Abstract
Mitochondrial dysfunction has been recognized as a significant cause of a number of serious multi-organ diseases. Tissues with a high metabolic demand such as brain, heart, muscle, CNS are often affected. Mitochondrial disease can be due to mutations in mitochondrial DNA (mtDNA) or in nuclear genes involved in mitochondrial function. There is no curative treatment for patients with mitochondrial disease. Given the lack of treatments and the limitations of prenatal and preimplantation diagnosis, attention has focused on prevention of transmission of mitochondrial disease through germline gene replacement therapy. Since mtDNA is strictly maternally inherited, two approaches have been proposed. In the first, the nuclear genome from the pronuclear stage zygote of an affected woman is transferred to an enucleated donor zygote. A second technique involves transfer of the metaphase II spindle from the unfertilized oocyte of an affected woman to an enucleated donor oocyte. Our group recently reported successful spindle transfer between human oocytes resulting in blastocyst development and embryonic stem cell derivation, with very low levels of heteroplasmy. In this review, we summarize these novel assisted reproductive techniques and their use to prevent transmission of mitochondrial disorders. The promises and challenges are discussed, focusing on their potential clinical application.
Keywords: mitochondria, nuclear transfer, gene replacement
Mitochondrial DNA Mutations and Human Disease
The mitochondria are intracellular organelles that provide an essential supply of cellular energy in the form of ATP generated via oxidative phosphorylation. Mitochondrial disease can be due to mutation in mtDNA or mutations in nuclear DNA involved in mitochondrial function. In addition, there is increasing evidence that acquired mtDNA mutations are involved in several chronic age-related diseases such as diabetes, cardiovascular disease, and Parkinson's Disease. (For review see 1)
The true prevalence of mtDNA disease is unknown. However, it is estimated that about 1 in 4,000 children are born in the U.S. with an inherited with mitochondrial disease. (2) Mitochondrial disease often affects high energy requiring tissues such as brain, muscle, liver, heart, kidney, and the CNS. These diseases are clinically heterogeneous but symptoms may include deafness, blindness, diabetes, muscle weakness, heart, kidney, and liver failure. There are a number of well-defined clinical syndromes. But, many patients do not fall into easily defined clinical groups.
The mitochondrial genome contains only 37 genes and mtDNA is maternally inherited. Each cell contains thousands of copies of mtDNA. Normal individuals are homoplasmic, that is all the mtDNA copies are identical. However, mitochondrial mutations may be either homoplasmic, in which all copies are mutated, or heteroplasmic, where the individual contains a mixture of mutated and wild-type DNA. Patients affected by mtDNA disease are usually heteroplasmic. Their tissues and cells have a mixture of wild-type and mutant mtDNA. The clinical phenotype depends on the ratio of mutated to wild-type mtDNA in affected cells and tissues. There is a threshold effect; that is, the level of abnormal mtDNA that causes mtDNA disease. This threshold varies by tissue and mutation type, but is usually in the range of 60–90%.
Treatment options are generally limited. Hence, preventive interventions that eliminate the likelihood of transmission of maternally inherited mitochondrial disease to offspring are being actively pursued.
Reproductive Options for Preventing Transmission of Mitochondrial Disease
The transmission of mtDNA is complex and poorly understood. The transmission of heteroplasmic mtDNA is complicated by selective genome replication and genetic bottleneck, resulting in marked variation in the levels of mutated mtDNA among the offspring of heteroplasmic mothers. (3) A woman with a low level of mtDNA heteroplasmy could transmit much higher levels to her children through the phenomenon known as the mitochondrial bottleneck.
