Abstract
The dose-dependent pharmacokinetics of itraconazole after intravenous (10, 20, or 30 mg/kg) and oral (10, 30, or 50 mg/kg) administration and the first-pass effects of itraconazole after intravenous, intraportal, intragastric, and intraduodenal administration at a dose of 10 mg/kg were evaluated in rats. After intravenous administration at a dose of 30 mg/kg, the area under the plasma concentration-time curve from time zero to infinity (AUC0-∞) was significantly greater than those at 10 and 20 mg/kg (1,090, 1,270, and 1,760 μg · min/ml for 10, 20, and 30 mg/kg, dose-normalized at 10 mg/kg). After oral administration, the AUC0-∞ was significantly different for three oral doses (380, 687, and 934 μg · min/ml for 10, 30, and 50 mg/kg, respectively, dose-normalized at 10 mg/kg). The extent of absolute oral bioavailability (F) was 34.9% after an oral dose at 10 mg/kg. The AUC0-∞ (or AUC0-8 h) values were comparable between intravenous and intraportal administration and between intragastric and intraduodenal administration, suggesting that the hepatic and gastric first-pass effects were almost negligible in rats. However, the AUC0-8 h values after intraduodenal and intragastric administration were significantly smaller than that after intraportal administration, approximately 30%, suggesting that the intestinal first-pass effect was approximately 70% of that of an oral dose of 10 mg/kg. The low F after oral administration of itraconazole at a dose of 10 mg/kg could be mainly due to the considerable intestinal first-pass effect.
The pharmacokinetics of itraconazole after oral administration to humans (10-12), rats (11, 22, 31), and rabbits, cats, and dogs (11) have been reported; however, data on the pharmacokinetics after intravenous administration to humans (12), dogs (11), and rats (22, 31) are scarce. It has been reported (12) that after oral administration of itraconazole to humans, the values for the area under the plasma concentration-time curve from time zero to infinity (AUC0-∞) for the drug were dose dependent (increased in proportion to dose increases); 50, 100, and 200 mg by capsule after a meal and 100 and 200 mg by capsule after breakfast. However, the dose-dependent pharmacokinetics of itraconazole after intravenous administration and the first-pass effects of the drug have not been reported. It has been reported (31) that the hepatic first-pass effect of itraconazole was estimated to be 18.5% in rats.
The purpose of this paper is to report the dose-dependent pharmacokinetics of itraconazole after intravenous (at doses of 10, 20, and 30 mg/kg) and oral (at doses of 10, 30, and 50 mg/kg) administration to rats and the intestinal first-pass effect of itraconazole after intravenous, intraportal, intragastric, and intraduodenal administration (at a dose of 10 mg/kg) to rats.
MATERIALS AND METHODS
Chemicals.
Sporanox intravenous solution (10 mg/ml as itraconazole as a solution in hydroxylpropyl-β-cyclodextrin [HP-β-CD], sorbitol, propylene glycol, HCl, NaOH, and water for injection; lot no. 01-D01A-IW), Sporanox oral solution (10 mg/ml as itraconazole as an oral solution in the same solution as used in the intravenous study; lot no. 02CB405), and powdered itraconazole, 7-hydroxyitraconazole, and R51012 (the internal standard in the high-performance liquid chromatographic [HPLC] assay) were supplied by Janssen Korea (Seoul, Korea). Dextran, HP-β-CD, the reduced form of NADP (NADPH as a tetrasodium salt), Tris buffer, and UDP glucuronic acid (as a trisodium salt) were purchased from Sigma Chemical Company (St. Louis, Mo.). Buffer solutions with pHs of 1 and 2 (HCl-KCl buffer) were purchased from Shinyo Pure Chemicals (Osaka, Japan). Other chemicals were of reagent grade or HPLC grade and used without further purification.
Animals.
Male Sprague-Dawley rats (weight, 260 to 340 g) were purchased from Charles River Company Korea (Biogenomics, Seoul, Korea). All rats were maintained in a clean room (Animal Center for Pharmaceutical Research, College of Pharmacy, Seoul National University, Seoul, Korea) at a temperature of between 20 and 23°C with 12-h light and dark cycles and a relative humidity of 50%. Rats were housed in metabolic cages (Tecniplast, Varese, Italy) supplied with filtered pathogen-free air and food (Samyang Company, Seoul, Korea) and water ad libitum. The Animal Care and Use Committee of College of Pharmacy, Seoul National University, approved the animal study protocol.
Stability of itraconazole in buffer solutions and human gastric juices.
A stock solution of itraconazole (dissolved in acetonitrile) was spiked (less than 10 μl/ml) in a glass test tube containing 5 to 10 ml of buffer solutions with pHs of 1 and 2 and gastric juice from five humans to make an itraconazole concentration of 0.5 μg/ml. After Vortex mixing, each test tube was placed in a water bath shaker kept at 37°C and at a rate of 50 oscillations per min (opm). After a 48-h incubation in pH solutions and a 4-h incubation in human gastric juices, a 50-μl aliquot was sampled from each test tube, and itraconazole concentrations were immediately analyzed by the HPLC method reported before (1). Human gastric juices were obtained from five patients before surgery at Seoul National University Hospital (Seoul, Korea) and had pHs of 3.90, 5.82, 3.19, 2.90, and 4.55.
Disappearance of itraconazole in rat tissue homogenate.
The procedures used to determine the disappearance of itraconazole in rat tissue homogenates were similar (19) to those reported previously (26). Approximately 1-g samples of rat liver, kidney, lung, spleen, stomach, and muscle (n = 5) were excised after cervical dislocation, rinsed with cold 0.9% NaCl injectable solution, blotted dry with tissue paper, and weighed. Metabolic activity was initiated by adding a 300-μl aliquot of the 9,000 × g supernatant fraction of each tissue homogenate (each tissue was homogenized with 4 volumes of 0.25 M sucrose; Ultra-Turrax T25; Janke and Kunkel, IKA-Labortechnik, Staufeni, Germany) to an Eppendorf tube containing a 570-μl aliquot of Tris buffer, a 10-μl (10 μg, the same solution as used in the intravenous study) aliquot of itraconazole, a 10-μl (1 mM) aliquot of NADPH, and a 10-μl (3.3 mM) aliquot of UDP glucuronic acid. To terminate enzyme activity, a 150-μl aliquot of 1 M NaOH was added to the reaction mixture after a 30-min incubation in a Thermomixer (Thermomixer 5436; Eppendorf, Hamburg, Germany) kept at 37°C and at a rate of 500 opm. After centrifugation, two 50-μl aliquots of each sample were stored in a −70°C freezer (Revco ULT 1490 D-N-S; Western Mednics, Asheville, N.C.) until HPLC analysis of itraconazole (1).
