Abstract
AIM:
To determine the range of tracheal collapse at end-expiration among chronic obstructive pulmonary disease (COPD) patients and to compare the extent of tracheal collapse between static end-expiratory and dynamic forced-expiratory multidetector-row computed tomography (MDCT).
MATERIALS AND METHODS:
After institutional review board approval and obtaining informed consent, 67 patients meeting the National Heart, Lung, and Blood Institute (NHLBI)/World Health Organization (WHO) Global Initiative for Chronic Obstructive Lung Disease (GOLD) criteria for COPD were sequentially imaged using a 64-detector-row CT machine at end-inspiration, during forced expiration, and at end-expiration. Standardized respiratory coaching and spirometric monitoring were employed. Mean percentage tracheal collapse at end-expiration and forced expiration were compared using correlation analysis, and the power of end-expiratory cross-sectional area to predict excessive forced-expiratory tracheal collapse was computed following construction of receiver operating characteristic (ROC) curves.
RESULTS:
Mean percentage expiratory collapse among COPD patients was 17±18% at end-expiration compared to 62±16% during forced expiration. Over the observed range of end-expiratory tracheal collapse (approximately 10–50%), the positive predictive value of end-expiratory collapse to predict excessive (≥80%) forced expiratory tracheal collapse was <0.3.
CONCLUSION:
COPD patients demonstrate a wide range of end-expiratory tracheal collapse. The magnitude of static end-expiratory tracheal collapse does not predict excessive dynamic expiratory tracheal collapse.
INTRODUCTION
Clinical and research protocols for multidetector-row computed tomography (MDCT) in patients with chronic obstructive pulmonary disease (COPD) commonly employ static end-expiratory imaging as a supplement to end-inspiratory imaging because it is the preferred method for assessing air-trapping in small airways disease. (1–5) Such information contributes to the quantitative phenotyping of COPD patients, which has the potential to guide management strategies in order to improve symptom relief while minimizing the risk of acute exacerbations and progression of disease. (6,7)
However, imaging during a forced expiratory manoeuvre is an increasingly accepted method for evaluating COPD patients with suspected hyperdynamic trachea due to either tracheomalacia (TM), characterized by weakness of the tracheobronchial cartilaginous structures, or excessive dynamic airway collapse (EDAC), defined as excessive bulging of the posterior membrane into the airway lumen during expiration without cartilage collapse. (8–14)
Accurate evaluation of tracheal dynamics is important because recent reports of selected patients with coexisting COPD and TM/EDAC have documented improved symptoms, quality of life, and functional status following central airway stabilization with silicone stents or tracheoplasty. (15–16) In order to guide radiologists in assessing tracheal dynamics using end-expiratory imaging, it would be helpful to know the range of tracheal collapse at end-expiration and whether the magnitude of “static” expiratory tracheal collapse predicts the magnitude of “dynamic” expiratory collapse.
Although previous studies have attempted to address these questions, they were limited by small sample size and lacked spirometric monitoring to ensure that MDCT acquisitions were obtained at the appropriate points in the respiratory cycle. (17–19) Thus, there is a need to more rigorously establish the range of end-expiratory tracheal collapse and to better define the relationships between end-expiratory and forced expiratory tracheal collapse in COPD patients.
The aims of the present study were twofold: (1) to determine the range of tracheal collapse at static end-expiration among patients with COPD; and (2) to compare the extent of tracheal collapse between static end-expiratory and dynamic forced expiratory MDCT.
MATERIALS AND METHODS
This study is an adjunct to a prospective 5-year research investigation of forced-expiratory tracheal dynamics in COPD that was approved by our hospital’s institutional review board (IRB) and was performed in compliance with Health Insurance Portability and Accountability Act guidelines. All patients provided written informed consent. The methodological design of this study, including descriptions of the imaging technique and breathing instructions, has been published previously in greater detail and is reviewed briefly below. (20,21) The research questions addressed by the study and the associated end-expiratory experimental methods are unique to this publication and were also IRB approved.