Genetic counseling is important to explain the genetic risks involved in spontaneous or assisted reproduction and the limits of prenatal and preimplantation testing. Preimplantation genetic diagnosis (PGD) have limited efficacy for mtDNA disease because of the uncertainty in predicting disease due to heteroplasmy and genetic bottleneck. PGD for mtDNA disease has been reported, (4–7) however, concerns remain if mutation loads detected in biopsied blastomeres or trophectoderm accurately represent the entire embryo. There are also uncertainties about correlation between mutation load and disease expression and severity. Another concern is that PGD may only reduce, but not eliminate, the risk of transmitting abnormal mtDNA that may lead to mitochondrial disease in subsequent generations.
Furthermore, PGD is not applicable to patients with homoplasmic mutations or high levels of heteroplasmy. For women with homoplasmic or high levels of heteroplasmic mtDNA mutations, currently the only option to ensure an unaffected child is whole oocyte donation. However, oocyte donation has the limitation of not maintaining the genetic link to the mother.
The limitations of PGD and whole oocyte donation have led to the search for alternative approaches to prevent mitochondrial disease transmission. These approaches involve the exchange of mitochondrial genome between gametes or embryos.
Cytoplasmic transfer was first proposed as a treatment for patients with infertility. Cytoplasmic transfer involves the transfer of a small portion of ooplasm, and hence mtDNA, from one oocyte to another. In 1997, Cohen et al reported the first cytoplasmic transfer in humans resulting in pregnancies. (8) This approach would likely not prevent the transmission of mitochondrial disease, since it does not remove the mutated mtDNA, but rather adds donor mitochondria, creating a heteroplasmic oocyte with both mitochondrial haplotypes. Moreover, the amount of healthy mtDNA that is transferred is relatively small.
Two other promising approaches have emerged more recently. With either method, any resulting child would inherit nuclear genetic material from both parents, while the mtDNA would be derived largely or perhaps exclusively from the oocyte provided by the healthy donor. These methods could avoid mitochondrial disease not just in the resulting child, but also in subsequent generations.
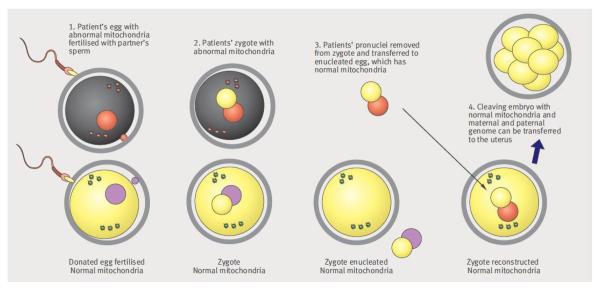
One of these approaches, termed pronuclear transfer, involves removal of both pronuclei from a zygote containing mtDNA mutations and transfer to the perivitelline space of a donated enucleated zygote. The pronuclei enclosed in a karyoplast are fused with the enucleated zygote by electric pulses or inactivated hemagglutinating virus of Japan (HVJ). The reconstructed zygote would contain the nuclear DNA material from one zygote, and cytoplasm and mtDNA predominantly from the other. (Fig 1) This has been successfully accomplished in the mouse model resulting in birth of normal offspring. (9)
Figure 1.
Pronuclear transfer technique*
*Image reproduced from Bredenoord AL and Braude P. Ethics of mitochondrial gene replacement: from bench to bedside. BMJ 2010;341.
Craven et al recently reported pronuclear transfer in human zygotes that were deemed abnormally fertilized. (10) They transferred pronuclei from one zygote into another enucleated zygote. These abnormally fertilized embryos were donated by patients undergoing in-vitro fertilization (IVF) for fertility treatment. Of the reconstructed embryos, 8.3% developed to the blastocysts stage. Genotype analysis revealed a low mtDNA carryover rate of <2%. But, the degree of carryover varied among blastomeres.
