Abstract
Binge drinking during adolescence and adulthood may have differential long-term effects on the brain. We investigated the long-term effects of chronic intermittent ethanol (CIE) exposure during adolescence and adulthood on impulsivity and anxiety-like behavior. Adolescent (adolescent-exposed) and adult (adult-exposed) rats were exposed to CIE/water on postnatal days (PND) 28-53 and PND146-171, respectively, and a 4-day ethanol/water binge on PND181-184 and PND271-274, respectively. During withdrawal from CIE and 4-day binge exposures, anxiety-like behavior and arousal were measured in the light-potentiated startle (LPS) and the acoustic startle (ASR) procedures, respectively. Impulsive choice was evaluated in the delay discounting task (DDT) at baseline and after ethanol challenges. Independent of age, ASR and LPS were decreased during withdrawal from CIE exposure. In contrast, LPS was increased in adult-exposed, but not adolescent-exposed, rats during withdrawal from the 4-day ethanol binge. CIE exposure had no effect on preference for the large delayed reward at baseline, independent of age. During DDT acquisition, CIE-exposed, compared with water-exposed rats, omitted more responses, independent of age, suggesting CIE-induced disruption of cognitive processes. Ethanol challenges decreased preference for the large reward in younger adolescent-exposed rats but had no effect in older adult-exposed rats independent of previous CIE/water exposure. Taken together, the present studies demonstrate that CIE withdrawal-induced decreases in anxiety and arousal were not age-specific. CIE exposure had no long-term effects on baseline impulsive choice. Subsequent ethanol exposure produced age-dependent effects on impulsivity (increased impulsivity in younger adolescent-exposed rats) and anxiety-like behavior (increased anxiety-like behavior in older adult-exposed rats).
Keywords: impulsivity, acoustic startle reflex, light-potentiated startle, ethanol withdrawal, acute ethanol challenge, delay discounting, adolescence
1. Introduction
The high level of alcohol binge drinking early in life (17% among adolescents aged 12-20 years and 40% among young adults aged 18-25 years) remains an important public health concern [1]. Younger drinkers (<25 years), categorized as type 2 alcoholics based on age of drinking onset and personality type [2], are characterized by high levels of impulsivity and novelty seeking and low levels of harm avoidance. In contrast, type 1 alcoholics (>25 years) start heavy drinking later in life and show low levels of novelty seeking and high levels of harm avoidance. Anxiety-like behavior is another behavioral trait that may be affected by drinking (for review, see [3]). Differences in personality traits including impulsivity and anxiety between type 1 and type 2 alcoholics may precede drinking or may result from heavy alcohol use during either adolescence or adulthood. However, studies investigating the long-term consequences of drinking during adolescence or adulthood on impulsivity and anxiety-related behaviors are very limited.
High self-reported impulsivity has been documented in alcohol-dependent adults [4], adolescents [5], and college students [6]. A small number of studies have investigated the impact of drinking on multiple aspects of impulsive behavior in laboratory settings. The delay discounting task (DDT) is commonly used in both human and animal studies to measure impulsive choice [7]. In this task, impulsivity is defined and measured as the preference for a smaller immediate reward over a larger delayed reward [8, 9]. Increased impulsive choice has been reported in adult abstinent alcoholics and heavy drinkers compared with light drinkers and control subjects [10-12]. Acute alcohol intoxication decreased impulsive choice in healthy undergraduate students [13], but increased impulsive choice in healthy adults [14] and non-dependent alcohol drinkers in a laboratory [15, 16] or bar setting [17] suggesting age-dependent effects of acute ethanol on impulsive choice. In experimental animals without a history of ethanol exposure, an acute ethanol challenge increased impulsive choice in non-selected adult rats [18-21] and in rats and mice bred for high, but not low ethanol drinking [22, 23]; but see [24]. However, the long-term effects of binge ethanol exposure during adolescence or adulthood on impulsive choice during adulthood have been largely unexplored.
Increased anxiety and an enhanced startle response are often associated with ethanol withdrawal [25-28]. In contrast to the majority of physical ethanol withdrawal symptoms that usually disappear within a few days, increased anxiety may last for months and even years, resulting in relapse to drinking [26, 29]. In both humans and animals, anxiety-like behavior can be assessed in the light-potentiated startle (LPS) procedure, in which startle responses are measured in successive sessions, during which the startle chambers are either dark or brightly lit. The startle response is potentiated by the aversive bright light in rodents. The degree to which light enhances startle reactivity is used as an operational measure of anxiety, and this response is selectively reduced by anxiolytic compounds [30, 31]. Work in our laboratory has shown that LPS was increased during spontaneous nicotine withdrawal in rats [32]. To the best of our knowledge, no studies have assessed LPS during ethanol withdrawal in rats with or without a history of previous ethanol exposure.
The aim of the present work was to investigate impulsive choice and anxiety-like behavior in rats exposed to chronic intermittent ethanol (CIE) during either adolescence or adulthood. To ensure ethanol exposure during the developmentally sensitive adolescent period, male Wistar rats were exposed to CIE throughout adolescence (PND 28-53), broadly defined from postnatal day (PND) 28 to PND42 or PND60 in males [33]. Adult rats were exposed to an identical CIE regimen during adulthood (PND146-171) to determine whether the effects of CIE exposure on impulsivity and anxiety are specific to ethanol exposure during adolescence. CIE exposure consisted of ethanol binges for two consecutive days at 48-h intervals of abstinence for 25 days. Similar intermittent ethanol administration regimens produced inflammatory brain damage and long-term alterations on cognitive and motor function [34] and resulted in tolerance to the hypnotic effects of an ethanol challenge in rats during adulthood [35]. Thus, we hypothesized that CIE exposure during adolescence or adulthood would have long-lasting effects on impulsivity and anxiety. The effects of CIE exposure on impulsivity were assessed using the DDT. We assessed impulsive choice under baseline conditions and after acute ethanol challenges in adult rats exposed to CIE during adolescence or adulthood. Anxiety-like behavior was assessed in the LPS procedure. This procedure also provides measures of the acoustic startle response (ASR). The ASR has been used to characterize arousal during ethanol withdrawal in both humans [25, 36] and rodents [37, 38]. LPS and ASR were assessed in both adult and adolescent rats during withdrawal from CIE exposure as well as during withdrawal from a 4-day ethanol binge in adulthood. Both CIE and 4-day ethanol binge exposures produced an average blood ethanol concentration (BEC) of 300 mg/dl, mimicking heavy alcohol use in humans [39].
2. Materials and methods
2.1. Animals
Two cohorts of 12 pregnant female Wistar rats (Charles River, Raleigh, NC, USA) arrived in the laboratory on gestational day 13. Male rats were weaned from each litter at 21 days of age (PND21; average body weight, 83.7±1.8 g), assigned to the adolescent experimental groups (water and CIE) and tested in two cohorts (n=50 total). Another 26 adult male Wistar rats (Charles River, Raleigh, NC, USA) arrived in the laboratory on PND 134 (average body weight, 361.4±4.2 g) and were assigned to the adult experimental groups (water and CIE). The experimental design is described below and presented in Figure 1. All of the rats were pair-housed and maintained in a humidity- and temperature-controlled vivarium under a reverse 12 h/ 12 h light/dark cycle (light off at 8:00 AM). Food and water were available ad libitum except during training and testing in the DDT. During behavioral training and testing in the DDT, the rats were food-deprived and received from 16 to 20 g/rat/day of food chow, including food pellets obtained during behavioral testing. The rats were fed 1 h after the experimental session. Training and testing occurred during the dark phase of the light/dark cycle. All of the experiments were in accordance with the guidelines of the American Association for the Accreditation of Laboratory Animal Care and National Research Council's Guide for Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee.
Figure 1.
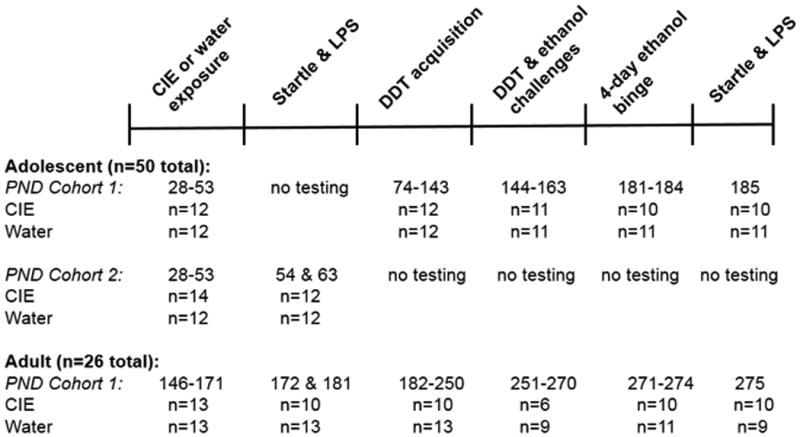
Diagram of experimental design that shows the sequence of exposure to ethanol, behavioral testing and the number of rats included in each experiment. See text for details regarding excluding rats from the statistical analyses or experiments. CIE, chronic intermittent ethanol; LPS, light-potentiated startle; ASR, acoustic startle response; DDT, delayed discounting task; PND, postnatal day; n, number of rats. See text for details.