Study population
Between October 2008 and November 2010, 100 patients with spirometrically confirmed COPD were enrolled into a study designed to define the prevalence of excessive dynamic airway collapse in this population. Patients with other risk factors for TM, including prior prolonged intubation, mediastinal radiation, tracheal surgery, or tracheal stent placement were excluded from study. Twenty-two of these patients underwent end-expiratory imaging during the initial visit following amendment of the original protocol to include end-expiratory imaging in addition to dynamic expiratory and end-inspiratory imaging sequences. Per the original study design, all COPD patients with excessive tracheal collapse (≥80%) at the time of the initial study visit as well as a randomly selected one-third of the remaining COPD participants were assigned to two annual repeat visits to assess longitudinal changes in tracheal dynamics. Among these participants, 45 underwent initial end-expiratory imaging at either the second annual visit (following protocol amendment, n=32) or the third annual visit (n=13). Thus, a total of 67 COPD participants underwent both dynamic and static end-expiratory imaging during the same visit.
Imaging technique
CT was performed using a 64–detector row machine (Light Speed VCT; General Electric Medical Systems, Waukesha, WI, USA) with the following parameters: 80 mA, 120 kVp, 0.625 mm detector collimation, and 0.5 s gantry rotation time. The scan length included the entire lungs.
Volumetric CT imaging of the entire lung was performed in the craniocaudal direction for both end-inspiratory (total lung capacity, TLC) and dynamic expiratory imaging (obtained during a forced expiratory manoeuvre). The total scanning time for each volumetric acquisition of the entire lungs was approximately 2.5 s. For both sequences, images were reconstructed at 2.5 mm thickness with 1.25 mm reconstruction intervals and transferred to a picture archiving communication system (PACS; Centricity, Version 3.1, General Electric Medical Systems) for analysis and interpretation. These parameters were selected based upon MDCT protocols validated by bronchoscopy for assessing tracheal collapsibility. (14) The mean effective dose for the two volumetric CT acquisitions combined was 2.2 mSv.
To minimize further radiation exposure, the additional end-expiratory acquisition was performed as a single 1.25 mm axial image 1 cm above the aortic arch. This anatomical level was selected because it is a standard site for measurement of tracheal dynamics in multiple previous studies. (18,19) Comparisons of dynamic and static expiratory airway collapse were made only at this anatomical level. The estimated whole-body dose equivalent for this additional image is 0.03 mSv.
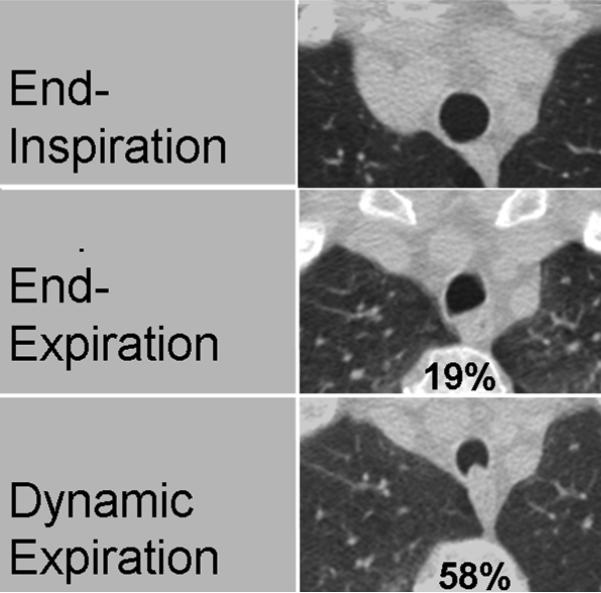
For all three imaging acquisitions, active respiratory coaching and spirometric monitoring were performed by an experienced respiratory physiologist in order to coordinate CT acquisitions with spirometric tracings of the respiratory cycle. (20) The order of imaging acquisitions was the same for all participants: end-inspiration (imaging at total lung capacity), dynamic forced expiration (imaging during forced exhalation), and static end-expiration (imaging at residual volume). Examples of the typical appearance of the trachea at end-inspiration, end-expiration, and during forced expiration are shown in Fig. 1.
