Abstract
A chromosome-encoded β-lactamase gene from Shewanella algae clinical isolate KB-1 was cloned and expressed in Escherichia coli. It encoded the Ambler class D enzyme OXA-55, sharing less than 55% identity with any other oxacillinases. Although conferring a narrow-spectrum β-lactam resistance phenotype, OXA-55 had carbapenem-hydrolyzing activity that mirrored the reduced susceptibility to imipenem observed in S. algae KB-1. Very similar oxacillinases were found in other S. algae isolates.
Bacteria of the genus Shewanella are gram-negative bacilli, facultatively anaerobic, belonging to the family Alteromonadaceae (25), that are widely distributed in marine and freshwater environments (23). Shewanella algae is a bacterial species recently identified (23, 24) that is closely related to Shewanella putrefaciens (formerly Pseudomonas putrefaciens) (25). Both S. putrefaciens and S. algae species are rare human pathogens (12, 15, 25).
Recently, we have characterized β-lactamase OXA-54 from S. oneidensis and we have shown that this Ambler class D enzyme hydrolyzed imipenem significantly (19). Three S. algae isolates were obtained from clinical samples during the last 2 years from our hospital, and the aim of the present study was to determine their β-lactamase content.
MATERIALS AND METHODS
Bacterial strains and plasmids.
S. algae clinical isolate KB-1 was isolated from an arm wound of a 10-year-old girl hospitalized at the Bicêtre hospital in 2001 (Kremlin-Bicêtre, France). S. algae KB-2 and KB-3 were from a peritoneum aspiration and from a deep pus sample of the flank, respectively, from patients also hospitalized at Bicêtre hospital. These isolates were identified by the API 32GN system (bioMérieux, Marcy l'Etoile, France) and by sequencing of 16S rRNA genes using primers 16S 8-27 (5′-AGAGTTTGATCHTGGYTYAGA-3′; H is A, T, or C and Y is C or T) and 16S 1512-1491 (5′-ACGGYTACCTTGTTACGACTTC-3′; Y is C or T) (9, 11, 23).
S. putrefaciens CIP 8040 (Institut Pasteur strain collection, Paris, France) was used as reference strain. Escherichia coli reference strain DH10B, and plasmid pBK-CMV (Stratagene, Amsterdam, The Netherlands) were used for cloning experiments, whereas rifampin-resistant E. coli JM109 was used for conjugation experiments (3).
Antimicrobial agents and MIC determinations.
The agents and their sources have been referenced elsewhere (17). MICs were determined by an agar dilution technique on Mueller-Hinton agar (Sanofi Diagnostics Pasteur, Paris, France) with an inoculum of 104 CFU per spot and were interpreted according to the guidelines of the National Committee for Clinical Laboratory Standards (14).
Plasmid DNA content and conjugation.
Plasmid DNA extraction of S. algae KB-1 was performed according to different methods as previously described (18). Direct transfer of β-lactam resistance markers into rifampin-resistant E. coli JM109 was attempted by liquid and solid mating-out assays and by electroporation of a putative plasmid DNA suspension of S. algae KB-1 into E. coli DH10B. Transconjugants and electroporants were selected on Trypticase soy (TS) agar plates containing rifampin (50 μg/ml) plus amoxicillin (50 μg/ml) or amoxicillin only, respectively.
Cloning experiments.
Whole-cell DNAs of S. algae KB-1, KB-2, and KB-3 were extracted as previously described (17). Cloning experiments were performed with Sau3AI-digested DNA of S. algae KB-1 and BamHI-restricted plasmid pBK-CMV, followed by expression of recombinant plasmids in E. coli DH10B as described previously (18). Transformants were selected on TS agar containing amoxicillin (100 μg/ml) and kanamycin (30 μg/ml). Antibiograms obtained by disk diffusion were performed with E. coli DH10B harboring recombinant plasmids, and sizes of the plasmid inserts were determined by restriction analysis (22). A recombinant plasmid, pS-1, obtained from cloning of Sau3AI-restricted fragments of S. algae KB-1, was retained for further analyses.
