Abstract
Estimates of 12‐month and lifetime prevalence and of lifetime morbid risk (LMR) of the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Text Revision (DSM‐IV‐TR) anxiety and mood disorders are presented based on US epidemiological surveys among people aged 13+. The presentation is designed for use in the upcoming DSM‐5 manual to provide more coherent estimates than would otherwise be available. Prevalence estimates are presented for the age groups proposed by DSM‐5 workgroups as the most useful to consider for policy planning purposes. The LMR/12‐month prevalence estimates ranked by frequency are as follows: major depressive episode: 29.9%/8.6%; specific phobia: 18.4/12.1%; social phobia: 13.0/7.4%; post‐traumatic stress disorder: 10.1/3.7%; generalized anxiety disorder: 9.0/2.0%; separation anxiety disorder: 8.7/1.2%; panic disorder: 6.8%/2.4%; bipolar disorder: 4.1/1.8%; agoraphobia: 3.7/1.7%; obsessive‐compulsive disorder: 2.7/1.2. Four broad patterns of results are most noteworthy: first, that the most common (lifetime prevalence/morbid risk) lifetime anxiety‐mood disorders in the United States are major depression (16.6/29.9%), specific phobia (15.6/18.4%), and social phobia (10.7/13.0%) and the least common are agoraphobia (2.5/3.7%) and obsessive‐compulsive disorder (2.3/2.7%); second, that the anxiety‐mood disorders with the earlier median ages‐of‐onset are phobias and separation anxiety disorder (ages 15–17) and those with the latest are panic disorder, major depression, and generalized anxiety disorder (ages 23–30); third, that LMR is considerably higher than lifetime prevalence for most anxiety‐mood disorders, although the magnitude of this difference is much higher for disorders with later than earlier ages‐of‐onset; and fourth, that the ratio of 12‐month to lifetime prevalence, roughly characterizing persistence, varies meaningfully in ways consistent with independent evidence about differential persistence of these disorders. Copyright © 2012 John Wiley & Sons, Ltd.
Keywords: anxiety disorders, mood disorders, lifetime morbid risk, prevalence, epidemiology
Introduction
Little agreement exists regarding the conventions for the time frame to use in reporting data from community epidemiological surveys on the prevalence of mental disorders. Some authors prefer to focus exclusively on estimates of recent prevalence in order to avoid the biases associated with selective recall (Costello et al., 2003; Jenkins et al., 2003). Others report the proportion of respondents who met criteria for mental disorder at some time in the past six months (Marcks et al., 2011; Park et al., 2011), 12 months (Andrade et al., 2012; McEvoy et al., 2011; Wittchen and Jacobi, 2005; Wittchen et al., 2011), or even at any time in their life (Carra et al., 2012; Pietrzak et al., 2011). In studies of lifetime disorders, furthermore, some researchers report estimates of lifetime prevalence (i.e. the proportion of people who have had the disorder at some time in their life), while others report estimates of lifetime morbid risk (LMR) (i.e. the proportion of people who will eventually develop the disorder at some time in their life whether or not they have a lifetime history at the time of assessment). While LMR is reported much less frequently than lifetime prevalence (Oakley Browne et al., 2006), it is important to appreciate that measures of LMR tell us not only about the proportion of the population that has so far experienced the disorder, but also about the additional proportion of the population that is expected (based on a projection from a survival model) to experience the disorder at some time in the future. The combined information about current and future cases is sometimes more important for policy planning purposes than information about cases that have occurred to date. Estimates of lifetime prevalence and LMR will typically be quite similar for disorders that usually start early in life, while the two estimates will diverge more and more for disorders with increasingly later ages‐of‐onset (Kessler et al., 2005a, 2007b).
Each of the earlier measures results in a different estimate that deals with a different time frame and, in the case of LMR, with information about age‐of‐onset (AOO) distributions. The inconsistent reporting of some but not all of these different estimates across studies has led to confusion about the most appropriate measures and has hampered synthesis of knowledge across studies. A number of issues need to be considered in determining which of the earlier measures to report for different purposes, although it is always possible to finesse the issue by reporting all of the earlier estimates. Perhaps the most contentious of these involves concerns about recall bias, leading some researchers to focus entirely on recent prevalence based on concerns about recall bias in measures with longer recall periods (Jenkins et al., 1997a). It is important to realize, though, that recall bias is a continuous gradient that does not end with a recall period of, say, one week (as in the Center for Epidemiologic Studies Depression (CES‐D) scale; Radloff, 1977) or one month (as in many reports of “current” prevalence in community epidemiological surveys; Jenkins et al., 1997b). Indeed, research on the use of daily diaries to collect more fine‐grained information about symptoms of mental disorders shows that recall bias exists even when the recall period is as short as one week (Zanni, 2007). Furthermore, research using the experience sampling method (ESM), in which diary respondents are signaled at random times of the day (either with a cell phone call or a beeper) to provide moment‐in‐time reports of mood, shows that recall bias exists even for a recall period as short as one day (Shiffman et al., 2008).
Another complicating factor in focusing on short recall periods is that the diagnostic classification of most mental disorders is based on criteria that typically comprise not only current symptoms but also symptoms that date back many months or even years. This makes it impossible or at least questionable to make diagnoses for many disorders based exclusively on data about current symptoms. For example, information about the lifetime occurrence of hypomania or mania is required to determine whether a current major depressive episode (MDE) is a major depressive disorder (MDD) or a depressive phase of a bipolar disorder (BPD). A diagnosis of generalized anxiety disorders (GADs) requires an assessment of symptoms over a six‐month time period. A diagnosis of dysthymic disorder requires an assessment of symptoms over a two‐year period. Diagnostic instruments for current disorders account for these issues by also assessing lifetime occurrence of some syndromes and collecting information about onset, recency, and duration of these syndromes to derive diagnoses. This being the case, we have to recognize that the search for symptom reports of mental disorders devoid of recall bias is doomed to failure. Indeed, as shown in the studies using moment‐in‐time ESM data collection, this is true even in the extreme case, such as with the assessment of premenstrual disorders, where daily diaries are used to chart symptoms over the full‐time period needed to meet diagnostic criteria (Futterman and Rapkin, 2006).
