Abstract
The use of phosphorus-containing flame retardants (PFRs) has increased over the past decade. Widespread human exposure has been reported, but information on the safety or potential health risks of PFRs is lacking. We assessed the relationship between urinary concentrations of two PFR metabolites [bis(1,3-dichloro-2-propyl) phosphate (BDCPP) and diphenyl phosphate (DPHP)] and semen quality, sperm motion parameters, and serum hormone levels in 33 men. BDCPP and DPHP concentrations were significantly greater in urine samples collected in the afternoon compared to those collected in the morning (p <0.05). In multivariable models, a number of statistically significant or suggestive associations were observed between the reproductive health measures and both PFR metabolites. While the study was limited by a small sample size, these results warrant further investigation in a larger study population. Additional studies on sources, pathways, and routes of PFR exposure, along with research on toxicokinetics and exposure measure utility, are also needed.
Keywords: Biomarker, Epidemiology, Exposure, Fertility, Reproduction, Thyroid
INTRODUCTION
Organophosphate chemicals have commonly been used as plasticizers and flame retardants in a range of materials and products over the past few decades. However, the use of phosphorus-containing as flame retardants (PFRs) has been increasing dramatically over the past decade with the recent bans or voluntary phase-outs of the widely used polybrominated diphenyl ethers (PBDE) and other persistent brominated flame retardants (BFRs).1, 2 Recent studies have reported evidence of widespread human exposure to PFRs,3–8 but information on the safety or potential health risks of common PFRs following human exposure is lacking. Studies of PFR exposure sources and pathways are also limited, but contact with dust in the home and other indoor environments is thought to be important as it is with the more intensely studied BFRs.9, 10 We previously reported that concentrations of two PFRs, tris(1,3,-dichloro-propyl) phosphate (TDCPP) and triphenyl phosphate (TPHP; formerly abbreviated as TPP),11 measured in house dust of men participating in a reproductive health study were similar in magnitude to those found for PBDEs, and that levels of these triesters in dust were associated with significant alterations in several reproductive health markers.6 TDCPP was associated with increased serum prolactin levels and decreased free androgen index and free T4 levels, while TPHP was associated with increased prolactin and decreased sperm concentration.
We also recently reported urinary concentrations of metabolites of TDCPP [bis(1,3-dichloro-2-propyl) phosphate (BDCPP)] and TPHP [diphenyl phosphate (DPHP)] in a subset of these men.7 Greater than 90% of the men had detectable concentrations of both BDCPP and DPHP, with concentrations spanning four orders of magnitude. However, TDCPP in house dust was only moderately correlated with urinary BDCPP from the same men (Spearman r = 0.3 to 0.5), and there was no correlation between TPHP and DPHP (Spearman r < 0.1). In this brief report we conducted an exploratory analysis of the association between urinary PFR metabolite concentrations and semen quality, sperm motion parameters, and reproductive and thyroid hormone levels in men. The comparison of effect estimates when using PFR biomarker versus house dust concentrations as the exposure variable may also provide indirect evidence for which exposure assessment approach may be associated with less measurement error and thus potentially considered preferred in future epidemiology studies.12
RESULTS
Data on urinary PFR metabolite concentrations and semen quality, sperm motion, and hormone measures were available for 33 men. Median (range) age and BMI were 35 (28 to 46) years and 25.8 (20.4 to 42.4) kg/m2, respectively. In preliminary bivariate analyses, there were no associations between urinary PFR metabolite concentrations and age or BMI. SG-corrected concentrations of BDCPP and DPHP were significantly greater in urine samples collected in the afternoon (BDCPP geometric mean = 384 pg/ml; DPHP geometric mean = 1090 pg/ml) compared to those collected in the morning (BDCPP geometric mean = 122 pg/ml; DPHP geometric mean = 265 pg/ml; p-values <0.05).
