Abstract
Glucokinase (GK) is activated by glucose binding to the substrate site, is inhibited by GK regulatory protein (GKRP) but stimulated by GK activator drugs (GKAs). To further explore the mechanisms of these processes we studied pure recombinant human GK (normal enzyme and a selection of 31 mutants) using steady state kinetics of the enzyme and tryptophan fluorescence (TF). TF studies of the normal binary GK/glucose complex corroborate recent crystallography showing that it exists in a closed conformation greatly different from the open conformation of the ligand free structure but indistinguishable from the ternary GK/glucose/GKA complex. GKAs did activate and GKRP did inhibit normal GK while its TF was doubled by glucose saturation. However, the enzyme kinetics, GKRP inhibition, TF enhancement by glucose and responsiveness to GKA of the selected mutants varied greatly. Two predominant response patterns were identified accounting for nearly all mutants: 1) GK mutants with a normal or close to normal response to GKA, normally low basal TF (indicating an open conformation), some variability of kinetic parameters (kcat, glucose S0.5, nH and ATP Km) but usually strong GKRP inhibition (13/31); and 2) GK mutants that are refractory to GKAs, exhibit relatively high basal TF (indicating structural compaction and partial closure), usually show strongly enhanced catalytic activity primarily due to lowering of the glucose S0.5 but with reduced or no GKRP inhibition in cases (14/31). These and pertinent literature data are best explained by envisioning a common allosteric regulator region with spatially non overlapping GKRP and GKA binding sites.
Keywords: Glucokinase, Glucose, Glucokinase Mutants, GKRP, GKA, Enzyme Kinetics, Tryptophan Fluorescence, Fluorescence Quantum Yield
INTRODUCTION
Substrate activation and allosteric regulation of enzymes and transporters are essential features of homeostasis in cells and organisms. Hemoglobin is the classical illustration of this principle in the physiological chemistry of oxygen [1]. Glucose activation of GK and allosteric inhibition by GKRP or activation by GKAs of the enzyme are other striking examples of these fundamental mechanisms [2–7], in this case of great importance for glucose homeostasis and the pharmacotherapy of diabetes mellitus. However, a full molecular understanding of such processes is frequently lacking, even for hemoglobin.
The present report is an attempt to contribute to our knowledge about GK regulation. GK, the low affinity isoform of hexokinases, plays a critical role in glucose homeostasis as the glucose sensor in pancreatic β-cells controlling glucose stimulated insulin release (GSIR) and, equally important, as regulator of hepatic glycolysis, glycogen synthesis and gluconeogenesis [4,5,7]. Regulation of the enzyme takes place at several levels including control of gene expression and protein degradation (on a time scale of minutes to hours) but also by substrate activation and allosteric modification (on a time scale of seconds to minutes) [5,7–10]. A single gene endowed with two promoters directs constitutive GK synthesis in the neuro-endocrine system including the pancreatic beta-cells and gonadotropes (regulated by the upstream promoter) or in the liver (regulated insulin dependently by the downstream promoter). Acute regulation of GK is mediated by its substrate glucose, by allosteric modifiers (GKRP, GKAs, the bifunctional enzyme F6P-2-P-kinase/phosphatase and the proapoptotic factor BAD [3–6,11,12] and by compartmental redistribution within the cell involving the nucleus, mitochondria, hormone granules and the Golgi apparatus [4,12,13]. Activation of GK by its substrate glucose in the physiological range of 4–12 mM is the basis of the enzyme’s cooperativity with regard to glucose and is caused by the process explained by a “mnemonic” or “ligand induced slow transition” mechanism during which GK changes from a low affinity open conformation to a high affinity closed conformation [2,14,15]. These two explanations have their corollary in the “induced fit” hypothesis or the concept of a ”preexisting equilibrium”. Accumulating evidence favors the idea of a “preexisting equilibrium” [16–19]. The transition process and its reversal is much slower than the catalytic cycle explaining the sigmoidal glucose dependency of the phosphorylation reaction. It is readily observable by TF recordings [15,20,21]. Superimposed onto this basic kinetic cooperativity with regard to glucose are allosteric control mechanism involving inhibition by GKRP and activation by GKAs. Inhibition by GKRP is a liver specific process, discovered by van Schaftingen [3,4]. GKRP is a 65 kD protein of unknown crystal structure. It is a primarily nuclear protein that binds to GK competitively with glucose and sequesters the inhibited enzyme in the nucleus. Its effectiveness is enhanced by F6P but counteracted by F1P which explains the stimulation of hepatic glycolysis by low levels of fructose. Glucose and F1P dissociate the nuclear GK/GKRP complex thus initiating translocation from the nucleus to the cytosol and thereby activating glycolysis and glycogen synthesis. The limited structural information is part of the reason that delineation of the interface between GK and GKRP remains disputed. The discovery of GKAs was reported in 2003 [5,6]. All currently known GKAs are pharmacological agents and the search for postulated physiological activators has been unsuccessful so far. GKAs are non essential allosteric modifiers of hepatic and islet GK. Countless patented GKA structures have a comparable pharmacophore with variable affinities to a well defined allosteric binding site of GK. GKAs lower the glucose S0.5 as much as tenfold and usually increase the kcat as much as twofold. They markedly increase the ATP Km at glucose below the glucose S0.5 and have variable strength in lowering the nH [5, 22]. As a result they enhance GSIR from the pancreas and stimulate hepatic glucose uptake and glycogen synthesis but curb glucose production. The most compelling evidence for the dominant role of GK in glucose homeostasis stems from the biochemical genetic analysis of more than 600 mutations in man [23] discovered in individuals with hyperinsulinism (HI) due to activating mutations and with diabetes mellitus due to inactivating mutations (mild forms when only one allele is affected in Maturity Onset Diabetes of the Young or MODY-2 and severe forms when both alleles are involved in Permanent Neonatal Diabetes Mellitus or PNDM). The recent demonstration of high efficacy of GKAs to lower blood glucose in patients with type 2 diabetes mellitus (T2DM) further underscores the medical relevance of GK’s role in glucose homeostasis [24].
In this study we have selected 31 GK mutants to explore the nature of the interaction between GK and its best known binding partners glucose, GKRP and GKAs. Enzyme kinetics and TF were used to investigate the actions of glucose, GKRP and GKA on function and structure of GK. Highly relevant published results were also considered and contributed critically to this exploration. The results of this investigation allowed us to conceptualize an allosteric regulatory region of the enzyme with clearly defined separate binding sites for GKRP and GKAs contrasted to the remote substrate binding domain with distinct contact areas for glucose and MgATP. The structural visualization of the glucose induced slow transition provides plausible corollaries for facilitating GKRP dissociation from and GKA binding to GK.
MATERIALS AND METHODS
Materials
D-(+)-glucose, SigmaUltra and of 95% purity by GC, was supplied by Sigma Chem. Co. (St. Louis, MO). The non fluorescent GKA (RO0274375-000) used here was discovered, synthesized and characterized by Hoffmann-La Roche, Nutley NJ (25). Its structure is shown below. Water used for all solutions was first deionized using Millipore reverse osmosis and then glass distilled.
Selection of GK mutants
We selected 31 GK mutants from our database of more than 100 well characterized mutants based primarily on kinetic criteria rather than being guided by the GK linked clinical phenotypes because the correlation between molecular features of the mutants and clinical manifestation is complicated by cell biological factors for example functional and structural instability [26]. The goal was to cover as wide a range as practical from strongly inhibited to strongly activated enzymes. For this purpose we used the GK “activity index” [27–29] or AI (kcat/S0.5nH) × (2.5/2.5 + ATP km) and achieved a 600 fold range from about 0.1–60 divided into two groups: thirteen enzymes with AIs from 0.09 to 1.60 including the control (AI = 1.37) and 19 enzymes with AIs from 2.08 to 57.3. This selection of enzymes featured the range of responsiveness to the allosteric modifiers GKA and GKRP from refractory to fully reactive required by the research plan. Extremes of mutational inactivation were avoided because very low D-glucose affinities and/or catalytic capacities decrease the quality of the biochemical analysis. And finally proteins with sufficient yields and high stability during storage were chosen to allow extensive kinetic studies and fluorescence analysis. Most of these enzymes have been studied and partially characterized before using their GST fusion proteins (but not pure GK) and have been described in previous publications [23, 27–31] which reported on the biochemical genetics of GK linked hyper- and hypoglycemia in man (wild type enzyme, V62M, S64P, S64Y, T65I, G68V, G68K, G72R, V91L, M197I, C213R, Y214C, C252Y, S263P, M298K, S336L, V389L, K414E, E442K, V452L, 454-alanine ins.,V455M and A456V). Seven were activating or inactivating mutants that had been designed in the course of the present or previous studies (M197E, M197E/A379T, M197L, Y214A, Y214A/V452A and Y215A). Two were recently identified in mice made diabetic by ENU treatment, i.e. K140E and P417R, and relevant biological data are being prepared for separate publication [32]. And finally, one was an incidental mutant that had been discovered in the course of GK mutagenesis experiments (A379T [30]).
Enzyme purification
Recombinant wild type and mutant human β-cell GKs were generated and expressed as GST fusion proteins in E. coli as previously described [21]. GST-GK fusion proteins were cleaved with factor Xa and submitted to another round of purification by removing GST with glutathione agarose and Factor Xa with Benzamidine Sepharose 6B following protocols provided by the manufacturer. Point mutations were introduced into the pGEX-3X vector using the QuickChange site-directed mutagenesis kit (Stratagene, La Jolla, California, USA). All mutants were transformed into E. coli cells and verified by DNA sequencing [21].
All mutant GK proteins were expressed in significant amounts as indicated by the yields in terms of mg protein per liter growth medium and were found to be essentially pure as demonstrated by the presence of a single band at 50 or 75 kDa on phast gel electrophoresis for GK or GST-GK, respectively. Purified GST-protein was stored at −80 °C, either with 50 mM glucose or in the absence of glucose in a medium containing 25 mM TRIS-HCl, 100 mM KCl, 30% glycerol and 2.5 mM dithiothreitol, pH 7.6 [21]. Immediately following cleavage and removal of GST, the enzyme was stored in buffer lacking glycerol but containing 50 mM glucose. Glucose was removed by dialysis and the enzyme (usually concentrated about twofold) was then stored in glucose and glycerol free buffer until used for kinetic analysis or spectrofluorimetry. Recombinant human GKRP was prepared as previously described [27,28].