How should the inevitability of recall bias be managed? As suggested earlier, exclusive focus on questions with a short recall period cannot be the answer, as even the shortest time interval will be subject to at least some recall bias. Furthermore, the use of very short recall periods reduces the practical value of data even if recall bias is de minimis, as there is little clinical or policy value in knowing only about symptoms over a very short recall period. Based on this realization, it has recently been suggested that the most useful policy‐relevant information on recent prevalence would focus on a 12‐month recall period due to the fact that most policy planning decisions are made on an annual basis (Wittchen and Jacobi, 2005; Wittchen et al., 2011). It would presumably also be of interest to have information on the distributions of persistence and severity of 12‐month cases (e.g. the proportion of MDEs occurring in the past 12 months that were both severe and lasted six month or longer). But if this is so, how should researchers deal with the fact that recall error will inevitably exist in responses to these questions? The most recent thinking about this question in the cognitive psychological literature on recall error in surveys suggests that two approaches can be used, both of them based on the realization that recall error varies substantially with the salience of the experiences respondents are being asked to recall (Eisenhower et al., 2004).
The first approach is to vary the recall period over which different questions are asked in order to adjust for variation in salience. For example, the US National Health Interview Survey asks about days out of role due to illness over a two‐week recall period, broken arms over a 12‐month recall period, and heart attacks over a lifetime recall period (Adams et al., 1999). The second approach is to vary the subtlety of the recall questions depending on variation in salience of the content. For example, the World Health Organization (WHO) Composite International Diagnostic Interview (CIDI), the diagnostic interview used in most community psychiatric epidemiological surveys around the world, asks fine‐grained questions about episodes of disorders that occurred over the 30 days before interview, more general questions about episodes that occurred in the 12 months before interview, and only very general questions about lifetime disorders (Kessler and Üstün, 2004). In addition, in cases where the CIDI seeks to obtain more detail about experiences that occurred at a time requiring long‐term recall, as in asking about AOO, special memory priming questions are used to help improve memory search and these questions are phrased in such a way as to allow for the range responses that arise in situations where some respondents have no clear memory of precise instances (Knäuper et al., 1999).
With regard to estimates of more long‐term prevalence, it has recently been suggested that the most useful policy‐relevant information would be about LMR rather than lifetime prevalence based on the former providing a more complete picture than the latter of long‐term population‐level disease burden (Wittchen and Jacobi, 2005; Wittchen et al., 2011). It is important to recognize, though, that while estimates of lifetime prevalence are calculated from observed data on the proportion of people in the population who ever experienced a given disorder at some time in their life up to the time of assessment, estimates of LMR are calculated by combining information on observed lifetime prevalence with data on predicted future onsets. Future onsets are predicted from any one of a number of somewhat different mathematical models that use information about conditional probabilities of first onset in different years of life obtained from people who already passed through those years of life (Fuchs et al., 2010). These models all assume that the experiences of currently younger people will either be the same as those of the older people who were used in estimating the conditional probabilities of first onset or will change in ways that can be predicted by trends in the experiences of these older people. Caution is needed in interpreting estimates of lifetime risk when there is reason to believe that the experiences of older and younger cohorts are quite different from each other. Furthermore, special efforts should be made whenever possible to base the lifetime risk projections used to calculate LMR on AOO curves that were constructed using short recall intervals and smoothing to reduce the effects of recall error and cohort effects (Eaton et al., 2012). In addition, AOO curves should be presented in conjunction with estimates of LMR because the distributional information in AOO curves can be helpful in targeting intervention efforts to the age ranges of highest onset risk (Kessler et al., 2007a).
Aims
In light of the earlier considerations, we present in this paper estimates of the 12‐month prevalence and LMR of a number of DSM‐IV‐TR (Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Text Revision) anxiety and mood disorders based on epidemiological surveys carried out on the US household population among people aged 13 and older. Although these surveys also assessed disruptive behavior disorders, the latter disorders are not considered here because the typically early AOO of disruptive behavior disorders makes the distinction between lifetime prevalence and LMR among adolescents‐adults much less striking for these disorders than for anxiety‐mood disorders. The presentation is intended for use in the upcoming DSM‐5 manual to allow for a more coherent and synchronous presentation of estimates than would otherwise be available. Prevalence estimates are presented by the age groups recently proposed by DSM‐5 workgroups as the most useful ones to consider for policy planning purposes. Earlier reports from these same surveys presented estimates of lifetime and recent prevalence and AOO distributions of these disorders for adolescents (Kessler et al., 2012) and adults (Kessler et al., 2005a, 2005b). However, data are reported here for the first time on estimates of prevalence, AOO distributions, and LMR for the combined samples of adolescents and adults. Minor discrepancies between some estimates reported here separately for adolescents or adults and the results reported in previous papers are due to improvements in data coding and weighting since earlier estimates were published.