DNA sequencing and protein analysis.
Both strands of the cloned DNA fragment of recombinant plasmid pS-1 were sequenced with an Applied Biosystems sequencer (ABI 377). The nucleotide and deduced protein sequences were analyzed with software available over the internet at the National Center for Biotechnology Information web site (http://www.ncbi.nlm.nih.gov). PCR experiments were performed as previously described (20) using specific primers OXA-55/1 (5′-CATCTACCTTTAAAATTCCC-3′) and OXA-55/2 (5′-AGCTGTTCCTGCTTGAGCAC-3′) for the blaOXA-55 gene to amplify and subsequently to sequence blaOXA-55-type genes from S. algae isolates KB-2 and KB-3.
IEF analysis.
Isoelectric focusing (IEF) analysis was performed with an Ampholine polyacrylamide gel (pH 3.5 to 9.5) (Amersham Pharmacia Biosciences, Orsay, France), as described previously (18) with culture extracts of S. algae KB-1, KB-2, and KB-3, S. putrefaciens CIP 8040T, and E. coli DH10B harboring recombinant plasmid pS-1.
β-Lactamase purification.
Culture of E. coli DH10B(pS-1) that produced OXA-55 was grown overnight at 37°C in 4 liters of TS broth containing 50 μg of ticarcillin per ml and 30 μg of kanamycin per ml. The protein extracts obtained were purified as described previously (20). Briefly, culture extracts were subjected to several purification steps, including ion-exchange chromatography with a 2- by 5-cm Q-Sepharose column (Amersham Pharmacia Biotech, Orsay, France) using a 30 mM Tris-HCl buffer (pH 8) followed by a 2- by 4-cm S-Sepharose column (Amersham Pharmacia Biotech) using a 20 mM BisTris buffer (pH 6.5). Elution of the β-lactamase was performed with a K2SO4 gradient (0 to 500 mM) in order to prevent potential activity inhibition by NaCl. The fractions containing the highest β-lactamase activity, determined by the nitrocefin test (Oxoid, Dardilly, France), were concentrated using Centrisart-C30 spin columns (Sartorius, Göttingen, Germany) and dialyzed against 100 mM phosphate buffer (pH 7.0).
Kinetic studies.
Purified β-lactamase was used for kinetic measurements performed at 30°C in 100 mM sodium phosphate (pH 7.0) (17). The kcat and Km values were determined by analyzing β-lactam hydrolysis under initial rate conditions with a UV spectrophotometer, as previously described (18). The 50% inhibitory concentrations (IC50) of clavulanic acid, tazobactam, sulbactam, and NaCl were determined (18). Various concentrations of these inhibitors were preincubated with purified enzyme for 3 min at 30°C to determine the concentrations that decreased the rate of hydrolysis of 100 μmol of cephalothin by 50%. Specific activities of protein extracts and purified β-lactamase from culture of E. coli DH10B(pS-1) were determined as described previously (2) with 100 μM cephalothin or imipenem as substrate. The protein purification rate was estimated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis SDS-PAGE analysis. The protein content was measured by the Bio-Rad DC protein assay.
Nucleotide sequence accession number.
The nucleotide sequence and deduced β-lactamase amino acid sequence reported in this work have been assigned to the GenBank and EMBL databases under accession no. AY343493.
RESULTS AND DISCUSSION
Strain identification, susceptibility testing, and IEF analysis.
Identification of clinical isolates KB-1, KB-2, and KB-3 with the API 32GN system resulted in identification of S. putrefaciens, but this biochemical method could not distinguish between S. putrefaciens and S. algae (25). Thus, 16S rRNA genes were amplified from isolates KB-1, KB-2, and KB-3 and results were compared to the sequence of 16S rRNA gene of S. putrefaciens CIP 8040 (8). Indeed, isolates KB-1, KB-2, and KB-3 belonged to the species S. algae. The sequence identity of 16S rRNA genes of these three isolates was 98%, whereas they shared 92% identity with the sequence of the 16S rRNA gene of S. putrefaciens CIP 8040T.