Biliary excretion after intravenous administration of itraconazole to rats.
The procedures used to determine biliary excretion were similar to those reported previously (25). In the early morning, the jugular vein and the bile duct of each rat were cannulated with polyethylene tubing (Clay Adams, Parsippany, N.J.) while each rat was lightly anesthetized with ether. Both cannulas were exteriorized to the dorsal side of neck and inserted into a wire coil for free movement of the rats. Each rat was housed individually in a rat metabolic cage (Daejong Scientific, Seoul, Korea). Itraconazole (the same solution as used in the intravenous study) at a dose of 30 mg/kg was infused over a 1-min period via the jugular vein of each rat (n = 9). The total injection volume was approximately 1.0 ml. Bile juice was collected for up to 24 h. After the exact volume of bile juice was measured, two 50-μl aliquots of each bile sample were stored in a −70°C freezer until HPLC analysis of itraconazole and 7-hydroxyitraconazole (1).
Intravenous and oral administration of itraconazole to rats.
The carotid artery (for blood sampling) and/or the jugular vein (only for the study of intravenous drug administration) of each rat was cannulated with polyethylene tubing (Clay Adams) under light ether anesthesia. Other procedures were similar to those reported previously (21). Experiments were started after 4 to 5 h of recovery from light ether anesthesia. Itraconazole at doses of 10 (n = 10), 20 (n = 13), and 30 (n = 12) mg/kg was administered via the jugular vein over a 1-min period (total injection volume was approximately 1 ml) to rats. Approximately 120 μl of blood sample was collected via the carotid artery at 0 (to serve as a control), 1 (at the end of the infusion), 5, 15, 30, 45, 60, 120, 180, 240, 360, 480, 720, 1,440, 1,800, 2,160, and 2,880 min after intravenous administration. After centrifugation, a 50-μl aliquot of plasma was stored in a −70°C freezer for HPLC analysis of itraconazole and 7-hydroxyitraconazole (1). An approximately 0.3-ml aliquot of heparinized 0.9% NaCl injectable solution (20 U/ml) was used to flush the cannula immediately after each blood sampling to prevent blood clotting.
After 48 h, each metabolic cage was rinsed with 10 ml of distilled water, and the rinses were combined with the 48-h urine sample. After the exact volume of the combined urine sample was measured, two 50-μl aliquots of the combined urine samples were stored in a −70°C freezer until HPLC analysis of itraconazole and 7-hydroxyitraconazole (1). At the same time (48 h), the entire gastrointestinal tract was removed, transferred to a beaker containing 100 ml of methanol (to facilitate extraction of itraconazole and 7-hydroxyitraconazole), and cut into small pieces with scissors. After manual shaking and stirring with a glass rod, two 50-μl aliquots of the supernatant were collected from each beaker and stored in a −70°C freezer until HPLC analysis of itraconazole and 7-hydroxyitraconazole (1).
Itraconazole at doses of 10 (n = 8), 30 (n = 10), and 50 (n = 10) mg/kg was administered orally (total oral volume was approximately 0.3, 0.9, and 1.5 ml, respectively) to rats with a feeding tube after overnight fasting with free access to water. An approximately 120-μl aliquot of blood was collected via the carotid artery at 0 (to serve as a control), 15, 30, 60, 120, 180, 240, 360, 480, 720, 1,440, 1,800, 2,160, and 2,880 min after oral administration. Urine samples were collected between 0 and 48 h. The plasma, urine, and gastrointestinal samples were handled similarly to those of the intravenous studies.
Measurement of hepatic first-pass effect of itraconazole.
The carotid artery and the jugular vein of each rat were cannulated under light ether anesthesia (21). At the same time, the pyloric vein was also cannulated (16) by the modified Suzuki method (30). The pyloric vein instead of the portal vein was cannulated to minimize impaired blood flow in the portal vein. Itraconazole (the same solution as used in the intravenous study) was infused over a 30-min period into the jugular vein and the pyloric vein after 4 to 5 h of recovery from light ether anesthesia with the assistance of an infusion pump (model 2400-006; Harvard Instrument, South Natick, Mass.) at a dose of 10 mg/kg for intravenous (n = 5) and intraportal (n = 6) administration. The total infusion volume was approximately 0.3 ml. At the same time, an equal volume (0.3 ml) of 20% HP-β-CD solution was also infused over a 30-min period via the pyloric vein for the intravenous study and via the jugular vein for the intraportal study. An approximately 120-μl aliquot of blood was collected via the carotid artery at 0 (to serve as a control), 15, 30 (at the end of the infusion), 31, 35, 45, 60, 90, 150, 210, 270, 390, 510, 750, 1,110, 1,470, 1,830, and 2,190 min after the start of infusion. After centrifugation, a 50-μl aliquot of plasma was kept in a −70°C freezer until HPLC analysis of itraconazole (1).
Measurement of gastric and intestinal first-pass effects of itraconazole.
Rats were fasted overnight with free access to water. The carotid artery and the pyloric vein of each rat were cannulated after abdominal incision under light ether anesthesia (20, 16, 17). For intraportal infusion (n = 5), 20% HP-β-CD (0.3 ml) was instilled into the stomach and duodenum with a 25-guage needle, and itraconazole (the same solution as used in the intravenous study) at a dose of 10 mg/kg was infused (0.3 ml) over a 30-min period into the pyloric vein with the assistance of an infusion pump. For intraduodenal instillation (n = 8), 20% HP-β-CD was instilled (0.3 ml) into the stomach and infused over a 30-min period via the pyloric vein, and itraconazole at a dose of 10 mg/kg was instilled (0.3 ml) into the duodenum. For intragastric instillation (n = 4), 20% HP-β-CD (0.3 ml) was infused over a 30-min period via the pyloric vein and instilled into the duodenum, and itraconazole at a dose of 10 mg/kg was instilled (0.3 ml) into the stomach. Other procedures were similar to those described for measuring the hepatic first-pass effect of itraconazole.
HPLC analysis of itraconazole and/or 7-hydroxyitraconazole.
