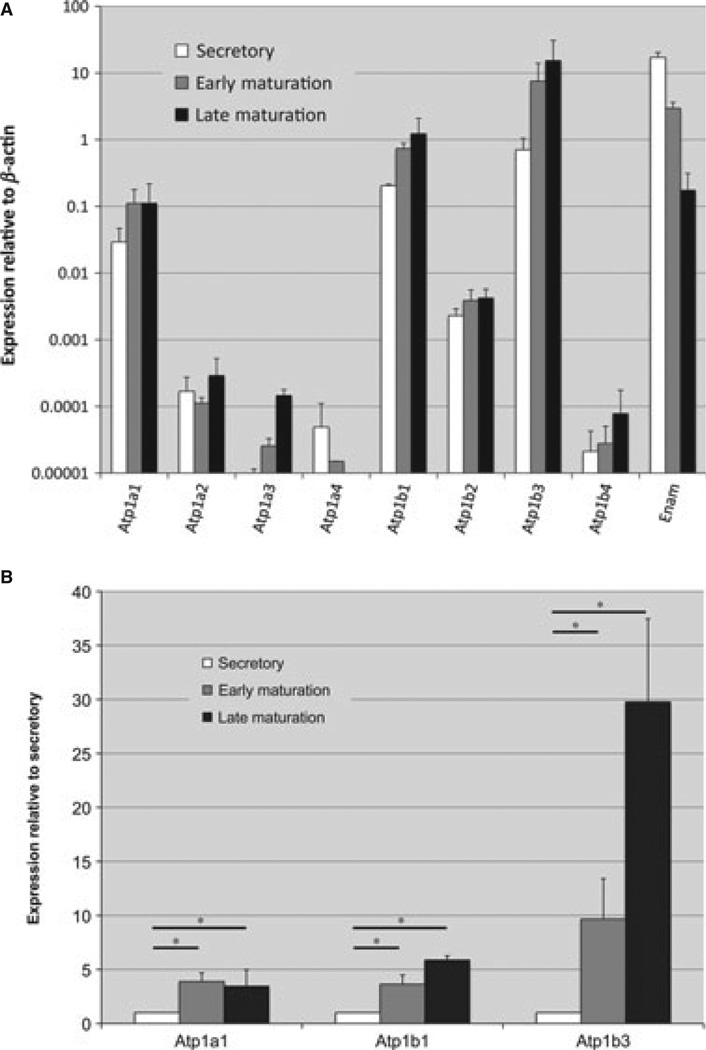
Rodent incisors grow continuously, such that regions from the apical end to the incisal end represent progressive developmental stages in enamel development. The enamel organ cells of mandibular incisors were divided into secretory, early-maturation, and late-maturation stages. Real-time PCR was performed on RNA isolated from enamel organ cells for each of the Na+/K+-ATPase α and β subunits and for Enam as a control. As expected, Enam expression decreased from secretory to maturation stages (11) (Fig. 1A). Among all eight subunits examined, mRNAs from Atp1a1, Atp1b1, and Atp1b3 were expressed at relatively high levels when the data were normalized to Actb transcripts (Fig. 1A). The mRNA profiles for Atp1a1, Atp1b1, and Atp1b3 were further plotted as fold change (i.e. early-maturation stage vs. secretory stage; and late-maturation stage vs. secretory stage) and an increase in expression of all three isoforms was evident in the transition from secretory to maturation stages (Fig. 1B). These data also indicate that the most highly expressed of all the subunits in late maturation is Atp1b3 (Fig. 1B).
Fig. 1.