MICs of β-lactams for S. algae KB-1 showed that it was resistant to cephalothin; had reduced susceptibility to amoxicillin, cefoxitin, cefuroxime, and imipenem; and was susceptible to the other β-lactams (Table 1). Addition of clavulanic acid and tazobactam did not significantly decrease MICs of β-lactams (Table 1). S. algae KB-2 and KB-3 had a similar resistance pattern (Table 1), whereas MICs of all β-lactams for S. putrefaciens CIP 8040 were lower than those for S. algae strains.
TABLE 1.
MICs of β-lactams for S. algae KB-1, KB-2, and KB-3; S. putrefaciens CIP 8040; and E. coli DH10B
| β-Lactam(s)a | MIC (μg/ml)
|
||||
|---|---|---|---|---|---|
| S. algae KB-1, KB-2, and KB-3 | S. putrefaciens CIP 8040 |
E. colib
|
|||
| DH10B(pS-1) | DH10B(pSon)c | DH10B | |||
| Amoxicillin | 16-32 | 32 | 128 | >512 | 4 |
| Amoxicillin + CLA | 8-16 | 32 | 128 | >512 | 4 |
| Ticarcillin | 2-4 | 4 | 512 | >512 | 4 |
| Ticarcillin + CLA | 2-4 | 4 | 256 | >512 | 4 |
| Piperacillin | 1 | 1 | 4 | 32 | 2 |
| Piperacillin + TZB | 1 | 1 | 4 | 32 | 2 |
| Cephalothin | 256-512 | 64 | 4 | 4 | 4 |
| Cefuroxime | 4 | 2 | 4 | 4 | 4 |
| Cefoxitin | 16 | 4 | 1 | 4 | 4 |
| Ceftazidime | 0.25 | 0.25 | 0.12 | 0.12 | 0.06 |
| Cefotaxime | 0.12 | 0.06 | 0.06 | 0.12 | 0.06 |
| Cefepime | 0.12 | 0.06 | 0.06 | 0.06 | 0.06 |
| Cefpirome | 0.06 | 0.06 | 0.06 | 0.25 | 0.06 |
| Moxalactam | 2 | 2 | 0.12 | 2 | 0.06 |
| Aztreonam | 0.25 | 0.25 | 0.06 | 0.06 | 0.12 |
| Imipenem | 4 | 1 | 0.25 | 1 | 0.06 |
| Meropenem | 0.25 | 0.06 | 0.06 | 0.12 | 0.06 |
CLA, clavulanic acid at a fixed concentration of 2 μg/ml; TZB, tazobactam at a fixed concentration of 4 μg/ml.
Recombinant plasmid pS-1 expressed β-lactamase OXA-55 from S. algae KB-1, whereas plasmid pSon expressed β-lactamase OXA-54 from S. oneidensis MR-1.
Data are from reference 19.
β-Lactamase extracts of S. algae KB-1, KB-2, and KB-3 submitted to IEF analysis gave one β-lactamase band with a pI of 8.6 (data not shown).
Cloning and sequence analysis of β-lactamase genes.
Clonings gave recombinant E. coli DH10B(pS-1) that displayed an inhibitor-resistant β-lactamase phenotype that hydrolyzed imipenem significantly (data not shown).
DNA sequence analysis of the 1,965-bp insert of pS-1 revealed an open reading frame of 870 bp that encoded a 289-amino-acid protein (OXA-55). The G+C content of this open reading frame was 54%, which was within the range of G+C content of genes of S. algae species (23) (Fig. 1). Downstream of the blaOXA-55 gene, a 375-bp sequence corresponding to the 3′-end part of a gene encoding a putative LysR-type transcriptional regulator was found. This sequence shared 72% nucleotide identity with the sequence located downstream of blaOXA-54 of S. oneidensis (19).
