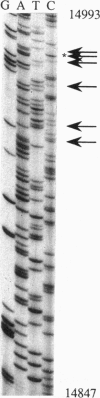
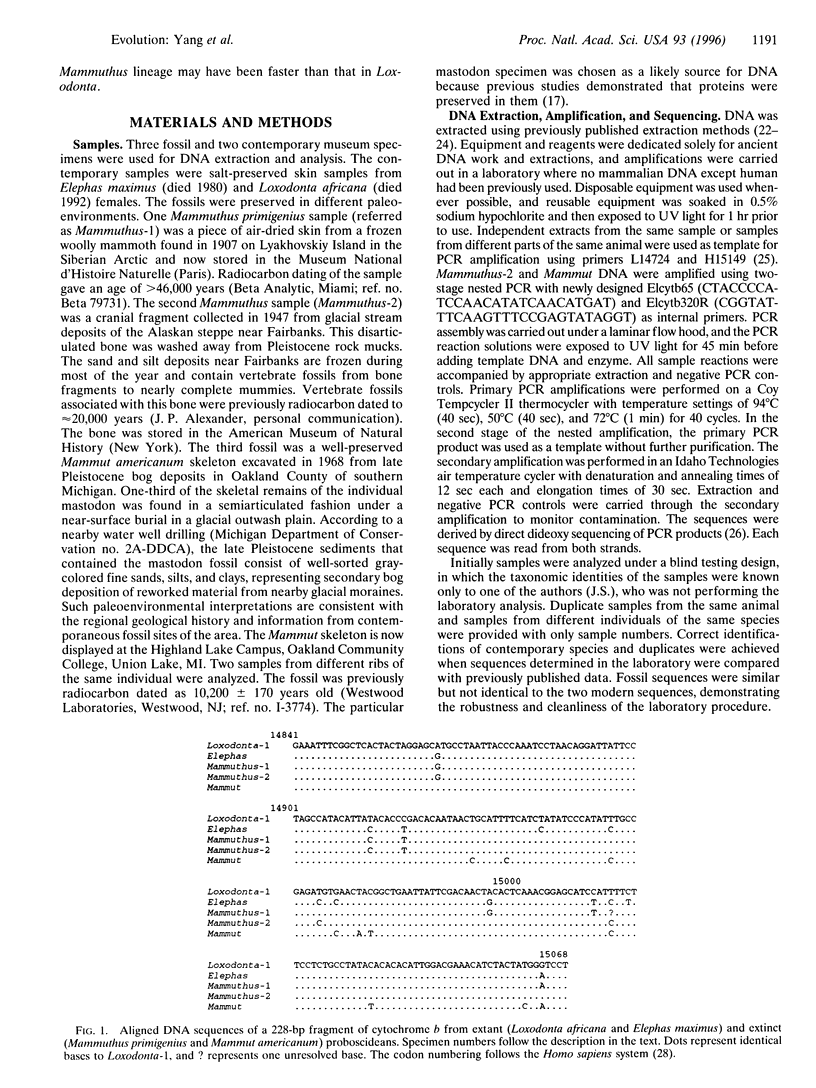
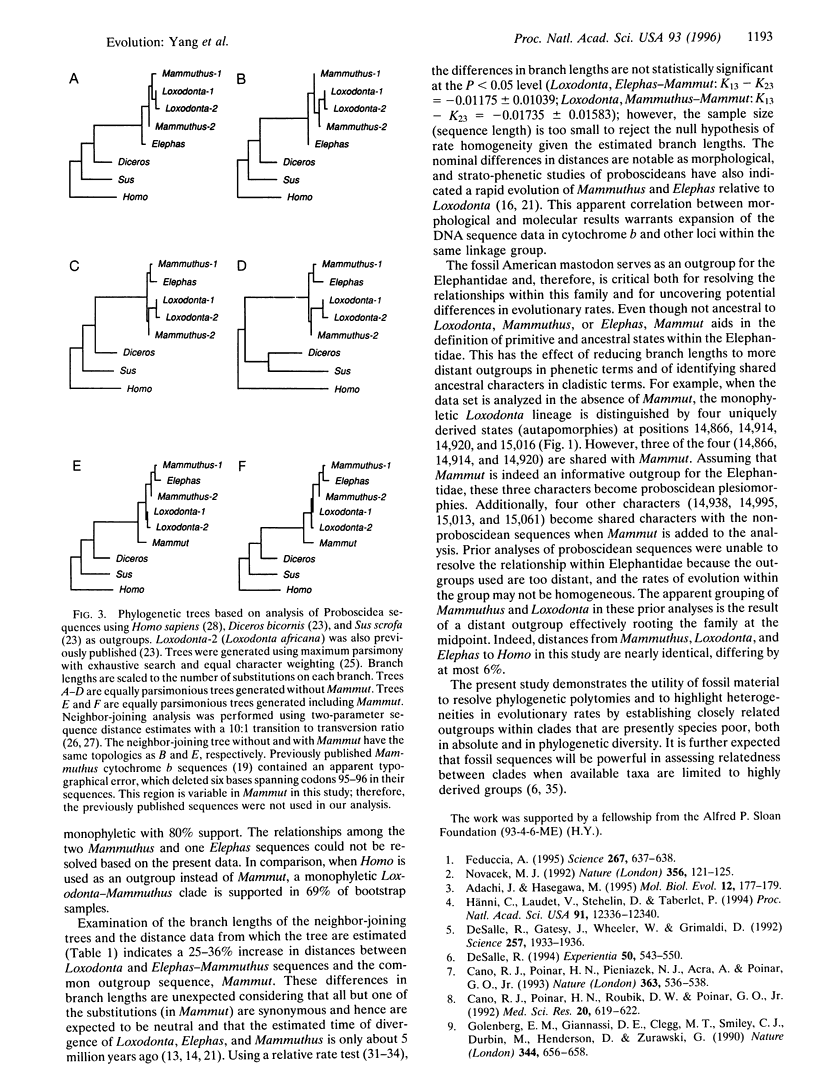
Abstract
DNA was extracted from the extinct American mastodon, the extinct woolly mammoth, and the modern Asian and African elephants to test the traditional morphologically based phylogeny within Elephantidae. Phylogenetic analyses of the aligned sequences of the mitochondrial gene cytochrome b support a monophyletic Asian elephant-woolly mammoth clade when the American mastodon is used as an outgroup. Previous molecular studies were unable to resolve the relationships of the woolly mammoth, Asian elephant, and African elephant because the sequences appear to have evolved at heterogeneous rates and inappropriate outgroups were used for analysis. The results demonstrate the usefulness of fossil molecular data from appropriate sister taxa for resolving phylogenies of highly derived or early radiating lineages.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adachi J., Hasegawa M. Phylogeny of whales: dependence of the inference on species sampling. Mol Biol Evol. 1995 Jan;12(1):177–179. doi: 10.1093/oxfordjournals.molbev.a040187. [DOI] [PubMed] [Google Scholar]
- Anderson S., Bankier A. T., Barrell B. G., de Bruijn M. H., Coulson A. R., Drouin J., Eperon I. C., Nierlich D. P., Roe B. A., Sanger F. Sequence and organization of the human mitochondrial genome. Nature. 1981 Apr 9;290(5806):457–465. doi: 10.1038/290457a0. [DOI] [PubMed] [Google Scholar]
- Barnard G. F., Puder M., Begum N. A., Chen L. B. PCR product sequencing with [alpha-33P] and [alpha-32P]dATP. Biotechniques. 1994 Apr;16(4):572–573. [PubMed] [Google Scholar]