Enzyme Kinetics
Kinetic properties of GK were determined as previously described [21,27,28]. GK activity was measured by spectrometry using an NADP+ coupled assay with glucose-6-phosphate dehydrogenase. The reaction medium contained: 6 mM MgCl2, 0.1 % bovine serum albumin, 150 mM KCl, 100 mM HEPES, 1 mM NADP+, 5 U/ml glucose-6-P dehydrogenase, 5 mM ATP, and 2 mM dithiothreitol. The following modifications to the protocol were made: In protocol A, kinetic studies were usually carried out with 11 glucose dilutions between 0–100 mM in the presence of 5 mM ATP. In protocol B, a glucose concentration at 10 × S0.5 was used but with varying ATP concentrations with 1 mM MgCl2 in excess. GKRP inhibition of GK and its mutants was assayed as described previously with minor modifications [27,28]. The assays were run at the corresponding glucose S0.5 and at 70% ATP saturation with 1 mM excess MgCl. Note that the pH of the buffer was 7.1 and the KCl was lowered to 25 mM compared to 150 mM in the routine GK assay. Because of limited availability of recombinant huGKRP and the large number of mutants we determined the relative inhibition at two GK/GKRP molar ratios (0.88 and 1.76) of the near linear portion of the dose response curve and report the mean value of the effects at nominally 1.26 excess of the inhibitor both in the absence and presence of 10 µM sorbitol-6-P.
Spectroscopic measurements
UV and visible absorption spectra were measured using a Hitachi Perkin-Elmer U-3000 spectrophotometer (Newtown, PA). Fluorescence intensity and spectra were measured with a Fluorolog-3-21 Jobin-Yvon Spex Instrument SA (Edison, NJ) equipped with a 450 W Xenon lamp for excitation and a cooled R2658P Hamamatsu photomultiplier tube for detection. Ninety-degree geometry was used for all measurements. 295 nm excitation wavelengths were used to observe fluorescence emission in the 290 nm – 500 nm range. Slit width was set to provide a band-pass 4 nm for excitation and 3 nm for emission. For time-dependent intensity glucose titrations a band-pass for excitation was 0.5 nm at λexc = 280 nm and for emission 12 nm at λem = 340 nm. A thermostatted cell holder maintained sample temperature. The glucose concentration titration curves were obtained by adding the sugar stepwise at increasing concentrations to the fluorescence cuvette. The stock concentration was 1 M and the pH was adjusted to 7.3. Stock solutions were prepared fresh before each titration but given sufficient time to anomerize The aliquots of the stock solution was added to the protein solution which was about 100 microgram/ml and contained 5 mM phosphate buffer with 100 mM KCl and 1 mM DTT, pH7.3.
Determination of Tryptophan Fluorescence Quantum Yield
Fluorescence quantum yields of tryptophan (Φ) were determined for GK wild type and mutants using the following equation [33]:
ΦS = ΦR (AR/AS) (FS/FR) (n2S/n2R)
The subscripts S and R refer to sample and reference standard, respectively; AR and AS denotes the Absorbances at the excitation wavelength (A<0.05 to avoid the inner filter effect). FS and FR denote the integral intensities of the recorded fluorescence spectra measured under identical instrument settings. nS and nR are the refractive indices. Fluorescence quantum yields were determined relative to N-acetyl-L-tryptophanamide (NATA, Φ = 0.14) in water as reference [34]
RESULTS AND DISCUSSION
1) Experimental Aspects: Functional comparison of GST-GK with GK after cleaving the fusion protein and pH dependencies of selected study parameters
Many previously published studies were performed with recombinant GST-GK and it was assumed that the pure, GST free authentic enzymes would be functionally indistinguishable from the fusion proteins [23,27–31]. This assumption remained to be comprehensively tested. In the present investigation we therefore prepared pure GKs by cleaving off the N-terminal GST tag from wild type and 31 mutants. We demonstrated more than 95% purity by the presence of a single 50 KD band on phast gel (not shown) and compared the characteristics of GST-GK with the corresponding pure enzymes (see the results of Tables 1 and 2 and On Line Supplemental Tables 1 and 2). This report expands to 43 cases the comparison of kinetics of GST-GK with pure GK following cleavage of the fusion protein. The present and published data show that removal of the N-terminal GST tag has little impact, but a single exception of Y214A/V452A as was discussed in an earlier publication is acknowledged [21]. Supplemental Figures 1 and 2 summarize that the kinetic and binding constants for the two preparations tend to agree since the kcat, the glucose S0.5, the ATP Km and the Hill coefficient nH are within experimental error comparable. This observation demonstrates that previously published biochemical genetic information and interpretations on "Glucokinase Disease" (almost entirely based on the analysis of GST tagged recombinant human glucokinase preparations) are biologically meaningful and that the present biophysical studies requiring pure enzyme in which the source of the TF signal is limited to the GK molecule are feasible.
Table 1.
Kinetic Parameters of WT and Mutant GK. Enzymes were Purified as GST Fusion proteins but then cleaved and stored in the Presence of 50mM D-Glucose
| Parameters | Yield (mg/L) |
kcat (sec−1) |
Glucose S0.5 (mM) |
nH | ATPKm (mM) |
GI |
|---|---|---|---|---|---|---|
| Mutants | ||||||
| WT (n=3) | 15.8±7.30 | 62.8±4.11 | 7.45±0.26 | 1.77±0.10 | 0.46±0.02 | 1.37±0.16 |
| V62M (n=4) | 29.4+−6.35 | 47.2+−12.6 | 5.9+−1.93 | 1.38+−0.18 | 0.521+−0.04 | 3.61+−1.41 |
| S64P (n=2) | 9.99 (11.1, 8.84) | 83.3 (83.7, 82.9) | 2.07 (1.94, 2.2) | 1.31 (1.32, 1.30) | 0.32 (0.35, 0.30) | 23.1 (24.8, 21.5) |
| S64Y (n=2) | 2.65 (2.2, 3.08) | 114 (111, 116) | 1.89 (1.96, 1.82) | 1.5 (1.56, 1.43) | 1.59 (1.63, 1.55) | 24.4 (23.1, 25.7) |
| T65I (n=2) | 28.8 (32.3, 25.3) | 22.6 (26.9, 18.2) | 1.69 (1.70, 1.68) | 1.30 (1.36, 1.24) | 0.57 (0.68, 0.45) | 8.29 (8.99, 7.58) |
| G68V (n=2) | 11.2 (11.1, 11.3) | 62.7 (56.1, 69.2) | 2.2 (2.2, 2.21) | 1.35 (1.42, 1.29) | 0.31 (0.30, 0.33) | 18.7 (17.2, 20.2) |
| G68K (n=2) | 25.3 (25.7, 24.8) | 43.2 (46.6, 39.8) | 2.34 (2.35, 2.32) | 1.33 (1.33, 1.32) | 0.39 (0.43, 0.35) | 10.9 (11.2, 10.5) |
| G72R (n=3) | 31.9 +−3.2 | 29.8 +−1.5 | 7.38 +−1.66 | 1.42 +−0.07 | 0.72 +−0.19 | 1.57+−0.5 |
| V91L (n=2) | 26.3 (26.7, 25.8) | 60.6 (70.3, 50.8) | 1.66 (1.78, 1.54) | 1.42 (1.56, 1.28) | 0.48 (0.42, 0.53) | 24.0 (27.9, 20.1) |
| K140E (n=4) | 14.4±1.51 | 40.0±1.36 | 10.8±1.13 | 1.57±0.13 | 0.35±0.08 | 0.78±0.08 |
| M197E (n=2) | 1.76 (1.92, 1.60) | 17.7 (19.9, 15.4) | 41.6 (46.2, 37.0) | 1.40 (1.47, 1.33) | 0.40 (0.47, 0.32) | 0.15 (0.16, 0.14) |
| M197I (n=2) | 6.59 (7.36, 5.82) | 58.1 (66.4, 49.8) | 2.49 (2.80, 2.18) | 1.80 (1.87, 1.73) | 1.39 (1.43, 1.35) | 6.33 (6.98, 5.68) |
| M197I-A397T (n=2) | 43.6 (48.4, 38.8) | 50.2 (53.9, 46.4) | 5.81 (6.34, 5.28) | 1.36 (1.42, 1.30) | 2.71 (2.81, 2.61) | 2.08 (2.18, 1.98) |
| M197L (n=2) | 34.0 (34.7, 33.2) | 62.6 (70.4, 54.7) | 4.03 (4.14, 3.91) | 1.60 (1.67, 1.53) | 1.31 (1.45, 1.17) | 4.05 (4.42, 3.67) |
| C213R (n=1) | 24.3 | 50.0 | 21.6 | 1.43 | 0.89 | 0.39 |
| Y214A (n=2) | 22.7 (23.4, 22.0) | 117 (123, 111) | 1.41 (1.48, 1.34) | 1.41 (1.47, 1.35) | 0.92 (0.94, 0.90) | 57.3 (59.0, 55.6) |
| Y214A/V452A (n=3) | 42.8±2.38 | 23.1±2.38 | 0.55±0.05 | 0.88±0.02 | 1.42±0.29 | 31.6±2.17 |
| Y214C (n=2) | 31.0 (33.2, 28.7) | 65.3 (71.0, 59.6) | 1.35 (1.39, 1.30) | 1.55 (1.60, 1.49) | 1.08 (1.25, 0.91) | 27.0 (28.0, 25.9) |
| Y215A (n=2) | 22.6 (23.8, 21.3) | 44.7 (47.5, 41.9) | 2.07 (2.10, 2.03) | 1.46 (1.48, 1.43) | 0.58 (0.59, 0.57) | 9.52 (10.1, 8.93) |
| C252Y (n=1) | 10.8 | 29.3 | 31.6 | 1.54 | 0.75 | 0.09 |
| S263P (n=2) | 22.9 (21.8, 24.0) | 44.6 (41.8, 47.4) | 9.70 (9.75, 9.64) | 1.66 (1.76, 1.56) | 0.63 (0.65, 0.61) | 0.89 (1.10, 0.68) |
| M298K (n=2) | 26.8 (31.4, 22.1) | 37.7 (35.3, 40.0) | 10.8 (11.2, 10.4) | 1.28 (1.31, 1.25) | 3.32 (3.48, 3.15) | 0.80 (0.80, 0.79) |
| S336L (n=2) | 15.2 (16.2, 14.1) | 2.46 (4.07, 0.84) | 4.75 (4.75, 4.75) | 0.95 (0.97, 0.93) | 12.6 (13.0, 12.1) | 0.11 (0.17, 0.04) |
| A379T (n=2) | 17.4 (18.4, 16.3) | 61.2 (64.7, 57.7) | 12.3 (12.4, 12.1) | 1.60 (1.65, 1.54) | 0.87 (0.88, 0.85) | 0.90 (0.94, 0.85) |
| V389L (n=2) | 20.3 (21.7,18.8) | 67.1(68.6, 65.5) | 3.45(3.63, 3.26) | 1.54(1.49, 1.58) | 0.76(0.78, 0.73) | 7.53(7.44, 7.61) |
| K414E (n=1) | 12.9 | 19.9 | 5.69 | 1.52 | 1.53 | 0.78 |
| P417R (n=4) | 4.47±0.96 | 48.3±7.40 | 6.59±0.32 | 1.52±0.04 | 2.08±0.15 | 1.60±0.14 |
| E442K (n=2) | 30.3 (31.8, 28.8) | 52.6 (57.4, 47.7) | 5.24 (5.53, 4.95) | 1.72 (1.84, 1.59) | 1.50 (1.57, 1.42) | 2.15 (2.20, 2.09) |
| V452L (n-2) | 5.42 (4.45, 6.38) | 122 (98.4, 145) | 2.27 (2.38, 2.17) | 1.46 (1.47,1.44) | 0.53 (0.57, 0.50) | 29 (20.8, 37.1) |
| 454-A (n=2) | 15.9 (16.0, 15.7) | 52.2 (57.3, 47.1) | 1.38 (1.46, 1.30) | 1.62 (1.64, 1.60) | 0.31 (0.32, 0.30) | 32.8 (39.8, 25.8) |
| V455M (n=2) | 23.8 (25.4, 22.2) | 61.8 (62.2, 61.3) | 3.24 (3.32, 3.16) | 1.62 (1.63, 1.60) | 0.38 (0.39, 0.36) | 6.68 (6.93, 6.42) |
| A456V (n=2) | 6.86 (7.14, 6.58) | 65.6 (69.6, 61.5) | 1.91 (2.17, 1.65) | 1.37 (1.41, 1.32) | 0.35 (0.36, 0.34) | 23.1 (23.9, 22.3) |
Table 2.