Methods
Sample
Data are based on the US National Comorbidity Survey Replication (NCS‐R; Kessler and Merikangas, 2004) and the Adolescent Supplement to that survey (NCS‐A; Merikangas et al., 2009). The NCS‐R design has been described in detail elsewhere (Kessler et al., 2004). In brief, the NCS‐R is based on a national face‐to‐face household survey of adults (ages 18+) designed to study prevalence and correlates of DSM‐IV disorders. The survey was administered in 2001–2003 and had two parts. Part I included an assessment of core DSM‐IV mental disorders administered to all respondents (n = 9282). Part II included questions about non‐core mental disorders (including some of those considered in this report), physical disorders, disease burden, and other correlates administered to all Part I respondents who met lifetime criteria for any Part I disorder plus a roughly one‐in‐three probability sub‐sample of other Part I respondents (n = 5692). The Part I–II response rate was 70.9%. Verbal informed consent was obtained before surveying. Consent procedures were approved by the Institutional Review Boards of both Harvard Medical School and the University of Michigan. The Part II sample, which is used in the current report, was weighted to adjust for differential probabilities of selection and under‐sampling of Part I respondents without DSM‐IV disorders. A weight was also used to adjust for small discrepancies between sample and population Census data on socio‐demographic and geographic variables. A more detailed discussion of NCS‐R sampling and weighting procedures is presented elsewhere (Kessler et al., 2004).
The NCS‐A design also has been described in detail elsewhere (Kessler et al., 2009a, 2009b). In brief, the NCS‐A is based on a national survey of adolescents (ages 13–17 at the time of sample selection, although a small number of respondents turned 18 before the date of their interview) who were interviewed February 2001–January 2004 in dual‐frame household and school samples in conjunction with the NCS‐R. The NCS‐A household sample included 904 adolescents (879 in school, 25 dropouts) from households in the NCS‐R (Kessler and Merikangas, 2004). The conditional adolescent response rate was 86.8%. The NCS‐A school sample included 9244 adolescents from a representative sample of schools in the adult sample areas. The conditional adolescent response rate was 82.6%. Written parent informed consent and written adolescent assent were obtained before surveying either adolescents or parents. Consent procedures were approved by the Institutional Review Boards of both Harvard Medical School and the University of Michigan. The NCS‐A household sample, despite comparatively small size, is important because it includes adolescents residing in areas where schools refused to participate. The high (72.0%) percent of non‐participating initially‐selected schools were replaced with matched replacement schools. Comparison of household sample respondents from non‐participating schools with school sample respondents from replacement schools found no evidence of bias in estimates of either disorder prevalence or correlates (Kessler et al., 2009a). One parent or surrogate (henceforth described as parents) of participating adolescents was asked to complete a self‐administered questionnaire about the adolescent's developmental history and mental health. The conditional response rate was 82.5–83.7% (household–school samples). The 6483 adolescent–parent pairs with both adolescent interviews and parent questionnaires, which is used in the current report, were weighted to adjust for differences in measured variables compared to incomplete pairs and were then post‐stratified to the distribution of the US population on a wide range of census socio‐demographic and geographic variables using methods discussed elsewhere (Kessler et al., 2009a, 2009b).
Diagnostic assessment
Adolescents in the NCS‐A were administered the fully‐structured CIDI Version 3.0 (Kessler and Üstün, 2004) modified to simplify language and use examples relevant to adolescents (Merikangas et al., 2009). The DSM‐IV/CIDI disorders considered include mood disorders (MDD, BPD I–II) and anxiety disorders [panic disorder, agoraphobia, social anxiety disorder, specific phobia, GAD, separation anxiety disorder (SAD), obsessive‐compulsive disorder (OCD), post‐traumatic stress disorder (PTSD)]. It should be noted that SAD is included here despite being listed in DSM‐IV among the Disorders Usually First Diagnosed in Infancy, Childhood, or Adolescence, as it is noted in the introduction to the DSM‐IV section on Anxiety Disorders that SAD is recognized to be an anxiety disorder.
All diagnoses were made using DSM‐IV distress/impairment criteria, organic exclusion rules, and diagnostic hierarchy rules except that panic disorder was assessed with or without a history of agoraphobia, agoraphobia was assessed with or without a history of panic, and we considered both MDE (i.e. with or without a history of BPD) and MDD (i.e. without a history of BPD). Dysthymic disorder was also assessed in the surveys but in a way that did not allow it to be clearly distinguished from major depression, leading us not to include dysthymic disorder in the analyses reported here. OCD was not assessed among adolescents. Adolescent interviews assessed all other disorders, while briefer parent questionnaires assessed only major depression based on evidence that parent reports play an important part in the diagnosis of depression (Braaten et al., 2001). Parent and adolescent reports were combined at the symptom level using an “or” rule. Exploratory analyses showed that the use of this “or” rule optimized concordance with diagnoses in the NCS‐A clinical reappraisal study (Kessler et al., 2009c).
As noted earlier, we focus on prevalence assessed in two time frames: the 12 months before interview and the lifetime. We also report projected estimates of LMR. The latter estimates were obtained by using reports about lifetime prevalence and retrospective reports about AOO to generate survival curves that were used to predict future risk of onset as of age 75 for all respondents who had not yet reached this age. The AOO curves are reported here in addition to the estimates of LMR. Special probing procedures that have been shown experimentally to increase recall of lifetime disorders (Kessler et al., 1998) were used in asking about lifetime prevalence. Another set of special probing procedures, which have been shown experimentally to increase the accuracy of AOO reports (Knäuper et al., 1999), was used in asking respondents with lifetime disorders to date the age when each of their lifetime disorders started.
An NCS‐A clinical reappraisal study documented good concordance between survey and clinical diagnoses based on the Schedule for Affective Disorders and Schizophrenia for School‐Age Children (K‐SADS) Lifetime Version (Kaufman et al., 1997). The same disorders were assessed in the NCS‐R with the adult version of the CIDI (Kessler and Üstün, 2004, 2008). This instrument used similar question wording and probing procedures as in the adolescent survey. A clinical reappraisal study that used blinded clinician‐administered reappraisal interviews based on the Structured Clinical Interview for DSM‐IV (SCID) Research Version, Non‐patient Edition (First et al., 2002) found generally good concordance with diagnoses for adults based on the CIDI (Haro et al., 2006).