2.2. Chronic intermittent ethanol, ethanol challenge, and 4-day ethanol binge exposures
Adolescent (PND28-53) and adult (PND146-171) rats were exposed to CIE or water administered intragastrically (IG) using stainless steel gavage needles (Roboz Surgical Instruments, Gaithersburg, MD, USA). The rats were administered 1-5 g/kg of a 25% (v/v) ethanol solution three times per day according to a 2-day on/2-day off regimen, for a total of seven 2-day binges. During CIE exposure, each subsequent ethanol dose was adjusted based on the behavioral intoxication score (see Supplementary Materials). Adolescent and adult control rats were administered sterile water IG according to the same regimen as the ethanol-treated rats.
Acute saline or ethanol challenge injections (0.5, 1, and 2 g/kg intraperitoneally [IP] in a volume of 1 ml/kg, 15 min before the session) were administered to the rats during adulthood (PND144-163 and PND251-270 in the adolescent and adult groups, respectively) once per week according to a within-subjects Latin-square experimental design.
After completion of the experiment with acute ethanol challenges, the rats were exposed to a single 4-day ethanol or water binge during adulthood (PND181-184 and PND271-274 in the adolescent and adult groups, respectively, 90-103 days after the termination of CIE exposure) counterbalanced with previous CIE/water exposure. The rats were administered 1-4 g/kg IG of a 25% (v/v) ethanol solution in sterile water via gavage twice per day with 6-h interval between injections for 4 days. During the ethanol binge, each subsequent ethanol dose was adjusted based on the behavioral intoxication score (see Supplementary Materials). Control rats were administered sterile water IG according to the same regimen as the ethanol-treated rats.
Blood samples (200 µl) were collected from the tip of the tail 60-90 min after the last ethanol dose on the 2nd, 4th, and 6th binge days during CIE exposure or the last injection on the 4th day of the 4-day ethanol binge. During ethanol challenge administration, blood samples were collected after the animals completed the test session in the DDT (˜2.5 h after the ethanol administration). Samples were centrifuged for 5 min at 1,800 × g and then stored at -80ºC. Blood ethanol concentrations were measured in serum using an AM1 fast alcohol analyzer calibrated with an external standard of 100 mg/dl (Analox Instruments, Lunenburg, MA, USA).
2.3. Light-potentiated startle and acoustic startle response: chambers and procedures
Eight acoustic startle chambers were used (SR-LAB, San Diego Instruments, San Diego, CA, USA). The startle chambers consisted of clear non-restrictive Plexiglas cylinders mounted on Plexiglas platforms and enclosed in ventilated sound-attenuated cubicles (20.5 cm length × 9 cm diameter). The startle chambers were equipped with high-frequency loudspeakers (SR-LAB, San Diego Instruments, San Diego, CA, USA) and fluorescent light bulbs (commercial electric model no. EDXO-23) that produced light intensities between 2700 and 3600 Lux. Movements within the cylinder were detected and transduced by a piezoelectric accelerometer attached to the platform, digitized, and stored by the operating computer using the SRLab program.
The LPS procedure was based on the procedure used previously in our laboratory [32]. One LPS measurement was based on four startle tests, termed blocks, which were conducted in two separate sessions of two blocks each. At the beginning of the startle tests (block 1 of the session), the rats were placed in the startle chamber and left undisturbed in the dark for 5 min. The 65 dB background white noise was presented during the 5 min acclimation period and throughout the test session. Thereafter, the rats were presented with 30 startle stimuli, 10 each at 90, 95, and 105 dB, with an average interstimulus interval of 30 s, presented in a pseudorandom order under dark conditions. These 30 stimuli constituted the first startle block. The second block of the session was initiated immediately after the first block. The second block was exactly the same as the first block, with the exception that it was presented either in the dark (dark→dark session) or in bright light (dark→light session). Thus, one full test of LPS consisted of two sequential sessions during testing, one dark→dark to measure the startle reflex and one dark→light to measure the startle-enhancing effects of the light. The order of sessions (block 2 in either the dark or light condition) was counterbalanced across animals within groups. The rats were tested 18-22 h after termination of CIE/water exposure. To assess the effects of ethanol withdrawal on LPS, the difference score of the peak startle value during the two sessions (i.e., dark→dark and dark→light) was calculated by subtracting the startle value of the first block from the startle value of the second block. The ASR was measured in the LPS procedure under dark→dark conditions (e.g., startle stimuli 90, 95 and 105 dB).
2.4. Delay discounting task: chambers and procedures
The discrete trials operant delay discounting procedure used in the present study was similar to the procedure developed by Evenden and Ryan (1996) [7] for two-lever boxes and modified by van Gaalen and colleagues [40] for the five-hole chambers. In this procedure, the sessions are organized in blocks of trials in which the fixed delays to the large reward are progressively increased throughout the session from 0 s to 60 s. This procedure allows the researcher to generate temporal-discounting functions within a single experimental session.
All of the testing was conducted in a set of 12 nine-hole test boxes (Med Associates, St. Albans, VT, USA). Each box consisted of a 25.5 width × 28.4 length × 28.7 height cm chamber enclosed in a sound-attenuating cubicle with a ventilator fan that provided air circulation and produced low-level background noise. Each chamber contained a curved rear wall with nine contiguous apertures. A photocell beam was located at the entrance of each aperture to detect nosepoke responses, and a 3 W stimulus light was located at the rear of each aperture. Metal inserts blocked every alternate hole, leaving open five holes for nosepoking. In the opposite wall, a magazine connected to a food dispenser permitted the automatic delivery of food pellets, with a photocell beam that detected head entries into the magazine. The apparatus was controlled by a computer that ran MedPC software.
Training and testing in the DDT is described in detail in our recently published work [41]. Briefly, in the discrete-trials choice procedure, the rats chose between one food pellet delivered immediately and four food pellets delivered after a delay. The position associated with the small and large reward was always the same for each rat and counterbalanced within each group. Failure to respond within 10-s resulted in the trial being recorded as an omission and a return to the intertrial interval (ITI) state until the next trial began. After delivery of the reward or the choice phase time elapsed, the cue lights were switched off, and an ITI commenced until the next trial was initiated. Nosepoking into non-illuminated holes during the test was recorded but had no consequences. The test sessions were divided into five blocks of 12 trials. Each block started with two forced trials in which, after initiating the trial with a nosepoke in the center hole, either the left or right hole was illuminated. For the next 10 trials, the animals had free choice. The delay for the large reward was increased after each block of 10 trials within each session according to the following progression: 0, 10 20, 40, 60 s. The ITI duration for all of the stages of delay discounting training was adjusted according to the delay duration (ITI duration = 100 s − [response latency + delay duration]). Thus, the delay duration was included in the ITI, and the trial duration was fixed at 100 s. The use of fixed ITI ensured that availability of the large and small rewards was equal in each trial. The session duration was fixed at 100 min.
Impulsive choice was calculated as the percentage of choice of the large reward for each delay block per session. High impulsive subjects discount the value of a large delayed reward and prefer a small immediate reward, whereas low impulsive subjects prefer large delayed rewards. The rats were trained in the DDT until delay-dependent choice for the large reward was stable between sessions. The rats were trained in the DDT until delay-dependent choice for the large reward was stable between sessions (i.e., < 10% variability over five consecutive days).
2.5. Experimental design
Experiments in the adolescent and adult experimental groups were conducted sequentially. Two cohorts of adolescent rats were used: Cohort 1 was tested in the DDT and the LPS after 4-day ethanol binge exposure, while Cohort 2 was tested in the LPS on days 1 and 10 of withdrawal from CIE exposure. One cohort of adult rats was tested in the LPS and DDT. The experimental design, including the sequences of all of the experimental procedures, ages of the rats and number of rats in each experimental group, are presented in Figure 1. Briefly, the rats were tested in the LPS procedure (Cohort 2 of adolescent rats and adult rats) on days 1 and 10 after termination of CIE exposure. Subsequently, the adult rats and adolescent rats (Cohort 1) were trained in the DDT. Once stable baseline performance was established (i.e., < 10% variability over five consecutive days), the ethanol/water challenges were administered 15 min before testing in the DDT according to a within-subjects Latin-square experimental design. The ethanol challenges occurred once per week. Finally, the rats were exposed to a 4-day ethanol/water binge and tested in the LPS procedure 24 h after the last ethanol/water administration (adult rats and Cohort 2 of adolescent rats).