Effect of Glucokinase activator (GKA) on recombinant human wild type and mutant Glucokinase (cleaved)
| Parameters | A (fold↑) |
Ec50 (uMA) |
B (fold↓) |
Ec50 (uMB) |
C (fold↑) |
Ec50 (uMC) |
nHill ctr/nHill act | ctrATPKm/actATPKm |
|---|---|---|---|---|---|---|---|---|
| GST-GCK | ||||||||
| WT (n=3) | 1.64±0.04 | 0.73±0.14 | 3.61±0.45 | 1.16±0.15 | 23.0±2.14 | 7.10±1.29 | 1.11±0.06 | 1.02±0.19 |
| V62M (n=2) | n.a. | n.a | n.a. | n.a. | n.a. | n.a. | 0.85 (0.91,0.78) | 1.04(1.36, 0.71) |
| S64P (n=20 | 0.72 (0.88, 0.55) | 7.52, na | 1.31 (1.28, 1.33) | 1.49 (0.74, 2.24) | 1.39 (1.33, 1.45) | 2.23 (1.34, 3.12) | 1.03 (1.00, 1.05) | 1.29 (1.29, 1.29) |
| S64Y (n=2) | Na, na | Na, na | 1.94 (2.12, 1.76) | 0.95 (0.54, 1.36) | 2.92 (3.19, 2.65) | 2.24 (2.40, 2.07) | 1.01 (0.9, 1.13) | 1.47 (1.49, 1.45) |
| T65I (n=2) | 2.01 (2.10, 1.92) | 4.47 (5.90, 3.03) | 1.67 (1.61, 1.72) | 1.55 (0.88, 2.21) | 4.37 (4.06, 4.67) | 6.34 (7.01, 5.67) | 0.96 (1.04, 0.88) | 0.98 (1.12, 0.83) |
| G68V (n=2) | 2.05 (2.00, 2.09) | 1.96 (2.16, 1.76) | 2.89 (2.86, 2.92) | 1.45 (1.40, 1.49) | 7.8 (7.45, 8.15) | 6.5 (6.35, 6.64 | 1.09 (1.09, 1.08) | 0.43 (0.47, 0.40) |
| G68K (n=2) | 2.14 (2.20, 2.07) | 2.82 (3.12, 2.52) | 3.30 (3.72, 2.88) | 1.71 (1.82, 1.60) | 14.7 (16.2, 13.1) | 7.70 (8.36, 7.03) | 0.99 (0.99, 0.99) | 0.62 (0.64, 0.60) |
| G72R (n=3) | 1.34 +−0.12 | 2.7 +−1.34 | 1.12 +−0.68 | 3.77 +−0.68 | 3.52 +−0.59 | 6.7 +−3.28 | 1.06 +−0.18 | 1.12 +−0.2 |
| V91L | 1.61 (1.68, 1.54) | 3.61 (5.12, 2.09) | 2.89 (2.97, 2.80) | 3.37 (4.23, 2.51) | 5.58 (5.88, 5.27) | 6.43 (6.43, 6.42) | 1.21 (1.38, 1.03) | 0.68 (0.79, 0.56) |
| K140E (n=4) | 1.73±0.07 | 1.87±0.70 | 4.52±0.48 | 1.68±0.51 | 22.7±1.69 | 10.2±1.35 | 0.99±0.10 | 1.14±0.17 |
| M197E (n=2) | 2.30 (2.39, 2.21) | 2.83 (3.72, 1.94) | 3.80 (3.92, 3.67) | 2.19 (2.25, 2.13) | 8.42 (11.6, 5.23) | 26.9 (32.4, 21.3) | 0.89 (0.94, 0.84) | 1.41 (1.44, 1.38) |
| M197I (n=2) | 1.68 (1.70, 1.66) | 1.01 (1.03, 0.98) | 3.36 (3.78, 2.94) | 1.98 (3.23, 0.72) | 16.1 (18.0, 14.1) | 3.88 (4.48, 3.27) | 1.44 (1.53, 1.35) | 1.30 (1.24, 1.34) |
| M197I-A397T (n=2) | 1.78 (1.90, 1.65) | 0.94 (1.09, 0.79) | 4.97 (5.43, 4.51) | 0.75 (0.90, 0.60) | 21.3 (21.3, 21.2) | 6.63 (7.00, 6.25) | 1.08 (1.10, 1.06) | 1.41 (1.48, 1.34) |
| M197L (n=2) | 1.69 (1.73, 1.65) | 1. 03 (1.20, 0.86) | 5.16 (5.88, 4.44) | 5.54 (8.02, 3.06) | 24.0 (27.9, 20.1) | 20.9 (34.8, 7.05) | 1.05 (1.19, 0.91) | 1.29 (1.26, 1.33) |
| C213R (n=1) | 1.30 | 1.97 | 3.97 | 1.44 | 6.94 | 2.41 | 0.89 | 1.48 |
| Y214A (n=2) | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | 0.96 (0.99, 0.92) | 0.98 (1.09, 0.87) |
| Y214A/V452A (n=3) | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | 0.89 ± 0.05 | 1.08 ± 0.06 |
| Y214C (n=2) | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | 0.96 (1.01, 0.91) | 1.07 (1.16, 0.97) |
| Y215A (n=2) | 1.84 (1.85, 1.82) | 2.33 (3.10, 1.55) | 3.43 (4.11, 3.74) | 1.34 (1.80, 0.87) | 9.46 (10.1, 8.82) | 9.01 (15.9, 2.11) | 1.23 (1.13, 1.33) | 0.96 (0.96, 0.96) |
| C252Y (n=1) | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | 1.00 | 1.21 |
| S263P (n=2) | 1.50 (1.44, 1.56) | 1.64 (2.09, 1.18) | 5.84 (6.74, 4.93) | 0.96 (0.83, 1.08) | 24.7 (21.6, 27.7) | 9.73 (9.95, 9.50) | 1.05 (1.17, 0.92) | 1.23 (1.25, 1.21) |
| M298K (n=2) | 1.32 (1.37, 1.26) | 0.32 (0.13, 0.50) | 10.1 (5.19, 15.0) | 0.47 (0.73, 0.21) | 11.2 (10.6, 11.7) | 10.3 (6.76, 13.9) | 0.86 (0.94, 0.77) | 0.70 (0.73, 0.66) |
| S336L (n=2) | 1.36 (1.38, 1.34) | 2.59 (3.47, 1.71) | 7.11 (7.20, 7.01) | 1.30 (1.35, 1.25) | 7.92 (8.00, 7.84) | 7.86 (9.64, 6.08) | 0.94 (0.96, 0.91) | 1.01 (1.01, 1.00) |
| A379T (n=2) | 1.60 (1.67, 1.52) | 2.16 (2.98, 1.34) | 4.65 (4.35, 4.95) | 1.66 (2.37, 0.94) | 18.6 (21.2, 16.0) | 12.4 (15.1, 9.65) | 1.13 (1.15, 1.13) | 1.13 (1.19, 1.07) |
| V389L (n=2) | 1.44 (1.37,1.5) | 1.6 (2.37,0.82) | 3.29 (3.59, 2.98) | 2.64 (3.23,2.04) | 8.57 (7.79,9.35) | 6.31 (6.76,5.86) | 1/11 (1.26,0.96) | 1.1 (1.03, 1.16) |
| K414E (n=1) | 1.60 | 1.13 | 5.74 | 1.93 | 25.1 | 11.1 | 1.07 | 0.95 |
| P417R (n=4) | 1.46±0.02 | 1.78±0.49 | 4.66±0.25 | 1.88±0.53 | 15.0±0.56 | 12.2±0.99 | 1.01±0.02 | 1.12±0.03 |
| E442K (n=2) | 1.73 (1.80, 1.63) | 1.08 (1.26, 0.90) | 3.36 (3.34, 3.37) | 1.37 (2.15, 0.58) | 15.1 (17.0, 13.1) | 7.40 (7.45, 7.34) | 1.15 (1.24, 1.06) | 1.09 (1.14, 1.03) |
| V452L (n=2) | 1.49, na | 1.20, na | 2.86 (2.97, 2.75) | 2.56 (2.35, 2.78) | 6.23 (6.42, 6.03) | 6.16 (5.00, 7.31) | 1.13 (1.11, 1.15) | 0.99 (0.87, 1.11) |
| 454-A (n=2) | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | 1.00 (1.33, 1.12) | 1.24 (1.23, 1.24) |
| V455M (n=2) | 1.30 (1.32, 1.27) | 3.08 (3.80, 2.35) | 1.23 (1.34, 1.11) | 0.45 (0.55, 0.34) | 2.85 (2.86, 2.84) | 5.47 (5.69, 5.24) | 0.97 (0.99, 0.94) | 0.95 (1.06, 0.84) |
| A456V (n=2) | n.a | n.a. | 3.60 (4.50, 2.70) | 1.23 (1.29, 1.16) | 7.55 (11.3, 3.80) | 8.15 (12.2, 4.10) | 0.95 (1.22, 0.68) | 1.57 (1.64, 1.50) |
Note: Defined as follows: A denotes the maximal fold drug effect on the kcat, B on the glucose affinity and C on the activity index.