Expression of Na+/K+-ATPase subunits at successive stages of enamel organ development. (A) Results of real-time PCR analyses for four α subunits (Atp1a1, Atp1a2, Atp1a3, and Atp1a4) and four β subunits (Atp1b1, Atp1b2, Atp1b3, and Atp1b4) of the Na+/K+-ATPase. The expression of each gene is compared within three stages of development (secretory, early maturation, and late maturation) in rat enamel organ epithelial cells, and normalized to β-actin. Enamelin (Enam), the expression of which is known to be high in secretory stage and low in the maturation stage, is used here as an mRNA quality indicator. For primer sequences, see Table 1. The averages and standard error of deviation were based on three independent real-time PCR amplifications using RNA from three different preparations. (B) The fold change relative to secretory stage was plotted for Atp1a1, Atp1b1, and Atp1b3. The Student’s t-test was used to compare the expression of each gene between secretory and early-maturation stages, and between secretory and late maturation-stages. *, significance at p < 0.05.
Protein expression levels of Na+/K+-ATPase α1, β1, and β3 subunits
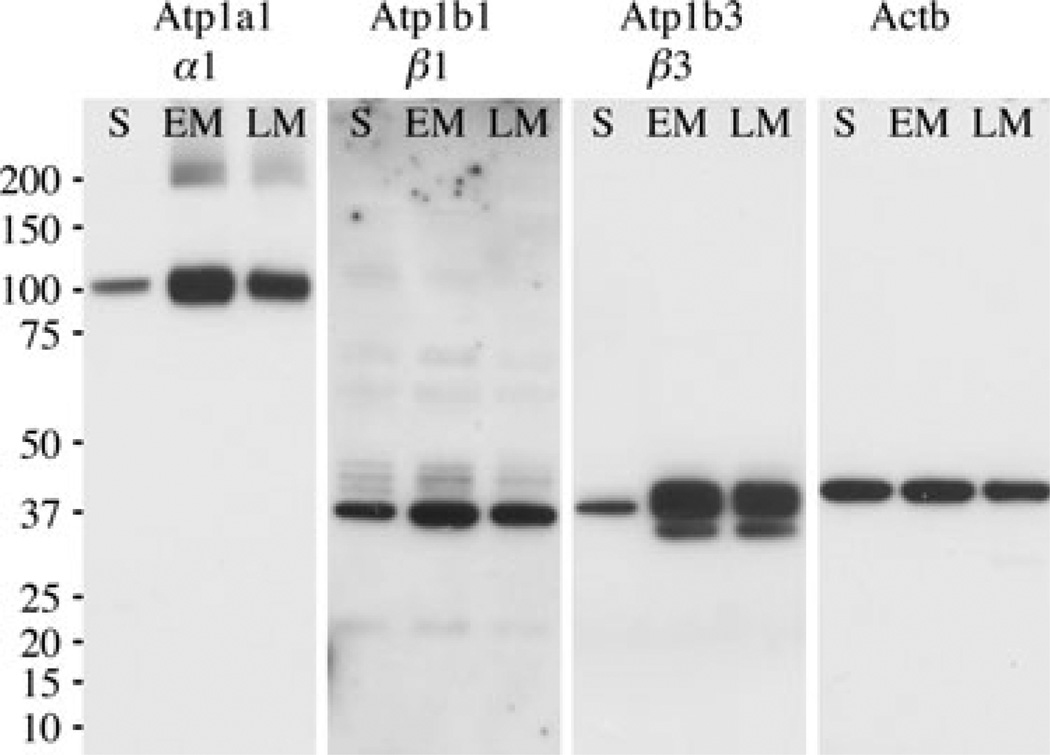
To confirm the expression of Na+/K+-ATPase α1, β1, and β3 subunits in enamel organ cells, total protein was extracted from secretory, early-maturation, and late-maturation stages and western blot analysis was performed (Fig. 2). Consistent with what was observed in real-time PCR, α1, β1, and β3 were all expressed in enamel organ cells and the level of expression clearly increased from secretory stage to early-maturation stage (Fig. 2). The multiple bands seen for β1 and β3 isoforms are typical of protein glycosylation, as previously described (12). Of note was that the protein expression level of all three subunits examined plateaued at the early-maturation stage (Fig. 2) as opposed to the levels of mRNA for Atp1b1 and Atp1b3, which increased gradually from secretory stage to late-maturation stage (Fig. 1B). This discrepancy between Atp1b1 and Atp1b3 mRNA and Atp1b1 and Atp1b3 protein profiles could be a result of differences in mRNA or protein stabilities, such as increased mRNA degradation during late-stage maturation.