The concentrations (or amounts) of itraconazole and/or 7-hydroxyitraconazole in the samples were determined by a slight modification of the HPLC method reported before (1). To a 2.2-ml Eppendorf tube containing a 50-μl aliquot of biological sample, a 50-μl aliquot of an internal standard (0.2 μg/ml dissolved in acetonitrile) and a 250-μl aliquot of 0.1 M carbonate buffer (pH 10) were added. After vortexing for 30 s, the mixture was extracted with 1 ml of tert-butyl methyl ether. The organic layer was evaporated under nitrogen at 65°C. The residue was then reconstituted with a 100-μl aliquot of the mobile phase, and a 75-μl aliquot was injected directly onto the HPLC column. The mobile phase, 20 mM KH2PO4 (pH 2)-acetonitrile-85% phosphoric acid (50:50:0.15, vol/vol/vol), was run at a flow rate of 2.0 ml/min, and the column effluent was monitored by a fluorescence detector set at an excitation wavelength of 260 nm and an emission wavelength of 364 nm. The retention times of 7-hydroxyitraconazole, itraconazole, and an internal standard were approximately 3, 6.2, and 9.3 min, respectively. The detection limits of itraconazole and 7-hydroxyitraconazole in plasma and urine were both 50 ng/ml. The coefficients of variation were generally low (below 8.21%).
Pharmacokinetic analysis.
The area under the plasma concentration-time curve form time zero to infinity (AUC0-∞) or for up to the last measured time, 48 h, in plasma (AUC0-48 h) was calculated by the trapezoidal rule method; this method utilized the logarithmic trapezoidal rule (3) for the calculation of the area during the declining-plasma level phase and the linear trapezoidal rule for the rising-plasma level phase. The area from the last datum point to time infinity (for the calculation of AUC0-∞) was estimated by dividing the last measured plasma concentration by the terminal rate constant.
Standard methods (9) were used to calculate the following pharmacokinetic parameters: the area under the first moment of the plasma concentration-time curve, mean residence time, apparent volume of distribution at steady state (Vss), and time-averaged total body (CL), renal (CLR), and nonrenal (CLNR) clearances (21).
The harmonic mean method was employed to calculate the mean values for Vss (4), terminal half-life (t1/2) (7), and each clearance (5).
Statistical analysis.
A P value of less than 0.05 was considered statistically significant with a t test between the two means for unpaired data or a Duncan's multiple-range test of the Social Package of Statistical Sciences (SPSS) posteriori analysis of variance among the three means for unpaired data. All results are expressed as means ± standard deviations.
RESULTS
Stability of itraconazole in buffer solutions and human gastric juices.
Itraconazole was stable in buffer solutions of pH of 1 or 2 for up to a 48-h incubation; 98.2 and 114% of the spiked amount of itraconazole were recovered after a 48-h incubation for buffer solutions having pHs of 1 and 2, respectively. The stability of itraconazole in buffer solutions having pHs of 3 to 14 could not be determined due to solubility problems. Itraconazole was also stable in human gastric juices for up to a 4-h incubation; 100, 101, 102, 110, and 94.5% of the spiked amount of itraconazole were recovered after a 4-h incubation for human gastric juices having pHs of 3.90, 5.82, 3.19, 2.90, and 4.55, respectively. This indicates that itraconazole is stable in acidic conditions, and that enzymatic degradation of itraconazole in human gastric juices is almost negligible.
Disappearance of itraconazole in rat tissue homogenate.
Approximately 7.00, 13.0, 18.7, 0, 17.4, and 15.6% of the spiked amount of itraconazole disappeared after a 30-min incubation of 1 μg of itraconazole with the 9,000 × g supernatant fraction of rat muscle, lung, liver, spleen, kidney, and stomach, respectively. These data suggested that the rat tissues studied did not have considerable metabolic activities for itraconazole.
Biliary excretion after intravenous administration to rats.
The 24-h biliary excretion of unchanged itraconazole and 7-hydroxyitraconazole after intravenous administration of itraconazole at a dose of 30 mg/kg was measured after bile duct cannulation in nine rats. The values were 0.230 ± 0.315 and 0.474 ± 0.372% of the itraconazole doses for itraconazole and 7-hydroxyitraconazole, respectively. These data indicated that the biliary excretion of itraconazole was almost negligible in rats.
Pharmacokinetics of itraconazole and 7-hydroxyitraconazole after intravenous administration of itraconazole to rats.
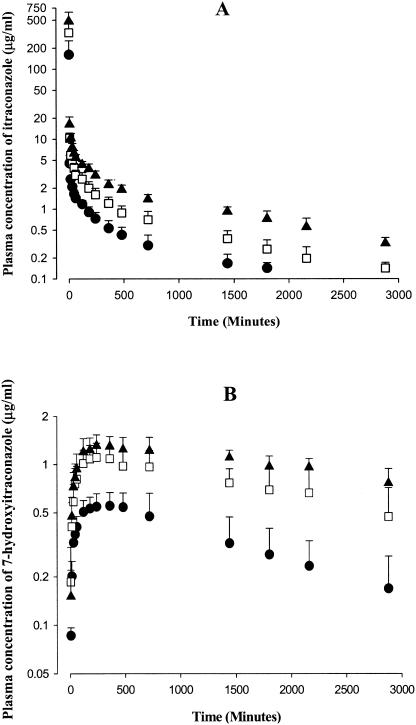
After a 1-min intravenous infusion of itraconazole at doses of 10, 20, and 30 mg/kg to rats, the mean arterial plasma concentrations of itraconazole declined in a polyexponential fashion for all three doses studied (Fig. 1A), with mean terminal t1/2s of 563, 767, and 1,050 min for 10, 20, and 30 mg/kg, respectively; the value at 30 mg/kg was significantly larger than that at 10 mg/kg (Table 1). Note that the dose-normalized (based on 10 mg/kg) AUC0-∞ values for itraconazole were dependent on the intravenous doses of itraconazole studied; the value at 30 mg/kg (1,760 ± 343 μg · min/ml) was significantly greater than those at 10 (1,090 ± 310 μg · min/ml) and 20 (1,270 ± 197 μg · min/ml) mg/kg (Table 1).
FIG. 1.
Mean arterial plasma concentration-time profiles of itraconazole (A) and 7-hydroxyitraconazole (B) after a 1-min intravenous infusion of itraconazole at doses of 10 (▪; n = 10), 20 (□; n = 13), and 30 (▴; n = 12) mg/kg to rats. Bars represent standard deviations.