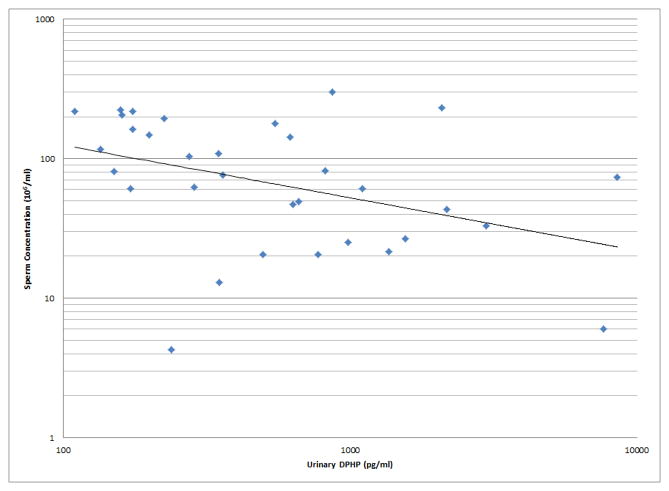
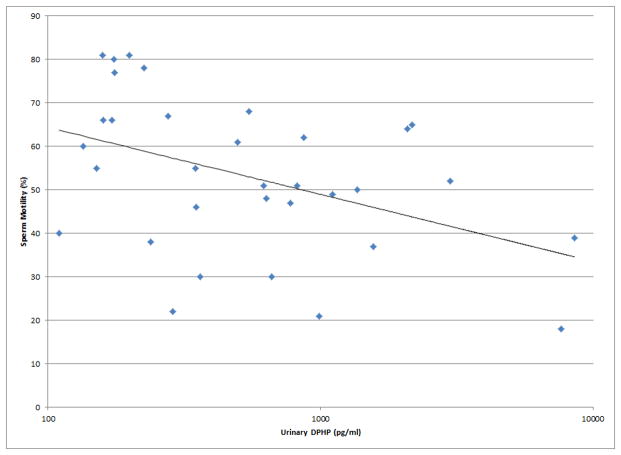
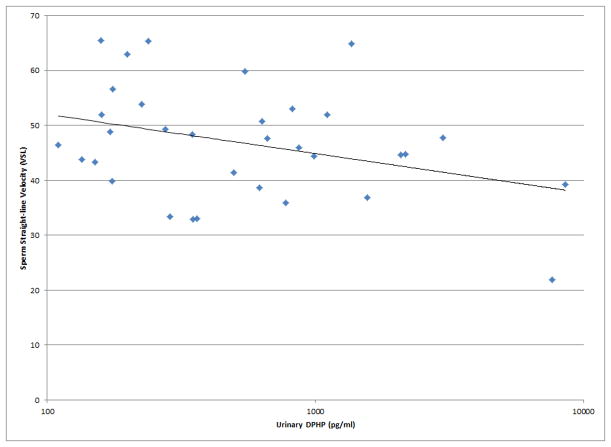
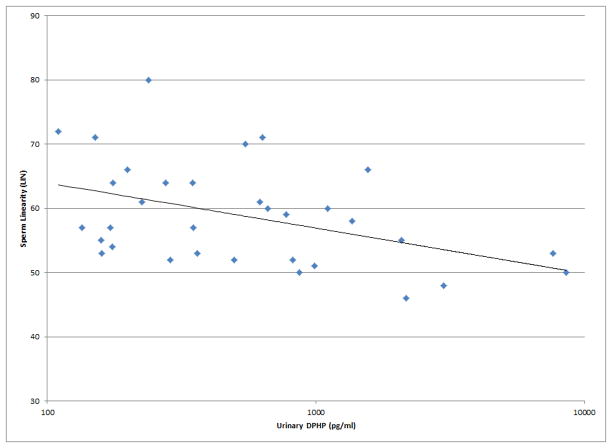
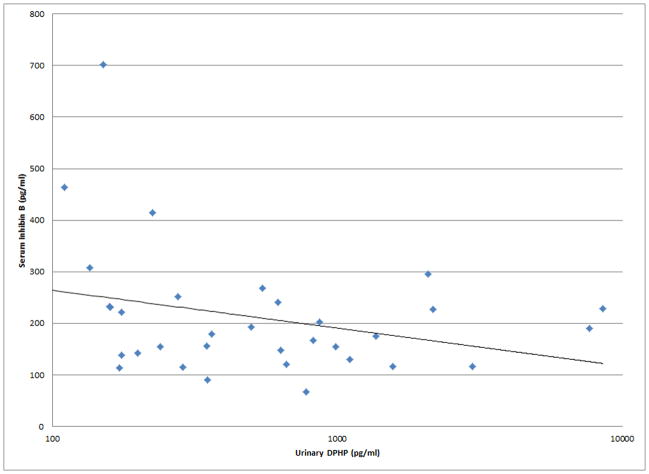
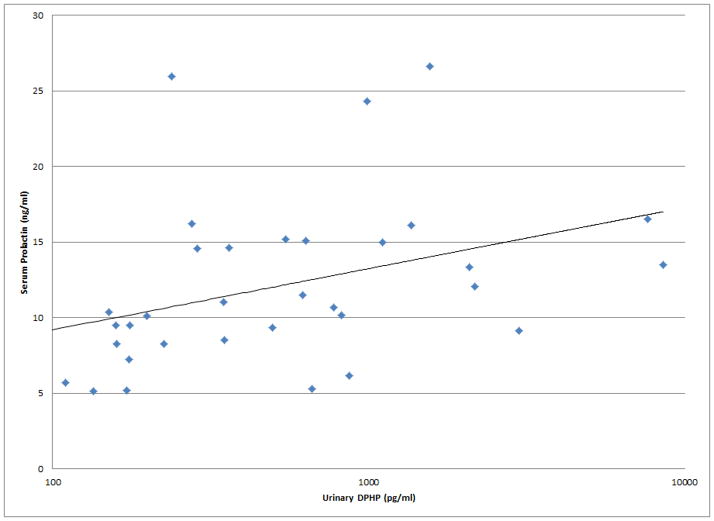
As shown in Figures 1 and 2, a number of moderately strong and statistically significant correlations were observed between the reproductive health measures and BDCPP (decreased sperm motility, VSL and LIN; increased TSH) or DPHP (sperm concentration, motility, VSL, LIN and inhibin b; increased prolactin). In multivariable models adjusted for age, BMI, time of day, and abstinence period (for semen quality and sperm motion parameter models only), many of these relationships remained (Table 1). Negative effect estimates for semen quality parameters were consistently large in relation to both urinary metabolites, although confidence intervals were wide. For BDCPP, an IQR increase in urinary metabolite concentration was