Figure 1.
Representative CT images of a 63-year-old woman with COPD demonstrate typical magnitudes of static end-expiratory tracheal collapse (19%) and of dynamic forced-expiratory collapse (58%). Expiratory tracheal morphology demonstrates relative flattening of the posterior membranous wall of the trachea at end-expiration and anterior bowing of this structure at forced expiration.
CT analysis and interpretation
Each study was reviewed and interpreted by a fellowship-trained thoracic radiologist with 17 years of experience in interpreting tracheal CT images. Images were reviewed using standard lung window settings (window level, –650 HU; window width, 1500 HU) using a PACS. (18,22) For each imaging sequence, measurements were obtained at a standard level of 1 cm above the aortic arch. Coronal and sagittal diameters were measured using electronic calipers. Using a previously validated technique, the cross-sectional area (CSA) of the airway lumen was measured by tracing the inner wall of the airway with an electronic tracing tool. (14,18) The percentage expiratory luminal collapse (%Collapse) was calculated by using the equation:
%Collapse = 100 × [1 – (area at expiration/area at end-inspiration)].
Statistical analysis
Descriptive data are reported as frequencies and cross tabulations for nominal measures, and means and standard deviations (SD) for continuous variables. The relationships among CSA of the tracheal lumen, changes in sagittal and coronal diameter of the tracheal lumen, static end-expiratory percentage expiratory tracheal collapse, and dynamic forced-expiratory percentage tracheal collapse were assessed by calculating correlation coefficients. The predictive characteristics of end-expiratory collapse on specified percentages of forced-expiratory collapse were assessed by measuring the area under receiver operating characteristic (ROC) curves and computing predictive power. All analyses were performed using SPSS statistical software (version 12: SPSS, Chicago, IL, USA).
RESULTS
The study population included 67 COPD patients whose characteristics are provided in Table 1. Over 70% of patients had moderate to severe COPD [National Heart, Lung, and Blood Institute (NHLBI)/World Health Organization (WHO) Global Initiative for Chronic Obstructive Lung Disease (GOLD) II–IV].
Table 1.
Patient characteristics
| Characteristic | n |
|---|---|
| Age 65.1 | (6.5a) |
| Male 38 | (57%) |
| Height (cm) | 169 (9.2a) |
| Weight (kg) | 86.4 (19.1a) |
| BMI 30.1 | (6a) |
| FEV1 (%Pred) |
67 (21a) |
| GOLD Stage I II III IV |
19 (28.4%) 36 (53.7%) 11 (16.4%) 1 (1.5%) |
SD
Average forced expiratory collapse (62±16%) was substantially greater than end-expiratory collapse (17±18%; Table 2a). Most of the forced expiratory change in area resulted from reduction in sagittal (51%) rather than coronal (34%) diameter, whereas change in sagittal (15%) and coronal (11%) diameters were similar at end exhalation. (Table 2b)
Table 2.
Mean inspiratory and expiratory areas: %Collapse and diameters
| Characteristic | n |
|---|---|
| Areas (mm2) and % Collapse | |
| Inspiratory area | 290 (69a) |
| Forced expiratory area | 110 (56) |
| End expiratory area | 239 (73) |
| Forced expiratory collapse | 62 (16) |
| End-expiratory collapse | 17 (18) |
| Diameters (mm) | |
| Inspiratory sagittal | 20 (3.1) |
| Forced expiratory sagittal | 9.8 (3.7) |
| End-expiratory sagittal | 16.9 (4.6) |
| Inspiratory coronal | 18 (3.4) |
| Forced expiratory coronal | 11.8 (3.6) |
| End-expiratory coronal | 16.3 (3.4) |
SD
Table 3 provides bivariate correlations between dynamic forced expiratory collapse and static end-expiratory measures including coronal and sagittal diameters, CSA, and %Collapse. Although some of these correlations are statistically significant at the conventional 0.05 probability level (two-tailed), the coefficient of determination (R2) was less than 9% for all comparisons. Not shown in the table, there was no significant correlation between end-expiratory collapse and percent predicted forced expiratory volume in 1 s (FEV1; p=0.691)
Table 3.