TABLE 1.
Pharmacokinetic parameters of itraconazole and 7-hydroxyitraconazole after a 1-min intravenous administration of itraconazole at doses of 10, 20, and 30 mg/kg to ratsa
| Parameter | Value at dose (mg/kg) of:
|
||
|---|---|---|---|
| 10 (n = 10) | 20 (n = 13) | 30 (n = 12) | |
| Body wt (g) | 310 ± 9.85 | 286 ± 9.39 | 303 ± 10.3 |
| Itraconazole | |||
| AUC0-∞b (μg · min/ml) | 1,090 ± 310 | 2,530 ± 394 | 5,270 ± 1,030c |
| Terminal t1/2 (min) | 563 ± 250 | 767 ± 352 | 1,050 ± 402d |
| Vss (ml/kg) | 5,070 ± 1,110 | 5,220 ± 2,150 | 6,170 ± 1,470 |
| MRT (min) | 585 ± 213 | 736 ± 264 | 1,150 ± 481c |
| CL (ml/min/kg) | 9.16 ± 3.55 | 7.92 ± 1.22 | 5.69 ± 1.07e |
| CLR (ml/min/kg) | 0.0631 ± 0.251 | 0.0833 ± 0.156 | 0.0628 ± 0.149 |
| CLNR (ml/min/kg) | 9.06 ± 3.51 | 7.60 ± 1.23 | 5.51 ± 1.13e |
| Ae0-48 h (% of dose) | 1.16 ± 0.664 | 2.24 ± 1.34 | 2.86 ± 2.53d |
| GI48 h (% of dose) | BDf | 0.335 ± 0.0372 | 0.348 ± 0.163 |
| 7-Hydroxyitraconazole | |||
| Cmaxb (μg/ml) | 0.603 ± 0.128 | 1.17 ± 0.224 | 1.39 ± 0.199c |
| Tmax (min) | 348 ± 202 | 323 ± 189 | 415 ± 352 |
| AUC0-48 hb (μg · min/ml) | 981 ± 318 | 2,200 ± 533 | 2,860 ± 429 |
| Ae0-48 h (% of dose) | 1.78 ± 1.44 | 1.01 ± 0.857 | 0.530 ± 0.646d |
| GI48 h (% of dose) | 0.697 ± 0.323 | 1.20 ± 0.841 | 0.978 ± 0.357 |
Abbreviations: AUC0-∞, total area under the plasma concentration-time curve from time zero to infinity; t1/2, half-life; Vss, apparent volume of distribution at steady state; MRT, mean residence time; CL, time-averaged total body clearance; CLR, time-averaged renal clearance; CLNR, time-averaged nonrenal clearance; Ae0-48 h, total amount excreted in urine as unchanged drug from time zero to 48 h; GI48 h, total amount recovered from the entire gastrointestinal tract as unchanged drug at 48 h; Cmax maximum plasma concentration; Tmax, time required to reach Cmax. Values are means ± standard deviations.
AUC0-∞, Cmax, and AUC0-48 h values were dose normalized to 10 mg/kg of itraconazole for statistical analysis.
The 30-mg/kg value was significantly different (P < 0.05) from those for 10 and 20 mg/kg.
The 30-mg/kg value was significantly different (P < 0.05) from those for 10 mg/kg.
Each group value was significantly different (P < 0.05).
BD, below detection limit.
As could be expected from the AUC0-∞ values for itraconazole, the CLs of itraconazole were also dependent on the intravenous doses of itraconazole studied. The CLs were 9.16, 7.92, and 5.69 ml/min/kg for 10, 20, and 30 mg/kg, respectively; each value was significantly different (Table 1). The dose-dependent CLs of itraconazole were due to dose-dependent CLNR values, because the CLR values were dose independent (Table 1). The CLNR values were 9.06, 7.60, and 5.51 ml/min/kg for 10, 20, and 30 mg/kg, respectively; each value was significantly different (Table 1). These data suggested that metabolism of itraconazole seemed to be saturated at high doses in rats. Moreover, other pharmacokinetic parameters of itraconazole listed in Table 1, such as mean residence time, the percentage of the intravenous dose of itraconazole recovered from the entire gastrointestinal tract at 48 h as unchanged drug (GI48 h), and the percentage of the intravenous dose of itraconazole excreted in 48-h urine samples as unchanged itraconazole (Ae0-48 h), were also dependent on the intravenous doses studied (Table 1). These data indicated that the pharmacokinetic parameters of itraconazole were dependent on the dose range studied (from 10 to 30 mg/kg). However, the Vss values for itraconazole were independent of the intravenous dose (Table 1). The Vss values, 5,070 to 6,170 ml/kg, were considerably high, suggesting that rat tissues have a good affinity for itraconazole. This could be expected because itraconazole has a very high lipophilicity (log partition coefficient in a system of n-octanol and an aqueous buffer solution of pH 8.1 was 5.66) (10).
The contribution of CLR of itraconazole to the CL of itraconazole was almost negligible; Ae0-48 h was less than 2.86% of an intravenous dose of itraconazole for all three intravenous doses studied (Table 1). The contribution of gastrointestinal (including biliary) excretion of unchanged itraconazole to CLNR of itraconazole also seemed to be negligible; the GI48 h was less than 0.348% for all three intravenous doses of itraconazole studied (Table 1). As mentioned earlier, biliary excretion of itraconazole was almost negligible, and itraconazole was stable in buffer solutions with pHs of 1 and 2 and in the combined gastric juices from five humans. These data suggested that intravenously administered itraconazole could be metabolized almost completely, and the CLNR values listed in Table 1 could represent metabolic clearance of itraconazole in rats.
After intravenous administration of itraconazole, 7-hydroxyitraconazole was detected from the first blood sampling time, 1 min, and reached its peak (Tmax) at 348, 323, and 415 min for 10, 20, and 30 mg/kg, respectively; the values were not significantly different (Fig. 1B and Table 1). After the peak plasma concentration was reached, the plasma concentrations of 7-hydroxyitraconazole declined slowly for up to 48 h, especially at 20 and 30 mg/kg (Fig. 1B). The dose-normalized (based on 10 mg/kg of itraconazole) AUC0-48 h values for 7-hydroxyitraconazole were independent of the intravenous dose of itraconazole; the values were 981 ± 318, 1,100 ± 267, and 953 ± 143 μg · min/ml for itraconazole at 10, 20, and 30 mg/kg, respectively (Table 1). Although the AUC0-∞ of itraconazole was dependent on the intravenous doses of itraconazole, AUC0-48 h of 7-hydroxyitraconazole was independent of the intravenous dose of itraconazole, suggesting that the formation of 7-hydroxyitraconazole did not contribute considerably to the dose-dependent AUC0-∞ of itraconazole. It has been reported that itraconazole was metabolized extensively into a very large number of metabolites (more than 30) in rats, dogs, and humans, each representing less than 1 to 5% of the administered dose (12).