associated with a suggestive (p=0.06) 15% decrease in sperm motility and a significant 37% decrease in sperm morphology. The negative relationship with LIN observed in the preliminary bivariate analysis was no longer statistically significant, though negative associations with sperm morphology and VCL, and a positive association with total T3, were found in the multivariable models. For DPHP, an IQR increase in urinary metabolite concentration was associated with a 57% decrease in sperm concentration and a 20% decrease in sperm motility. The positive association with prolactin observed in the preliminary analysis was confounded by time of day of sample collection and was no longer significant in the adjusted model. As with BDCPP, a positive association between DPHP and total T3 was observed in the multivariable analysis.
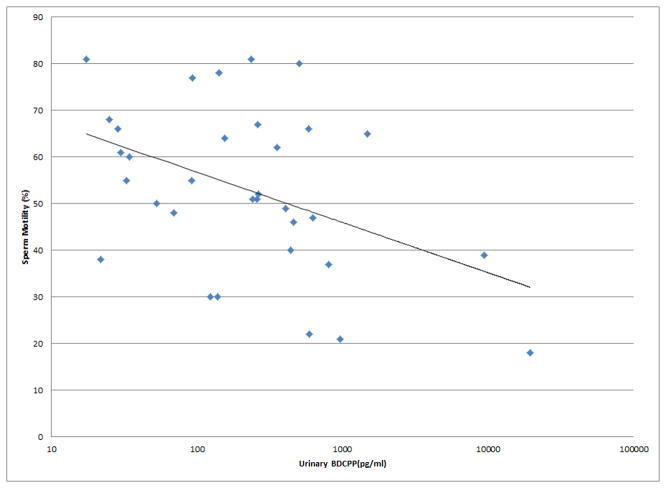
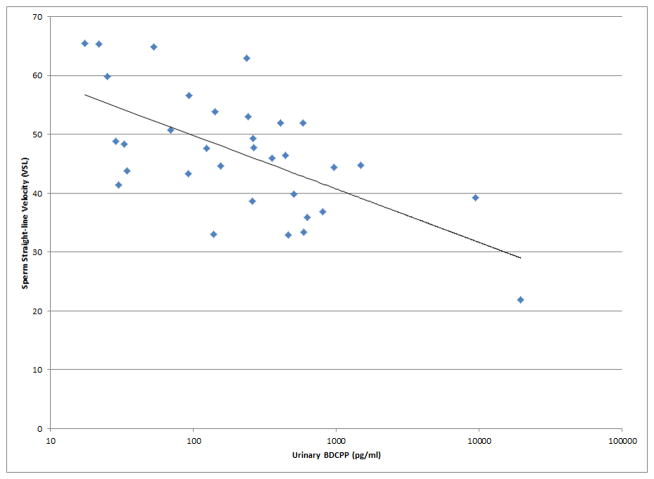
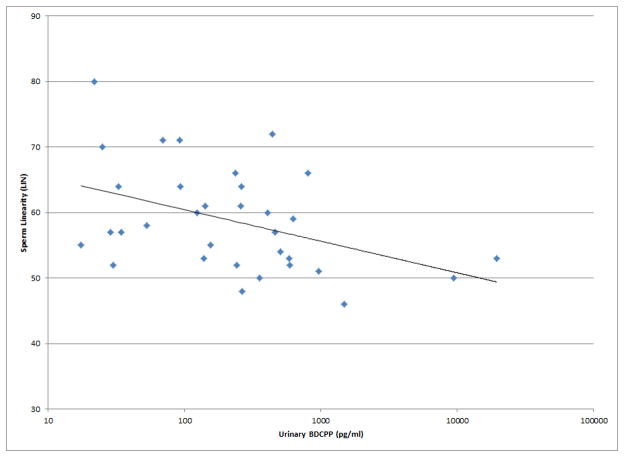
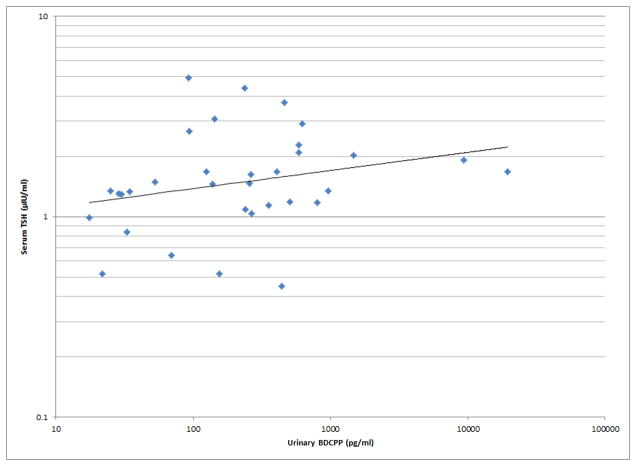
Figure 1.
Selected scatterplots of SG-corrected urinary BDCPP concentration in relation to a) sperm motility (rs = −0.38, p<0.05); b) sperm straight line velocity (rs = −0.57, p<0.01); c) sperm linearity (rs = −0.43, p<0.05); d) serum TSH (rs = 0.33, p<0.1).
Figure 2.
Selected scatterplots of SG-corrected urinary DPHP concentration in relation to a) sperm concentration (rs = −0.44, p<0.1); b) sperm motility (rs = −0.43, p<0.05); c) sperm straight line velocity (rs = −0.57, p<0.01); d) sperm linearity (rs = −0.44, p<0.05); e) serum inhibin b (rs = −0.28, p=0.2); f) serum prolactin (rs = 0.50, p<0.01).
Table 1.
Adjusteda regression coefficients (95% confidence intervals) for percent change (relative to population median) in semen quality parameter, sperm motion parameter, reproductive hormone level, or thyroid hormone level associated with an interquartile range (IQR) increase in specific gravity corrected BDCPP or DPHP concentration in urine (N=33).