Ec50 values are half maximal drug concentrations for the effect on the Kcat, the glucose affinity, and the activity index.
Pure GK was also used to obtain essential information not previously recorded, i.e., the pH dependencies of the relative quantum yields of the glucose induced fluorescence increase and of the effect that GKAs have on the glucose KDs of the wild type enzyme (see supplemental Figures 3 and 4 and also supplemental Tables 3 and 4). Strong pH dependencies were observed for both parameters with optima in the physiological range.
2) Effects of point mutations on GK characteristics including GKA responsiveness
The physiological substrate D-glucose was used for a comprehensive characterization of the 32 pure GK protein species. The studies included basic kinetics, kinetics influenced by GKA activation or GKRP inhibition and characteristics of substrate binding with TF (Tables 1–5). The results of the wide ranging analysis summarized in these tables are recorded in the order of the linear sequence of human islet GK as the least biased data base presentation. A full data set for practically every enzyme was obtained with the exception of GKRP inhibition data for S336L because of its prohibitively low kcat and relatively high ATP Km. The responsiveness to GKA activation and GKRP inhibition varied greatly ranging from normal to totally refractory as anticipated (for enzymes which had been studied before using GST fusion proteins) or predicted by extrapolation based on the nature of the mutation. The comprehensive glucose binding studies using TF made possible by employing pure GK add a new dimension to the kinetic analysis of this set of mutants. All TF based glucose binding curves were hyperbolic in agreement with previous reports (Supplemental Figure 5) but the spectra and the quantum yields of the basal, unliganded state and the glucose induced relative fluorescence increases and maximal intensities varied greatly among the different enzymes. Examples of these glucose titrations and of the fluorescence emission spectra are shown in supplemental Figures 5 and 6. The impact of a particular mutation on the basal fluorescence and the glucose induced fluorescence increase can be surprisingly small considering the magnitude of the functional change it causes, as seen in the case of the activating V455M (compare the corresponding kinetic and binding constants of Tables 1–5 and supplemental Figure 6A with 6C). This can be contrasted to cases with dramatic changes of basal and glucose induced fluorescence of enzymes that are kinetically not very far from normal (e.g. G72R and S263P as apparent from Tables 1–5 and supplemental Figure 6B and 6D). It is remarkable that only 5 of the 31 mutants (M197I, M197L, V389L, E442K and V455M) have a normal or close to normal emission maximum of 326–327 nm in the basal state in contrast to the others which show red shifts to as high as 332–333 nm and that all but 2 mutants (T65I and C252Y) show a significant blue shift upon glucose saturation (Table 5 and examples in Figure 6E and 6F). Previous studies with single tryptophan enzymes suggest that the red shifts of the emission maxima in the basal state are due to the influence of W99 and W167 but that the process of glucose binding is then responsible for the rebound to the blue by the altered TF spectrum of W167 [21]. We arrive at this interpretation from quantitatively analyzing the basal and glucose dependent TF spectra of the three glucokinase mutants which retain only a single tryptophan. The TF spectra of the free and glucose saturated native enzyme can be explained by simple additivity of the individual spectra of the single tryptophan mutants [21]. The result is indeed remarkable in view of the fact that two tryptophan substitutions are needed to generate mutants containing only one tryptophan which causes significant functional changes. The results imply that the backbone structures of these mutants are maintained. All together these studies strengthen our interpretation that extrapolations from the spectra to the backbone structures of the open (ligand free) and closed (glucose saturated) conformations are valid.
Table 5.
Average quantum yields of Trp fluorescence of GK and GK mutants
| GK- enzymes | ϕaverage no glucose |
ϕaverage with glucose |
Em. max no glucose |
Em. max with glucose |
|---|---|---|---|---|
| WT | 0.094 | 0.187 | 326 | 326 |
| V62M | 0.136 | 0.187 | 331 | 327 |
| S64P | 0.124 | 0.172 | 332 | 327 |
| S64Y | 0.126 | 0.191 | 329 | 327 |
| T65I | 0.140 | 0.198 | 328 | 328 |
| G68K | 0.138 | 0.200 | 332 | 328 |
| G68V | 0.130 | 0.190 | 330 | 327 |
| G72R | 0.143 | 0.184 | 331 | 327 |
| V91L | 0.139 | 0.190 | 330 | 327 |
| K140E | 0.100 | 0.136 | 329 | 327 |
| M197E | 0.094 | 0.123 | 331 | 328 |
| M197I | 0.098 | 0.186 | 327 | 326 |
| M197I/A379T | 0.096 | 0.145 | 331 | 327 |
| M197L | 0.096 | 0.195 | 326 | 325 |
| C213R* | 0.119 | 0.155 | 332 | 329 |
| Y214A | 0.108 | 0.196 | 328 | 326 |
| Y214A/V452A* | 0.122 | 0.170 | 328 | 325 |
| Y214C | 0.118 | 0.173 | 328 | 326 |
| Y215A | 0.129 | 0.180 | 330 | 326 |
| C252Y* | 0.123 | 0.147 | 333 | 331 |
| S263P | 0.126 | 0.155 | 332 | 328 |
| M298K | 0.117 | 0.193 | 328 | 326 |
| S336L | 0.100 | 0.141 | 329 | 327 |
| A379T | 0.092 | 0.151 | 331 | 327 |
| V389L | 0.102 | 0.161 | 326 | 326 |
| K414E | 0.132 | 0.163 | 332 | 329 |
| P417R | 0.098 | 0.133 | 331 | 328 |
| E442K | 0.110 | 0.174 | 327 | 325 |
| V452L | 0.117 | 0.180 | 329 | 327 |
| 454-Ala | 0.131 | 0.185 | 330 | 325 |
| V455M | 0.102 | 0.191 | 327 | 326 |
| A456V | 0.102 | 0.153 | 330 | 325 |
GKA RO0274375 (RO-cpdA) was used in the present experiments because previous kinetic and biophysical studies from this laboratory on the mechanism of action of GKAs were done with this compound and because it is not fluorescent [25]. We were unable to study GKA/GKRP interactions directly because of limited supply of the inhibitor. However, GKAs of a great variety of structures were employed in the published reports that are relevant for the present study because this interaction was directly addressed. Five of these which address the critical issue of glucose dependency of GKA binding to the enzyme deserve discussion here [35–39]. In the present study RO0274375 did not change TF of wild type GK in the absence of glucose using standard assay conditions which is interpreted as evidence that the drug does not bind. This finding is in agreement with two other studies using structurally different GKAs and different methodologies [36,37]. In a third study glucose independent drug binding was demonstrated at high GKA concentrations based on increased TF of GK with two different GKAs, one of them fluorescent but with fluorescence characteristics allowing differentiation from TF. It was also demonstrated that the EC50 and KD of GKAs decrease markedly with increasing glucose concentrations [35]. It is perhaps not surprising that GKA structure affects the glucose dependency of drug binding. However, as important as differences of GKA structures might be, incubation conditions and mutations of GK greatly influence the glucose dependency of GKA binding. For example inclusion of 20% glycerol in the buffer or mutation of W99 have a profound impact allowing RO-cpdA binding in the absence of glucose, again judging from the effect on TF (Zelent et al., to be published). The present conclusion that GKRP and GKA binding to GK are mutually exclusive is very strongly supported by these reports based on studies with other, structurally distinct GKAs [36–39]. Structure Activity Relationship studies of GKA/GKRP interaction in affecting GK have to be greatly expanded to develop a full understanding of this biochemically and pharmacologically significant question.
3) Testing the hypothesis that mutational activation of GK may lead to partial closure of the superopen GK conformation and might thereby interfere with allosteric regulation of the enzyme
It had been noticed earlier that certain mutants (C213R, Y214A/V452A and C252Y) have a high basal fluorescence and lack responsiveness to GKA [21]. This observation led to the speculation that a high basal TF could be a manifestation of a partially closed, compact conformation of the protein, reducing “pari passu” the responsiveness to allosteric modification by GKRP and GKAs. The present study is a test of this hypothesis which offered a constructive framework for interpreting the large mass of experimental data. It was therefore explored whether a correlation exists between the enzyme’s TF, both basal and glucose induced, and GK’s catalytic capacity (kcat/glucose S0.5), GKA responsiveness (as measured by the effect of the activator on the glucose KD and its S0.5) and responsiveness to the physiological inhibitor GKRP (Figure 1 graphically summarizes the results presented in Tables 1–5). The GKA effect on the glucose KD expressed as KD(CTRL)/KD(GKA) and plotted in the order of descending effectiveness was used as basis of comparison because this parameter is the simplest and does not involve the complex chemistry of catalysis and thus seemed to be most suitable for this purpose. A comparison of panels A–E of Figure 1 suggests strongly that the hypothesis is supported by the evidence. In general it is evident from comparing the response pattern depicted in panel A with those of panels B–E that good correlations or remarkable trends of correlations do exist between the glucose binding data and the other parameters.