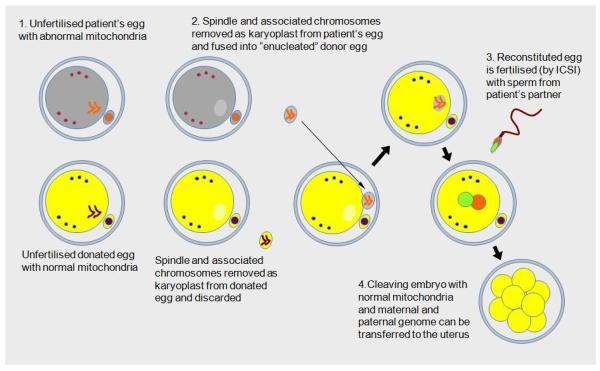
An alternative approach, termed spindle transfer, uses micromanipulation techniques to transfer the nuclear genetic material (the spindle with maternally-derived chromosomes attached) from one unfertilized oocyte to another from which its own nuclear material has been removed. (Fig 2) The reconstituted oocyte is then fertilized to allow embryo development.
Figure 2.
Spindle transfer technique*
*Image reproduced from www.hfea.gov.uk/6372.html
Recently, Tachibana et al demonstrated the first successful generation of healthy offspring by spindle transfer in the nonhuman primate model. (11) The spindles were isolated and transferred into enucleated mature oocytes with minimal mtDNA carryover. The reconstructed oocytes were fertilized and four healthy offspring were born with less than 1% carryover mtDNA. Spindle transfer was also successfully carried out after oocyte cryopreservation resulting in the birth of healthy monkey offspring. (12)
Using a similar approach, we also recently reported successful spindle transfer between human MII oocytes resulting in blastocyst development and embryonic stem cell (ESCs) derivation, with very low levels of heteroplasmy. (12) Although there was a slightly higher rate of abnormal fertilization, the remaining embryos developed to blastocyst and yielded ESCs similar to control embryos and had normal euploid karyotypes with exclusively donor mtDNA (<1% mtDNA carryover).
A follow-up study by Paull et al also demonstrated maternal spindle transfer with human oocytes, although these were parthenogenically activated rather than fertilized. (13) Abnormal oocyte activation was prevented using cooling. The mtDNA carryover rate was < 1%. ESCs and their differentiated phenotypes showed normal mitochondrial function.
The long-term safety and efficacy of these techniques in humans is unknown and further clinical research is necessary. However, animal studies including in nonhuman primates are encouraging and no abnormalities were seen during long-term follow up observations to adulthood. (12) The safety issues of these techniques include reducing the amount of mutated mtDNA carryover, methods to avoid abnormal fertilization and possible nuclear-to-mtDNA incompatibility concerns.
As mentioned above, both these techniques are associated with some degree of mtDNA carryover during spindle or pronuclear transfer, and thus, possible persistence of mutated mtDNA. On average, 1% of mitochondrial heteroplasmy was detected in monkey and human spindle transfer embryos and offspring. (11–13) In human pronuclear transfer embryos, up to 2% of carryover was observed. Furthermore, a considerable amount of mitochondrial heteroplasmy was observed among blastomeres of the same embryo. (10)
In the majority of mitochondrial diseases, the threshold of mutated to wild-type DNA that must be reached before clinical features are observed is high. Thus, low levels of mutant mtDNA transferred during transplantation are unlikely to cause disease. Any early segregation of a very low level of mutant mtDNA is unlikely to be a problem for children born as a result of maternal spindle or pronuclear transfer. There is a slight risk however, that the mutated genotype may segregate to specific tissues or the germline affecting future generations. There is potential concern for subsequent generations given that a female child born after these techniques may have a proportion of oocytes with a significant level of mutated mtDNA due to mitochondrial bottleneck.
It will be important to determine the safety of these techniques with regard to embryo development. While studies in animals are reassuring, the Craven study noted approximately 50% reduced embryo development following pronuclear transfer. (10) As noted, these studies were performed using abnormally fertilized embryos. Thus, further study using normally fertilized embryos is warranted. Similarly, while studies of spindle transfer in the rhesus monkey are reassuring, the human studies revealed a higher rate of abnormal fertilization in the spindle transfer embryos compared to control embryos. (11,12) Despite this however, the yield of normal, euploid spindle transfer blastocysts per ovarian stimulation cycle, was sufficient to be clinically useful.