| BDCPPb | DPHPb | |||
|---|---|---|---|---|
|
| ||||
| % Change (95% CI) | p-value | % Change (95% CI) | p-value | |
|
| ||||
| Sperm concentrationb | −27.3 (−57.5, 22.0) | 0.22 | −57.2 (−77.8, −18.8) | 0.01 |
| Sperm motility | −14.9 (−30.5, 0.8) | 0.06 | −20.3 (−41.1, 0.5) | 0.055 |
| Sperm morphology | −36.7 (−70.6, −3.1) | 0.03 | −28.1 (−76.1, 19.9) | 0.24 |
|
| ||||
| Straight-line velocity (VSL) | −18.7 (−27.8, −9.7) | 0.0002 | −19.0 (−32.7, −5.3) | 0.009 |
| Curvilinear velocity (VCL) | −14.1 (−24.1, −4.2) | 0.007 | −7.2 (−22.2, −7.8) | 0.33 |
| Linearity (LIN) | −5.1 (−11.5, 1.3) | 0.11 | −12.6 (−20.1, −5.2) | 0.002 |
|
| ||||
| FSHb | 7.2 (−15.8, 36.7) | 0.55 | 15.5 (−16.1, 59.0) | 0.35 |
| LHb | −2.4 (−20.0, 19.4) | 0.81 | 21.1 (−6.2, 56.5) | 0.14 |
| Inhibin B | −5.4 (−40.2, 29.5) | 0.76 | −37.6 (−82.2, 7.0) | 0.095 |
| Testosteronec | −2.2 (−13.2, 8.8) | 0.68 | 9.8 (−4.6, 24.3) | 0.18 |
| Estradiol | 5.0 (−18.2, 28.3) | 0.66 | 5.4 (−25.9, 36.6) | 0.73 |
| SHBGb | −9.5 (−25.8, 10.5) | 0.34 | −16.1 (−36.1, 10.1) | 0.20 |
| FAIb | 4.1 (−14.7, 27.0) | 0.68 | 27.1 (−0.6, 61.6) | 0.06 |
| Prolactinb | 6.2 (−13.0, 29.6) | 0.53 | 19.2 (−7.7, 56.5) | 0.18 |
|
| ||||
| Free T4 | 0.7 (−3.2, 4.7) | 0.70 | 3.9 (−1.0, 8.9) | 0.12 |
| Total T3 | 6.6 (1.6, 12.8) | 0.02 | 7.8 (−0.2, 15.8) | 0.054 |
| TSHb | 40.3 (11.4, 77.1) | 0.006 | 25.1 (−11.2, 77.6) | 0.19 |
Adjusted for age, BMI, and time of sample collection. Models for semen quality and sperm motion parameters additionally adjusted for abstinence period.
Variable ln-transformed in statistical analysis
Models for testosterone also adjusted for ln-transformed SHBG
Since urinary DPHP and house dust TPHP concentrations6 were both negatively associated with sperm concentration but not correlated with one another, a secondary analysis was conducted where both urinary DPHP concentrations and TPHP concentrations in house dust were included in the same model with the other covariates. Both exposure variables were statistically significant in this model, where the negative association with urinary DPHP was stronger than that for dust TPHP. An IQR increase in urinary DPHP was associated with a 56% (95% CI −76%, −22%; p=0.007) decrease in sperm concentration, whereas an IQR increase in dust TPHP was associated with a 20% (95% CI −33%, −4%; p=0.02) decrease in sperm concentration.
DISCUSSION
In the present exploratory study, we report a number of statistically significant or suggestive relationships between urinary biomarkers of two PFRs and markers of male reproductive health. While experimental toxicology studies of these chemicals in the peer-reviewed literature are limited, there is some laboratory evidence to support our findings. High doses of TDCPP and TPHP have been associated with histopathologic abnormalities of the male reproductive tract and reduced male fertility in rats.13, 14 More recently, both PFRs were found to cause changes in hormone levels through altered steroidogenesis or estrogen metabolism in zebrafish,15–17 and have been shown to alter thyroid signaling mechanisms in vitro and in vivo.17–19 We also found that PFR metabolite concentrations were much greater in urine samples collected in the afternoon compared to those collected in the morning, which may provide important clues for human exposure sources, patterns and toxicokinetics. For example, since the half-life of these chemicals is on the order of several hours, it may suggest that diet may be an important exposure pathway, that activity (e.g., leading to dust aerosolization) in the home may be an important contributor for exposure beyond simply spending time in the home (e.g., during sleep), or perhaps more likely that exposures outside the home (e.g., automobile or workplace) among these men may have been greater than those experienced while inside the home. However, more detailed time-activity data than were collected in this study would be needed in order to investigate these differences further.