FIG. 1.
Nucleotide sequence of a 1,516-bp fragment of recombinant plasmid pS-1 containing the blaOXA-55 gene and the 3′-end part of a gene encoding a putative LysR-type transcriptional regulator. The deduced amino acid sequence is designated in the single-letter code below the nucleotide sequence, and conserved residues of serine-based β-lactamases are underlined. The stars indicate stop codons.
Genetic location of β-lactamase gene.
Plasmid extraction of S. algae KB-1 as well as transformation and conjugation experiments failed, suggesting a chromosomal location of this gene that was strengthened by the presence of similar surrounding DNA sequences to those surrounding the β-lactamase gene identified in S. oneidensis.
Sequence analysis of β-lactamase OXA-55.
The 289-amino-acid protein OXA-55 shared some identity with several Ambler class D enzymes. β-Lactamase OXA-55 had 55% amino acid identity with OXA-54 from S. oneidensis (19), 41% with OXA-10, 39% with OXA-23 and -27 from A. baumannii, and 33% with the cluster OXA-24, -25, -26, and -40 from A. baumannii (1, 4, 5, 10, 11, 16).
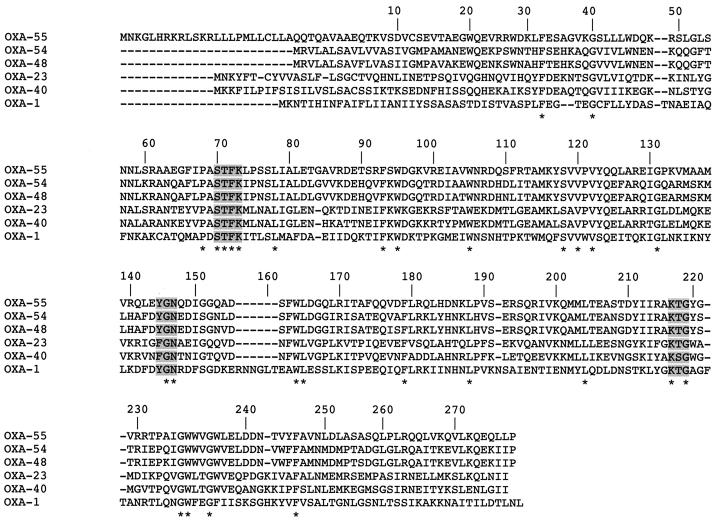
Two conserved motifs, S-T-F-K and K-T-G, were identified at class D β-lactamase (DBL) positions 70 to 73 and 216 to 218, respectively (7). These motifs are involved in the function of the serine active site of oxacillinases (6, 13). The typical motif YGN of oxacillinases was found at DBL positions 144 to 146, as observed for OXA-54, whereas the oxacillinases with carbapenem-hydrolyzing properties identified in A. baumannii (OXA-23 to -27 and OXA-40) possessed an FGN motif at this position (1, 5, 10, 11, 16). However, as we have found previously, the phenylalanine residue at position 144 is not critical for imipenem hydrolysis of those oxacillinases (11).
A detailed analysis of the amino acid sequence of OXA-55 did not identify any specific amino acid residue that could explain the carbapenem-hydrolyzing property of this enzyme (Fig. 2).
FIG. 2.
Comparison of the amino acid sequence of OXA-55 to those of oxacillinases OXA-54 from S. oneidensis MR-1 (19), OXA-48 from K. pneumoniae 11978 (21), OXA-23 from A. baumannii 6B92 (10), OXA-40 from A. baumannii CLA-1 (11), and OXA-1 from E. coli K10-35 (13). The shaded boxes and stars indicate conserved and highly conserved residues among oxacillinases, and stars alone indicate highly conserved residues. Numbering of β-lactamases is according to the DBL system (7).