Fig. 2.
Expression of α1, β1 and β3 protein subunits of Na+/K+-ATPase in enamel organ of rat incisors. Western blot analyses are shown using protein extracts from secretory (S), early maturation (EM), and late maturation (LM) stages of rat enamel organ epithelial cells and antibodies recognizing α1, β1, β3, and Actb (β-actin; a loading control). Protein weight marker in kDa is indicated on the left.
Immunolocalization of Na+/K+-ATPase α1, β1, and β3 subunits during amelogenesis
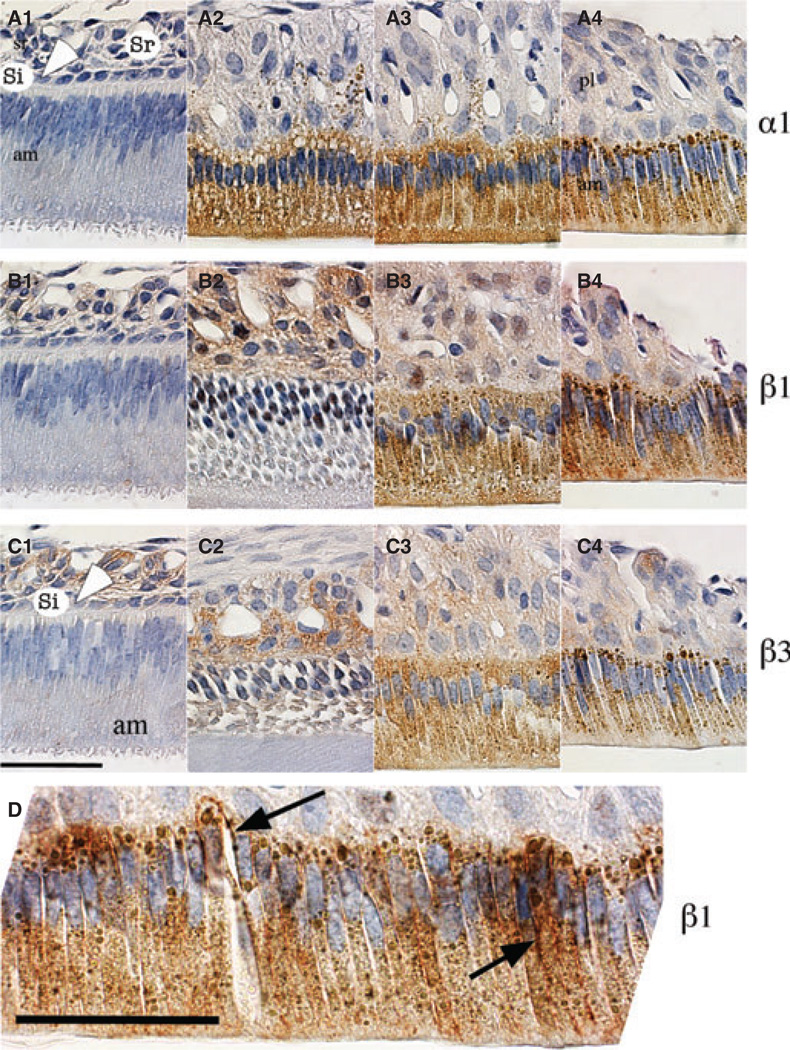
Immunohistochemistry was performed to examine the expression profiles in different enamel organ cell types. Our data show that expression of the α1 subunit was undetectable in secretory stage (Fig. 3A1) and was observed in ameloblasts, and to a lesser extent in papillary layer cells, during early maturation (Fig. 3A2, A3). In late maturation, α1 was expressed primarily in ameloblasts (Fig. 3A4). The expression patterns for β1 and β3 showed a similar distribution with weak expression noted in the entire enamel epithelial organ during the secretory stage and a noticeable increase in papillary layer cells in early maturation stage (Fig. 3B1,B2, C1,C2). The strongest expression levels for the β1 and β3 subunits were in ameloblasts in the late-maturation stage (Fig. 3B3,B4,C3,C4).