2.6. Statistical analysis
All of the analyses were conducted using SPSS 19 software and appropriate analyses of variance (ANOVAs). The sources of significant two-way or three-way interactions were examined by simple main-effect analyses and post-hoc comparisons using Fisher's Least Significant Difference (LSD) test. The Mauchley's sphericity test for repeated measures was applied if violations of homogeneity were detected. Epsilon adjustments for non-sphericity were performed using the Greenhouse-Geisser's epsilon test with the uncorrected values for degrees of freedom reported. The level of significance was set at the level p < 0.05.
Both age groups were included in the ANOVAs to evaluate the effects of the factors Age and CIE exposure as the between-subjects factors. Appropriate repeated-measures ANOVAs evaluated the effects of CIE exposure and Age on test-specific repeated variables. Correlational analyses were performed for a matrix of ethanol intoxication scores and BECs using Spearman's rank correlation. Additionally, body weight was used as a covariate for all of the ANCOVAs performed on the startle data.
For the DDT data, the primary dependent measure was percent choice for the large reward, which was calculated for each delay block within each session. Baseline impulsive choice was calculated as the average choice in each trial block during the last five days of testing under baseline conditions. Omission errors were also assessed.
3. Results
3.1. Ethanol dose, body weights, behavioral intoxication scores and blood ethanol concentrations during chronic intermittent ethanol exposure
During CIE exposure, two rats from the adolescent group and three rats from the adult group died before completion of ethanol exposure, resulting in 48 rats in the adolescent group (cohort 1 and cohort 2 combined) and 23 rats in the adult group that were included in the statistical analyses. In the analyses reported in this section, the data from both adolescent cohorts were combined (see rationale in the Supplementary Materials).
The ANOVAs on the ethanol dose administered revealed significant main effects of Age (F(1,32)=27.8, p<0.0001) and Days of binge (F(6,192)=5.11, p<0.0001) and a Days of binge × Age interaction (F(6,192)=13.8, p<0.0001; Fig.S1C in the Supplementary Materials). During CIE exposure, adolescent and adult rats received a total ethanol dose of 25.9±0.4 g/kg/binge and 21.71±0.7 g/kg/binge, respectively. Across ethanol binges during CIE exposure, adolescent animals received a significantly larger ethanol dose compared with adult animals (LSD post hoc test, p<0.05; Fig.S1C in the Supplementary Materials).
Data analyses performed on body weights, blood ethanol concentrations and behavioral intoxication scores during CIE exposure are described in detail in the Supplementary Materials and presented in Supplementary Figure S1.
3.2. Light-potentiated startle and acoustic startle response during days 1 and 10 of withdrawal from chronic intermittent ethanol exposure
Light-potentiated startle is minimally affected by body weight because the data are expressed as a difference in responses within the same subject. Therefore, body weights were not used as a covariate for these analyses. Overall ANOVAs on the LPS data obtained during days 1 and 10 of withdrawal from CIE exposure revealed a main effect of Age (F(1,43)=9.1, p<0.01), a nearly significant main effect of Pulse Intensity (F(2,86)=2.9, p<0.058), and significant Age × Pulse Intensity interaction (F(2,86)=6.4, p<0.05) but no effect of CIE exposure or Day of withdrawal and no other interactions. Less variability in startle amplitudes was observed at a pulse intensity of 90 dB compared with the 95 and 105 dB pulse intensities. A separate ANOVA on the LPS data at a 90 dB pulse intensity revealed a significant effect of CIE exposure (F(1,43)=4.8, p <0.05) but no effect of Age or Withdrawal Day and no interactions. Post hoc comparisons indicated that LPS was significantly decreased in CIE-exposed rats compared with water-exposed rats (LSD post-hoc test, p<0.05; Fig. 2B).
Figure 2.
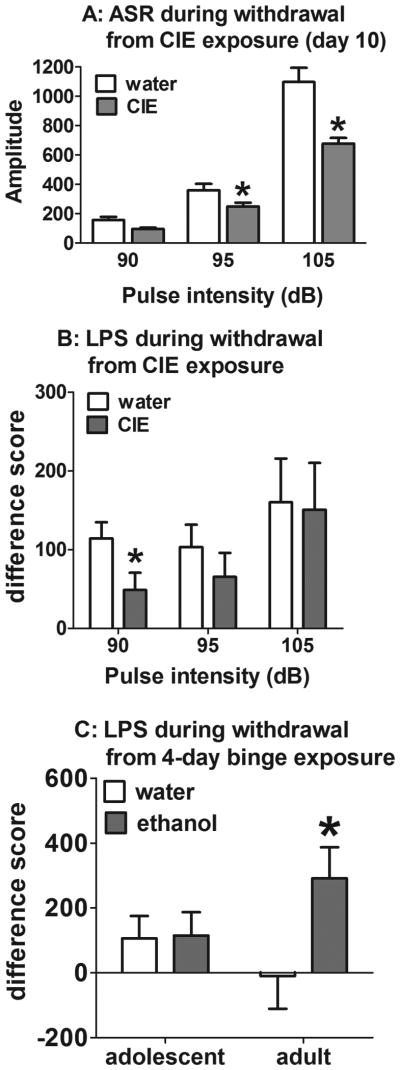
Acoustic startle response (A) and light-potentiated startle (B) during withdrawal from CIE exposure and light-potentiated startle during 24-h withdrawal from a 4-day binge exposure (C). The data are expressed as mean ± SEM. *p < 0.05, significant difference between CIE/ethanol- and water-exposed rats (LSD test). In A and B, data from adolescent and adult experimental groups were combined because there was no effect of Age indicated in the ANOVA.
Considering that differences in body weights between adult and adolescent rats may influence the magnitude of the startle response, ANOVAs on body weights obtained during days 1 and 10 of withdrawal from CIE exposure were performed (see Supplementary Materials for detailed analyses). Based on statistically significant differences in body weights between adult and adolescent rats, the ASR data obtained in the LPS procedure were analyzed separately for days 1 and 10 of withdrawal, with body weights on the corresponding withdrawal day as a covariate in each analysis. The ANCOVA on startle data obtained on day 1 of withdrawal revealed a significant Pulse intensity × Body weight interaction (F(2,84)=4.3, p<0.05) but no other significant main effects or interactions (data not shown). The ANCOVA on startle data obtained on day 10 of withdrawal revealed no Pulse intensity × Body weight interaction, indicating that body weight did not affect the ASR on day 10 of withdrawal. The ANCOVA revealed significant effects of CIE exposure (F(1,42)=7.8, p<0.01) and Pulse intensity (F(2,84)=3.9, p<0.05) and a Pulse intensity × CIE exposure interaction (F(2,84)=9.6, p<0.01) but no effect of Age. Regardless of age, the startle amplitude was decreased in CIE-exposed rats at 95 and 105 dB pulse intensities on day 10 of withdrawal compared with water-exposed rats (LSD post-hoc test, p< 0.01; Fig. 2A).
3.3. Light-potentiated startle and acoustic startle response during withdrawal from a 4-day ethanol binge exposure in rats with previous chronic intermittent ethanol/water exposure
Before the initiation of the 4-day binge exposure, the body weights of the rats did not differ between experimental groups (adolescent-exposed group: 478.2±12.2 g; adult-exposed group: 505.2±13.4 g). During the 4-day binge, both adolescent-exposed and adult-exposed rats received a similar cumulative ethanol dose (25.7±0.7 g/kg and 27.6±0.82 g/kg, respectively). Data analysis performed on intoxication scores during the 4-day ethanol binge exposure are described in detail in the Supplementary Materials.
The ANOVAs on LPS data during withdrawal from the 4-day ethanol or water binge revealed a significant effect of Pulse intensity (F(2,72)=4.3, p<0.05) but no other main effects or interactions. However, a nearly significant main effect of 4-day ethanol binge was found (F(1,36)=3.4, p<0.07), with a nearly significant 4-day binge × Age interaction (F(1,36)=3.0, p<0.09). To further explore any potential age-related differences in LPS during withdrawal, separate ANOVAs on LPS data in the adult-exposed and adolescent-exposed groups were performed. In the adult-exposed group, the ANOVA revealed a significant effect of 4-day ethanol binge (F(1,17)=4.7, p<0.05) but no effect of Pulse intensity and no interaction. Post-hoc comparisons confirmed that LPS was significantly increased in ethanol-exposed rats compared with water-exposed rats (LSD post-hoc test, p<0.05; Fig. 2C). In the adolescent-exposed group, the ANOVA revealed no significant main effects or interactions (Fig. 2C).