Overall, the number, magnitude and strength of statistically significant or suggestive associations between urinary PFR metabolites and measures of male reproductive health were greater than our previous report assessing house dust PFR concentrations in relation to these outcomes in the same cohort.6 Despite the lack of correlation (r < 0.1) we recently reported between TPHP concentrations in house dust and DPHP concentrations in urine,7 in the present study we found that urinary DPHP was associated with decreased sperm concentration and increased serum prolactin (though the association with prolactin was confounded by time of day of sample collection since prolactin levels are also higher in samples collected in the afternoon20) in patterns consistent with our previous report on house dust concentrations of TPHP.6 Although peculiar, there may be several possible explanations for consistent relationships between these endpoints and two exposure assessment approaches that were not correlated with one another: 1) the significant results for either house dust or urinary biomarker concentrations (or both) are due to chance findings; 2) Both TPHP and DPHP are bioactive, and there may be other uses (e.g., as a plasticizer) and sources of DPHP besides TPHP in house dust,21 thus both measures may be associated with the same outcome measure but not with each other; 3) DPHP may be a metabolite of other chemicals in addition to TPHP (e.g., certain alkylated triphenyl phosphate isomers used as flame retardants, among others22) that may be associated with these endpoints; 4) Since TPHP exposure may come from sources or locations outside the home (e.g., offices, automobiles), perhaps the two measures reflect differing aspects of exposure and both were associated with substantial but differing error in relation to true exposure. The latter possible explanations may be supported by the observation that both house dust TPHP and urinary DPHP were significantly associated with sperm concentration when included in the same multivariable model. If possibility #4 were to in fact explain our observations, the true association between TPHP exposure and sperm concentration could be quite strong.
Likewise, for TDCPP/BDCPP, the significant associations we previously reported between house dust concentrations and free T4, FAI and prolactin, but lack of relationship between these measures and urinary metabolite concentrations reported here, may signify either chance associations with house dust or that house dust may be associated with less exposure measurement error when considering the exposure window of importance for these hormones (i.e., dust may represent a more stable, long-term measure of exposure if house dust is a primary source of exposure and dust may not be as susceptible to confounding by time of day of sample collection compared to urinary PFR metabolites). On the other hand, in the present study we report associations between urinary BDCPP and semen quality parameters, sperm motion parameters, total T3, and TSH that were not evident in our previous analysis of house dust. This too may reflect either chance findings, evidence that urinary biomarkers of PFRs may be associated with less error compared to dust when studying these outcome variables, or some combination of these and other possible explanations. As suggested above, it may also be possible that the two exposure measures capture different aspects of exposure and measurement error in consideration of overall health effects associated with true TDCPP/BDCPP exposure or biologically effective dose.
In conclusion, this brief report describes, as far as we are aware, the first human study of relationships between biomarkers of exposure to PFRs and male reproductive health. While the study was limited by a small sample size, our compelling results warrant further investigation in a larger study population. Additional studies on sources, pathways, and routes of PFR exposure, along with research on toxicokinetics and exposure measure utility, are also needed.
MATERIALS & METHODS
The present analysis was conducted among a subset of men participating in an ongoing study of environmental factors in reproductive health. Subject recruitment and participation has been described previously.4, 23 Briefly, men between 18 and 54 years of age were recruited from the Andrology Laboratory of Massachusetts General Hospital. Men included in this sub-study were enrolled in years 2003 and 2004. Exclusion criteria included prior vasectomy or current use of exogenous hormones. All men participating in the study provided written informed consent, and institutional review board approval was obtained from each participating institution.
A urine, serum and semen sample was collected from each participant at their enrollment clinic visit. Following the clinic visit, the men were given detailed instructions to send the collection bag from their home vacuum cleaner to study team in a pre-paid mailer. In the laboratory, after dust was obtained from the vacuum bags and passed through a 150 μm screen sieve, TDCPP and TPHP concentrations were measured using gas chromatography-mass spectrometry (GC/MS).4, 6 DBCPP and DPHP, metabolites of TDCPP and TPHP, respectively, were measured in the urine samples via atmospheric pressure chemical ionization liquid chromatography-tandem mass spectrometry (ACPI-LC/MS/MS).7, 24 Specific gravity (SG) was also measured in each urine sample using a digital handheld refractometer.
Semen samples were analyzed for sperm concentration and motion parameters [sperm motility, straight line velocity (VSL), curvilinear velocity (VCL), and linearity (LIN)] by a computer-aided semen analyzer as described previously (Meeker and Stapleton 2010; Meeker et al. 2011).6, 25 Sperm morphology was assessed using on two slides per specimen viamicroscope using an oil-immersion 100x objective.6, 25 Serum samples were analyzed for follicle-stimulating hormone (FSH), luteinizing hormone (LH), inhibin b, testosterone, estradiol, sex hormone binding globulin (SHBG), prolactin, free T4, total T3, and thyrotropin (TSH) using commercially available immunoassays, also described previously.6, 23 Finally, the free androgen index (FAI) was calculated as the molar ratio of total testosterone to SHBG.