Analysis methods
Twelve‐month prevalence within age groups was estimated using simple cross‐tabulation methods. AOO reports were projected to estimate lifetime risk as of age 75 using the two‐part actuarial method implemented in SAS v9.1 (SAS Institute Inc., 2001). The actuarial method differs from the more familiar Kaplan–Meier (Kaplan and Meier, 1958) method in using a more accurate way of estimating the timing of onsets within a given year (Halli et al., 1992). This method assumes constant conditional risk of onset at a given year of life across cohorts. LMR was estimated as of age 75 from the projections in the survival curves. Standard errors of these projections were based on simulations using the jackknife repeated replication method (Kish and Frankel, 1974) implemented in a SAS macro (SAS Institute Inc., 2001). All significance tests were evaluated at the 0.05 level with two‐sided tests.
Results
Lifetime prevalence
The most prevalent lifetime syndrome considered here is MDE (16.6%), while the most prevalent disorders are specific phobia (15.6%), MDD (14.4%), and social phobia (10.7%) (Tables 1 and 2). The 2.2% difference between MDE and MDD is due to the fact that MDE but not MDD includes respondents with BPD. The prevalence of BPD (2.5%) is somewhat higher than the difference between MDE and MDD (2.2%) because some people with BPD have a history of only manic episodes without ever having an episode of MDE. Most people with a history of either MDE or MDD have had recurrent episodes rather than only a single lifetime episode (12.2% versus 4.4% for MDE; 10.3% versus 4.1% for MDD). Other than specific and social phobias, the anxiety disorders with the highest lifetime prevalence are SAD (6.7%) and PTSD (5.7%). The less common anxiety disorders include GAD (4.3%), panic disorder with or without agoraphobia (PD; 3.8%), agoraphobia with or without panic disorder (2.5%), and OCD (2.3%).
Table 1.
Lifetime prevalence of DSM‐IV‐TR/CIDI anxiety disorders by age and gender in the National Comorbidity Survey Replication (NCS‐R) and Adolescent Supplement (NCS‐A)
| Ages 13–17 | Ages 18–64 | Ages 65+ | Total (ages 13+)a | |||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Female | Male | Total | Female | Male | Total | Female | Male | Total | Female | Male | Total | |||||||||||||
| Percent | (SE) | Percent | (SE) | Percent | (SE) | Percent | (SE) | Percent | (SE) | Percent | (SE) | Percent | (SE) | Percent | (SE) | Percent | (SE) | Percent | (SE) | Percent | (SE) | Percent | (SE) | |
| Panic disorderb | 2.5 | (0.3) | 2.1 | (0.4) | 2.3 | (0.3) | 7.0* | (0.4) | 3.3 | (0.4) | 5.2 | (0.3) | 2.5 | (0.7) | 1.6 | (0.6) | 2.1 | (0.5) | 4.8* | (0.3) | 2.7 | (0.3) | 3.8 | (0.2) |
| Generalized anxiety disorder | 2.8 | (0.5) | 1.6 | (0.4) | 2.2 | (0.3) | 7.7* | (0.4) | 4.6 | (0.5) | 6.2 | (0.3) | 4.8* | (0.6) | 1.3 | (0.5) | 3.3 | (0.4) | 5.5* | (0.3) | 3.1 | (0.3) | 4.3 | (0.2) |
| Agoraphobiac | 3.7* | (0.6) | 1.7 | (0.4) | 2.7 | (0.4) | 3.2* | (0.3) | 2.0 | (0.3) | 2.6 | (0.2) | 1.5 | (0.5) | 0.7 | (0.4) | 1.2 | (0.3) | 3.2* | (0.3) | 1.8 | (0.2) | 2.5 | (0.2) |
| Social phobia | 11.2* | (1.1) | 6.2 | (0.7) | 8.6 | (0.6) | 14.2* | (0.7) | 11.8 | (0.6) | 13.0 | (0.5) | 7.1 | (0.9) | 5.1 | (1.2) | 6.3 | (0.7) | 12.3* | (0.6) | 8.9 | (0.5) | 10.7 | (0.4) |
| Specific phobia | 23.0* | (1.3) | 17.1 | (0.6) | 20.0 | (1.0) | 17.5* | (0.6) | 9.9 | (0.6) | 13.8 | (0.4) | 9.1* | (1.2) | 3.6 | (0.7) | 6.8 | (0.7) | 18.7* | (0.6) | 12.3 | (0.6) | 15.6 | (0.5) |
| Separation anxiety disorder | 9.5* | (0.9) | 5.9 | (1.0) | 7.7 | (0.5) | 8.2* | (0.6) | 4.7 | (0.5) | 6.6 | (0.4) | 1.9 | (0.4) | 1.3 | (0.8) | 1.6 | (0.5) | 8.3* | (0.5) | 5.2 | (0.4) | 6.7 | (0.3) |
| Post‐traumatic stress disorder | 6.9* | (0.8) | 2.3 | (1.2) | 4.5 | (0.4) | 11.7* | (0.8) | 4.0 | (0.3) | 8.0 | (0.5) | 2.5* | (0.6) | 0.4 | (0.2) | 1.6 | (0.3) | 8.5* | (0.6) | 2.8 | (0.3) | 5.7 | (0.3) |
| Obsessive‐compulsive disorder | —d | —d | —d | 3.6* | (0.6) | 1.8 | (0.4) | 2.7 | (0.4) | 0.6 | (0.5) | 0.3 | (0.3) | 0.5 | (0.4) | 3.0 | (0.5) | 1.6 | (0.3) | 2.3 | (0.3) | |||
| Any anxiety disorder | 38.3 | (1.6) | 26.8 | (1.0) | 32.4 | (1.0) | 40.4 | (1.1) | 26.4 | (1.1) | 33.7 | (0.9) | 17.7 | (1.4) | 11.1 | (1.6) | 14.9 | (1.3) | 37.3 | (0.9) | 25.6 | (0.8) | 31.6 | (0.7) |
| (n) | (3219) | (3024) | (6243) | (2978) | (2245) | (5223) | (446) | (263) | (709) | (6643) | (5532) | (12175) | ||||||||||||
The NCS‐A (ages 13–17) and NCS‐R (ages 18+) samples were combined without weighting to adjust for the higher probability of selection of adolescents than adults in the two surveys.