Fig. 3.
Immunolocalization of α1, β1, and β3 subunits in enamel organ of rat incisors. Sagittal sections of maxillary incisors from 4-wk-old rats were stained with antibodies against subunit α1 (panels A1–A4), β1 (panels B1–B4), and β3 (panels C1–C4) and were counterstained with haematoxylin. Differential localization is shown at secretory (panels A1, B1, and C1), early-maturation (panels A2–3, B2–3, and C2–3) and late-maturation (panels A4, B4, and C4) stages of enamel organ development. Enamel organ cells, ameloblasts (am), stratum intermedium (si, and white arrowhead), stellate reticulum (sr), and papillary layer cells (pl) are labelled. (D) Magnified image of late-maturation-stage ameloblasts illustrating the basolateral (black arrow) and cytoplasm expression, possibly associated with the endoplasmic reticulum, of the β1 subunit. Scale bars = 50 µm.
In summary, the expression of α1, β1, and β3 subunits in ameloblasts showed a gradual increase from the secretory stage to the late-maturation stage, whereas in the outer layer cells, stratum intermedium and stellate reticulum in the secretory stage and papillary layer in the maturation stage, the expression of these three subunits seemed to increase from secretory to maturation, peak at early maturation, and then decrease gradually in late maturation. This was consistent with the results of western blotting, which compared the three stages of combined enamel organ cells, showing that expression of protein for each of the three subunits plateaued at the early-maturation stage.
Within ameloblasts, the localization of all three subunits was primarily cytoplasmic and occasionally concentrated along the basolateral membranes, and this distribution is consistent with cytoplasmic localization to the endoplasmic reticulum (ER) of kidney cells (13, 14). To highlight this epitope distribution, an enlarged image for the β1 subunit is shown (Fig. 3D). No consistent staining was detected at the apical membrane of ameloblasts, although some fields suggested the presence of α and β subunits in the ruffled border of ameloblasts (Fig. 3A3,B3).
Discussion
We have shown that the Na+/K+-ATPase α1, β1, and β3 subunits are expressed in enamel organ, most notably in the basolateral membranes, and/or in the basal portion of the cytoplasm in maturation-stage ameloblasts. In an early study by Garant & Sasaki (15), where an enzyme-based histochemical methodology was used to locate Na+/K+-ATPase pump activity in enamel organ cells, this pump activity was sensitive to ouabain and was most noticeable in the cells of the stratum intermedium and in papillary layer cells of both secretory-stage and transition-stage ameloblasts, with little expression evident in ameloblasts. However, since that study was published (15), it has been shown that rats have both ouabain-sensitive (α2 and α3) and ouabain-resistant (α1) forms of Na+/K+-ATPase (16, 17). Thus, the rat enamel-organ Na+/K+-ATPase activity identified previously in this enzymatic-based study (15) may have excluded the ouabain-resistant α1 subunit profile, which is the focus of our report in rat ameloblasts using isotope-specific antibody. A more recent study, by Josephsen et al. (18), showed that one of the α subunits of Na+/K+-ATPase localized almost exclusively to the papillary layer cells of the enamel organ, which clearly contrasts with the data obtained here, although it was unclear which one of the α subunits was recognized by the antibody used in this earlier study (18).