Statistical analysis was conducted using SAS version 9.2 (SAS Institute, Inc., Cary, NC) in a manner consistent with our previous report on relationships between PFRs in dust and male reproductive health measures.6 Briefly, continuous variables were assessed for normality and proper transformations [e.g., natural log (ln)] were carried out prior to further statistical analysis. Preliminary bivariate relationships between variables were assessed by constructing scatter plots and calculating Spearman rank correlation coefficients (rs). We then utilized multivariable linear regression to assess relationships between urinary PFR metabolite concentrations and semen quality/sperm motion parameters and serum levels of reproductive/thyroid hormones while accounting for potential confounding variables (age, BMI, time of day of sample collection). To improve interpretability and to allow for comparisons with our earlier dust analysis, regression coefficients were expressed as a percent change in semen or hormone measure (relative to the population median) associated with an interquartile range (IQR) increase in urine PFR metabolite concentration.
References
- 1.USEPA; Agency USEP, editor. Furniture Flame Retardancy Partnership: Environmental Profiles of Chemical Flame-Retardant Alternatives for Low-Density Polyurethane Foam. Washington, DC: 2005. [Google Scholar]
- 2.Van den Eede N, Dirtu AC, Neels H, Covaci A. Analytical developments and preliminary assessment of human exposure to organophosphate flame retardants from indoor dust. Environment international. 2011;37:454–61. doi: 10.1016/j.envint.2010.11.010. [DOI] [PubMed] [Google Scholar]
- 3.van der Veen I, de Boer J. Phosphorus flame retardants: Properties, production, environmental occurrence, toxicity and analysis. Chemosphere. 2012;88:1119–53. doi: 10.1016/j.chemosphere.2012.03.067. [DOI] [PubMed] [Google Scholar]
- 4.Stapleton HM, Klosterhaus S, Eagle S, Fuh J, Meeker JD, Blum A, Webster TF. Detection of organophosphate flame retardants in furniture foam and U.S house dust. Environmental science & technology. 2009;43:7490–5. doi: 10.1021/es9014019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stapleton HM, Klosterhaus S, Keller A, Ferguson PL, van Bergen S, Cooper E, Webster TF, Blum A. Identification of flame retardants in polyurethane foam collected from baby products. Environmental science & technology. 2011;45:5323–31. doi: 10.1021/es2007462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Meeker JD, Stapleton HM. House dust concentrations of organophosphate flame retardants in relation to hormone levels and semen quality parameters. Environmental health perspectives. 2010;118:318–23. doi: 10.1289/ehp.0901332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Meeker JD, Cooper EM, Stapleton HM, Hauser R. Urinary metabolites of organophosphate flame retardants: temporal variability and correlations with house dust concentrations. Environmental health perspectives. 2013;121:580–5. doi: 10.1289/ehp.1205907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carignan CC, McClean MD, Cooper EM, Watkins DJ, Fraser AJ, Heiger-Bernays W, Stapleton HM, Webster TF. Predictors of tris(1,3-dichloro-2-propyl) phosphate metabolite in the urine of office workers. Environment international. 2013;55:56–61. doi: 10.1016/j.envint.2013.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Johnson PI, Stapleton HM, Sjodin A, Meeker JD. Relationships between polybrominated diphenyl ether concentrations in house dust and serum. Environmental science & technology. 2010;44:5627–32. doi: 10.1021/es100697q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stapleton HM, Eagle S, Sjodin A, Webster TF. Serum PBDEs in a North Carolina Toddler Cohort: Associations with Handwipes, House Dust, and Socioeconomic Variables. Environmental health perspectives. 2012;120:1049–54. doi: 10.1289/ehp.1104802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bergman A, Ryden A, Law RJ, de Boer J, Covaci A, Alaee M, Birnbaum L, Petreas M, Rose M, Sakai S, Van den Eede N, van der Veen I. A novel abbreviation standard for organobromine, organochlorine and organophosphorus flame retardants and some characteristics of the chemicals. Environment international. 2012;49:57–82. doi: 10.1016/j.envint.2012.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Armstrong B. Exposure measurement error: Consequences and design issues. In: Nieuwenhuijsen MJ, editor. Exposure Assessment in Occupational and Environmental Epidemiology. New York: Oxford University Press; 2004. pp. 181–200. [Google Scholar]