Correlation of static end-expiratory measures with forced expiratory collapse
| Static measure | Ra |
p- Value |
|---|---|---|
| End-expiratory collapse | 0.244 | 0.047 |
| End-expiratory area | −0.146 | 0.237 |
| End expiratory sagittal diameter | −0.293 | 0.016 |
| Insp–exp sagittal difference | 0.246 | 0.045 |
| End-expiratory coronal diameter | −0.070 | 0.573 |
| Insp–exp coronal difference | 0.277 | 0.023 |
Correlation with dynamic collapse
Insp–exp, inspiratory–expiratory.
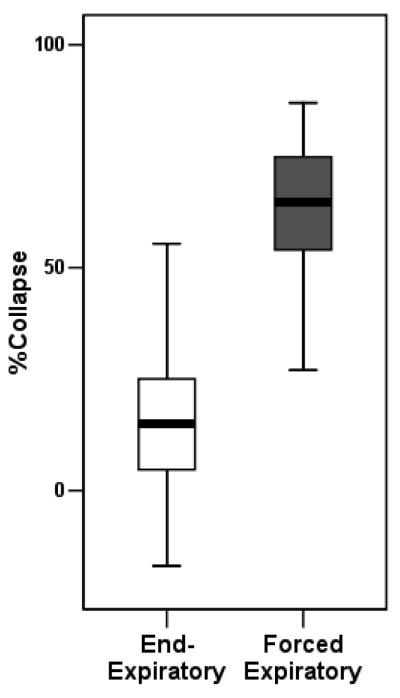
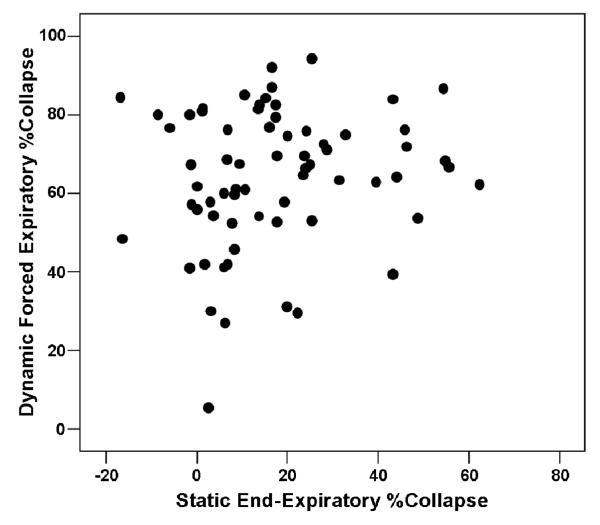
The median, interquartile, and total range of forced expiratory (dynamic) and end-expiratory (static) %Collapse are shown in Fig. 2, and a scatter plot of forced- on end-expiratory tracheal collapse is provided in Fig. 3. Graphically, there is no apparent positive association between forced- and end-expiratory collapse, as is evident by the low coefficient of determination (R2 =0.059).
Figure 2.
Box plot showing median value, interquartile- and total range of forced expiratory (dynamic) and end-expiratory %Collapse.
Figure 3.
Scatter plot of forced expiratory collapse by end-expiratory collapse.
To examine the predictive characteristics of end-expiratory tracheal collapse, we computed the area under the ROC curve and determined the positive predictive power for values of end-expiratory tracheal collapse ranging from 10–50%. Fifteen (22.4%) of 67 COPD patients in this sample had ≥80% forced expiratory collapse. Using this value as a diagnostic indicator, the area under the ROC curve was 0.418 (p=0.336). The sensitivity and specificity of various degrees of end-expiratory collapse to predict excessive forced expiratory collapse are shown in Table 4. Positive predictive power was <0.3 for end-expiratory values in the range of 10–50% tracheal collapse. Only one of four patients (25%) with end-expiratory collapse greater than 50% also demonstrated ≥80% forced expiratory collapse.
Table 4.