The body weights of the rats did not differ between experimental groups (see Supplementary Materials), therefore, body weights were not used as a covariate in subsequent data analyses. The ANOVAs on the ASR data revealed a significant effect of Pulse intensity (F(2,66)= 176.5, p<0.0001) but no effect of CIE exposure, Age, or 4-day ethanol binge and no interactions (data not shown).
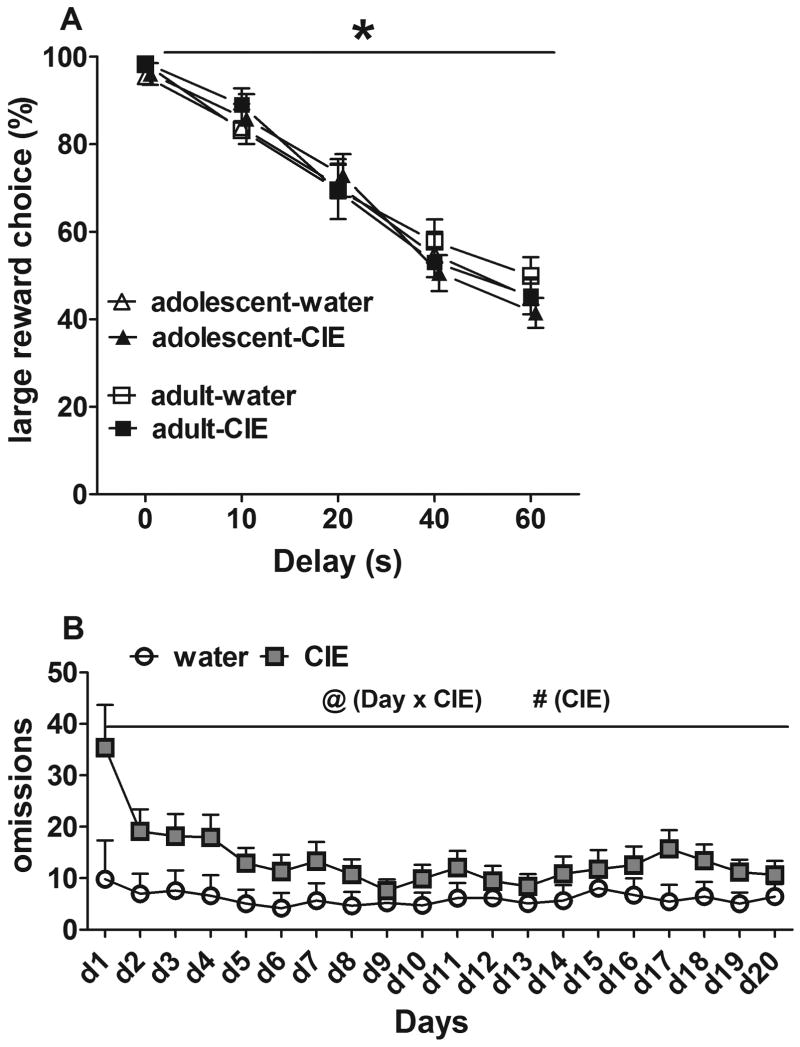
3.4. Delay discounting task acquisition
The ANOVAs on impulsive choice data during 20 training sessions revealed a significant effect of Delay (F(4,156)=162.9, p<0.0001), a Delay × Day interaction (F(76,2964)=2.7, p<0.0001), and a Delay × Day × CIE exposure interaction (F(76,2964)=1.9, p<0.0001). Rats exposed to CIE or water during adulthood or adolescence acquired the DDT with no differences between experimental groups in preference for the large reward (Fig. 3A). The ANOVA on omissions during acquisition of the DDT revealed significant main effects of CIE exposure (F(1,41)=3.8, p<0.05) and Day (F(19,779)=3.9, p<0.001) and Day × CIE exposure (F(19,779)=2.06, p<0.01) and Day × Age (F(19,779)=2.9, p<0.05) interactions, with no main effect of Age. Independent of age, CIE exposure increased the number of omissions throughout training (Fig. 3B).
Figure 3.
Rats' performance in the delayed discounting task. (A) Average preference for the large reward during the last 5 days of baseline performance. *p < 0.05, significant effect of the factor Delay (ANOVA). (B) Omission errors during task acquisition. @p < 0.05, significant Day × CIE exposure interaction (ANOVA); #p < 0.01, significant main effect of CIE exposure (ANOVA). The data are expressed as mean ± SEM.
3.5. Ethanol challenges in the delay discounting task
During ethanol challenges in the DDT, two rats from the adolescent-exposed group and one rat from the adult-exposed group became sick and were excluded from the analyses. Further, six animals from the adult-exposed group (three water-exposed rats and three ethanol-exposed rats) were unable to complete the DDT test session after the 1 g/kg ethanol challenge because of motor impairments and were excluded from the analysis. In addition, the data obtained during the last 60 s delay were not included in the analyses, because a majority of the rats were unable to complete the test session after 1 g/kg ethanol challenge. Finally, ethanol challenge at the highest dose (2 g/kg) completely disrupted performance in all rats, therefore, these data were not included in the analyses.
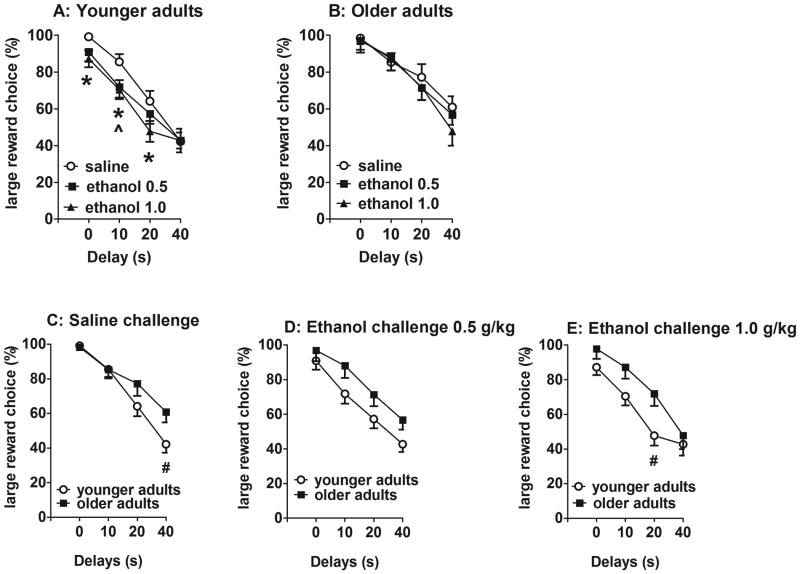
The ANOVAs on preference for the large reward revealed significant effects of Age (F(1,33)=5.6, p<0.05) and Delay (F(3,99)=121.4, p<0.0001), and Delay × CIE exposure (F(3,99)=3.2, p<0.05) and Delay × Ethanol challenge × Age (F(6,198)=2.4, p<0.05) interactions. Pairwise comparisons within each age group showed decreased preference for the large reward after 1.0 g/kg ethanol compared with saline at the 0, 10, and 20 s delays and after 0.5 g/kg at the 10 s delay only in adolescent-exposed animals independent of CIE/water exposure (LSD test, p<0.05; Fig. 4A). Ethanol challenges had no effect on preference for the large reward in adult-exposed animals independent of CIE/water exposure (Fig. 4B). Pairwise comparisons between younger (adolescent-exposed) and older (adult-exposed) rats showed that younger rats had overall decreased preference for the large reward compared with older rats (Fig. 4C-E), with significant differences observed after saline at the 40 s delay (LSD test, p<0.05; Fig. 4C) and after 1.0 g/kg ethanol at the 20 s delay (LSD test, p<0.05; Fig. 4E) indicating age-dependent effects of ethanol on impulsivity with no effect of previous CIE/water exposure. The analyses of indifference points revealed significant effects of CIE exposure (F(1,87)=9.29, p<0.01) and Age (F(1,87)=7.16, p<0.01) but no effect of Ethanol challenge and no interactions (data not shown). The analyses of omissions showed significant effects of Ethanol challenge (F(2,120)=12.01, p<0.0001) and Age (F(1,120)=11.36, p<0.01) and a significant Ethanol Challenge × Age interaction (F(2,120)=3.76, p<0.05) but no effect of CIE exposure and no other interactions. Pairwise comparisons showed that the highest ethanol dose of 1 g/kg significantly increased omissions in all of the animals, with a significantly greater effect in the adult-exposed group compared with the adolescent-exposed group, independent of CIE exposure (data not shown).
Figure 4.