Figure 1.
This Figure provides a summary of the experimental data. Panel A shows the GKA responsiveness of all 32 enzymes (wt and 31 mutants, 24 of these disease causing and the rest incidental or designed). GKA responsiveness is expressed as the ratio of KDs in the absence and presence of drug levels near saturating for the wild type enzyme (20 µM). The data are presented in two groups: normal or retaining a significant response (white) vs. uniformly low or absent response (black). Panel B shows the results of TF measurements in the absence (white and black) and presence (grey) of saturating levels of glucose expressed in terms of relative quantum yield. Note that 15/15 of GKA refractory mutants have a high basal quantum yield and that the majority of these is also GKRP resistant (11/15). Note also that there are 4 outliers in the left group with high basal TF, three of which are known to be structurally unstable (thermolabile). Panel C also shows GKA responsiveness, however based on steady state activity measurements of enzyme activity in the presence and absence of GKA. However the S0.5 with saturating GKA present was obtained from dose dependency studies with the drug level extending to saturation instead of using a fixed drug concentration as in panels A and B. Panel D shows the kcat/S0.5 ratios as a measure of catalytic capacity of the enzymes studied here. Note the value is high for all spontaneous mutants causing hyperinsulinemic hypoglycemia in heterozygous carriers. Panel E shows relative GKRP inhibition of GK as measured by steady state kinetic analysis with GKRP in 1.26 fold excess over GK present at 10 nanoM. These measurements were made in the absence (white and black) and in the presence of 10 µM sorbitol-6-P which enhances the effect of GKRP (gray bars). The six outliers are considered in the discussion.
A careful inspection of the proposed correlations of response patterns reveals apparent exceptions (Figure 1). Explanations are offered in the following in an attempt to deal with this issue. The correlation of patterns 1A and 1B shows 4 apparent exceptions: C213R, M298K, S263P and K414E. These mutations are all mildly inhibitory and cause an increase of basal fluorescence without altering GKA or GKRP responsiveness significantly. In addition S263P and M298K have been found to be more thermolabile than any other instability mutant known to date [32]. However, information is insufficient to explain the basal fluorescence increase nor is it sufficient to conclude that it is an expression of structural compaction. Comparing panels 1A and 1C correlations are apparently less than ideal for V389L and M298K. V389L (an HI causing activating mutation) and M298K (an inactivating, very thermo labile mutant causing MODY) are outside the allosteric activator region and their exceptional sensitivity to GKA has little impact on the conclusions. Also note that the glucose S0.5 is a parameter that is derived from the far more complex process of chemical catalysis than that expressed by the glucose KD which is undoubtedly reflected in subtle differences of their GKA responsiveness. Comparing panels 1A and 1D it appears that V62M, T65I, G72R and C252Y (all part of or close to the allosteric activator site) are outliers. However, lack of extensive activation (GKs 2, 5 and 8) or even reduction (GK 20) of catalytic capacity caused by substitution of amino acids in the allosteric activator site does not contradict the conclusions in view of the complex nature of the kcat/S0.5 expression. Interpreting the results of GKRP inhibition studies is perhaps the most complicated and challenging. The results with mutants K140E, M197E, V452L, G68K, Y214C and Y214A don’t seem to fit when panels 1A and 1E are compared. Decreased effectiveness of GKRP can have many causes: 1) The mutation may interfere with binding of GKRP to one of the two separate contact patches of GK considered here integral to the inhibitor binding site without influencing the GKA site which regulates conformational transitions. This seems to be the case for K140E and M197E both resulting in a charge change without affecting basal fluorescence or GKA responsiveness; 2) Activating mutations V452L, Y214C and Y214A may be effective in interfering with GKA binding and sufficiently strong to cause partial closure of GK conformation (as apparent by the increased basal fluorescence) but not strong enough to prevent GKRP binding and GK inhibition at low glucose levels. We have no plausible explanation for the exceptional behavior of G68K. Finally, it should be kept in mind that the different GK conformations considered here are probably present in a preexisting equilibrium which could be subtly altered by differential ligand binding [16–19]. It seems therefore that on the whole the pattern of GKRP inhibition of the present set of mutants is plausibly related to the other patterns. On balance we conclude that the correlations of patterns 1A – 1E are strong enough to support our views about the allosteric mechanisms underlying GKRP inhibition and GKA activation of GK discussed below.
4) Structural information on GK contributes greatly to the understanding of the present results
Current views about GK are greatly influenced by the crystal structures described by Kamata and colleagues [40] showing a ligand free “superopen” conformation which is contrasted to a “closed” ternary GK/glucose/GKA complex Recent crystallographic studies have greatly amplified this knowledge base by solving the structures of the binary GK/glucose complex (3IDH.pdb, also [41]), the ternary GK/glucose/MgANP complex (3FGU.pdb) and finally of the quaternary GK/glucose/MgANP/GKA complex (3ID8.pdb). This new crystallographic evidence suggests strongly that all glucose containing complexes are practically indistinguishable as illustrated here by the nucleotide free complexes when compared to the open structure of Kamata (Figure 2). The evidence from TF measurements demonstrates also that the binary GK/glucose complex does indeed resemble very closely the closed ternary GK/glucose/GKA because the fluorescence characteristics are practically the same (not shown). Based on this background we projected the mutated amino acids shown here to interfere with GKA action and also of those mutants that impair GK inhibition by GKRP onto the open and closed configurations, the latter with GKA present (Figures 3A and B; Figures 4A and B) and contrasted them to the projections of mutants ineffective in this regard (Figures 3C and D; Figures 4C and 4D). We also included in this graphic representation highly relevant published results of a mutational analysis of GKRP inhibition of Xenopus Laevis or human glucokinase showing that the amino acids E51, E52, H141, K142, K143 and K144 are involved in GKRP action and contrasting them with 12 others that are not which includes A114I, T116Q, M121G, Y125H, V154C, R155H, H156Q, E157T, D158N, T346V, L349R, R553Q [42,43]. The binding site for GKAs has been well delineated and includes the following amino acids: V62, R63, M210, I211, Y214, Y215, M235, V452 and V455 [5]. The results are striking. The projections show that GKA and GKRP bind to a common region of GK (termed allosteric regulator region) but interact with clearly separate domains in this region and that glucose binding to the substrate site affects the conformations of these domains differentially in a manner which causes the GKRP binding patches to separate and presumably dislocate the inhibitor while opening the GKA binding domain so that the activator has access to its contact amino acids. The concerted motion of these subdomains is depicted in Figure 5.
Figure 2.
Three GK structures in equilibrium. GK in the super open conformation (A, IV4t.pdb), in the binary closed structure (B, 3IDC.pdb) and the conformation of the closed ternary complex (C, IV4S.pdb) are shown. The tryptophans are in red, glucose is green and GKA is blue.
Figure 3.
This figure was prepared to show the location of those amino acids which are implicated in GKA and GKRP binding (A and B) as contrasted to amino acids which are not (C and D). The structures are viewed from the side. We used in addition to our own results (red, blue and light blue) pertinent data on 18 mutants (gray) reported in the literature [42,43]). Note that the activating mutants M197I, V389L and E442K are located outside the allosteric activator site as currently conceptualized. The locations of the three tryptophans are indicated. Open (ligand free on the left) and closed (plus glucose in green and GKA in yellow on the right) structures are shown. Most notable are the opening of the GKA binding site upon addition of glucose (compare locations of amino acids in red of the left with their locations on the right) on one hand and the separation of the two GKRP contact patches of amino acids when glucose plus GKA bind (E51/E52 from K140, H141, K142, K143, L144 and M197) on the other. It should be realized that any structural change that might be associated with a mutation (e.g. partial cleft closure) is ignored for lack of direct information. Note that T346, L349 and R353 of the xenopus laevis enzyme are replaced by the corresponding amino acids K,Y and S in human glucokinase.
Figure 4.
This figure is designed in the same way as Figure 4 except the orientation is different.. Our own data are in blue or red and those from the literature in gray. The locations of tryptophans are indicated. Glucose is green and the GKA yellow. Remarkably, the amino acids not involved in GKA or GKRP binding (action) are randomly scattered in the periphery of the protein and are absent from the "allosteric regulator region". This is most convincing by viewing 3C and 4C. Also notable is the fact that the tops of the large and the small lobes show no evidence of involvement in GKA or GKRP binding (action).
Figure 5.
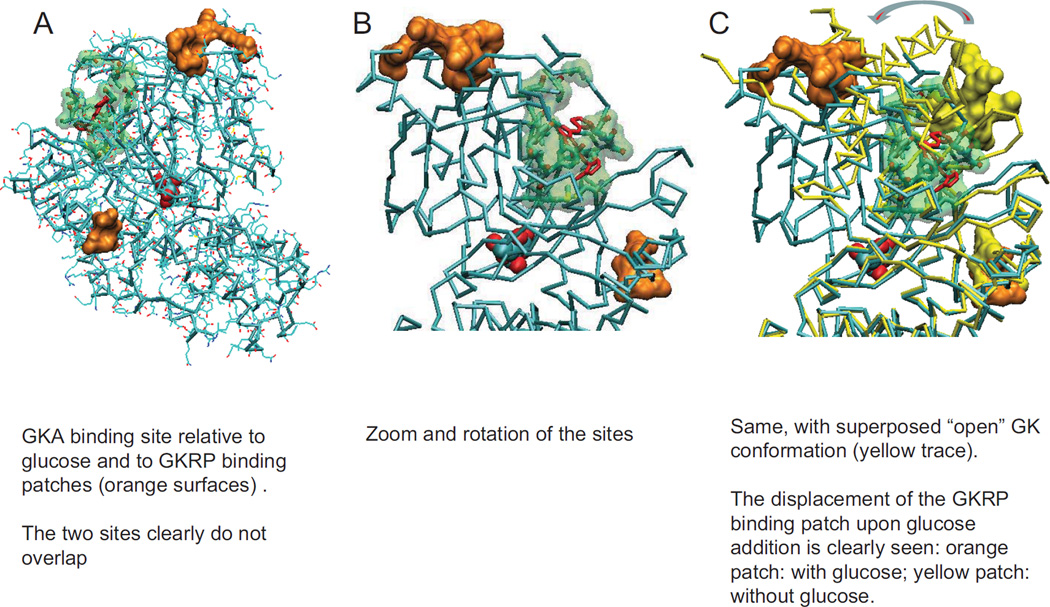
Glucokinase GKA binding site relative to glucose and to GKRP binding patches. A and B represent the closed ternary structures and C depicts the transition from the open to the closed conformation illustrating the large spatial separation of the two GKRP binding patches.