Pharmacokinetics of itraconazole and 7-hydroxyitraconazole after oral administration of itraconazole to rats.
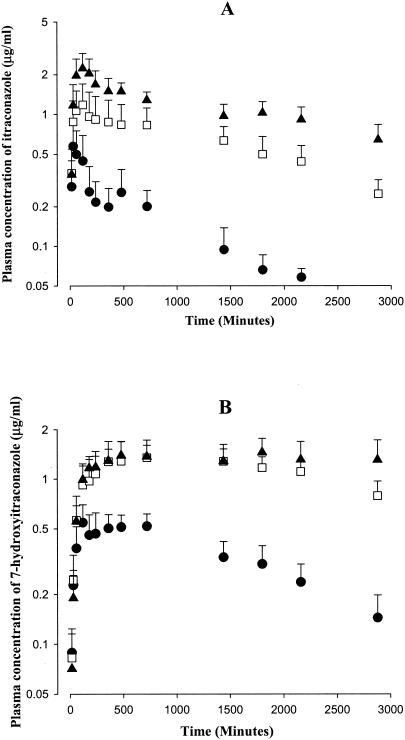
After oral administration of itraconazole at doses of 10, 30, and 50 mg/kg to rats, itraconazole was absorbed rapidly from the gastrointestinal tract; the drug was detected in plasma from the first blood sampling time, 15 min (Fig. 2A). After peak plasma concentration was reached (Tmax, 158 to 324 min, Table 2), the mean arterial plasma concentrations of itraconazole declined (Fig. 2A), with mean terminal t1/2s of 639, 864, and 1,490 min for 10, 30, and 50 mg/kg, respectively; the value at 50 mg/kg was significantly longer than those at 10 and 30 mg/kg (Table 2). Note that the AUC0-∞ values for itraconazole were also dependent on the oral dose of itraconazole; the dose-normalized (based on 10 mg/kg) AUC0-∞ values for itraconazole were significantly different among the three oral doses of itraconazole; the AUC0-∞ values for itraconazole were 380 ± 76.3, 687 ± 217, and 934 ± 194 μg · min/ml for 10, 30, and 50 mg/kg, respectively. Moreover, other pharmacokinetic parameters of itraconazole, such as the maximum plasma concentration and GI48 h, were also dependent on the oral dose of itraconazole (Table 2). These data indicated that the pharmacokinetic parameters of itraconazole were also dependent on the oral doses studied, 10 to 50 mg/kg, in rats.
FIG. 2.
Mean arterial plasma concentration-time profiles of itraconazole (A) and 7-hydroxyitraconazole (B) after oral administration of itraconazole at doses of 10 (▪; n = 8), 30 (□; n = 10), and 50 (▴; n = 10) mg/kg to rats. Bars represent standard deviations.
TABLE 2.
Pharmacokinetic parameters of itraconazole and 7-hydroxyitraconazole after an oral administration of itraconazole at doses of 10, 30, and 50 mg/kg to ratsa
| Parameter | Value at dose (mg/kg) of:
|
||
|---|---|---|---|
| 10 (n = 8) | 30 (n = 10) | 50 (n = 10) | |
| Body wt (g) | 264 ± 13.7 | 274 ± 7.47 | 264 ± 4.12 |
| Itraconazole | |||
| AUC0-∞b (μg · min/ml) | 380 ± 76.3 | 2,060 ± 651 | 4,670 ± 969d |
| Terminal t1/2 (min) | 639 ± 182 | 864 ± 223 | 1,490 ± 616c |
| Cmaxb (μg/ml) | 0.658 ± 0.224e | 1.25 ± 0.450 | 2.34 ± 0.570 |
| Tmax (min) | 158 ± 201 | 273 ± 429 | 324 ± 532 |
| CLR (ml/min/kg) | BDf | 0.00850 ± 0.00168 | 0.0128 ± 0.0334 |
| Ae0-48 h (% of dose) | BD | 0.0543 ± 0.0152 | 0.270 ± 0.256 |
| GI48 h (% of dose) | 1.30 ± 0.686 | 1.55 ± 1.43 | 5.91 ± 4.34c |
| F (%) | 34.9 | 63.0 | 85.7 |
| 7-Hydroxyitraconazole | |||
| Cmaxb (μg/ml) | 0.615 ± 0.0963 | 1.51 ± 0.335 | 1.56 ± 0.306d |
| Tmax (min) | 345 ± 268e | 960 ± 617 | 1,060 ± 735 |
| AUC0-48 hb (μg · min/ml) | 1,000 ± 171 | 3,280 ± 572 | 3,690 ± 764c |
| Ae0-48 h (% of dose) | BD | 0.0405 ± 0.0142 | 0.124 ± 0.0890 |
| GI48 h (% of dose) | 0.773 ± 0.170 | 0.728 ± 0.246 | 0.848 ± 0.399 |
See Table 1, footnote a.
AUC0-∞, Cmax, and AUC0-48 h values were normalized to 10 mg/kg of itraconazole for statistical analysis.
The 50-mg/kg value was significantly different (P < 0.05) from those at 10 and 30 mg/kg.
Each group value was significantly different (P < 0.05).
The 10-mg/kg value was significantly different (P < 0.05) from those at 30 and 50 mg/kg.
BD, below detection limit.
Since the pharmacokinetic parameters of itraconazole were dose dependent after both intravenous and oral administration, the F could also be dose dependent; the values were 34.9, 63.0, and 85.7% for 10, 30, and 50 mg/kg, respectively, based on the AUC0-∞ after an intravenous dose of 10 mg/kg (Table 2). Although more than 98% of the orally administrated itraconazole at a dose of 10 mg/kg was absorbed from the rat gastrointestinal tract, the extent of absolute oral bioavailability (F) was quite low (34.9%), suggesting that the first-pass (gastric, intestinal, and/or hepatic) effects of itraconazole could be considerable after a low oral dose.