Delayed reward choice (%) in response to ethanol/saline challenges in younger adult rats (adolescent-exposed rats; A) or older adult rats (adult-exposed rats; B) regardless of previous CIE/water exposure.*p < 0.05, significant difference between saline and 1 g/kg ethanol (Fisher's LSD test). ^p < 0.05, significant difference between saline and 0.5 g/kg ethanol (Fisher's LSD test). The delayed reward choice (%) in younger adult (adolescent-exposed) rats compared with older adult (adult-exposed) rats in response to saline (C), 0.5 g/kg ethanol (D), and 1.0 g/kg ethanol (E) challenges is shown. #p < 0.05, significant difference between younger and older adult rats independent of previous CIE/water exposure (LSD test). The data are expressed as mean ± SEM.
The ANOVA on the BEC data revealed a significant effect of Ethanol challenge dose (F(2,105)=28.49, p<0.0001) but no effects of CIE exposure or Age and no interactions. The average BECs (˜2.5 h after ethanol administration) across all of the experimental groups were 6.2±1.15 mg/dl and 18.4±5.8 mg/dl after ethanol doses of 0.5 and 1 g/kg, respectively. During ethanol challenges, a positive correlation was found between BECs and behavioral intoxication scores in rats exposed to CIE during adolescence and adulthood (60% and 77%, respectively).
4. Discussion
Our findings demonstrate that withdrawal from chronic intermittent ethanol exposure either during adolescence or adulthood decreased both ASR and LPS, indicating decreased arousal and anxiety-like behavior, respectively. In contrast, withdrawal from exposure to the 4-day ethanol binge during adulthood increased LPS in older adult rats with previous CIE/water exposure during adulthood, indicating increased anxiety-like behavior. Exposure to a 4-day ethanol binge had no effect on LPS in younger adult rats with previous CIE/water exposure during adolescence, indicating diminished sensitivity to ethanol withdrawal-induced increases in anxiety. Chronic intermittent ethanol exposure had no long-term effect on baseline impulsive choice. During acquisition of the DDT, all of the rats with previous CIE exposure showed increased omissions, independent of age, suggesting that CIE exposure disrupted cognitive processes. Exposure to ethanol challenges in adulthood increased impulsive choice in younger adolescent-exposed rats but had no effect in older adult-exposed rats, regardless of previous CIE/water exposure.
The effects of chronic intermittent ethanol and 4-day binge exposures on body weights and behavioral intoxication scores are discussed in the Supplementary Materials.
4.1. Light-potentiated startle and acoustic startle response during withdrawal from chronic intermittent ethanol and 4-day ethanol binge exposures
This is the first study that evaluated the effects of ethanol withdrawal on anxiety-like behavior in the LPS task in adult and adolescent rats. During withdrawal from CIE exposure, LPS was decreased in both adolescent-exposed and adult-exposed rats, indicating decreased anxiety. Similarly, adult rats exposed to ethanol during adolescence exhibited decreased anxiety-like behavior in the open field conflict test [42, 43] and elevated plus maze [44]. Furthermore, in the social interaction test, no anxiety response was observed after exposure to 5-15 days of a continuous or intermittent ethanol liquid diet [45, 46] in either adult or adolescent rats. In contrast to CIE exposure and consistent with the literature that largely showed increased anxiety during ethanol withdrawal in both humans and rodents in various tests (for review, see [3, 47], withdrawal from a single 4-day ethanol binge during adulthood resulted in increased anxiety, reflected in increased LPS in adult-exposed rats. These findings indicate that the temporal pattern or duration of ethanol exposure (seven intermittent 2-day binges vs. a single 4-day binge) may have opposite effects on anxiety associated with ethanol withdrawal in adults. Interestingly, however, exposure to a 4-day ethanol binge had no effect on LPS in adolescent-exposed rats. The lack of effect of withdrawal from a 4-day ethanol binge on LPS in adolescent-exposed rats can be attributed to decreased ethanol withdrawal sensitivity that extended from adolescence into adulthood in the adolescent ethanol exposed rats (“lock in” effect of ethanol exposure during adolescence).
The age-dependent effects of the 4-day ethanol binge on LPS may suggest differential long-term alterations in the brains of adolescent-exposed and adult-exposed rats that can be related to the chronic mild stress experienced during CIE/water exposure. Repeated exposure to intragastric gavage injections has been shown to increase plasma corticosterone levels [48]. Exposure to predictable chronic mild stress during adolescence had antidepressant- and anxiolytic-like effects in adulthood [49, 50]. Furthermore, mice exposed to the stress of chronic social isolation combined with ethanol during adolescence showed decreased anxiety-like behavior in the elevated plus maze compared with control mice or mice with identical manipulations during adulthood [51]. Thus, exposure to 2-day ethanol binges followed by 2-day ethanol withdrawal for seven cycles may have attenuated the stress response and reduced anxiety in both adult and adolescent rats. However, the long-term effects of CIE/water exposure, manifested as decreased LPS during withdrawal from a 4-day ethanol binge, were observed only in adolescent-exposed rats. These findings may indicate that the effects of chronic mild stress extended from adolescence into adulthood in the adolescent ethanol exposed rats, again suggesting “lock in” effects.
The ASR is a measure of behavioral reactivity to external stimuli [52] that may reflect hyperarousal during ethanol withdrawal. ASR was decreased during withdrawal from CIE exposure and unchanged during withdrawal from a 4-day ethanol binge in both adolescent-exposed and adult-exposed rats. A reduced ASR magnitude during withdrawal from CIE exposure could reflect an anhedonic state associated with the stress of repeated ethanol withdrawals. Supporting this possibility, repeated exposure to restraint stress decreased the ASR in rats [53]. Furthermore, in humans, low baseline ASR has been reported in patients with depressive symptoms and anhedonia [54].
The literature on the effects of ethanol withdrawal on ASR in both humans and animals is inconsistent. Similar to our findings with CIE exposure, ASR was decreased during withdrawal from ethanol vapor exposure in both adolescent and adult Sprague-Dawley rats [37, 38]. Other studies performed in adult rats of inbred and outbred strains exposed to various ethanol regimens demonstrated increased [55-60] or unchanged [61, 62] ASR during ethanol withdrawal. In humans, increased ASRs have been observed in alcohol-dependent patients during acute withdrawal and in early-onset alcohol-dependent patients after protracted withdrawal [25, 36]. In contrast, other studies reported reduced ASRs in alcohol-dependent individuals during protracted abstinence [63]. These discrepant findings in both animals and humans may be attributable to procedural differences in startle assessment between laboratories (e.g., pulse intensities; assessments of the ASR under dark or light conditions) and ethanol exposure regimens. Alternatively, these findings may suggest that ASR may not be a reliable measure of arousal during ethanol withdrawal.
One of the neurobiological systems that mediates ethanol withdrawal, including ethanol withdrawal-related anxiety, is corticotropin-releasing factor (CRF) [64-66]. For example, during ethanol withdrawal, decreased anxiety was correlated with decreased CRF levels in the central nucleus of the amygdala in rats with a history of adolescent ethanol exposure [44, 67]. Additionally, cholinergic hypoactivity has also been observed in adolescent [42] and adult [68, 69] rats during ethanol withdrawal and was correlated with decreased anxiety and behavioral disinhibition [42]. Therefore, both decreased CRF function and cholinergic hypoactivity could contribute to the decreased anxiety observed during withdrawal from CIE exposure in adult and adolescent rats, and during withdrawal from the 4-day ethanol binge in adolescent-exposed rats during adulthood.
In contrast, increased anxiety observed in adult-exposed rats after exposure to a single 4-day ethanol binge may be attributable to increased CRF levels during ethanol withdrawal [70]. Neuroanatomical studies showed that CRF is co-localized in glutamatergic and GABAergic afferents and synapses with dopaminergic and non-dopaminergic neurons in the ventral tegmental area and extended amygdala (for review, see [71]. Therefore, complex interactions between CRF and multiple neurotransmitter systems (e.g., dopamine, γ-aminobutyric acid, and glutamate) may occur during ethanol withdrawal that could differentially affect anxiety-like behavior during adolescence and adulthood, but these effects have yet to be demonstrated.
4.2. Chronic intermittent ethanol exposure and impulsive choice
CIE exposure during adolescence or adulthood had no effect on baseline preference for the large reward. Similarly, our previous work showed no long-term effects of exposure to a 4-day ethanol binge during adolescence (PND33-36) on baseline levels of impulsive action, another form of impulsivity assessed by premature responding in the 5-choice serial reaction time task (5-CSRTT; [72]). Importantly, extensive training under familiar experimental conditions in both the DDT and 5-CSRTT may not allow for the detection of baseline differences in impulsivity. Interestingly, however, during acquisition of the DDT, CIE-exposed rats made more omission errors than water-exposed rats, regardless of age. Increased omission errors could indirectly suggest that CIE exposure had long-term disruptive effects on cognitive processes including impairments in both attention and learning, during acquisition of a new task. Considering that the DDT is a food-motivated task, increases in omission errors may be related to altered motivation for food reward in CIE-exposed rats. However, similar CIE exposure during either adolescence or adulthood had no effect on motivation to respond for a food reward assessed by fixed- and progressive-ratio schedules during adulthood [73], suggesting that CIE exposure impaired cognitive function rather than caused alterations in motivation for food.