This mechanism envisioning a circumscribed allosteric regulator region with separate but interdependent binding sites for GKAs and GKRP located in the circumscribed domain where the two main lobes of the enzyme are joined together (concept A) as described above in Figures 3 and 4 and summarized in Figure 5 is not compatible with another recent proposal (concept B) which identified different essential GKRP binding patches, one at the tip of the large lobe (L350/N355) and the other at the inner aspect of the substrate binding area (L58/N204) even though both mechanisms postulate that glucose binding is responsible for a conformational change that dissociates the inhibitor from the enzyme [44,45]. These contradictory concepts were developed with greatly different research strategies which may explain the diverging results and conclusions. Concept A is based on the outcome of a mutational analysis of GKA activation and GKRP inhibition using TF and solution kinetic analysis of recombinant GK and a large number of its mutants (mostly identified in MODY-2 and HI patients and from an extensive study with Xenopus Laevis GK), whereas concept B is based largely on cell biological evaluation of GK mutants that were designed as guided by random peptide phage display library screening for binding partners of GKRP and GK. The present mechanism is compelling because it was developed from a large database compiled from two independent complementary studies which both used the highly robust methodologies of enzyme kinetic analysis and tryptophan spectrofluorimetry coupled with wide ranging site directed mutagenesis of GK and because it is internally highly coherent. It is important that unresolved questions arising from these two, mechanistically diverging concepts be conclusively settled by further critical examination.
The interpretation of the present results from extensive TF studies of pure recombinant human islet GK in solution is obviously grounded in the remarkable results and the views of crystallographers but it leads to a significant progress in the structural characterization of the enzyme. The present TF data strongly suggest that the backbone structure of the binary GK/glucose complex is the same as that of the ternary GK/glucose/GKA complex because the TF spectra are virtually indistinguishable, i.e. the shape of the emission spectra, the wavelengths of the maxima and their quantum yields are the same. The conclusion is warranted because TF is an extremely sensitive indicator of even minor structural changes of GK as amply demonstrated in this and other published or yet to be published results. Identity of TF is thus very strong evidence for identity of molecular backbone structure [21,46]. The high sensitivity of TF in this particular case is explained by the ideal locations of the enzyme’s three trypytophans, particularly of W167 which closely reflects structural events in the glucose binding site and of W99 which mirrors dependably even minor local changes in the allosteric activator site while W257 fluorescence is barely affected by most GK modifications. Extensive studies with mutated GKs retaining only one of the three tryptophans have been invaluable in the individual characterization of these intramolecular fluorescent probes [21]. TF measurements of the open form of many mutant enzymes studied here clearly show that activation of the enzyme by single point mutations in the allosteric activator site is frequently associated with a greatly enhanced quantum yield and a red shift of the emission maximum which is interpreted as evidence for the existence of a compacted, partially closed structure of the apoenzyme. We speculate that W99 is the predominant localized source of this TF increase and that glucose binding elicits the additional fluorescence augmentation and blue shift of the spectrum by affecting W167. It is not unreasonable to speculate that such a partially closed GK structure could normally exist as a low abundance intermediate conformation in equilibrium with the open apoenzyme and a fully closed structure favored by formation of the glucose bound binary complex and that suitable amino acid substitutions change the distribution of these multiple conformations helping to explain the increase in substrate affinity typical for these mutants. Molecular dynamics calculations and rapid mixing studies of the TF increase during the glucose induced slow transition from the open to the closed conformation have generated evidence for the existence of several intermediates [16–19]. It is not unreasonable to propose that the high basal TF of activated and perhaps other mutants (e.g. C213R, M298K, S263P and K414E) represent one or more of these hypothetical intermediates in the pathway from the open to the closed conformation. Inclusion of the GK/GKRP interaction in the concept of a preexisting equilibrium of multiple GK conformations would then also offer explanations for apparent exceptions in the correlations of patterns 1B with 1E as an expression of effective sequestration of GK in the form of binary GK/GKRP complexes (e.g.G68K, Y214C and Y214A).
GENERAL CONCLUSIONS AND FUTURE OUTLOOK
Glucokinase is activated pharmacologically by a novel class of GKAs [5,6] and is inhibited physiologically by the liver specific GKRP [3,4]. We have used mutational analysis to advance the characterization of the enzyme's binding sites for GKAs and GKRP. Effects of a GKA (RO0274375) and recombinant human GKRP (huGKRP) on steady state kinetics of recombinant human islet GK were studied and TF was used to determine the glucose KD (both in the presence and absence of GKA). TF was also used to extract structural information on GK and its mutants in its open ligand free form (the apoenzyme), in the closed glucose bound conformation (the binary enzyme/glucose complex) and in the closed GKA activated, glucose containing structure (the ternary enzyme/glucose/GKA complex) by measuring the quantum yield and spectral characteristics under these conditions which was essential for the interpretation of the large data base (see also [21]). All together we compared 31 mutants with wild type GK and also evaluated highly relevant results on mutant enzymes reported in the literature.
Our results and evidence from the literature support the view that both GKA and GKRP bind to a GK allosteric regulator region [5,6,22,35–39], that their binding is mutually exclusive [5,33,35], that GKA binding is glucose dependent [5] but that GKRP inhibition of GK is competitive with glucose [3,4]. The view is based on the finding that point mutations in this regulator region have highly predictable effects on the enzyme conformation as shown by the TF data, on enzyme kinetics and GK's responsiveness to activator and inhibitor. Two characteristic GK profiles predominate accounting for nearly all mutants. 1) Mutants with a normal or close to normal GKA response (13/31) usually exhibiting the low basal TF of wild type enzyme (interpreted to indicate an open relaxed conformation of GK), show some variability in catalytic activity but are usually strongly inactivated by GKRP. 2) Mutants which are refractory to a GKA (14/31) usually exhibiting a high basal TF (interpreted as evidence for structural compaction and partial closure of GK), are usually strongly activated (i.e. they have an increased kcat/S0.5 ratio) and show greatly reduced or no inhibition by GKRP.
The effects of amino acid substitutions on GK are direct, indirect or may result from a dual mechanism. Mutations of known GKA contact amino acids or close neighbors (V62M, S64P/Y, T65I, S68K/V, G72R, Y214C, Y215A, C252Y and 454-A-insert) are probably directly activated by the substitution resulting in structural changes that then cause a higher basal TF and refractoriness to both the activator and inhibitor. GKRP resistance in another group of mutants (E51S, E52K, K140E, H141G, K142P, K143H, L144M and M197E) is also best explained by a localized direct effect of the substituted amino acid, an interpretation supported by the fact that the mutants studied here comprehensively (K140E and M197E) show normal basal TF and GKA reactivity. GKs in a large third group of these mutants have normal basal TF and retain their response to GKA and GKRP. Note again that TF and GKA pharmacology are not available for the Xenopus Laevis GKs which provide an important part of the argument [42,43]. Plausible explanations for a few outliers were offered above..
It is concluded that the GKA and GKRP contact areas are both located in the same GK allosteric regulator region situated on the outer aspects of the protein's hinge between the two lobes but that these ligand specific sites do not overlap (Figure 5). They flank two sides of the back of GK. The location of the GKA binding pocket has been characterized crystallographically involving at least 9 amino acids of the loop between beta-1 to beta-2, and in the helices alpha 5 and 13 [5,40]. The proposed GKRP binding area is situated opposite to the GKA site involving at least 8 amino acids assembled in two separate contact patches (present results and [42]). In a concerted manner both binding sites undergo very large changes as a result of the glucose dependent global conformational transition associated with enzyme activation and manifested by a marked increase of TF. In the course of this process GKAs gain access to their receptor site by opening of the V62-G72 loop which is blocked off in the ligand free enzyme and as it seems partly because W99 is moved out of the way [21], whereas GKRP loses its grip on GK because critical contact amino acids are pushed apart (E51 and E52 are now widely separated from K140-L144 and M197). This mechanism explains the mutually exclusive binding of activator and inhibitor and also demonstrates again that glucose is the prime mover.
The experimental results and the discussion of this study contribute to the emerging understanding of structure/function relationships of GK and its physiological and pharmacological regulators and modifiers. However, a complete understanding of the complexities of GK function and structure will remain elusive until the interactions with other significant binding partners are also explored both by solution biochemistry, crystallography and cell biological methods. Reaching this goal is of high biological and medical importance because GK is an essential player in glucose homeostasis serving as glucose sensor in the insulin producing pancreatic beta cells and as regulator with high control strength for hepatic glucose metabolism and also because it is the target of GKAs, a new class of antidiabetic agents with medical potential.
Supplementary Material
Table 3.
Effect of 1.26 molar excess of human GKRP on human WT and Mutant of GK in the absence and presence of sorbitol-6-phosphate (relative inhibition of GK activity)
| No | GK - enzyme | GKRP alone | GKRP plus 10 µM S6P |
|---|---|---|---|
| 1 | WT | 32.5 | 55 |
| 2 | V62M | 8.5 | 11.5 |
| 3 | S64P | 5 | 4 |
| 4 | S64Y | 19 | 15 |
| 5 | T65I | 7.5 | 23 |
| 6 | G68K | 15 | 32.5 |
| 7 | G68V | 2 | 20.5 |
| 8 | G72R | 5 | 6.5 |
| 9 | V91L | 6 | 22.5 |
| 10 | K140E | 9 | 10.5 |
| 11 | M197E | 13.5 | 18.5 |
| 12 | M197I | 47.5 | 59.5 |
| 13 | M197I/A379T | 18 | 37 |
| 14 | M197L | 15.5 | 37 |
| 15 | C213R | 19.5 | 43 |
| 16 | Y214A | 24.5 | 53 |
| 17 | Y214A/V452A | 11.5 | 20.5 |
| 18 | Y214C | 19.5 | 41 |
| 19 | Y215A | 6 | 4.5 |
| 20 | C252Y | 4 | 4 |
| 21 | S263P | 19 | 49.5 |
| 22 | M298K | 20 | 44 |
| 23 | S336L | n.d. | n.d. |
| 24 | A379T | 17 | 53 |
| 25 | V389L | 67 | 75 |
| 26 | K414E | 13.5 | 49 |
| 27 | P417R | 10.5 | 39.5 |
| 28 | E442K | 14 | 40.5 |
| 29 | V452L | 19 | 38.5 |
| 30 | 454-Ala | 0.5 | 17 |
| 31 | V455M | 26 | 52 |
| 32 | A456V | 32.5 | 55 |
Table 4.