With or without agoraphobia.
With or without a history of panic disorder
Obsessive‐compulsive disorder was not assessed among adolescents.
Significant gender difference within the sub‐sample.
Table 2.
Lifetime prevalence of DSM‐IV‐TR/CIDI mood disorders by age and gender in the National Comorbidity Survey Replication (NCS‐R) and Adolescent Supplement (NCS‐A)
| Ages 13–17 | Ages 18–64 | Ages 65+ | Total (ages 13+)a | |||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Percent | Male | Total | Female | Male | Total | Female | Male | Total | Female | Male | Total | |||||||||||||
| Percent | (SE) | Percent | (SE) | Percent | (SE) | Percent | (SE) | Percent | (SE) | Percent | (SE) | Percent | (SE) | Percent | (SE) | Percent | (SE) | Percent | (SE) | Percent | (SE) | Percent | (SE) | |
| Major depressive episode | ||||||||||||||||||||||||
| Single | 3.7 | (0.6) | 2.5 | (0.5) | 3.1 | (0.4) | 6.4* | (0.4) | 4.7 | (0.3) | 5.6 | (0.3) | 4.2 | (0.6) | 3.0 | (0.9) | 3.7 | (0.5) | 5.2* | (0.3) | 3.7 | (0.3) | 4.4 | (0.2) |
| Recurrent episodes | 13.1* | (1.1) | 6.0 | (0.8) | 9.5 | (0.8) | 18.8* | (0.7) | 11.8 | (0.7) | 15.4 | (0.6) | 8.8* | (1.5) | 2.3 | (0.7) | 6.1 | (0.9) | 15.6* | (0.6) | 8.7 | (0.5) | 12.2 | (0.4) |
| Total | 16.8* | (1.3) | 8.5 | (0.9) | 12.6 | (0.9) | 25.2* | (0.7) | 16.5 | (0.8) | 20.9 | (0.6) | 13.0* | (1.3) | 5.3 | (1.2) | 9.8 | (0.9) | 20.7* | (0.6) | 12.3 | (0.6) | 16.6 | (0.5) |
| Major depressive disorder | ||||||||||||||||||||||||
| Single | 3.4 | (0.5) | 2.4 | (0.5) | 2.9 | (0.4) | 6.0* | (0.4) | 4.2 | (0.4) | 5.2 | (0.3) | 4.2 | (0.6) | 3.0 | (0.9) | 3.7 | (0.5) | 4.8* | (0.2) | 3.4 | (0.3) | 4.1 | (0.2) |
| Recurrent episodes | 10.8* | (1.1) | 4.8 | (0.7) | 7.7 | (0.8) | 16.1* | (0.7) | 10.1 | (0.7) | 13.2 | (0.6) | 8.3* | (1.4) | 1.9 | (0.6) | 5.6 | (0.8) | 13.2* | (0.6) | 7.3 | (0.5) | 10.3 | (0.4) |
| Total | 14.2* | (1.2) | 7.2 | (0.8) | 10.6 | (0.8) | 22.1* | (0.7) | 14.4 | (0.7) | 18.3 | (0.6) | 12.6* | (1.2) | 4.9 | (1.2) | 9.3 | (0.8) | 18.1* | (0.6) | 10.7 | (0.6) | 14.4 | (0.5) |
| Bipolar disorder | ||||||||||||||||||||||||
| BPD I | 0.3 | (0.2) | 0.1 | (0.0) | 0.2 | (0.1) | 1.3 | (0.2) | 0.9 | (0.2) | 1.1 | (0.2) | 0.1 | (0.1) | 0.4 | (0.3) | 0.2 | (0.1) | 0.8 | (0.1) | 0.5 | (0.1) | 0.6 | (0.1) |
| BPD II | 2.8 | (0.5) | 2.8 | (0.5) | 2.8 | (0.4) | 1.6 | (0.2) | 1.2 | (0.2) | 1.4 | (0.1) | 0.4 | (0.2) | 0.0 | (0.0) | 0.2 | (0.1) | 1.9 | (0.2) | 1.8 | (0.2) | 1.8 | (0.2) |
| BPD I or II | 3.1 | (0.5) | 2.8 | (0.5) | 3.0 | (0.4) | 2.9 | (0.3) | 2.1 | (0.2) | 2.5 | (0.2) | 0.5 | (0.2) | 0.4 | (0.3) | 0.4 | (0.2) | 2.7 | (0.3) | 2.2 | (0.2) | 2.5 | (0.2) |
| Any mood disorder | 18.2 | (1.3) | 10.8 | (1.2) | 14.4 | (1.0) | 25.7 | (0.7) | 16.9 | (0.8) | 21.4 | (0.5) | 13.1 | (1.3) | 5.3 | (1.2) | 9.8 | (0.9) | 21.5 | (0.6) | 13.5 | (0.7) | 17.5 | (0.5) |
| (n) | (3,219) | (3,024) | (6,243) | (2,978) | (2,245) | (5,223) | (446) | (263) | (709) | (6,643) | (5,532) | (12,175) | ||||||||||||
The NCS‐A (ages 13–17) and NCS‐R (ages 18+) samples were combined without weighting to adjust for the higher probability of selection of adolescents than adults in the two surveys.