A history of CIE exposure during either adolescence or adulthood had no effect on impulsive choice in response to acute ethanol challenges during adulthood. Surprisingly, the effects of acute ethanol on impulsive choice were age-dependent. Specifically, in older adults (PND251-270; adult-exposed rats) acute ethanol had no effect on impulsive choice. In contrast, younger adult rats (PND144-163; adolescent-exposed rats) exhibited increased impulsive choice after ethanol at the highest challenge dose (1 g/kg). However, age-related preexisting differences in impulsive choice may affect ethanol reactivity in the DDT. Studies in humans showed that older adults are less sensitive to immediate reward than younger adults [74]. Similarly, in the present study, younger adolescent-exposed rats exhibited increased impulsive choice at the longer 40 s delay compared with older adult-exposed rats after saline. However, ethanol increased impulsive choice in rats pre-selected as high- and low-impulsive [19], suggesting that preexisting differences may not change the effects of ethanol on delay discounting.
In addition, the age-related differences in response to an ethanol challenge on impulsive choice may be explained by differential sensitivity to the sedative effects of ethanol [75]. Notably, 1.0 g/kg ethanol disrupted performance in the DDT in older adult-exposed rats to a larger extent (e.g., six rats were excluded from the analyses) than in younger adolescent-exposed rats (none of the rats were excluded). Therefore, higher sensitivity to ethanol in older adult-exposed rats resulted in either disrupted performance in the DDT at higher ethanol doses or no effect on impulsive choice at lower ethanol doses.
5. Conclusions
Our findings indicate that withdrawal from exposure to CIE decreased anxiety-like behavior in both adult and adolescent rats. Exposure to a single ethanol binge in adulthood increased anxiety-like behavior in rats previously exposed to CIE or water during adulthood, whereas it had no effect in rats previously exposed to CIE/water during adolescence. These findings suggest that drinking during adulthood may be promoted by increased anxiety in rats exposed to ethanol during adulthood and by decreased sensitivity to ethanol withdrawal (e.g., decreased anxiety) in rats exposed to ethanol during adolescence. Furthermore, CIE during either adolescence or adulthood had no long-term effect on baseline impulsive choice assessed in the DDT. Independent of age, CIE exposure disrupted cognitive processes in adulthood, reflected in increased omissions during acquisition of the DDT. Ethanol challenges increased impulsive choice in younger adults (adolescent-exposed rats) but not in older adults (adult-exposed rats), independent of history of CIE/water exposure. These results indicate that adolescent intermittent ethanol exposure may increase the vulnerability to develop alcohol dependence during adulthood because of impaired attentional processes and increased impulsive choice during re-exposure to ethanol at a younger age.
Supplementary Material
Highlights.
CIE exposure and age had no effect on baseline impulsive choice.
Ethanol increased impulsivity in younger adult rats regardless of CIE exposure.
CIE withdrawal-induced decreases in anxiety and arousal were not age-specific.
Subsequent ethanol withdrawal produced age-dependent increases in anxiety.
Acknowledgments
This work was supported by National Institutes of Health grant U01-AA019970-NADIA Project to AM and a UC MEXUS-CONACYT fellowship to JMT. The NIH and UC MEXUS-CONACYT had no role in the study design, data collection, data analysis, data interpretation, writing of the report, or decision to submit the article for publication.
The authors would like to thank Dr. Xia Li and Mrs. Jessica Benedict for excellent technical assistance with LPS data collection and analyses, and Mr. Michael Arends for outstanding editorial assistance.
Abbreviations
- CIE
chronic intermittent ethanol
- PND
postnatal day
- LPS
light-potentiated startle
- BEC
blood ethanol concentration
- DDT
delay discounting task
Footnotes
Conflict of interest: SS, NB and JMT have nothing to disclose. AM has received contract research support from Bristol-Myers Squibb Co., Forest Laboratories and Astra-Zeneca, and honoraria/consulting fees from AbbVie during the past 3 years. There are no actual or potential financial conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Substance Abuse and Mental Health Services Administration. 2011 http://www.samhsa.gov.
- 2.Cloninger CR. Neurogenetic adaptive mechanisms in alcoholism. Science. 1987;236:410–6. doi: 10.1126/science.2882604. [DOI] [PubMed] [Google Scholar]
- 3.Heilig M, Egli M, Crabbe JC, Becker HC. Acute withdrawal, protracted abstinence and negative affect in alcoholism: are they linked? Addict Biol. 2010;15:169–84. doi: 10.1111/j.1369-1600.2009.00194.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jakubczyk A, Klimkiewicz A, Mika K, Bugaj M, Konopa A, Podgorska A, et al. Psychosocial predictors of impulsivity in alcohol-dependent patients. J Nerv Ment Dis. 2013;201:43–7. doi: 10.1097/NMD.0b013e31827aaf9d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Soloff PH, Lynch KG, Moss HB. Serotonin, impulsivity, and alcohol use disorders in the older adolescent: a psychobiological study. Alcohol Clin Exp Res. 2000;24:1609–19. [PubMed] [Google Scholar]
- 6.Benjamin L, Wulfert E. Dispositional correlates of addictive behaviors in college women: binge eating and heavy drinking. Eat Behav. 2005;6:197–209. doi: 10.1016/j.eatbeh.2003.08.001. [DOI] [PubMed] [Google Scholar]
- 7.Evenden JL, Ryan CN. The pharmacology of impulsive behaviour in rats: the effects of drugs on response choice with varying delays of reinforcement. Psychopharmacology (Berl) 1996;128:161–70. doi: 10.1007/s002130050121. [DOI] [PubMed] [Google Scholar]
- 8.Evenden JL. Varieties of impulsivity. Psychopharmacology (Berl) 1999;146:348–61. doi: 10.1007/pl00005481. [DOI] [PubMed] [Google Scholar]
- 9.Ainslie G. Specious reward: a behavioral theory of impulsiveness and impulse control. Psychol Bull. 1975;82:463–96. doi: 10.1037/h0076860. [DOI] [PubMed] [Google Scholar]
- 10.Petry NM. Delay discounting of money and alcohol in actively using alcoholics, currently abstinent alcoholics, and controls. Psychopharmacology (Berl) 2001;154:243–50. doi: 10.1007/s002130000638. [DOI] [PubMed] [Google Scholar]
- 11.Vuchinich RE, Simpson CA. Hyperbolic temporal discounting in social drinkers and problem drinkers. Exp Clin Psychopharmacol. 1998;6:292–305. doi: 10.1037//1064-1297.6.3.292. [DOI] [PubMed] [Google Scholar]
- 12.Mitchell JM, Fields HL, D'Esposito M, Boettiger CA. Impulsive responding in alcoholics. Alcohol Clin Exp Res. 2005;29:2158–69. doi: 10.1097/01.alc.0000191755.63639.4a. [DOI] [PubMed] [Google Scholar]
- 13.Ortner CN, MacDonald TK, Olmstead MC. Alcohol intoxication reduces impulsivity in the delay-discounting paradigm. Alcohol Alcohol. 2003;38:151–6. doi: 10.1093/alcalc/agg041. [DOI] [PubMed] [Google Scholar]
- 14.Dougherty DM, Mathias CW, Marsh-Richard DM, Furr RM, Nouvion SO, Dawes MA. Distinctions in Behavioral Impulsivity: Implications for Substance Abuse Research. Addictive disorders & their treatment. 2009;8:61–73. doi: 10.1097/ADT.0b013e318172e488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dougherty DM, Marsh-Richard DM, Hatzis ES, Nouvion SO, Mathias CW. A test of alcohol dose effects on multiple behavioral measures of impulsivity. Drug Alcohol Depend. 2008;96:111–20. doi: 10.1016/j.drugalcdep.2008.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Reynolds B, Richards JB, de Wit H. Acute-alcohol effects on the Experiential Discounting Task (EDT) and a question-based measure of delay discounting. Pharmacol Biochem Behav. 2006;83:194–202. doi: 10.1016/j.pbb.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 17.Moore SC, Cusens B. Delay discounting predicts increase in blood alcohol level in social drinkers. Psychiatry Res. 2010;179:324–7. doi: 10.1016/j.psychres.2008.07.024. [DOI] [PubMed] [Google Scholar]