Binding constants, mM, and Relative Fluorescence Increase for D-glucose using cleaved GK and GK Mutants in the absence and presence of 20µM GKA measured by Trp Fluorescence
| GK - enzyme | KD D-glucose |
KD-GKA 20µM GKA, and D-glucose |
KD / KD-GKA |
|---|---|---|---|
| WT | 7.39 (0.83)** | 0.87 (0.71) | 8.49 |
| V62M | 5.45 (0.31) | 5.03 (0.27) | 1.08 |
| S64P | 3.48 (0.30) | 3.01 (0.26) | 1.16 |
| S64Y | 1.93 (0.47) | 1.38 (0.42) | 1.4 |
| T65I | 0.21 (0.53) | 0.20 (0.51) | 1.05 |
| G68K | 0.62 (0.64) | 0.46 (0.47) | 1.35 |
| G68V | 0.54 (0.44) | 0.37 (0.40) | 1.46 |
| G72R | 5.12 (0.17) | 4.67 (0.18) | 1.1 |
| V91L | 0.32 (0.26) | 0.22 (0.25) | 1.45 |
| K140E | 3.64 (0.34) | 0.87 (0.31) | 4.18 |
| M197E | 52.0 (0.24) | 12.8 (0.23) | 4.06 |
| M197I | 2.09 (0.44) | 0.55 (0.29) | 3.8 |
| M197I/A379T | 3.51 (0.40) | 0.67 (0.30) | 5.24 |
| M197L | 3.03 (0.80) | 0.95 (0.61) | 3.19 |
| C213R* | 31.6 (0.23) | 3.17 (0.63) | 9.97 |
| Y214A | 1.06 (0.70) | 1.01 (0.63) | 1.05 |
| Y214A/V452A* | 0.25 (0.31) | 0.25 (0.30) | 1 |
| Y214C | 1.52 (0.48) | 1.38 (0.44) | 1.1 |
| Y215A | 1.35 (0.28) | 0.86 (0.28) | 1.57 |
| C252Y* | 222 (0.0.8) | 221 (0.09) | 1 |
| S263P | 8.52 (0.22) | 1.29 (0.21) | 6.6 |
| M298K | 13.8 (0.93) | 2.09 (0.68) | 6.6 |
| S336L | 5.18 (0.45) | 1.14 (0.42) | 4.54 |
| A379T | 10.49 (0.47) | 2.70 (0.39) | 3.88 |
| V389L | 1.83 (0.67) | 0.27 (0.43) | 6.78 |
| K414E | 4.70 (0.15) | 0.80 (0.12) | 5.88 |
| P417R | 6.10 (0.34) | 0.95 (0.28) | 6.42 |
| E442K | 4.79 (0.41) | 1.05 (0.31) | 4.56 |
| V452L | 1.92 (0.50) | 1.06 (0.43) | 1.81 |
| 454-Ala | 1.23 (0.30) | 0.86 (0.30) | 1.43 |
| V455M | 2.82 (0.77) | 0.90 (0.70) | 3.13 |
| A456V | 1.79 (0.33) | 0.71 (0.22) | 2.52 |
GK mutants from [21].
Fluorescence increase indicated in italic.
ABBREVIATIONS
- GK
Glucokinase
- GKA
Glucokinase Activator Drug
- GST
Glutathione S-Transferase
- GKRP
Glucokinase Regulatory Protein
- GSIR
Glucose Stimulated Insulin Release
- ATP
Adenosine 5’-triphosphate
- BAD
a proapoptotic factor
- MODY-2
Maturity Onset Diabetes of the Young
- PNDM
Permanent Neonatal Diabetes Mellitus
- TF
Tryptophan Fluorescence
REFERENCES
- 1.Yonetani T, Laberge M. Protein dynamics explain the allosteric behaviors of hemoglobin. Biochim. Biophys. Acta. 2008;1784:1146–1158. doi: 10.1016/j.bbapap.2008.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cornish-Bowden A, Cardenas ML. Glucokinase: A Monomeric Enzyme with Positive Cooperativity. In: Matschinsky FM, Magnuson MA, editors. Glucokinase and Glycemic Disease: From Basics to Novel Therapeutics. Frontiers in Diabetes. Vol. 16. Basel: Karger; 2004. pp. 125–134. [Google Scholar]
- 3.Vandercammen A, Van Schaftingen E. The mechanism by which rat liver glucokinase is inhibited by the regulatory protein. Eur. J. Biochem. 1990;191:483–489. doi: 10.1111/j.1432-1033.1990.tb19147.x. [DOI] [PubMed] [Google Scholar]
- 4.Agius L. Glucokinase and molecular aspects of liver glycogen metabolism. Biochem. J. 2008;414:1–18. doi: 10.1042/BJ20080595. [DOI] [PubMed] [Google Scholar]
- 5.Matschinsky FM. Assessing the potential of glucokinase activators in diabetes therapy. Nat. Rev. Drug Discov. 2009;8:399–416. doi: 10.1038/nrd2850. [DOI] [PubMed] [Google Scholar]
- 6.Grimsby J, Sarabu R, Corbett WL, Haynes NE, Bizzarro FT, Coffey JW, Guertin KR, Hilliard DW, Kester RF, Mahaney PE, Marcus L, Qi L, Spence CL, Tengi J, Magnuson MA, Chu CA, Dvorozniak MT, Matschinsky FM, Grippo JF. Allosteric activators of glucokinase: potential role in diabetes therapy. Science. 2003;301:370–373. doi: 10.1126/science.1084073. [DOI] [PubMed] [Google Scholar]
- 7.Matschinsky FM, Ellerman JE .Metabolism of glucose in the islets of Langerhans. J. Biol. Chem. 1968;243:2730–2736. [PubMed] [Google Scholar]
- 8.Postic C, Shiota M, Magnuson MA. Cell-specific roles of glucokinase in glucose homeostasis. Recent Prog. Horm. Res. 2001;56:195–218. doi: 10.1210/rp.56.1.195. [DOI] [PubMed] [Google Scholar]
- 9.Iynedjian PB. Molecular physiology of mammalian glucokinase. Cell. Mol. Life Sci. 2008;66:27–42. doi: 10.1007/s00018-008-8322-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bedoya FJ, Matschinsky FM, Shimizu T, O’Neil JJ, Appel MC. Differential regulation of glucokinase activity in pancreatic islets and liver of the rat. J. Biol. Chem. 1986;261:10760–10764. [PubMed] [Google Scholar]
- 11.Langer S, Kaminski MT, Lenzen S, Baltrusch S. Endogenous activation of glucokinase by 6-phosphofructo-2 kinase/fructose-2,6-bisphosphatase is glucose dependent. Mol. Endocrinol. 2010;24:1988–1997. doi: 10.1210/me.2010-0115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Danial NN, Walensky LD, Zhang C-Y, Choi CS, Fisher JK, Molina AJA, Datta SR, Pitter KL, Bird GH, Wikstrom JD, Deeney JT, Robertson K, Morash J, Kulkarni A, Neschen S, Kim S, Greenberg ME, Corkey BE, Shirihai OS, Shulman GI, Lowell BB, Korsmeyer SJ. Dual role of proapoptotic BAD in insulin secretion and beta cell survival. Nature Medicine. 2008;14:144–153. doi: 10.1038/nm1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sorenson RL, Stout LE, Brelje TC, Jetton T, Matschinsky FM. Immunohistochemical evidence for the presence of glucokinase in the gonadotropes and thyrothropes of the anterior pituitary gland of rat and monkey. J. Histochem. Cytochem. 2007;55:555–566. doi: 10.1369/jhc.6A7117.2007. [DOI] [PubMed] [Google Scholar]
- 14.Neet KE, Ainslie GR. Hysteretic enzymes. Methods Enzymol. 1980;64:192–226. doi: 10.1016/s0076-6879(80)64010-5. [DOI] [PubMed] [Google Scholar]
- 15.Lin SX, Neet KE. Demonstration of a slow conformational change in liver glucokinase by fluorescence spectroscopy. J. Biol. Chem. 1990;265:9670–9675. [PubMed] [Google Scholar]
- 16.Kim YB, Kalinowski SS, Marcinkeviciene J. A pre-steady state analysis of ligand binding to human glucokinase: evidence for a preexisting equilibrium. Biochemistry. 2007;46:1423–1431. doi: 10.1021/bi0617308. [DOI] [PubMed] [Google Scholar]
- 17.Zhang J, Li C, Chen K, Zhu W, Shen X, Jiang H. Conformational transition pathway in the allosteric process of human glucokinase. Proc. Natl Acad. Sci. USA. 2006;103:13368–13373. doi: 10.1073/pnas.0605738103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Larion M, Salinas RK, Bruschweiler-Li L, Bruschweiler R, Miller BG. Direct Evidence of Conformational Heterogeneity in Human Pancreatic Glucokinase from High-Resolution Nuclear Magnetic Resonance. Biochemistry. 2010;49:7969–7971. doi: 10.1021/bi101098f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Larion M, Miller BG. Global fit analysis of glucose binding curves reveals a minimal model for kinetic cooperativity in human glucokinase. Biochemistry. 2010;49:8902–8911. doi: 10.1021/bi1008672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Heredia VV, Thomson J, Nettleton D, Sun S. Glucose-induced conformational changes in glucokinase mediate allosteric regulation: transient kinetic analysis. Biochemistry. 2006;45:7553–7562. doi: 10.1021/bi060253q. [DOI] [PubMed] [Google Scholar]
- 21.Zelent B, Odili S, Buettger C, Shiota C, Grimsby J, Taub R, Magnuson MA, Vanderkooi JM, Matschinsky FM. Sugar binding to recombinant wild-type and mutant glucokinase monitored by kinetic measurement and tryptophan fluorescence. Biochem. J. 2008;413:269–280. doi: 10.1042/BJ20071718. [DOI] [PubMed] [Google Scholar]
- 22.Matschinsky FM, Zelent B, Doliba NM, Kaestner KH, Vanderkooi JM, Grimsby J, Berthel SJ, Sarabu R. Research and Development of Glucokinase Activators for Diabetes Therapy: Theoretical and practical Aspects. In: Schwanstecher M, editor. Handbook of Experimental Pharmacology 203: Diabetes Perspectives in Drug Therapy. Berlin-Heidelberg: Springer Verlag; 2011. pp. 357–401. [DOI] [PubMed] [Google Scholar]