Also, visualization of the spindle requires exposure to polarized light birefringence, the safety of which is unknown. In addition, the occurrence of aberrant chromosome segregation at metaphase II during oocyte maturation is increased in aged oocytes. (14) Therefore, techniques must be developed to minimize the risk of aneuploidy. Both techniques require the use of reversible cytoskeletal inhibitors and hemagglutinating virus of Japan, the safety of which has not been rigorously tested in human oocytes or embryos. Tachibana et al demonstrated no detectable viral genome in both derived ESCs and in placental tissue of spindle transfer offspring. (11)
There are logistical challenges as well. Both pronuclear and spindle transfer require synchronization of ovarian stimulation of the affected woman and donor so that the oocyte retrievals occur on the same day. Due to individual variation in response to ovarian stimulation, this may be challenging. Therefore, the efficacy of the techniques using cryopreserved oocytes and embryos will have to be considered.
Vitrification of oocytes may offer a solution. Tachibana et al showed that in the rhesus spindle transfer model, the cytoplasm is more sensitive to vitrification-induced damage than the spindle. (12) Paull et al found that they could freeze isolated karyoplasts and use these in maternal spindle transfer after thawing. (13) These findings suggest that fresh cytoplast is essential for successful spindle transfer, and that vitrified oocytes from carriers of mtDNA mutations can be used for spindle transfer in a clinical setting.
Concern has also been raised about potential incompatibility between the nuclear and mtDNA genomes. Studies in intraspecies crossing in insects or inbred mice indicate that foreign mitochondrial genotypes can affect expression of nuclear genes and health outcomes. (15) While any new technique is associated with some risk, we believe that the lack of any evidence of mitochondrial-nuclear “mismatch” within heterogeneous species like humans as a cause of disease is reassuring. Furthermore, there are now multiple reports of the health status of the offspring born after “unmatched” mitochondrial replacement in macaques and all have shown no difference between these offspring and controls suggesting that harmful interactions are unlikely to occur in humans. (11,12,16) However, since deleterious effects of mitochondrial gene replacement may not be evident until later into adulthood, long term follow up is essential.
Assisted reproductive technology techniques have been associated with an increased incidence of epigenetic abnormalities such as Beckwith-Wiedemann Syndrome and Angelman Syndrome. (17) The question of whether nuclear transfer techniques increase the risk of epigenetic abnormalities is unknown. Studies in animal models so far are reassuring.
Ethical Issues and Future Research
Mitochondrial gene replacement raises a number of ethical concerns. (18) Although the results with the two techniques are promising, further research needs to be done with regards to safety and efficacy before application to clinical practice. Nonhuman primate (NHP) research is necessary due to ethical challenges of conducting reproductive research in humans and concern about the safety of new reproductive technologies and their impact on further generations. NHP models have been valuable preclinical experimental systems for assessing novel assisted reproductive techniques in reproductive medicine. Furthermore, primate ESCs are important for understanding the mechanisms of stem cell differentiation and are valuable for understanding the therapeutic potential of differentiated human ESC lines.
Mitochondrial gene replacement is especially challenging because the technique involves modification of the germline and modifications would be transmitted to subsequent generations. Reproductive research is unique in that although the patient undergoes the intervention, the potential risk is to the offspring. Although results in NHPs are reassuring, some effects may not manifest for many years.
In vitro research using human embryos is controversial, as are the creation of embryos specifically for research, and the financial compensation of oocyte donors. One advantage of spindle transfer over pronuclear transfer is that donor oocytes need not be fertilized, which would avoid the creation and destruction of embryos for the sole purpose of medical treatment.