The real-time PCR and western blot data in the present study are highly supportive of our IHC data and suggest that of the four Na+/K+-ATPase α subunits, only the α1 subunit is expressed at any appreciable level in the enamel organ. We showed that Na+/K+-ATPase α1, β1, and β3 subunits were expressed primarily in a-meloblasts in the late-maturation stage. We hypothesize that just like the enterocytes in intestine and the epithelium lining in kidney tubules (19), ameloblasts form a continuous sheet of cells capable of performing a classical transepithelial transport function. Na+ is pumped out of the ameloblasts, establishing a high osmolarity in the intercellular spaces between adjacent ameloblasts, thus allowing water to diffuse into this intercellular space from the enamel matrix and subsequently enter the intercellular tissue fluid circulating within the papillary layer. This could help to explain, in part, water removal from the enamel matrix during enamel maturation. There may also be a role for membrane-bound aquaporins, such as AQP4 and AQP5, known to be expressed in earlier stages of amelogenesis (20). The role of individual aquaporins in amelogenesis warrants further investigation. This published report on aquaporins in dental hard-tissue development examined only secretory-stage amelogenesis, and the authors noted that because of the limited specimens examined, no conclusions could be made relative to the process of enamel (or dentin) biomineralization (20).
The export of Na+ and the coupled import of K+ by the Na+/K+-ATPase can also create an Na+ gradient that can support secondary active transport, which couples the kinetic energy of Na+ moving down its concentration gradient to the movement of other cations in an opposing direction. In ameloblasts this could be a working relationship with Na+ and Ca2+ import and export activities. Yet, in the maturation stage of amelogenesis, Ca2+ is required to support continued volumetric growth of HA crystallites. The presence of sodium–calcium exchangers (NCX1 and NCX3), and also the sodium/calcium/potassium exchanger, NCKX4, at the apical membrane of maturation ameloblasts, probably mediates Ca2+ export (21, 22). All three calcium transporters require a transmembrane sodium gradient (23). Our data show a dramatic increase in expression of the Na+/K+-ATPase in a-meloblasts from secretory stage to maturation stage, consistent with the Ca2+-export profiles of enamel cells that are up to four times higher in maturation stage, again when compared with secretory-stage amelogenesis (7, 24). It is important to point out that Na+/K+-ATPase is expressed at the basolateral membrane, whereas NCX1, NCX3, and NCKX4 are primarily expressed at apical and/or apicolateral membranes. This arrangement, for example with Na+/K+-ATPase and NCKX4 at opposing poles, would allow for the transcellular movements of both Na+ (import at the apical pole and export at the basolateral membrane) and K+ (export at the apical and import at the basolateral), which could partially drive the net extrusion of Ca2+ into the maturing enamel matrix (25). On the other hand, Ca2+ pumped out into the intercellular spaces between ameloblasts is able to access the enamel in smooth-ended ameloblasts (SA) because tight junctions at the distal (enamel-facing) end of the ruffle-ended ameloblasts (RA) are relaxed in SA (26). Also, as a consequence of removing the tight junction seal in SA, Na+ can diffuse to the extracellular space adjacent to the apical membrane to fuel the Na+–Ca+ exchangers there.
Moreover, the Na+/K+-ATPase, through its α subunit, interacts with the inositol 1,4,5-triphosphate (IP3) receptor (ITPR1), a receptor that mediates calcium release from the ER. The binding of IP3 to the IP3 receptor leads to Ca2+ release through the opening of calcium channels on the ER membrane (27, 28). It could be hypothesized that the interaction with the Na+/K+-ATPase may potentially change the gating property of IP3 receptors, affecting Ca2+ release. Considering the huge demand for calcium during enamel maturation and the high level of Na+/K+-ATPase expression, regulation of the interaction between Na+/K+-ATPase and ITPR1 may represent a fundamental mechanism for release of Ca2+ during amelogenesis.
Acknowledgements
The authors would like to thank Mrs Janice Bea for proofing and editing the manuscript. The authors would also like to thank the three anonymous reviewers for their comments and suggestions to improve the manuscript. This work was supported by grants DE013404 and DE019629 (MLP) and DE022799 (RSL) from the National Institutes of Health.
Footnotes
Conflicts of interest – The authors declare no potential conflicts of interest with respect to the authorship and/or publication of this article.