Significant gender difference within the sub‐sample.
All the earlier disorders are more prevalent among women than men, most of them significantly so. Most, but not all, of these disorders are estimated to have higher lifetime prevalence among adults in the age range 18–64 than adolescents (ages 13–17) with the exceptions of BPD II, agoraphobia, specific phobia, and SAD. The only substantive reason for lifetime prevalence being higher among younger than older people is rising prevalence in more recent cohorts. But methodological factors could also create such an age inversion. This could be due either to sample selection bias (i.e. more bias against people with than without lifetime disorders being in the sample than with increasing age), reporting bias (i.e. greater failure to report lifetime disorder due either to forgetting or conscious non‐disclosure with increasing age), or classification bias (i.e. differential classification error in the CIDI by age). We have no empirical basis for adjudicating among these different possibilities with the data presented here. However, it is noteworthy that controversy exists about the misclassification of BPD among youth (e.g. misinterpretation of symptoms of attention‐deficit/hyperactivity disorder as due to BPD) that might account for the high prevalence estimate of BPD among adolescents (Youngstrom et al., 2008). And it is also noteworthy that both specific phobia and SAD are disorders that typically start early in life and might remit prior to adulthood, raising the possibility of especially high rates of under‐reporting among adults.
In addition, all the disorders considered here are estimated to have lower lifetime prevalence among older (ages 65+) than adults in the age range 18–64. This could be due to cohort effects; that is, to a genuine increase in lifetime prevalence of disorders among adults in more recent cohorts. Or it could be due to sample selection bias caused either by early mortality or sufficient morbidity related to a history of mental disorders which makes it impossible to participate in a survey or to be excluded from the sample (e.g. living in a nursing home). Effects of mental disorders on physical disorders (Scott et al., 2007; Scott et al., 2009) and mortality (Grossardt et al., 2009; Lefèvre et al., 2011) have been documented in the literature, increasing the plausibility of this methodological interpretation of the age‐related differences in lifetime prevalence estimates. Another possibility, though, is that older adults are more likely than younger adults to forget or consciously fail to disclose lifetime mental disorders. Given that the magnitude of some prevalence differences are larger than we would expect based exclusively on effects of differential morbidity and mortality, it is likely that either increases in true prevalence in more recent cohorts and/or an increase in under‐reporting among the elderly are involved to at least some degree in accounting for the lower lifetime prevalence estimates among older adults.
Age‐of‐onset (AOO) distributions
The AOO distributions were standardized to remove information about between‐disorder differences in LMR so as to visualize the age ranges of highest risk for each disorder. Two patterns can be seen clearly in the resulting representation (Figures 1, 2, 3). First, median AOO (i.e. the 50th percentile on the AOO curves) is earliest for the phobias and SAD (ages 15–17) and latest for PD (age 23), MDE (age 25), and GAD (age 30). Second, the AOO distribution is much more concentrated for some disorders than others. The disorder with by far the narrowest inter‐quartile range (IQR; i.e. the number of years between the 25th and 75th percentiles of the AOO distribution) is SAD (three years), while three of the four disorders with the next narrowest IQRs are the phobias (6–11 years). MDE, GAD, and PD, at the other extreme, have IQRs in the range 23–27 years.
Figure 1.
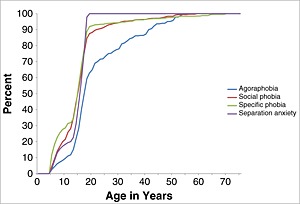
Standardized age‐of‐onset distributions of DSM‐IV‐TR/CIDI anxiety disorders with the earliest ages‐of‐onset (agoraphobia, social phobia, specific phobia and separation anxiety) by age in the National Comorbidity Survey Replication (NCS‐R) and Adolescent Supplement (NCS‐A) (n = 12,175).
Figure 2.
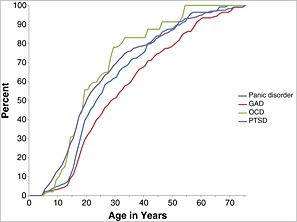
Standardized age‐of‐onset distributions of DSM‐IV‐TR/CIDI anxiety disorders with later ages‐of‐onset (PD, GAD, OCD and PTSD) by age in the National Comorbidity Survey Replication (NCS‐R) and Adolescent Supplement (NCS‐A) (n = 12,175)
Figure 3.
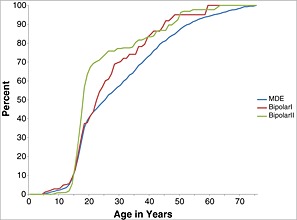
Standardized age‐of‐onset distributions of DSM‐IV‐TR/CIDI mood disorders (MDE, BPD I and BPD II) by age in the National Comorbidity Survey Replication (NCS‐R) and Adolescent Supplement (NCS‐A) (n = 12,175)
Lifetime morbid risk (LMR)
Projected LMR as of age 75 based on the AOO distributions is consistently higher than lifetime prevalence across all disorders (Tables 3 and 4). However, the ratios of the two estimates vary considerably across disorders both among adolescents and adults in the 18–64 age range. Comparison of the two estimates for respondents older than 64 makes less sense, as lifetime prevalence estimates among these older respondents are so low as to suggest that the model used to estimate LMR is inappropriate for this segment of the population. Focusing, then, on adolescents and adults ages 18–64, we see that the disorders with the highest ratios of estimated lifetime prevalence to projected LMR in both sub‐samples are SAD (75–88%) and the phobias (66–110% for adolescents; 71–100% for adults). These high ratios suggest that the vast majority of both the adolescents and the adults who will ever have these disorders already had onsets by the time of our survey. This reflects the early ages‐of‐onset of these disorders seen in Figure 1. In the case of adolescent specific phobia, furthermore, the ratio of current lifetime prevalence to estimated LMR being above 100% indicates that the model assumptions used to project LMR are inconsistent with the observed data.