Sensitivity and specificity of end-expiratory collapse to predict excessive forced expiratory collapse (≤80%)
| %End-expiratory collapse |
Sensitivity | Specificity |
|---|---|---|
| 10 | 0.60 | 0.44 |
| 20 | 0.20 | 0.60 |
| 30 | 0.13 | 0.79 |
| 40 | 0.13 | 0.85 |
| 50 | 0.07 | 0.94 |
DISCUSSION
A wide range of static end-expiratory tracheal collapse was observed among patients with COPD. The results of the present study clearly demonstrate that the magnitude of static end-expiratory tracheal collapse does not predict the extent of dynamic expiratory collapse.
During maximal forced expiration, with development of highly negative transmural pressure in the airway segment downstream from the site of flow limitation (choke point), COPD patients can demonstrate substantial intrathoracic tracheal narrowing. (23) To the extent that airway elasticity and airway wall characteristics are affected by COPD, there may be less tethering force on central airways and one might also expect a substantially reduced tracheal CSA at static end-exhalation. However, any tendency for reduced end-expiratory tracheal CSA among COPD patients may be offset by larger end-expiratory volume. Although the average residual volume among COPD patients was found to be 122% of the predicted value, there was no significant association between static end-expiratory tracheal collapse and percent predicted residual volume (R2 = 0.012). Given that elevated residual volume is indicative of COPD severity, this finding is consistent with previously published results showing no significant correlation between dynamic expiratory tracheal collapse and FEV1. (21)
Although previous studies have shown that dynamic expiratory tracheal collapse is greater than collapse measured at static end-expiration, these studies did not directly address the question of whether the magnitude of static end-expiratory tracheal collapse predicts the extent of dynamic expiratory collapse. (17,19) Importantly, the results of the present study indicate that the magnitude of static end-expiratory tracheal collapse is not predictive of the severity of dynamic expiratory collapse. Thus, from a practical perspective, for COPD patients in whom there is a high clinical suspicion for excessive expiratory tracheal collapse, imaging with dynamic expiratory CT may be indicated, regardless of the degree of tracheal collapse observed at end-expiration.
There are several potential limitations of the present study. First, the trachea was evaluated at only one anatomical level. This level was selected to enable direct comparison to previous published studies. (14,24–26) Because the trachea is a tubular structure with similar compliance throughout most of its intrathoracic course, this factor did not bias the results. (27) It has been previously reported that there were no cases of highly localized forced-expiratory tracheal collapse in this sample, and that there was close agreement between mid- and lower tracheal %Collapse. Second, there was no attempt to distinguish among different patterns of central airway collapse, such as predominant reduction in coronal versus sagittal diameter versus more concentric circumferential collapse. Further study focusing on different patterns of collapse may identify relationships between static and dynamic measures that were not detected. Third, no direct measurements of tracheal pressure were obtained during the study. However, in a recent study, forced expiratory efforts and dynamic tracheal %Collapse were highly reproducible when accompanied by active respiratory coaching and spirometric monitoring (28). Fourth, behavioural factors, such as voluntary glottal inhibition of maximal forced exhalation or glottal closure at end exhalation, could not be completely controlled. Such behaviour may explain why one participant demonstrated a greater magnitude of tracheal collapse at end-expiration than during forced expiration. Finally, as the respiratory manoeuvres were acquired in a standard order (end-inspiration, dynamic forced expiration, end-expiration) without randomization of the manoeuvres, the volunteer could potentially become tired during the last sequence, which could influence the results. However, the findings are consistent with those of a previous study in which the sequence of respiratory manoeuvres was end-inspiration, end-expiration, and dynamic forced expiration. (17) Furthermore, expired volume was monitored to assure CT acquisition at residual volume. Thus, it is unlikely that the sequence of expiratory imaging influenced the present results.
In summary, COPD patients display a wide range of tracheal collapse at end-expiration. The magnitude of end-expiratory tracheal collapse is not associated with the magnitude of forced expiratory tracheal collapse. Moreover, no threshold of end-expiratory collapse reliably predicts excessive dynamic tracheal collapse. Therefore, dynamic expiratory imaging should be considered when there is high clinical suspicion, for excessive expiratory tracheal collapse such as TM.