References
- 1.Blanco G. The NA/K-ATPase and its isozymes: what we have learned using the baculovirus expression system. Front Biosci. 2005;10:2397–2411. doi: 10.2741/1705. [DOI] [PubMed] [Google Scholar]
- 2.Therien AG, Blostein R. Mechanisms of sodium pump regulation. Am J Physiol Cell Physiol. 2000;279:C541–C566. doi: 10.1152/ajpcell.2000.279.3.C541. [DOI] [PubMed] [Google Scholar]
- 3.Glynn IM. A hundred years of sodium pumping. Annu Rev Physiol. 2002;64:1–18. doi: 10.1146/annurev.physiol.64.081501.130716. [DOI] [PubMed] [Google Scholar]
- 4.Kaplan JH. Biochemistry of Na. K-ATPase. Annu Rev Biochem. 2002;71:511–535. doi: 10.1146/annurev.biochem.71.102201.141218. [DOI] [PubMed] [Google Scholar]
- 5.Blanco G. Na, K-ATPase subunit heterogeneity as a mechanism for tissue-specific ion regulation. Semin Nephrol. 2005;25:292–303. doi: 10.1016/j.semnephrol.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 6.Geering K. FXYD proteins: new regulators of Na-K-ATPase. Am J Physiol Renal Physiol. 2006;290:F241–F250. doi: 10.1152/ajprenal.00126.2005. [DOI] [PubMed] [Google Scholar]
- 7.Smith CE. Cellular and chemical events during enamel maturation. Crit Rev Oral Biol Med. 1998;9:128–161. doi: 10.1177/10454411980090020101. [DOI] [PubMed] [Google Scholar]
- 8.Lacruz RS, Smith CE, Bringasjr P, Chen YB, Smith SM, Snead ML, Kurtz I, Hacia JG, Hubbard MJ, Paine ML. Identification of novel candidate genes involved in mineralization of dental enamel by genome-wide transcript profiling. J Cell Physiol. 2012;227:2264–2275. doi: 10.1002/jcp.22965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nanci A. Chapter 7: Enamel composition, formation, and structure. In: Dolan JJ, editor. Ten Cate’s oral histology: development, structure, and function. 7th edn. St. Louis, MO, USA: Mosby Elsevier; 2008. pp. 141–190. [Google Scholar]
- 10.Garant PR, Sasaki T, Colflesh PE. Na-K-ATPase in the enamel organ: localization and possible roles in enamel formation. Adv Dent Res. 1987;1:267–275. doi: 10.1177/08959374870010021601. [DOI] [PubMed] [Google Scholar]
- 11.Lacruz RS, Smith CE, Moffatt P, Chang EH, Bromage TG, Bringas P, Jr, Nanci A, Baniwal SK, Zabner J, Welsh MJ, Kurtz I, Paine ML. Requirements for ion and solute transport, and pH regulation during enamel maturation. J Cell Physiol. 2012;227:1776–1785. doi: 10.1002/jcp.22911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Geering K. The functional role of beta subunits in oligomeric P-type ATPases. J Bioenerg Biomembr. 2001;33:425–438. doi: 10.1023/a:1010623724749. [DOI] [PubMed] [Google Scholar]
- 13.Gatto C, McLoud SM, Kaplan JH. Heterologous expression of Na(+)-K(+)-ATPase in insect cells: intracellular distribution of pump subunits. Am J Physiol Cell Physiol. 2001;281:C982–C992. doi: 10.1152/ajpcell.2001.281.3.C982. [DOI] [PubMed] [Google Scholar]
- 14.Galva C, Artigas P, Gatto C. Nuclear Na+/K+-ATPase plays an active role in nucleoplasmic Ca2+ homeostasis. J Cell Sci. 2012;125:6137–6147. doi: 10.1242/jcs.114959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Garant PR, Sasaki T. Ultracytochemistry of ouabain-sensitive K+-dependent p-nitrophenyl phosphatase in rat incisor enamel organ. Anat Rec. 1986;216:1–9. doi: 10.1002/ar.1092160102. [DOI] [PubMed] [Google Scholar]