Table 3.
Lifetime morbid risk (LMR) and the ratio of lifetime prevalence to morbid risk (LT/LMR) of DSM‐IV‐TR/CIDI anxiety disorders by age in the National Comorbitiy Survey Replication (NCS‐R) and Adolescent Supplement (NCS‐A)
| LMR | LT/LMR | ||||
|---|---|---|---|---|---|
| Percent | (SE) | 13–17 | 18–64 | Total (ages 13+)a | |
| Panic disorderb | 6.8 | (0.4) | 0.3 | 0.8 | 0.5 |
| Generalized anxiety disorder | 9.0 | (0.5) | 0.2 | 0.7 | 0.5 |
| Agoraphobiac | 3.7 | (0.3) | 0.7 | 0.7 | 0.7 |
| Social phobia | 13.0 | (0.5) | 0.7 | 1.0 | 0.8 |
| Specific phobia | 18.4 | (0.5) | 1.1 | 0.8 | 0.8 |
| Separation anxiety disorder | 8.7 | (0.4) | 0.9 | 0.8 | 0.8 |
| Post‐traumatic stress disorder | 10.1 | (0.6) | 0.4 | 0.8 | 0.6 |
| Obsessive‐compulsive disorder | 2.7 | (0.4) | —d | 1.0 | 0.9 |
| Any anxiety disorder | 41.7 | (0.8) | 0.8 | 0.8 | 0.8 |
| (n) | (12,175) | (6243) | (5223) | (12,175) | |
The NCS‐A (ages 13–17) and NCS‐R (ages 18+) samples were combined without weighting to adjust for the higher probability of selection of adolescents than adults in the two surveys.
With or without agoraphobia.
With or without a history of panic disorder.
Obsessive‐compulsive disorder was not assessed among adolescents.
Table 4.
Lifetime morbid risk (LMR) and the ratio of lifetime prevalence to morbid risk (LT/LMR) of DSM‐IV‐TR/CIDI mood disorders by age in the National Comorbidity Survey Replication (NCS‐R) and Adolescent Supplement (NCS‐A)
| LMR | LT/LMR | ||||
|---|---|---|---|---|---|
| Percent | (SE) | 13–17 | 18–64 | Total (ages 13+)a | |
| Major depressive episode | 29.9 | (0.6) | 0.4 | 0.7 | 0.6 |
| Bipolar disorder | |||||
| BPD I | 1.1 | (0.2) | 0.1 | 1.0 | 0.6 |
| BPD II | 2.9 | (0.3) | 1.0 | 0.5 | 0.6 |
| BPD I or II | 4.1 | (0.4) | 0.7 | 0.6 | 0.6 |
| Any mood disorder | 30.7 | (0.7) | 0.5 | 0.7 | 0.6 |
| (n) | (12,175) | (6243) | (5223) | (12,175) | |
The NCS‐A (ages 13–17) and NCS‐R (ages 18+) samples were combined without weighting to adjust for the higher probability of selection of adolescents than adults in the two surveys.
The ratios of lifetime prevalence to LMR are much lower for MDE, GAD, PD, and PTSD among adolescents (24–45%), while these ratios are higher among adults (69–79%) even though they remain lower than the ratios reported in the last paragraph for the early‐onset anxiety disorders. This reflects the later ages‐of‐onset seen for these disorders than for the early‐onset anxiety disorders in Figure 2. The higher ratios for these disorders among adults than adolescents reflect the fact that many first onsets occur in the middle years of life. Indeed, a comparison of cumulative lifetime prevalence estimates as of age 64 with those as of age 17 in Figures 2 and 3 shows that an estimated 69–74% of all lifetime cases of MDE, GAD, and PD have first onsets in the age range 18–64. It is interesting to note that the proportion is lower for PTSD (55%) due to the much higher proportion of lifetime cases of PTSD (42%) than the other three disorders in this group (20% of GAD; 27% of MDE and PD) that occur either in childhood or adolescence. The higher proportion of lifetime cases of PTSD than the other three disorders that have first onsets in childhood/adolescence reflects the fact that many traumatic life experiences have occurred for the first time in childhood‐adolescence (Cisler et al., 2012; Fairbank and Fairbank, 2009). An exception to this general pattern of higher ratios among adults than adolescents occurs for BPD. This could reflect the possible over‐diagnosis of BPD among adolescents that was noted earlier in this section.
Twelve‐month prevalence
The most prevalent disorders in the 12‐months before interview are the same as those in the lifetime: specific phobia (12.1%), social phobia (7.4%), and MDD (7.1%; Tables 5 and 6). The other disorders considered here have much lower 12‐month prevalence, from a high of 3.7% for PTSD to lows of 1.2% for OCD (not assessed among adolescents) and SAD, and 1.7% for agoraphobia. The rank‐order of 12‐month prevalence estimates is very similar to that of lifetime estimates, but there are a few inversions. For example, social phobia has higher 12‐month prevalence than MDD, while the reverse is true for lifetime prevalence. This inversion reflects the more persistent course of social phobia than MDD, which can be seen indirectly in the fact that the ratio of 12‐month to lifetime prevalence is roughly 70% for social phobia compared to roughly 50% for MDD.
Table 5.