After oral administration of itraconazole, 7-hydroxyitraconazole was detected in plasma from the first blood sampling time, 15 min (Fig. 2B), and reached its peak (Tmax) at 345, 960, and 1,060 min for 10, 30, and 50 mg/kg, respectively; these values were significantly different (Table 2). After the peak plasma concentration had been reached, the concentrations of 7-hydroxyitraconazole in plasma declined slowly for up to 48 h, especially at 30 and 50 mg/kg (Fig. 2B). Moreover, the dose-normalized (based on 10 mg/kg of itraconazole) AUC0-48 h values for 7-hydroxyitraconazole were dependent on the oral dose of itraconazole; the values were 1,000 ± 171, 1,090 ± 191, and 738 ± 153 μg · min/ml for itraconazole at 10, 30, and 50 mg/kg, respectively (Table 2). The value at 50 mg/kg was significantly smaller than those at 10 and 30 mg/kg, and this could be due to saturable first-pass effects of itraconazole for the formation of 7-hydroxyitraconazole in rats.
Measurement of hepatic first-pass effect of itraconazole in rats.
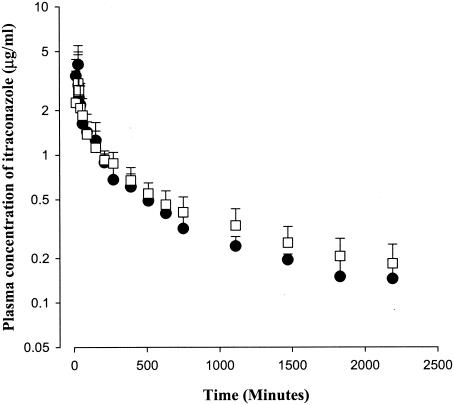
After a 30-min intravenous and intraportal administration of itraconazole to rats at a dose of 10 mg/kg, the mean arterial plasma concentrations declined in a polyexponential fashion for both routes of administration (Fig. 3), with mean terminal half-lives of 1,070 ± 976 and 937 ± 295 min for intravenous and intraportal administration, respectively; these values were not significantly different. The AUC0-∞ values for itraconazole were also not significantly different between the two routes of administration, 1,210 ± 220 and 1,270 ± 228 μg · min/ml for intravenous and intraportal administration, respectively. These data indicated that the hepatic first-pass effect of itraconazole was not considerable in rats. The CL (8.30 ± 1.36 and 8.46 ± 1.85 ml/min/kg, respectively), mean residence time (1,570 ± 1,190 and 1,320 ± 358 min, respectively), and Vss (10,100 ± 492 and 9,280 ± 2,580 ml/kg, respectively) of itraconazole were not significantly different between the two routes of administration.
FIG. 3.
Mean arterial plasma concentration-time profiles of itraconazole after a 30-min intravenous (▪; n = 5) and intraportal (□; n = 6) infusion at a dose of 10 mg/kg to rats. Bars represent standard deviations.
Measurement of gastric and intestinal first-pass effects of itraconazole in rats.
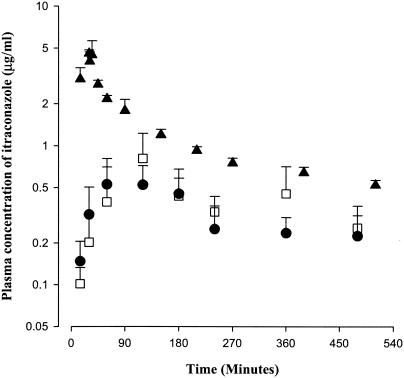
In this experiment, most of the rats died between 24 and 48 h, possibly due to stress. Hence, the AUC ratios of itraconazole between oral administration (Fig. 2) and intraportal infusion (Fig. 3) were measured for up to 6 h, 8 h, and infinity. The AUCoral/AUCintraportal ratios of itraconazole were very similar; the values were 24.1 (112 ± 51.3/465 ± 84.6), 26.5 (139 ± 51.3/525 ± 93.2), and 29.9% (380 ± 76.3/1,270 ± 228) for 6 h, 8 h, and infinity, respectively. Therefore, in the present study, blood samples were collected for up to 8 h. The mean arterial plasma concentration-time curves of itraconazole after intraportal infusion and intragastric and intraduodenal instillation at a dose of 10 mg/kg to rats are shown in Fig. 4. The AUC0-8 h values for itraconazole were not significantly different between the intragastric (175 ± 85.6 μg · min/ml) and intraduodenal (152 ± 50.6 μg · min/ml) routes of administration, suggesting that the gastric first-pass effect of itraconazole was almost negligible in rats. However, the values were significantly smaller (70.1 and 74.0% decrease, respectively) than those after intraportal administration (585 ± 15.8 μg · min/ml), indicating that the intestinal first-pass effect of itraconazole was considerable (approximately 71.1% [(100 − 1.30) × (0.701 + 0.740)/2] of the oral dose) in rats.
FIG. 4.
Mean arterial plasma concentration-time profile of itraconazole after intraportal (▴, n = 5), intragastric (▪, n = 4), and intraduodenal (□, n = 8) administration of the drug at a dose of 10 mg/kg to rats. Bars represent standard deviations.
DISCUSSION
Our data demonstrate that itraconazole shows a poor renal excretion ratio in rats. Considering the CLR values for itraconazole (Table 1) and the reported kidney blood flow rate of 36.8 ml/min/kg (6) and hematocrit of approximately 45% (27) in rats, the estimated renal extraction ratios (CLR of itraconazole/kidney plasma flow rate, only for urinary excretion of unchanged itraconazole) were 0.312, 0.412, and 0.310% for 10, 20, and 30 mg/kg, respectively. The mean protein binding of itraconazole to fresh rat plasma (n = 5) at an itraconazole concentration of 10 μg/ml was 99.1 ± 2.35% with an equilibrium dialysis technique (29). Considering the mean protein binding value and CLR values for itraconazole (Table 1) in rats, the estimated renal clearances of itraconazole as free (unbound in plasma proteins) drug were 7.01, 9.26, and 6.98 ml/min/kg for 10, 20, and 30 mg/kg, respectively. The values were somewhat faster than the reported glomerular filtration rate in rats, 5.24 ml/min/kg (6), suggesting that renal secretion of itraconazole was not considerable in rats.