- 23.Osbak KK, Colclough K, Saint-Martin C, Beer NL, Bellanné-Chantelot C, Ellard S, Gloyn AL. Update on mutations in glucokinase (GCK), which cause maturity-onset diabetes of the young, permanent neonatal diabetes, and hyperinsulinemic hypoglycemia. Hum. Mutat. 2009;30:1512–1526. doi: 10.1002/humu.21110. Review. [DOI] [PubMed] [Google Scholar]
- 24.Bonadonna RC, Heise T, Arbet-Engels C, Kapitza C, Avogaro A, Grimsby J, Zhi J, Grippo JF, Balena R. Piragliatin (RO4389620), a Novel Glucokinase Activator, Lowers Plasma Glucose Both in the Postabsorptive State and after a Glucose Challenge in Patients with Type 2 Diabetes Mellitus: A Mechanistic Study. J. Clin. Endocrinol. Metab. 2010 Aug 25; doi: 10.1210/jc.2010-1041. as. [DOI] [PubMed] [Google Scholar]
- 25.Haynes NE, Corbett WL, Bizarro FT, Guertin KR, Hilliard DW, Holland GW, Kester RF, Mahaney PE, Qi L, Spence CL, Tengi J, Dvorozniak MT, Railkar A, Matschinsky FM, Grippo JF, Grimsby J, Sarabu R. Discovery, Structure-Activity Relationships and Efiicacy of Glucokinase Activator (2R)-3-Cyclopentyl-2-(4-methanosulfonylphenyl)-N-thiazol-2-yl-propionamide (R)O0281675) J. Med. Chem. 2010;53:3618–3625. doi: 10.1021/jm100039a. [DOI] [PubMed] [Google Scholar]
- 26.Arden C, Trainer A, de la Iglesia N, Scougall ET, Gloyn AL, Lange AJ, Shaw JA, Matschinsky FM, Agius L. Cell biological assessment of glucokinase mutations V62M and G72Rin pancreatic beta-cells: evidence for cellular instability of catalytic activity. Diabetes. 2007;56:1773–1782. doi: 10.2337/db06-1151. [DOI] [PubMed] [Google Scholar]
- 27.Gloyn AL, Odili S, Zelent D, Buettger C, Castleden HA, Steele AM, Stride A, Shiota C, Magnuson MA, Lorini R, d'Annunzio G, Stanley CA, Kwagh J, van Schaftingen E, Veiga-da-Cunha M, Barbett F, Dunten P, Han Y, Grimsby J, Taub R, Ellard S, Hattersley AT, Matschinsky FM. Insights into the structure and regulation of glucokinase from a novel mutation (V62M), which causes maturity-onset diabetes of the young. J Biol Chem. 2005;280:14105–14113. doi: 10.1074/jbc.M413146200. [DOI] [PubMed] [Google Scholar]
- 28.Sagen JV, Odili S, Bjørkhaug L, Zelent D, Buettger C, Kwagh J, Stanley C, Dahl-Jørgensen K, de Beaufort C, Bell GI, Han Y, Grimsby J, Taub R, Molven A, Søvik O, Njølstad PR, Matschinsky FM. From clinicogenetic studies of maturity-onset diabetes of the young to unraveling complex mechanisms of glucokinase regulation. Diabetes. 2006;55:1713–1722. doi: 10.2337/db05-1513. [DOI] [PubMed] [Google Scholar]
- 29.Gloyn AL, Odili S, Buettger C, Njølstad PR, Shiota C, Magnuson MA, Matschinsky FM. Glucokinase and the Regulation of Blood Sugar. A Mathematical Model Predicts the Threshold for Glucose Stimulated Insulin Release for GCK Gene Mutations that Cause Hyper- and Hypoglycemia. In: Matschinsky FM, Magnuson MA, editors. Glucokinase and Glycemic Disease: From Basics to Novel Therapeutics. Frontiers in Diabetes. Vol. 16. Basel: Karger; 2004. pp. 92–109. [Google Scholar]
- 30.Sayed S, Langdon DR, Odili S, Chen P, Buettger C, Schiffman AB, Suchi M, Taub R, Grimsby J, Matschinsky FM, Stanley CA. Extremes of clinical and enzymatic phenotypes in children with hyperinsulinism caused by glucokinase activating mutations. Diabetes. 2009;58:1419–1427. doi: 10.2337/db08-1792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Beer NL, Tribble ND, Colclough K, Arundel P, Grimsby J, Chik C, Ellard S, Gloyn AL. EASD Meeting. Stockholm: 2010. Naturally occurring glucokinase mutations at the same amino acid residue cause opposite clinical phenotypes of hypo- and hyperglycaemia. Abstract #495. [Google Scholar]
- 32.Fenner D, Odili S, Hong HK, Kobayashi Y, Kohsaka A, Vitatemas MH, Chen P, Zelent B, Grimbsby J, Takahashi JS, Matschinsky FM, Bass J. ENU-Induction, Biochemical Genetics, and Pharmacogenetics of Glucokinase Diabetes in the Mouse. J. Biol. Chem. 2011 doi: 10.1074/jbc.M111.269100. (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lakowicz JR. Principles of Fluorescence Spectroscopy. 3rd ed. New York: Springer; 2006. [Google Scholar]
- 34.Szabo AG, Rayner DM. Fluorescence decay of tryptophan conformers in aqueous solution. J. Am. Chem. Soc. 1980;102:554–563. [Google Scholar]
- 35.Antoine M, Boutin JA, Ferry G. Binding Kinetics of Glucose and Allosteric Activators to Human Glucokinase Reveal Multiple Conformational States. Biochemistry. 2009;48:5466–5482. doi: 10.1021/bi900374c. [DOI] [PubMed] [Google Scholar]
- 36.Ralph EC, Thomson J, Almaden J, Sun S. Glucose modulation of glucokinase activation by small molecules. Biochemistry. 2008;47:5028–5036. doi: 10.1021/bi702516y. [DOI] [PubMed] [Google Scholar]
- 37.Heredia VV, Carlson TJ, Garcia E, Sun S. Biochemical Basis of Glucokinase Activation and the Regulation by Glucokinase Regulatory Protein in Naturally Occurring Mutants. J. Biol. Chem. 2006;281:40201–40207. doi: 10.1074/jbc.M607987200. [DOI] [PubMed] [Google Scholar]
- 38.Anderka O, Boyken J, Aschenbach U, Batzer A, Boscheinen O, Schmoll D. Biophysical characterization of the interaction between hepatic glucokinase and its regulatory protein: impact of physiological and pharmacological effectors. J Biol Chem. 2008;283:31333–31340. doi: 10.1074/jbc.M805434200. [DOI] [PubMed] [Google Scholar]
- 39.Futamura M, Hosaka H, Kadotani A, Shimazaki H, Sasaki K, Ohyama S, Nishimura T, Eiki J, Nagata Y. An allosteric activator of glucokinase impairs the interaction of glucokinase and glucokinase regulatory protein and regulates glucose metabolism. J. Biol. Chem. 2006;281:37668–37674. doi: 10.1074/jbc.M605186200. [DOI] [PubMed] [Google Scholar]
- 40.Kamata K, Mitsuya M, Nishimura T, Eiki J, Nagata Y. Structural basis for allosteric regulation of the monomeric allosteric enzyme human glucokinase. Structure (Camb.) 2004;12:429–438. doi: 10.1016/j.str.2004.02.005. [DOI] [PubMed] [Google Scholar]
- 41.Dunten P, Swain A, Kammlott U, Crowther R, Lukacs CM, Levin W, Reik L, Grimsby J, Corbett WL, Magnuson MA, Matschinsky FM, Grippo JF. Crystal Structure of Human Liver Glucokinase Bound to a Small Molecule Allosteric Activator. In: Matschinsky FM, Magnuson MA, editors. Glucokinase and Glycemic Disease: From Basics to Novel Therapeutics. Frontiers in Diabetes. Vol 16. Basel: Karger; 2004. pp. 145–154. [Google Scholar]
- 42.Veiga-da-Cunha M, Courtois S, Michel A, Gosselain E, Van Schaftingen E. Amino acid conservation in animal glucokinases. Identification of residues implicated in the interaction with the regulatory protein. J. Biol. Chem. 1996;271:6292–6297. doi: 10.1074/jbc.271.11.6292. [DOI] [PubMed] [Google Scholar]
- 43.Veiga-da-Cunha M, Xu LZ, Lee YH, Marotta D, Pilkis SJ, Van Schaftingen E. Effect of mutations on the sensitivity of human beta-cell glucokinase to liver regulatory protein. Diabetologia. 1996;39:1173–1179. doi: 10.1007/BF02658503. [DOI] [PubMed] [Google Scholar]
- 44.Baltrusch S, Tiedge M. Glucokinase regulatory network in pancreatic beta-cells and liver. Diabetes. 2006;55(Suppl. 2):S55–S64. [Google Scholar]
- 45.Baltrusch S, Francini F, Lenzen S, Tiedge M. Interaction of glucokinase with the liver regulatory protein is conferred by leucine-asparagine motifs of the enzyme. Diabetes. 2005;54:2829–2837. doi: 10.2337/diabetes.54.10.2829. [DOI] [PubMed] [Google Scholar]
- 46.Molnes J, Bjørkhaug L, Søvik O, Njølstad PR, Flatmark T. Catalytic activation of human glucokinase by substrate binding: residue contacts involved in the binding of D-glucose to the super-open form and conformational transitions. The FEBS Journal. 2008;275:2467–2481. doi: 10.1111/j.1742-4658.2008.06391.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.