It is critical to continue studies in animal models and human tissues in vitro to provide further safety information on nuclear transfer techniques. More research is needed to understand how mitochondrial bottleneck and segregation occur in humans. It will also be necessary to study human ESC lines generated by mtDNA replacement (12) to confirm the lack of incompatibility between the nuclear and mtDNA genomes. In addition, it will be necessary to analyze epigenetic and gene expression normalcy in human blastocysts and ESCs derived by mtDNA transfer. Further studies in vitrified human oocytes will also need to be conducted.
Although extensive animals and preclinical human studies are important, clinical trials are essential. There may be important differences between human and macaque oocytes and the macaque may not be a fully predictable model for the human. Without some risk, several assisted reproductive technology innovations such as IVF, ICSI, and PGD would never have come to fruition. These techniques should only be used to avoid serious mitochondrial disease in carefully selected patients. Patients and oocyte donors must provide informed consent. And, it will be important that the children born following mitochondrial replacement be followed long term to monitor any effects on children born and future generations.
The Nuffield Council on Bioethics in the United Kingdom concluded that if mitochondrial gene replacement techniques proved to be acceptable safe, research and use would be ethical to use conducted under appropriate regulatory oversight (http://www.nuffieldbioethics.org/…/Mitochondrial_DNA_disorders_summary_web.pdf). Rigorous review by an institutional research board and embryonic stem cell oversight committees is absolutely warranted. Adequate informed consent, long term follow up of resulting offspring, and a transparent public process are essential.
Legislation regarding embryo research varies considerably between different countries. In the UK, the Secretary of State for Health recently commissioned the Human Fertilization & Embryology Authority (HFEA) to convene an expert scientific panel to review methods to prevent mitochondrial disease. The report on the assessment of the safety and efficacy of mitochondrial replacement can be found at www.hfea.gov.uk/6372.html. HFEA found broad public support for mitochondrial replacement. The panel concluded that the techniques of maternal spindle transfer and pronuclear transfer are potentially useful for a group of patients whose offspring have severe or lethal genetic mtDNA disease. The panel urged that additional research be undertaken to provide further data on efficacy and safety of these techniques before their clinical use. The panel also recommended long term follow-up of any children born as a result of these techniques. In June 2013, the UK government announced its decision to proceed with the draft regulation, which will enable the use of mitochondrial replacement techniques to be used for patient treatment.
Although no federal law bans human embryo research in the United States, there are restrictions on funding. Federal funding, under the Dickey-Wicker amendment, prohibits the creation of human embryos for research purposes or research in which a human embryo is harmed or destroyed. Several states, such as California and New York, provide funding support for embryonic stem cell research. However, some states, such as California, ban compensation of oocyte donors for research. Funding and regulatory barriers pose the risk that the U.S. will lag behind other countries such as the UK in this important area of research and novel therapeutics.
REFERENCES
- 1.Taylor RW, Turnbull DM. Mitochondrial DNA mutations in human disease. Nat Rev Genet. 2005;6:389–402. doi: 10.1038/nrg1606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schaefer AM, Taylor RW, Turnbull DM, Chinnery PF. The epidemiology of mitochondrial disorders--past, present and future. Biochim Biophys Acta. 2004;1659:115–20. doi: 10.1016/j.bbabio.2004.09.005. [DOI] [PubMed] [Google Scholar]