- 16.O’brien WJ, Lingrel JB, Wallick ET. Ouabain binding kinetics of the rat alpha two and alpha three isoforms of the sodium-potassium adenosine triphosphate. Arch Biochem Biophys. 1994;310:32–39. doi: 10.1006/abbi.1994.1136. [DOI] [PubMed] [Google Scholar]
- 17.Berrebi-Bertrand I, Maixent JM, Christe G, Lelievre LG. Two active Na+/K+-ATPases of high affinity for ouabain in adult rat brain membranes. Biochim Biophys Acta. 1990;1021:148–156. doi: 10.1016/0005-2736(90)90027-l. [DOI] [PubMed] [Google Scholar]
- 18.Josephsen K, Takano Y, Frische S, Praetorius J, Nielsen S, Aoba T, Fejerskov O. Ion transporters in secretory and cyclically modulating ameloblasts. A new hypothesis for cellular control of preeruptive enamel maturation. Am J Physiol Cell Physiol. 2010;299:C1299–C1307. doi: 10.1152/ajpcell.00218.2010. [DOI] [PubMed] [Google Scholar]
- 19.Diamond JM, Bossert WH. Standing-gradient osmotic flow. A mechanism for coupling of water and solute transport in epithelia. J Gen Physiol. 1967;50:2061–2083. doi: 10.1085/jgp.50.8.2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Felszeghy S, Modis L, Nemeth P, Nagy G, Zelles T, Agre P, Laurikkala J, Fejerskov O, Thesleff I, Nielsen S. Expression of aquaporin isoforms during human and mouse tooth development. Arch Oral Biol. 2004;49:247–257. doi: 10.1016/j.archoralbio.2003.09.011. [DOI] [PubMed] [Google Scholar]
- 21.Okumura R, Shibukawa Y, Muramatsu T, Hashimoto S, Nakagawa K, Tazaki M, Shimono M. Sodium-calcium exchangers in rat ameloblasts. J Pharmacol Sci. 2010;112:223–230. doi: 10.1254/jphs.09267fp. [DOI] [PubMed] [Google Scholar]
- 22.Hu P, Lacruz RS, Smith CE, Smith SM, Kurtz I, Paine ML. Expression of the sodium/calcium/potassium exchanger, NCKX4, in ameloblasts. Cells Tissues Organs. 2012;196:501–509. doi: 10.1159/000337493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Guerini D. The Ca2+ pumps and the Na+/Ca2+ exchangers. Biometals. 1998;11:319–330. doi: 10.1023/a:1009210001608. [DOI] [PubMed] [Google Scholar]
- 24.Hubbard MJ. Calcium transport across the dental enamel epithelium. Crit Rev Oral Biol Med. 2000;11:437–466. doi: 10.1177/10454411000110040401. [DOI] [PubMed] [Google Scholar]
- 25.Lacruz RS, Smith CE, Kurtz I, Hubbard MJ, Paine ML. New paradigms on the transport functions of maturationstage ameloblasts. J Dent Res. 2013;92:122–129. doi: 10.1177/0022034512470954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bartlett JD, Smith CE. Modulation of cell-cell junctional complexes by matrix metalloproteinases. J Dent Res. 2013;92:10–17. doi: 10.1177/0022034512463397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tian J, Xie ZJ. The Na-K-ATPase and calcium-signaling microdomains. Physiology (Bethesda) 2008;23:205–211. doi: 10.1152/physiol.00008.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Reinhard L, Tidow H, Clausen MJ, Nissen P. Na(+), K (+)-ATPase as a docking station: protein-protein complexes of the Na(+), K (+)-ATPase. Cell Mol Life Sci. 2013;70:205–222. doi: 10.1007/s00018-012-1039-9. [DOI] [PMC free article] [PubMed] [Google Scholar]