Dose-dependent pharmacokinetic parameters of itraconazole (especially AUC0−∞ values) were observed after both intravenous (Table 1) and oral (Table 2) administration to rats. Hence, the low dose of 10 mg/kg was arbitrarily chosen to measure the first-pass effects of itraconazole in rats. The F value was 34.9% at a dose of 10 mg/kg (Table 2). Since 1.30% of orally administered itraconazole at a dose of 10 mg/kg was unabsorbed from gastrointestinal tract for up to 48 h (23), approximately 65% [F (0.349) = extent of absorption (1 − 0.0130) × availability through gastrointestinal tract and liver (0.349/0.987), hence extent of first-pass effect (0.646) = 1 − (0.349/0.987)] of orally administered itraconazole at a dose of 10 mg/kg could be eliminated (mainly by metabolism) by the first-pass effect.
After intravenous administration of itraconazole at doses of 10 to 30 mg/kg to rats, the CL values (5.69 to 9.16 ml/min/kg based on plasma data; Table 1) were considerably smaller than the reported cardiac output in rats, 296 ml/min/kg, based on blood data (6). This suggested that the first-pass effect of itraconazole in the lung and heart is negligible or absent in rats.
The AUC0-∞ values for itraconazole at a dose of 10 mg/kg were comparable between intraportal and intravenous administration and between intragastric and intraduodenal administration, suggesting that the hepatic and gastric first-pass effects of itraconazole are almost negligible in rats. However, after intragastric and intraduodenal instillation of itraconazole at a dose of 10 mg/kg, the AUC0-∞ values for itraconazole were 29.9 and 26.0%, respectively, of that after intraportal administration, suggesting that the intestinal first-pass effect of itraconazole are approximately 70% of the oral dose in rats. Considerable intestinal first-pass effects of furosemide (13), azosemide (16), YH439 (a new hepatoprotective agent) (17), YJA-20379-8 (a new proton pump inhibitor) (18), ipriflavone (20), bumetanide (14), KR-31543 (a new neuroprotective agent for ischemia-reperfusion damage) (24), SR-4668 (a candidate for diabetic neuropathy) (15), KR-60436 (a new reversible proton pump inhibitor) (32), and DA-7867 (a new oxazolidinone antibiotic) (2) in rats, and midazolam (28) and saquinavir (8) in humans have been reported.
In conclusion, after oral administration of itraconazole at a dose of 10 mg/kg to rats, the F value was approximately 35% and the intestinal first-pass effect was approximately 70% of the oral dose. Hence, the low F of itraconazole at a dose of 10 mg/kg could be due mainly to considerable intestinal first-pass effects. At a high oral dose of itraconazole, the F value increased with increasing doses (Table 2), and hence, the intestinal first-pass effect could be decreased by increasing oral doses of itraconazole.
Acknowledgments
This study was supported in part by the 2003 BK21 Project for Medicine, Dentistry, and Pharmacy.
REFERENCES
- 1.Allenmark, S., D. J. Touw, and P. N. F. C. de Goede. 1990. Rapid method of the analysis of itraconazole and 7-hydroxyitraconazole in serum by high-performance liquid chromatography. J. Chromatogr. 523:203-206. [DOI] [PubMed] [Google Scholar]
- 2.Bae, S. K., W.-S. Chung, E. J. Kim, J. K. Rhee, J. W. Kwon, W. B. Kim, and M. G. Lee. 2004. Pharmacokinetics of DA-7867, a new oxazolidinone, after intravenous and oral administration to rats: intestinal first-pass effect. Antimicrob. Agents Chemother. 48:659-662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chiou, W. L. 1978. Critical evaluation of potential error in pharmacokinetic studies using the linear trapezoidal rule method for the calculation of the area under the plasma level-time curve. J. Pharmacokinet. Biopharm. 6:539-546. [DOI] [PubMed] [Google Scholar]
- 4.Chiou, W. L. 1979. New calculation method for mean apparent drug volume of distribution and application to rational dosage regimen. J. Pharm. Sci. 68:1067-1069. [DOI] [PubMed] [Google Scholar]
- 5.Chiou, W. L. 1980. New calculation method of mean total body clearance of drugs and its application to dosage regimens. J. Pharm. Sci. 69:90-91. [DOI] [PubMed] [Google Scholar]
- 6.Davies, B., and T. Morris. 1993. Physiological parameters in laboratory animals and humans. Pharm. Res. 10:1093-1095. [DOI] [PubMed] [Google Scholar]
- 7.Eatman, F. B., W. A. Colburn, H. G. Boxenbaum, H. N. Posmanter, R. E. Weinfeld, R. Ronfeld, L. Weissman, J. D. Moore, M. Gibaldi, and S. A. Kaplan. 1977. Pharmacokinetics of diazepam following multiple-dose oral administration to healthy human subjects. J. Pharmacokinet. Biopharm. 5:481-494. [DOI] [PubMed] [Google Scholar]
- 8.Fitzsimmons, M. E., and J. M. Collins. 1997. Selective biotransformation of the human immunodeficiency virus protease inhibitor, saquinavir by human small intestine cytochrome P450 3A4: Potential contribution to high first-pass metabolism. Drug Metab. Dispos. 25:256-266. [PubMed] [Google Scholar]
- 9.Gibaldi, M., and D. Perrier. 1982. Pharmacokinetics, 2nd ed. Marcel Dekker, Inc., New York, N.Y.
- 10.Hardin, T. C., J. R. Graybill, R. Fetchick, R. Woestenborghs, M. G. Rinaldi, and J. G. Kuhn. 1988. Pharmacokinetics of itraconazole following oral administration to normal volunteers. Antimicrob. Agents Chemother. 32:1310-1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Heykants, J., M. Michiels, W. Meuldermans, J. Monbaliu, K. Lavrijsen, A. van Peer, J. C. Levron, R. Woestenborghs, and G. Cauwenberg. 1987. The pharmacokinetics of itraconazole in humans: an overview, p. 223-229. In R. A. Promtling (ed.), Recent trends in the discovery, development and evaluation of antifungal agents. Prous Science Publishers, Barcelona, Spain.