PCR experiments using primers annealing the blaOXA-55 gene allowed us to identify two blaOXA-55-type β-lactamase genes from S. algae isolates KB-2 and KB-3. The sequence of these β-lactamases shared at least 98% identity with that of S. algae KB-1 (data not shown).
The very likely chromosomal location of blaOXA-55 and the presence of almost identical genes in other S. algae isolates argue for OXA-55-type enzymes being naturally produced by S. algae.
Susceptibility testing of recombinant strains.
MICs of β-lactams for E. coli DH10B(pS-1) showed that it was resistant to amoxicillin and ticarcillin and had a reduced susceptibility to imipenem (Table 1). A similar low level of resistance to carbapenems has been reported for other carbapenem-hydrolyzing enzymes once expressed in E. coli (3, 5, 10, 11, 20). Clavulanic acid and tazobactam did not restore β-lactam activities significantly when OXA-55 was expressed (Table 1).
Kinetic analysis of OXA-55.
After purification from culture extracts of E. coli DH10B(pS-1), the specific activity of β-lactamase OXA-55 against cephalothin was determined to be 0.5 U/mg of protein and its purification factor was 30-fold. Protein purity was estimated to be >95% by sodium dodecyl sulfate-polyacrylamide gel electrophoresis analysis (data not shown). β-Lactamase OXA-55 had a narrow hydrolytic spectrum, including penicillins and narrow-spectrum cephalosporins (Table 2). Hydrolysis of oxacillin and cloxacillin was detected as observed for most oxacillinases (13) but not for other carbapenem-hydrolyzing oxacillinases (1, 4, 5, 10, 11, 16).
TABLE 2.
Kinetic parameters and relative kcat/Km values of purified β-lactamase OXA-55 from S. algae KB-1, OXA-54 from S. oneidensis MR-1, and OXA-40 from A. baumannii CLA-1a
| Substrate | OXA-55
|
OXA-54 (relative kcat/Km)b | OXA-40 (relative kcat/Km)c | |||
|---|---|---|---|---|---|---|
| kcat (s−1) | Km (μM) | kcat/Km (mM−1 · s−1) | Relative kcat/Km | |||
| Benzylpenicillin | 4 | 25 | 160 | 100 | 100 | 100 |
| Ampicillin | 8 | 500 | 15 | 10 | 6 | 9 |
| Ticarcillin | 1 | 20 | 50 | 31 | 6 | 9 |
| Piperacillin | 3 | 110 | 30 | 17 | 4 | 23 |
| Cephalothin | 0.6 | 70 | 10 | 5 | 0.5 | 23 |
| Cephaloridine | 10 | 750 | 15 | 9 | 1.5 | 2 |
| Cefotaxime | ND | — | — | — | 0.4 | — |
| Ceftazidime | 2 | 4,700 | 0.5 | 0.25 | — | 5 |
| Cefepime | ND | — | — | — | 0.1 | — |
| Cefpirome | 0.1 | 670 | 0.1 | 0.06 | 2 | — |
| Oxacillin | 5 | 390 | 10 | 6 | 23 | 1 |
| Cloxacillin | 0.5 | 190 | 3 | 1.5 | — | — |
| Aztreonam | ND | — | — | — | — | — |
| Imipenem | 0.1 | 20 | 5 | 3 | 10 | 7 |
| Meropenem | 0.05 | 500 | 0.1 | 0.06 | 0.02 | — |
Data are means of three independent experiments. Standard deviations were within 10% of the means. Relative values were calculated according to the value obtained for benzylpenicillin, which was set at 100. ND, no detectable hydrolysis (< 0.01 s−1). —, not determinable.
Data are from reference 19.
Data are from reference 11.