- Cano R. J., Poinar H. N., Pieniazek N. J., Acra A., Poinar G. O., Jr Amplification and sequencing of DNA from a 120-135-million-year-old weevil. Nature. 1993 Jun 10;363(6429):536–538. doi: 10.1038/363536a0. [DOI] [PubMed] [Google Scholar]
- DeSalle R., Gatesy J., Wheeler W., Grimaldi D. DNA sequences from a fossil termite in Oligo-Miocene amber and their phylogenetic implications. Science. 1992 Sep 25;257(5078):1933–1936. doi: 10.1126/science.1411508. [DOI] [PubMed] [Google Scholar]
- DeSalle R. Implications of ancient DNA for phylogenetic studies. Experientia. 1994 Jun 15;50(6):543–550. doi: 10.1007/BF01921723. [DOI] [PubMed] [Google Scholar]
- Feduccia A. Explosive evolution in tertiary birds and mammals. Science. 1995 Feb 3;267(5198):637–638. doi: 10.1126/science.267.5198.637. [DOI] [PubMed] [Google Scholar]
- Golenberg E. M., Giannasi D. E., Clegg M. T., Smiley C. J., Durbin M., Henderson D., Zurawski G. Chloroplast DNA sequence from a miocene Magnolia species. Nature. 1990 Apr 12;344(6267):656–658. doi: 10.1038/344656a0. [DOI] [PubMed] [Google Scholar]
- Hagelberg E., Clegg J. B. Isolation and characterization of DNA from archaeological bone. Proc Biol Sci. 1991 Apr 22;244(1309):45–50. doi: 10.1098/rspb.1991.0049. [DOI] [PubMed] [Google Scholar]
- Hagelberg E., Sykes B., Hedges R. Ancient bone DNA amplified. Nature. 1989 Nov 30;342(6249):485–485. doi: 10.1038/342485a0. [DOI] [PubMed] [Google Scholar]
- Hagelberg E., Thomas M. G., Cook C. E., Jr, Sher A. V., Baryshnikov G. F., Lister A. M. DNA from ancient mammoth bones. Nature. 1994 Aug 4;370(6488):333–334. doi: 10.1038/370333b0. [DOI] [PubMed] [Google Scholar]