- 12.Heykants, J., A. van Peer, V. van de Velde, P. van Rooy, W. Meuldermans, K. Lavrijsen, R. Woestenborghs, J. van Cutsem, and G. Cauwenbergh. 1989. The clinical pharmacokinetics of itraconazole: an overview. Mycoses 32(Suppl. 1):67-87. [DOI] [PubMed] [Google Scholar]
- 13.Kim, E. J., K. S. Han, and M. G. Lee. 2000. Gastrointestinal first-pass effect of furosemide in rats. J. Pharm. Pharmacol. 152:1337-1343. [DOI] [PubMed] [Google Scholar]
- 14.Kim, E. J., K. S. Han, and M. G. Lee. 2000. Intestinal first-pass effect of bumetanide in rats. Int. J. Pharm. 194:193-199. [DOI] [PubMed] [Google Scholar]
- 15.Kim, E. J., S. K. Bae, H. J. Kim, Y. G. Kim, S.-O. Kim, D. H. Lee, H. Lim, and M. G. Lee. 2003. Dose-independent pharmacokinetics of a candidate for diabetic neuropathy, SR-4668, after intravenous and oral administration to rats: Intestinal first-pass effect. J. Pharm. Sci. 92:1112-1124. [DOI] [PubMed] [Google Scholar]
- 16.Kim, J., S. H. Kim, and M. G. Lee. 1997. Liver and gastrointestinal first-pass effects of azosemide in rats. J. Pharm. Pharmacol. 49:878-883. [DOI] [PubMed] [Google Scholar]
- 17.Kim, J., K. S. Han, J. W. Lee, and M. G. Lee. 1998. Hepatic and intestinal first-pass effects of a new hepatoprotective agent, YH439, in rats. Res. Commun. Mol. Pathol. Pharmacol. 102:125-136. [PubMed] [Google Scholar]
- 18.Kim, J., E. J. Kim, K. S. Han, M. S. Chang, and M. G. Lee. 1999. Gastrointestinal first-pass effect of YJA-20379-8, a new reversible proton pump inhibitor, in rats. J. Pharm. Pharmacol. 51:1031-1036. [DOI] [PubMed] [Google Scholar]
- 19.Kim, S. H., W. B. Kim, and M. G. Lee. 1999. Pharmacokinetic changes of a new carbapenem, DA-1131, after intravenous administration to spontaneously hypertensive rats and deoxycorticosterone acetate-salt-induced hypertensive rats. Drug Metab. Dispos. 27:710-716. [PubMed] [Google Scholar]
- 20.Kim, S. H., and M. G. Lee. 2002. Pharmacokinetics of ipriflavone, an isoflavone derivative, after intravenous and oral administration to rats: hepatic and intestinal first-pass effects. Life Sci. 70:1299-1315. [DOI] [PubMed] [Google Scholar]
- 21.Kim, S. H., Y. M. Choi, and M. G. Lee. 1993. Pharmacokinetics and pharmacodynamics of furosemide in protein-calorie malnutrition. J. Pharmacokinet. Biopharm. 21:1-17. [DOI] [PubMed] [Google Scholar]
- 22.Lee, A. K., C. Y. Ahn, E. J. Kim, J. W. Kwon, S. G. Kim, S. J. Chung, C-K. Shim, and M. G. Lee. 2003. Effects of cysteine on the pharmacokinetics of itraconazole in rats with protein-calorie malnutrition. Biopharm. Drug Dispos. 24:63-70. [DOI] [PubMed] [Google Scholar]
- 23.Lee, M. G., and W. L. Chiou. 1983. Evaluation of potential causes for the incomplete bioavailability of furosemide: gastric first-pass metabolism. J. Pharmacokinet. Biopharm. 11:623-640. [DOI] [PubMed] [Google Scholar]
- 24.Lee, M. H., S. K. Bae, E. J. Kim, Y. G. Kim, S.-O. Kim, D. H. Lee, H. Lim, S.-E. Yoo, and M. G. Lee. 2003. Dose-independent pharmacokinetics of a new neuroprotective agent for ischemia-reperfusion damage, KR-31543, after intravenous and oral administration to rats: hepatic and intestinal first-pass effects. J. Pharm. Sci. 92:190-201. [DOI] [PubMed] [Google Scholar]
- 25.Lee, S. H., and M. G. Lee. 1996. Pharmacokinetics and pharmacodymanics of azosemide after intravenous and oral administration to rats: Absorption from various GI segments. J. Pharmacokinet. Biopharm. 24:551-568. [DOI] [PubMed] [Google Scholar]
- 26.Litterst, C. L., E. G. Mimnaugh, R. L. Regan, and T. E. Gram. 1975. Comparison of in vitro drug metabolism by lung, liver, and kidney of several common laboratory species. Drug Metab. Dispos. 3:259-265. [PubMed] [Google Scholar]
- 27.Mitruka, B. M., and H. M. Rawnsley. 1981. Clinical biochemical and hematological reference values in normal experimental animals and normal humans, 2nd ed. Masson Publishing USA Inc., New York, N.Y.
- 28.Paine, M. F., D. D. Shen, K. L. Kunze, J. D. Perkins, C. L. Marsh, J. P. McVicar, D. M. Barr, B. S. Gillies, and K. E. Thummel. 1996. First-pass metabolism of midazolam by the human intestine. Clin. Pharmacol. Ther. 60:14-24. [DOI] [PubMed] [Google Scholar]
- 29.Shim, H. J., E. J. Lee, S. H. Kim, M. Yoo, J. W. Kwon, W. B. Kim, and M. G. Lee. 2000. Factors influencing the protein binding of a new phosphodiesterase V inhibitor, DA-8159, using an equilibrium dialysis technique. Biopharm. Drug Dispos. 21:285-291. [DOI] [PubMed] [Google Scholar]
- 30.Xu, Z.-X., J. P. Tang, M. Badr, and S. Melethil. 1992. Kinetics of aluminum in rats. III: Effect of route of administration. J. Pharm. Sci. 81:160-163. [DOI] [PubMed] [Google Scholar]
- 31.Yoo, S. D., E. Kang, H. Jun, B. S. Shin, K. C. Lee, and K. H. Lee. 2000. Absorption, first-pass metabolism, and disposition of itraconazole in rats. Chem. Pharm. Bull. 48:798-801. [DOI] [PubMed] [Google Scholar]
- 32.Yu, S. Y., S. K. Bae, E. J. Kim, Y. G. Kim, S.-O. Kim, D. H. Lee, H. Lim, and M. G. Lee. 2003. Dose-independent pharmacokinetics of a new reversible proton pump inhibitor, KR-60436, after intravenous and oral administration to rats: gastrointestinal first-pass effect. J. Pharm. Sci. 92:1592-1603. [DOI] [PubMed] [Google Scholar]