The overall catalytic activity of OXA-55 is less robust than that of other carbapenem-hydrolyzing oxacillinases, including OXA-54 from S. oneidensis (1, 5, 10, 11, 16, 19). Nevertheless, the ability of OXA-55 to hydrolyze imipenem was similar to that of OXA-54 and OXA-40, with relative kcat/Km values compared to benzylpenicillin of 3, 10, and 7%, respectively (Table 2). Hydrolysis of imipenem by OXA-55 was low: its catalytic activity (5 mM−1 · s−1) was lower than that reported for OXA-40 from Acinetobacter baumannii CLA-1 (15 mM−1 · s−1) (11). Hydrolysis of meropenem was detected, although at a lower level than that of imipenem.
Activity inhibition measured by determination of IC50s showed that OXA-55 was weakly inhibited by clavulanic acid (700 μM), tazobactam (12 μM), and sulbactam (300 μM), like most oxacillinases (13).
OXA-55 activity was inhibited by NaCl (IC50, 48 mM), like most oxacillinases except those possessing an FGN motif at DBL positions 144 to 146—i.e., several carbapenem-hydrolyzing oxacillinases (1, 4, 5, 10, 11, 16).
The β-lactamase OXA-55 from S. algae may constitute as well a reservoir for oxacillinase genes able to spread through a mobilization process like those of S. oneidensis. Indeed, the plasmid-mediated carbapenem-hydrolyzing β-lactamase OXA-48, which is almost identical to the chromosome-encoded β-lactamase of S. oneidensis, has been identified very recently in a Klebsiella pneumoniae clinical isolate (21).
Acknowledgments
This work was funded by a grant from the Ministère de l'Education Nationale et de la Recherche (UPRES-EA3539), Université Paris XI, Paris, France. L.P. is a researcher from INSERM, France.
REFERENCES
- 1.Afzal-Shah, M., N. Woodford, and D. M. Livermore. 2001. Characterization of OXA-25, OXA-26, and OXA-27, molecular class D β-lactamases associated with carbapenem resistance in clinical isolates of Acinetobacter baumannii. Antimicrob. Agents Chemother. 45:583-588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aubert, D., L. Poirel, J. Chevalier, S. Leotard, J.-M. Pages, and P. Nordmann. 2001. Oxacillinase-mediated resistance to cefepime and susceptibility to ceftazidime in Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 45:1615-1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bellais, S., D. Girlich, A. Karim, and P. Nordmann. 2002. EBR-1, a novel Ambler subclass B1 β-lactamase from Empedobacter brevis. Antimicrob. Agents Chemother. 46:3223-3227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bonnet, R., H. Marchandin, C. Chanal, D. Sirot, R. Labia, C. De Champs, E. Jumas-Bilak, and J. Sirot. 2002. Chromosome-encoded class D β-lactamase OXA-23 in Proteus mirabilis. Antimicrob. Agents Chemother. 46:2004-2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bou, G., A. Oliver, and J. Martinez-Beltran. 2000. OXA-24, a novel class D β-lactamase with carbapenemase activity in an Acinetobacter baumannii clinical strain. Antimicrob. Agents Chemother. 44:1556-1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bush, K., G. A. Jacoby, and A. A. Medeiros. 1995. A functional classification scheme for β-lactamases and its correlation with molecular structure. Antimicrob. Agents Chemother. 39:1211-1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Couture, F., J. Lachapelle, and R. C. Levesque. 1992. Phylogeny of LCR-1 and OXA-5 with class A and class D β-lactamases. Mol. Microbiol. 6:1693-1705. [DOI] [PubMed] [Google Scholar]
- 8.Devulder, G., G. Perriere, F. Baty, and J. P. Flandrois. 2003. BIBI, a bioinformatics bacterial identification tool. J. Clin. Microbiol. 41:1785-1787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dominguez, H., B. F. Vogel, L. Gram, S. Hoffmann, and S. Schaebel. 1996. Shewanella alga bacteremia in two patients with lower leg ulcers. Clin. Infect. Dis. 22:1036-1039. [DOI] [PubMed] [Google Scholar]