- Higuchi R., Bowman B., Freiberger M., Ryder O. A., Wilson A. C. DNA sequences from the quagga, an extinct member of the horse family. Nature. 1984 Nov 15;312(5991):282–284. doi: 10.1038/312282a0. [DOI] [PubMed] [Google Scholar]
- Hänni C., Laudet V., Stehelin D., Taberlet P. Tracking the origins of the cave bear (Ursus spelaeus) by mitochondrial DNA sequencing. Proc Natl Acad Sci U S A. 1994 Dec 6;91(25):12336–12340. doi: 10.1073/pnas.91.25.12336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Höss M., Päbo S., Vereshchagin N. K. Mammoth DNA sequences. Nature. 1994 Aug 4;370(6488):333–333. doi: 10.1038/370333a0. [DOI] [PubMed] [Google Scholar]
- Irwin D. M., Kocher T. D., Wilson A. C. Evolution of the cytochrome b gene of mammals. J Mol Evol. 1991 Feb;32(2):128–144. doi: 10.1007/BF02515385. [DOI] [PubMed] [Google Scholar]
- Li W. H., Tanimura M. The molecular clock runs more slowly in man than in apes and monkeys. Nature. 1987 Mar 5;326(6108):93–96. doi: 10.1038/326093a0. [DOI] [PubMed] [Google Scholar]
- Novacek M. J. Mammalian phylogeny: shaking the tree. Nature. 1992 Mar 12;356(6365):121–125. doi: 10.1038/356121a0. [DOI] [PubMed] [Google Scholar]
- Soltis P. S., Soltis D. E., Smiley C. J. An rbcL sequence from a Miocene Taxodium (bald cypress). Proc Natl Acad Sci U S A. 1992 Jan 1;89(1):449–451. doi: 10.1073/pnas.89.1.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu C. I., Li W. H. Evidence for higher rates of nucleotide substitution in rodents than in man. Proc Natl Acad Sci U S A. 1985 Mar;82(6):1741–1745. doi: 10.1073/pnas.82.6.1741. [DOI] [PMC free article] [PubMed] [Google Scholar]