- 18.Olmstead MC, Hellemans KG, Paine TA. Alcohol-induced impulsivity in rats: an effect of cue salience? Psychopharmacology (Berl) 2006;184:221–8. doi: 10.1007/s00213-005-0215-0. [DOI] [PubMed] [Google Scholar]
- 19.Poulos CX, Parker JL, Le DA. Increased impulsivity after injected alcohol predicts later alcohol consumption in rats: evidence for "loss-of-control drinking" and marked individual differences. Behav Neurosci. 1998;112:1247–57. doi: 10.1037//0735-7044.112.5.1247. [DOI] [PubMed] [Google Scholar]
- 20.Tomie A, Aguado AS, Pohorecky LA, Benjamin D. Ethanol induces impulsive-like responding in a delay-of-reward operant choice procedure: impulsivity predicts autoshaping. Psychopharmacology (Berl) 1998;139:376–82. doi: 10.1007/s002130050728. [DOI] [PubMed] [Google Scholar]
- 21.Evenden JL, Ryan CN. The pharmacology of impulsive behaviour in rats VI: the effects of ethanol and selective serotonergic drugs on response choice with varying delays of reinforcement. Psychopharmacology (Berl) 1999;146:413–21. doi: 10.1007/pl00005486. [DOI] [PubMed] [Google Scholar]
- 22.Wilhelm CJ, Mitchell SH. Rats bred for high alcohol drinking are more sensitive to delayed and probabilistic outcomes. Genes Brain Behav. 2008;7:705–13. doi: 10.1111/j.1601-183X.2008.00406.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Oberlin BG, Grahame NJ. High-alcohol preferring mice are more impulsive than low-alcohol preferring mice as measured in the delay discounting task. Alcohol Clin Exp Res. 2009;33:1294–303. doi: 10.1111/j.1530-0277.2009.00955.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wilhelm CJ, Mitchell SH. Acute ethanol does not always affect delay discounting in rats selected to prefer or avoid ethanol. Alcohol Alcohol. 2012;47:518–24. doi: 10.1093/alcalc/ags059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Krystal JH, Webb E, Grillon C, Cooney N, Casal L, Morgan CA, 3rd, et al. Evidence of acoustic startle hyperreflexia in recently detoxified early onset male alcoholics: modulation by yohimbine and m-chlorophenylpiperazine (mCPP) Psychopharmacology (Berl) 1997;131:207–15. doi: 10.1007/s002130050285. [DOI] [PubMed] [Google Scholar]
- 26.Roelofs SM. Alcohol. Vol. 2. Fayetteville, NY: 1985. Hyperventilation, anxiety, craving for alcohol: a subacute alcohol withdrawal syndrome; pp. 501–5. [DOI] [PubMed] [Google Scholar]
- 27.Carlson RW, Kumar NN, Wong-Mckinstry E, Ayyagari S, Puri N, Jackson FK, et al. Alcohol withdrawal syndrome. Critical care clinics. 2012;28:549–85. doi: 10.1016/j.ccc.2012.07.004. [DOI] [PubMed] [Google Scholar]
- 28.Pittman B, Gueorguieva R, Krupitsky E, Rudenko AA, Flannery BA, Krystal JH. Multidimensionality of the Alcohol Withdrawal Symptom Checklist: a factor analysis of the Alcohol Withdrawal Symptom Checklist and CIWA-Ar. Alcohol Clin Exp Res. 2007;31:612–8. doi: 10.1111/j.1530-0277.2007.00345.x. [DOI] [PubMed] [Google Scholar]
- 29.De Soto CB, O'Donnell WE, De Soto JL. Long-term recovery in alcoholics. Alcohol Clin Exp Res. 1989;13:693–7. doi: 10.1111/j.1530-0277.1989.tb00406.x. [DOI] [PubMed] [Google Scholar]
- 30.de Jongh R, Groenink L, van Der Gugten J, Olivier B. The light-enhanced startle paradigm as a putative animal model for anxiety: effects of chlordiazepoxide, flesinoxan and fluvoxamine. Psychopharmacology (Berl) 2002;159:176–80. doi: 10.1007/s002130100914. [DOI] [PubMed] [Google Scholar]
- 31.Walker DL, Davis M. Light-enhanced startle: further pharmacological and behavioral characterization. Psychopharmacology (Berl) 2002;159:304–10. doi: 10.1007/s002130100913. [DOI] [PubMed] [Google Scholar]
- 32.Jonkman S, Risbrough VB, Geyer MA, Markou A. Spontaneous nicotine withdrawal potentiates the effects of stress in rats. Neuropsychopharmacology. 2008;33:2131–8. doi: 10.1038/sj.npp.1301607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Spear LP. The adolescent brain and age-related behavioral manifestations. Neurosci Biobehav Rev. 2000;24:417–63. doi: 10.1016/s0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- 34.Pascual M, Blanco AM, Cauli O, Minarro J, Guerri C. Intermittent ethanol exposure induces inflammatory brain damage and causes long-term behavioural alterations in adolescent rats. Eur J Neurosci. 2007;25:541–50. doi: 10.1111/j.1460-9568.2006.05298.x. [DOI] [PubMed] [Google Scholar]
- 35.Matthews DB, Tinsley KL, Diaz-Granados JL, Tokunaga S, Silvers JM. Alcohol. Vol. 42. Fayetteville, NY: 2008. Chronic intermittent exposure to ethanol during adolescence produces tolerance to the hypnotic effects of ethanol in male rats: a dose-dependent analysis; pp. 617–21. [DOI] [PubMed] [Google Scholar]
- 36.Schellekens AF, Mulders PC, Ellenbroek B, de Jong CA, Buitelaar JK, Cools A, et al. Early-onset alcohol dependence increases the acoustic startle reflex. Alcohol Clin Exp Res. 2012;36:1075–83. doi: 10.1111/j.1530-0277.2011.01700.x. [DOI] [PubMed] [Google Scholar]
- 37.Slawecki CJ, Ehlers CL. Enhanced prepulse inhibition following adolescent ethanol exposure in Sprague-Dawley rats. Alcohol Clin Exp Res. 2005;29:1829–36. doi: 10.1097/01.alc.0000183024.47167.27. [DOI] [PubMed] [Google Scholar]
- 38.Slawecki CJ, Roth J, Gilder A. Neurobehavioral profiles during the acute phase of ethanol withdrawal in adolescent and adult Sprague-Dawley rats. Behavioural brain research. 2006;170:41–51. doi: 10.1016/j.bbr.2006.01.023. [DOI] [PubMed] [Google Scholar]
- 39.Urso T, Gavaler JS, Van Thiel DH. Blood ethanol levels in sober alcohol users seen in an emergency room. Life Sci. 1981;28:1053–6. doi: 10.1016/0024-3205(81)90752-9. [DOI] [PubMed] [Google Scholar]
- 40.van Gaalen MM, van Koten R, Schoffelmeer AN, Vanderschuren LJ. Critical involvement of dopaminergic neurotransmission in impulsive decision making. Biol Psychiatry. 2006;60:66–73. doi: 10.1016/j.biopsych.2005.06.005. [DOI] [PubMed] [Google Scholar]
- 41.Kayir H, Semenova S, Markou A. Baseline impulsive choice predicts the effects of nicotine and nicotine withdrawal on impulsivity in rats. Prog Neuropsychopharmacol Biol Psychiatry. 2014;48:6–13. doi: 10.1016/j.pnpbp.2013.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ehlers CL, Criado JR, Wills DN, Liu W, Crews FT. Periadolescent ethanol exposure reduces adult forebrain ChAT+IR neurons: correlation with behavioral pathology. Neuroscience. 2011;199:333–45. doi: 10.1016/j.neuroscience.2011.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ehlers CL, Liu W, Wills DN, Crews FT. Periadolescent ethanol vapor exposure persistently reduces measures of hippocampal neurogenesis that are associated with behavioral outcomes in adulthood. Neuroscience. 2013;244:1–15. doi: 10.1016/j.neuroscience.2013.03.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gilpin NW, Karanikas CA, Richardson HN. Adolescent binge drinking leads to changes in alcohol drinking, anxiety, and amygdalar corticotropin releasing factor cells in adulthood in male rats. PLoS One. 2012;7:e31466. doi: 10.1371/journal.pone.0031466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Breese GR, Overstreet DH, Knapp DJ, Navarro M. Prior multiple ethanol withdrawals enhance stress-induced anxiety-like behavior: inhibition by CRF1- and benzodiazepine-receptor antagonists and a 5-HT1a-receptor agonist. Neuropsychopharmacology. 2005;30:1662–9. doi: 10.1038/sj.npp.1300706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wills TA, Knapp DJ, Overstreet DH, Breese GR. Sensitization, duration, and pharmacological blockade of anxiety-like behavior following repeated ethanol withdrawal in adolescent and adult rats. Alcohol Clin Exp Res. 2009;33:455–63. doi: 10.1111/j.1530-0277.2008.00856.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kliethermes CL. Anxiety-like behaviors following chronic ethanol exposure. Neurosci Biobehav Rev. 2005;28:837–50. doi: 10.1016/j.neubiorev.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 48.Zhou Y, Franck J, Spangler R, Maggos CE, Ho A, Kreek MJ. Reduced hypothalamic POMC and anterior pituitary CRF1 receptor mRNA levels after acute, but not chronic, daily “binge” intragastric alcohol administration. Alcohol Clin Exp Res. 2000;24:1575–82. [PubMed] [Google Scholar]