- 13.Latendresse JR, Brooks CL, Flemming CD, Capen CC. Reproductive toxicity of butylated triphenyl phosphate and tricresyl phosphate fluids in F344 rats. Fundam Appl Toxicol. 1994;22:392–9. doi: 10.1006/faat.1994.1044. [DOI] [PubMed] [Google Scholar]
- 14.NRC. Toxicological Risks of Selected Flame-Retardant Chemicals. Washington, DC: National Research Council, National Academies Press; 2000. [PubMed] [Google Scholar]
- 15.Liu X, Ji K, Choi K. Endocrine disruption potentials of organophosphate flame retardants and related mechanisms in H295R and MVLN cell lines and in zebrafish. Aquatic toxicology. 2012;114–115:173–81. doi: 10.1016/j.aquatox.2012.02.019. [DOI] [PubMed] [Google Scholar]
- 16.Liu X, Ji K, Jo A, Moon HB, Choi K. Effects of TDCPP or TPP on gene transcriptions and hormones of HPG axis, and their consequences on reproduction in adult zebrafish (Danio rerio) Aquatic toxicology. 2013;134–135:104–11. doi: 10.1016/j.aquatox.2013.03.013. [DOI] [PubMed] [Google Scholar]
- 17.Liu C, Wang Q, Liang K, Liu J, Zhou B, Zhang X, Liu H, Giesy JP, Yu H. Effects of tris(1,3-dichloro-2-propyl) phosphate and triphenyl phosphate on receptor-associated mRNA expression in zebrafish embryos/larvae. Aquatic toxicology. 2013;128–129:147–57. doi: 10.1016/j.aquatox.2012.12.010. [DOI] [PubMed] [Google Scholar]
- 18.Crump D, Chiu S, Kennedy SW. Effects of tris(1,3-dichloro-2-propyl) phosphate and tris(1-chloropropyl) phosphate on cytotoxicity and mRNA expression in primary cultures of avian hepatocytes and neuronal cells. Toxicological sciences : an official journal of the Society of Toxicology. 2012;126:140–8. doi: 10.1093/toxsci/kfs015. [DOI] [PubMed] [Google Scholar]
- 19.Wang Q, Liang K, Liu J, Yang L, Guo Y, Liu C, Zhou B. Exposure of zebrafish embryos/larvae to TDCPP alters concentrations of thyroid hormones and transcriptions of genes involved in the hypothalamic-pituitary-thyroid axis. Aquatic toxicology. 2013;126:207–13. doi: 10.1016/j.aquatox.2012.11.009. [DOI] [PubMed] [Google Scholar]
- 20.Meeker JD, Singh NP, Hauser R. Serum concentrations of estradiol and free T4 are inversely correlated with sperm DNA damage in men from an infertility clinic. Journal of andrology. 2008;29:379–88. doi: 10.2164/jandrol.107.004416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Makiguchi K, Satoh T, Kakuchi T. Diphenyl Phosphate as an Efficient Cationic Organocatalyst for Controlled/Living Ring-Opening Polymerization of delta-Valerolactone and epsilon-Caprolactone. Macromolecules. 2011;44:1999–2005. [Google Scholar]
- 22.Stapleton HM, Sharma S, Getzinger G, Ferguson PL, Gabriel M, Webster TF, Blum A. Novel and high volume use flame retardants in US couches reflective of the 2005 PentaBDE phase out. Environmental science & technology. 2012;46:13432–9. doi: 10.1021/es303471d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Meeker JD, Godfrey-Bailey L, Hauser R. Relationships between serum hormone levels and semen quality among men from an infertility clinic. Journal of andrology. 2007;28:397–406. doi: 10.2164/jandrol.106.001545. [DOI] [PubMed] [Google Scholar]
- 24.Cooper EM, Covaci A, van Nuijs AL, Webster TF, Stapleton HM. Analysis of the flame retardant metabolites bis(1,3-dichloro-2-propyl) phosphate (BDCPP) and diphenyl phosphate (DPP) in urine using liquid chromatography-tandem mass spectrometry. Analytical and bioanalytical chemistry. 2011;401:2123–32. doi: 10.1007/s00216-011-5294-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Meeker JD, Yang T, Ye X, Calafat AM, Hauser R. Urinary concentrations of parabens and serum hormone levels, semen quality parameters, and sperm DNA damage. Environmental health perspectives. 2011;119:252–7. doi: 10.1289/ehp.1002238. [DOI] [PMC free article] [PubMed] [Google Scholar]