ACKNOWLEDGEMENTS
This study was supported by NIH Grant 5R01HL84331-2.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Sundaram B, Chughtai AR, Kazerooni EA. Multidetector high-resolution computed tomography of the lungs: protocols and applications. J Thorac Imaging. 2010;25:125–41. doi: 10.1097/RTI.0b013e3181d9ca37. [DOI] [PubMed] [Google Scholar]
- 2.Wright CD. Tracheomalacia. Chest Surg Clin N Am. 2003;13:349–57. doi: 10.1016/s1052-3359(03)00036-x. [DOI] [PubMed] [Google Scholar]
- 3.Loeve M, Lequin MH, deBruijne, et al. Cystic fibrosis: are volumetric ultra-low-dose expiratory CT scans sufficient for monitoring related lung disease? Radiology. 2009;253:223–9. doi: 10.1148/radiol.2532090306. [DOI] [PubMed] [Google Scholar]
- 4.Ochs RA, Petkovska I, Kim HJ, et al. Prevalence of tracheal collapse in an emphysema cohort as measured with end-expiration CT. Acad Radiol. 2009;16:46–53. doi: 10.1016/j.acra.2008.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yamashiro T, Estepar RSJ, Matsuoka s, et al. Intrathoracic tracheal volume and collapsibility on inspiratory and end-expiratory CT scans: correlations with lung volume and pulmonary function in 85 smokers. Acad Radiol. 2011;18:299–305. doi: 10.1016/j.acra.2010.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lynch DA, Al-Qaisi MA. Quantitative computed tomography of chronic obstructive pulmonary disease. J Thorac Imaging. 2013;28:284–290. doi: 10.1097/RTI.0b013e318298733c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boiselle PM, Crapo JD, Han MK, et al. Expert opinion: how can quantitative CT benefit patients with COPD and idiopathic interstitial pneumonias? J Thorac Imaging. 2013;28:263. doi: 10.1097/RTI.0b013e3182a11afd. [DOI] [PubMed] [Google Scholar]
- 8.Murgu SD, Colt HG. Tracheobronchomalacia and excessive dynamic airway collapse. Clin Chest Med. 2013;34:527–555. doi: 10.1016/j.ccm.2013.05.003. [DOI] [PubMed] [Google Scholar]
- 9.Brillet PY, Fetita CI, Saragaglia A, et al. Investigation of airways using MDCT for visual and quantitative assessment in COPD patients. Int J COPD. 2008;3:97–107. doi: 10.2147/copd.s2302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ridge CA, O'Donnell CR, Lee EY, Majid A, Boiselle PM. Tracheobronchomalacia: current concepts and controversies. J Thorac Imaging. 2011;26:278–89. doi: 10.1097/RTI.0b013e3182203342. [DOI] [PubMed] [Google Scholar]
- 11.Fraser RS, Colman N, Müller NL, Paré PD. Upper airway obstruction. In: Fraser RS, Paré PD, editors. Synopsis of Diseases of the Chest. 4th. Saunders; Philadelphia, PA: 1999. pp. 2042–2046. (1999) [Google Scholar]
- 12.Boiselle PM, Feller-Kopman D, Ashiku S, Week D, Ernst A. Tracheobronchomalacia: evolving role of dynamic multislice helical CT. Radiol Clin North Am. 2003;41:627–36. doi: 10.1016/s0033-8389(03)00023-x. [DOI] [PubMed] [Google Scholar]
- 13.Carden KA, Boiselle PM, Waltz DA, Ernst A. Tracheomalacia and tracheobronchomalacia in children and adults: an in-depth review. Chest. 2005;127:984–1005. doi: 10.1378/chest.127.3.984. [DOI] [PubMed] [Google Scholar]
- 14.Lee KS, Sun MR, Ernst A, Feller-Kopman D, Majid A, Boiselle PM. Comparison of dynamic expiratory CT with bronchoscopy for diagnosing airway malacia: a pilot evaluation. Chest. 2007;31:758–64. doi: 10.1378/chest.06-2164. [DOI] [PubMed] [Google Scholar]