- 10.Donald, H. M., W. Scaife, S. G. B. Amyes, and H. K. Young. 2000. Sequence analysis of ARI-1, a novel OXA β-lactamase, responsible for imipenem resistance in Acinetobacter baumannii 6B92. Antimicrob. Agents Chemother. 44:196-199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Héritier, C., L. Poirel, D. Aubert, and P. Nordmann. 2003. Genetic and functional analysis of the chromosome-encoded carbapenem-hydrolyzing oxacillinase OXA-40 of Acinetobacter baumannii. Antimicrob. Agents Chemother. 47:268-273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Khashe, S., and J. M. Janda. 1998. Biochemical and pathogenic properties of Shewanella alga and Shewanella putrefaciens. J. Clin. Microbiol. 36:783-787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Naas, T., and P. Nordmann. 1999. OXA-type β-lactamases. Curr. Pharm. Design 5:865-879. [PubMed] [Google Scholar]
- 14.National Committee for Clinical Laboratory Standards. 2001. Performance standards for antimicrobial susceptibility testing. Eleventh informational supplement. M100-S11. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 15.Paccalin, M., G. Grollier, G. Le Moal, F. Rayeh, and C. Camiade. 2001. Rupture of a primary aortic aneurysm infected with Shewanella alga. Scand. J. Infect. Dis. 33:774-775. [DOI] [PubMed] [Google Scholar]
- 16.Paton, R., R. S. Miles, J. Hood, and S. G. B. Amyes. 1993. ARI-1: β-lactamase mediated imipenem resistance in Acinetobacter baumannii. Int. J. Antimicrob. Agents 2:81-88. [DOI] [PubMed] [Google Scholar]
- 17.Philippon, L. N., T. Naas, A.-T. Bouthors, V. Barakett, and P. Nordmann. 1997. OXA-18, a class D clavulanic acid-inhibited extended-spectrum β-lactamase from Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 41:2188-2195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Poirel, L., D. Girlich, T. Naas, and P. Nordmann. 2001. OXA-28, an extended-spectrum variant of OXA-10 β-lactamase from Pseudomonas aeruginosa and its plasmid- and integron-located gene. Antimicrob. Agents Chemother. 45:447-453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Poirel, L., C. Héritier, and P. Nordmann. 2004. Chromosome-encoded Ambler class D β-lactamase of Shewanella oneidensis as a progenitor of carbapenem-hydrolyzing oxacillinase. Antimicrob. Agents Chemother. 48:348-351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Poirel, L., C. Héritier, I. Podglajen, W. Sougakoff, L. Gutmann, and P. Nordmann. 2003. Emergence in Klebsiella pneumoniae of a chromosome-encoded SHV β-lactamase that compromises the efficacy of imipenem. Antimicrob. Agents Chemother. 47:755-758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Poirel, L., C. Héritier, V. Tolün, and P. Nordmann. 2004. Emergence of oxacillinase-mediated resistance to imipenem in Klebsiella pneumoniae. Antimicrob. Agents Chemother. 48:15-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sambrook, J., and D. W. Russell. 2001. Molecular cloning: a laboratory manual, 3rd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 23.Simidu, U., K. Kita-Tsukamoto, T. Yasumoto, and M. Yotsu. 1990. Taxonomy of four marine bacterial strains that produce tetrodotoxin. Int. J. Syst. Bacteriol. 40:331-336. [DOI] [PubMed] [Google Scholar]
- 24.Truper, H. G., and L. D. Clari. 1997. Taxonomic note: necessary correction of specific epithets formed as substantives (nouns) “in apposition.” Int. J. Syst. Bacteriol. 47:908-909. [Google Scholar]
- 25.Vogel, B. F., K. Jorgensen, H. Christensen, J. E. Olsen, and L. Gram. 1997. Differentiation of Shewanella putrefaciens and Shewanella alga on the basis of whole-cell protein profiles, ribotyping, phenotypic characterization, and 16S rRNA gene sequence analysis. Appl. Environ. Microbiol. 63:2189-2199. [DOI] [PMC free article] [PubMed] [Google Scholar]