- 49.Suo L, Zhao L, Si J, Liu J, Zhu W, Chai B, et al. Predictable chronic mild stress in adolescence increases resilience in adulthood. Neuropsychopharmacology. 2013;38:1387–400. doi: 10.1038/npp.2013.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wilkin MM, Waters P, McCormick CM, Menard JL. Intermittent physical stress during early-and mid-adolescence differentially alters rats' anxiety- and depression-like behaviors in adulthood. Behav Neurosci. 2012;126:344–60. doi: 10.1037/a0027258. [DOI] [PubMed] [Google Scholar]
- 51.Conrad KL, Winder DG. Alcohol. Vol. 45. Fayetteville, NY: 2011. Altered anxiety-like behavior and long-term potentiation in the bed nucleus of the stria terminalis in adult mice exposed to chronic social isolation, unpredictable stress, and ethanol beginning in adolescence; pp. 585–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Braff DL, Geyer MA, Swerdlow NR. Human studies of prepulse inhibition of startle: normal subjects, patient groups, and pharmacological studies. Psychopharmacology (Berl) 2001;156:234–58. doi: 10.1007/s002130100810. [DOI] [PubMed] [Google Scholar]
- 53.Conti LH, Printz MP. Rat strain-dependent effects of repeated stress on the acoustic startle response. Behavioural brain research. 2003;144:11–8. doi: 10.1016/s0166-4328(03)00061-5. [DOI] [PubMed] [Google Scholar]
- 54.Mneimne M, McDermut W, Powers AS. Affective ratings and startle modulation in people with nonclinical depression. Emotion. 2008;8:552–9. doi: 10.1037/a0012827. [DOI] [PubMed] [Google Scholar]
- 55.Macey DJ, Schulteis G, Heinrichs SC, Koob GF. Time-dependent quantifiable withdrawal from ethanol in the rat: effect of method of dependence induction. Alcohol. 1996;13:163–70. doi: 10.1016/0741-8329(95)02030-6. [DOI] [PubMed] [Google Scholar]
- 56.Rassnick S, Koob GF, Geyer MA. Responding to acoustic startle during chronic ethanol intoxication and withdrawal. Psychopharmacology (Berl) 1992;106:351–8. doi: 10.1007/BF02245417. [DOI] [PubMed] [Google Scholar]
- 57.Vandergriff J, Kallman MJ, Rasmussen K. Moxonidine, a selective imidazoline-1 receptor agonist, suppresses the effects of ethanol withdrawal on the acoustic startle response in rats. Biol Psychiatry. 2000;47:874–9. doi: 10.1016/s0006-3223(00)00229-8. [DOI] [PubMed] [Google Scholar]
- 58.Pohorecky LA, Cagan M, Brick J, Jaffe SL. The startle response in rats: effect of ethanol. Pharmacol Biochem Behav. 1976;4:311–6. doi: 10.1016/0091-3057(76)90247-1. [DOI] [PubMed] [Google Scholar]
- 59.Pohorecky LA, Roberts P. Development of tolerance to and physical dependence on ethanol: daily versus repeated cycles treatment with ethanol. Alcohol Clin Exp Res. 1991;15:824–33. doi: 10.1111/j.1530-0277.1991.tb00609.x. [DOI] [PubMed] [Google Scholar]
- 60.Pohorecky LA, Roberts P. Daily dose of ethanol and the development and decay of acute and chronic tolerance and physical dependence in rats. Pharmacol Biochem Behav. 1992;42:831–42. doi: 10.1016/0091-3057(92)90037-g. [DOI] [PubMed] [Google Scholar]
- 61.Sandbak T, Rimol LM, Jellestad FK, Murison R. Relating acoustic startle reactivity and plasticity to alcohol consumption in male Wistar rats. Physiol Behav. 2000;68:723–33. doi: 10.1016/s0031-9384(99)00239-5. [DOI] [PubMed] [Google Scholar]
- 62.Chester JA, Blose AM, Froehlich JC. Acoustic startle reactivity during acute alcohol withdrawal in rats that differ in genetic predisposition toward alcohol drinking: effect of stimulus characteristics. Alcohol Clin Exp Res. 2004;28:677–87. doi: 10.1097/01.alc.0000125345.19665.09. [DOI] [PubMed] [Google Scholar]
- 63.Marin M, Ponce G, Martinez-Gras I, Koeneke A, Curivil P, Jimenez-Arriero MA, et al. Impairments of prepulse inhibition of the startle response in abstinent alcoholic male patients. Alcohol Alcohol. 2012;47:545–51. doi: 10.1093/alcalc/ags055. [DOI] [PubMed] [Google Scholar]
- 64.Koob GF. The role of CRF and CRF-related peptides in the dark side of addiction. Brain Res. 2010;1314:3–14. doi: 10.1016/j.brainres.2009.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Koob GF, Zorrilla EP. Neurobiological mechanisms of addiction: focus on corticotropin-releasing factor. Curr Opin Investig Drugs. 2010;11:63–71. [PMC free article] [PubMed] [Google Scholar]
- 66.Gilpin NW. Alcohol. Vol. 46. Fayetteville, NY: 2012. Corticotropin-releasing factor (CRF) and neuropeptide Y (NPY): effects on inhibitory transmission in central amygdala, and anxiety- & alcohol-related behaviors; pp. 329–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wills TA, Knapp DJ, Overstreet DH, Breese GR. Interactions of stress and CRF in ethanol-withdrawal induced anxiety in adolescent and adult rats. Alcohol Clin Exp Res. 2010;34:1603–12. doi: 10.1111/j.1530-0277.2010.01245.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hodges H, Allen Y, Sinden J, Mitchell SN, Arendt T, Lantos PL, et al. The effects of cholinergic drugs and cholinergic-rich foetal neural transplants on alcohol-induced deficits in radial maze performance in rats. Behav Brain Res. 1991;43:7–28. doi: 10.1016/s0166-4328(05)80048-8. [DOI] [PubMed] [Google Scholar]
- 69.Floyd EA, Young-Seigler AC, Ford BD, Reasor JD, Moore EL, Townsel JG, et al. Alcohol. Vol. 14. Fayetteville, NY: 1997. Chronic ethanol ingestion produces cholinergic hypofunction in rat brain; pp. 93–8. [DOI] [PubMed] [Google Scholar]
- 70.Criado JR, Liu T, Ehlers CL, Mathe AA. Prolonged chronic ethanol exposure alters neuropeptide Y and corticotropin-releasing factor levels in the brain of adult Wistar rats. Pharmacol Biochem Behav. 2011;99:104–11. doi: 10.1016/j.pbb.2011.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.George O, Le Moal M, Koob GF. Allostasis and addiction: role of the dopamine and corticotropin-releasing factor systems. Physiol Behav. 2012;106:58–64. doi: 10.1016/j.physbeh.2011.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Semenova S. Attention, impulsivity, and cognitive flexibility in adult male rats exposed to ethanol binge during adolescence as measured in the five-choice serial reaction time task: the effects of task and ethanol challenges. Psychopharmacology (Berl) 2012;219:433–42. doi: 10.1007/s00213-011-2458-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Risher ML, Fleming RL, Boutros N, Semenova S, Wilson WA, Levin ED, et al. Long-term effects of chronic intermittent ethanol exposure in adolescent and adult rats: radial-arm maze performance and operant food reinforced responding. PLoS One. 2013;8:e62940. doi: 10.1371/journal.pone.0062940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Eppinger B, Nystrom LE, Cohen JD. Reduced sensitivity to immediate reward during decision-making in older than younger adults. PLoS One. 2012;7:e36953. doi: 10.1371/journal.pone.0036953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.York JL, Chan AW. Age effects on chronic tolerance to ethanol hypnosis and hypothermia. Pharmacol Biochem Behav. 1994;49:371–6. doi: 10.1016/0091-3057(94)90436-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.