- 15.Ernst A, Majid A, Feller-Kopman D, et al. Airway stabilization with silicone stents for treating adult tracheobronchomalacia: a prospective observational study. Chest. 2007;132:609–616. doi: 10.1378/chest.06-2708. [DOI] [PubMed] [Google Scholar]
- 16.Majid A, Guerrero J, Gangadharan S, et al. Tracheobronchoplasty for severe tracheobronchomalacia: a prospective outcome analysis. Chest. 2008;134:801–807. doi: 10.1378/chest.08-0728. [DOI] [PubMed] [Google Scholar]
- 17.Aquino SL, Shepard JA, Ginns LC, et al. Acquired tracheomalacia: detection by expiratory CT scan. J Comput Assist Tomogr. 2001;25:394–9. doi: 10.1097/00004728-200105000-00011. [DOI] [PubMed] [Google Scholar]
- 18.Baroni RH, Feller-Kopman D, Nishino M, et al. Tracheobronchomalacia: comparison between end-expiratory and dynamic expiratory CT for evaluation of central airway collapse. Radiology. 2005;235:635–41. doi: 10.1148/radiol.2352040309. [DOI] [PubMed] [Google Scholar]
- 19.Ferretti GR, Jankowski A, Perrin MA, et al. Multi-detector CT evaluation in patients suspected of tracheobronchomalacia: comparison of end-expiratory with dynamic expiratory volumetric acquisitions. Eur J Radiol. 2008;68:340–6. doi: 10.1016/j.ejrad.2007.08.029. [DOI] [PubMed] [Google Scholar]
- 20.Boiselle PM, O'Donnell CR, Bankier AA, et al. Tracheal collapsibility in healthy volunteers during forced expiration: assessment with multidetector CT. Radiology. 2009;252:255–62. doi: 10.1148/radiol.2521081958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Boiselle PM, Michaud G, Roberts D, et al. Dynamic expiratory tracheal collapse in COPD: correlation with clinical and physiologic parameters. Chest. 2012;142:1539–1544. doi: 10.1378/chest.12-0299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Boiselle PM. Tracheomalacia, functional imaging of the large airways with multidetector-row CT. In: Boiselle PM, White CS, editors. New Techniques in Cardiothoracic Imaging. Informa; New York, NY: 2007. pp. 177–185. [Google Scholar]
- 23.Loring SH, O’Donnell CR, Feller-Kopman DJ, Ernst A. Central airway mechanics and flow limitation in acquired tracheobronchomalacia. Chest. 2007;131:1118–1124. doi: 10.1378/chest.06-2556. [DOI] [PubMed] [Google Scholar]
- 24.Stern EJ, Graham CM, Webb WR, Gamsu G. Normal trachea during forced expiration: dynamic CT measurements. Radiology. 1993;187:27–31. doi: 10.1148/radiology.187.1.8451427. [DOI] [PubMed] [Google Scholar]
- 25.Boiselle PM, Ernst A. Tracheal morphology in patients with tracheomalacia: prevalence of inspiratory lunate and expiratory frown shapes. J Thorac Imaging. 2006;21:190–196. doi: 10.1097/01.rti.0000213647.42041.d0. [DOI] [PubMed] [Google Scholar]
- 26.Gamsu G, Webb WR. Computed tomography of the trachea: normal and abnormal. AJR Am J Roentgenol. 1982;139:321–326. doi: 10.2214/ajr.139.2.321. [DOI] [PubMed] [Google Scholar]
- 27.Griscom NT. Diseases of the trachea, bronchi, and smaller airways. Radiol Clin North Am. 1993;31:605–615. [PubMed] [Google Scholar]
- 28.Boiselle PM, O'Donnell CR, Loring SH, Bankier AA. Reproducibility of forced expiratory tracheal collapse: assessment with MDCT in healthy volunteers. Acad Radiol. 2010;17:1186–1189. doi: 10.1016/j.acra.2010.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]