- 3.Brown DT, Samuels DC, Michael EM, Turnbull DM, Chinnery PF. Random genetic drift determines the level of mutant mtDNA in human primary oocytes. Am J Hum Genet. 2001;68:533–6. doi: 10.1086/318190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Steffann J, Frydman N, Gigarel N, Burlet P, Ray PF, Fanchin R, Feyereisen E, Kerbrat V, Tachdgian G, Bonnefont JP, Frydman R, Munnich A. Analysis of mtDNA variant segregation during early human embryonic development: a tool for successful NARP preimplantation diagnosis. J Med Genet. 2006;43:244–247. doi: 10.1136/jmg.2005.032326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Steffann J, Gigarel N, Corcos J, Bonniere M, Encha-Razavi F, Sinico M, Prevot S, Dumez Y, Yamgnane A, Frydman R, Munnich A, Bonnefont JP. Stability of the m.8993T->G mtDNA mutation load during human embryofetal development has implications for the feasibility of prenatal diagnosis in NARP syndrome. J Med Genet. 2007;44:664–669. doi: 10.1136/jmg.2006.048553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thorburn DR, Wilton L, Stock-Myer S. Healthy baby girl born following preimplantation genetic diagnosis for mitochondrial DNA m.8993T>G mutation. Mol Genet Metab. 2009;98:5–6. [Google Scholar]
- 7.Treff NR, Campos J, Tao X, Levy B, Ferry KM, Scott RT., Jr. Blastocyst preimplantation genetic diagnosis (PGD) of a mitochondrial DNA disorder. Fertil Steril. 2012;98:1236–40. doi: 10.1016/j.fertnstert.2012.07.1119. [DOI] [PubMed] [Google Scholar]
- 8.Cohen J, Scott R, Schimmel T, Levron J, Willadsen S. Birth of infant after transfer of anucleate donor oocyte cytoplasm into recipient eggs. Lancet. 1997;350:186–7. doi: 10.1016/S0140-6736(05)62353-7. [DOI] [PubMed] [Google Scholar]
- 9.McGrath J, Solter D. Nuclear transplantation in the mouse embryo by microsurgery and cell fusion. Science. 1983;220:1300–2. doi: 10.1126/science.6857250. [DOI] [PubMed] [Google Scholar]
- 10.Craven L, Tuppen HA, Greggains GD, Harbottle SJ, Murphy JL, Cree LM, et al. Pronuclear transfer in human embryos to prevent transmission of mitochondrial DNA disease. Nature. 2010;465:82–5. doi: 10.1038/nature08958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tachibana M, Sparman M, Sritanaudomchai H, Ma H, Clepper L, Woodward J, et al. Mitochondrial gene replacement in primate offspring and embryonic stem cells. Nature. 2009;461:367–72. doi: 10.1038/nature08368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tachibana M, Amato P, Sparman M, Woodward J, Sanchis DM, Ma H, et al. Towards germline gene therapy of inherited mitochondrial diseases. Nature. 2013;493:627–31. doi: 10.1038/nature11647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Paull D, Emmanuele V, Weiss KA, Treff N, Stewart L, Hua H, et al. Nuclear genome transfer in human oocytes eliminates mitochondrial DNA variants. Nature. 2013;493:632–7. doi: 10.1038/nature11800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Battaglia DE, Goodwin P, Klein NA, Soules MR. Influence of maternal age on meiotic spindle assembly in oocytes from naturally cycling women. Hum Reprod. 1996;11:2217–22. doi: 10.1093/oxfordjournals.humrep.a019080. [DOI] [PubMed] [Google Scholar]
- 15.Reinhardt K, Dowling DK, Morrow EH. Medicine. Mitochondrial replacement, evolution, and the clinic. Science. 2013;341:1345–6. doi: 10.1126/science.1237146. [DOI] [PubMed] [Google Scholar]
- 16.Lee HS, Ma H, Juanes RC, Tachibana M, Sparman M, Woodward J, Ramsey C, Xu J, Kang EJ, Amato P, Mair G, Steinborn R, Mitalipov S. Rapid mitochondrial DNA segregation in primate preimplantation embryos precedes somatic and germline bottleneck. Cell Rep. 2012;1:506–15. doi: 10.1016/j.celrep.2012.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Manipalviratn S, DeCherney A, Segars J. Imprinting disorders and assisted reproductive technology. Fertil Steril. 2009;91:305–15. doi: 10.1016/j.fertnstert.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bredenoord AL, Braude P. Ethics of mitochondrial gene replacement: from bench to bedside. BMJ. 2010;341:c6021. doi: 10.1136/bmj.c6021. [DOI] [PubMed] [Google Scholar]