Twelve‐month prevalence (12M) and the ratios of 12‐month to lifetime prevalence (12M/LT) and lifetime morbid risk (12M/LMR) of DSM‐IV‐TR/CIDI anxiety by age in the National Comorbidity Survey Replication (NCS‐R) and Adolescent Supplement (NCS‐A)
| 13–17 | 18‐64 | 65+ | Total (ages 13+)a | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 12M | 12M/LT | 12M | 12M/LT | 12M | 12M/LT | 12M | 12M/LT | 12M/LMR | |||||
| Percent | (SE) | Estimated | Percent | (SE) | Estimated | Percent | (SE) | Estimated | Percent | (SE) | Estimated | Estimated | |
| Panic disorderb | 1.9 | (0.3) | 0.8 | 3.1 | (0.2) | 0.6 | 0.7 | (0.2) | 0.3 | 2.4 | (0.1) | 0.6 | 0.3 |
| Generalized anxiety disorder | 0.9 | (0.1) | 0.4 | 2.9 | (0.2) | 0.5 | 1.2 | (0.3) | 0.4 | 2.0 | (0.1) | 0.5 | 0.2 |
| Agoraphobiac | 2.0 | (0.3) | 0.8 | 1.7 | (0.1) | 0.6 | 0.4 | (0.2) | 0.4 | 1.7 | (0.1) | 0.7 | 0.5 |
| Social phobia | 7.9 | (0.6) | 0.9 | 8.0 | (0.4) | 0.6 | 2.7 | (0.4) | 0.4 | 7.4 | (0.3) | 0.7 | 0.6 |
| Specific phobia | 16.3 | (0.9) | 0.8 | 10.1 | (0.5) | 0.7 | 5.0 | (0.6) | 0.7 | 12.1 | (0.4) | 0.8 | 0.7 |
| Separation anxiety disorder | 1.5 | (0.2) | 0.2 | 1.0 | (0.1) | 0.2 | 0.0 | (0.0) | 0.0 | 1.2 | (0.1) | 0.7 | 0.1 |
| Post‐traumatic stress disorder | 3.6 | (0.4) | 0.8 | 4.4 | (0.4) | 0.5 | 0.4 | (0.1) | 0.3 | 3.7 | (0.3) | 0.6 | 0.4 |
| Obsessive‐compulsive disorder | —d | —d | —d | 1.3 | (0.3) | 0.5 | 0.3 | (0.2) | 0.7 | 1.2 | (0.3) | 0.5 | 0.4 |
| Any anxiety disorder | 25.2 | (1.0) | 0.8 | 21.3 | (0.6) | 0.6 | 7.6 | (0.9) | 0.5 | 22.2 | (0.5) | 0.7 | 0.5 |
| (n) | (6243) | (5223) | (709) | (12,175) | |||||||||
The NCS‐A (ages 13–17) and NCS‐R (ages 18+) samples were combined without weighting to adjust for the higher probability of selection of adolescents than adults in the two surveys.
With or without agoraphobia.
With or without a history of panic disorder.
Obsessive‐compulsive disorder was not assessed among adolescents.
Table 6.
Twelve‐month prevalence (12M) and the ratios of 12‐month to lifetime prevalence (12M/LT) and lifetime morbid risk (12M/LMR) of DSM‐IV‐TR/CIDI mood disorders by age in the National Comorbidity Survey Replication (NCS‐R) and Adolescent Supplement (NCS‐A)
| 13–17 | 18–64 | 65+ | Total (ages 13+)a | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 12M | 12M/LT | 12M | 12M/LT | 12M | 12M/LT | 12M | 12M/LT | 12M/LMR | |||||
| Percent | (SE) | Estimated | Percent | (SE) | Estimated | Percent | (SE) | Estimated | Percent | (SE) | Estimated | Estimated | |
| Major depressive episode | |||||||||||||
| Single | 1.8 | (0.3) | 0.6 | 1.6 | (0.1) | 0.3 | 0.7 | (0.2) | 0.2 | 1.6 | (0.1) | 0.4 | ‐‐ |
| Recurrent episodes | 7.3 | (0.8) | 0.8 | 7.7 | (0.3) | 0.5 | 2.0 | (0.4) | 0.3 | 7.0 | (0.4) | 0.6 | ‐‐ |
| Total | 9.1 | (0.8) | 0.7 | 9.3 | (0.4) | 0.4 | 2.6 | (0.4) | 0.3 | 8.6 | (0.4) | 0.5 | 0.3 |
| Major depressive disorder | |||||||||||||
| Single | 1.6 | (0.3) | 0.6 | 1.3 | (0.1) | 0.3 | 0.7 | (0.2) | 0.2 | 1.4 | (0.1) | 0.3 | ‐‐ |
| Recurrent episodes | 5.8 | (0.7) | 0.8 | 6.4 | (0.3) | 0.5 | 1.7 | (0.3) | 0.3 | 5.7 | (0.3) | 0.6 | ‐‐ |
| Total | 7.4 | (0.8) | 0.7 | 7.7 | (0.3) | 0.4 | 2.3 | (0.3) | 0.2 | 7.1 | (0.4) | 0.5 | ‐‐ |
| Bipolar disorder | |||||||||||||
| BPD I | 0.2 | (0.1) | 0.9 | 0.7 | (0.1) | 0.6 | 0.1 | (0.1) | 0.5 | 0.4 | (0.1) | 0.7 | 0.4 |
| BPD II | 2.1 | (0.3) | 0.8 | 1.0 | (0.1) | 0.7 | 0.1 | (0.1) | 0.6 | 1.4 | (0.1) | 0.7 | 0.5 |
| BPD I or II | 2.3 | (0.3) | 0.8 | 1.7 | (0.2) | 0.7 | 0.2 | (0.1) | 0.5 | 1.8 | (0.1) | 0.7 | 0.4 |
| Any mood disorder | 10.4 | (0.8) | 0.7 | 9.9 | (0.4) | 0.5 | 2.6 | (0.4) | 0.3 | 9.4 | (0.4) | 0.5 | 0.3 |
| (n) | (6243) | (5223) | (709) | (12,175) | |||||||||
The NCS‐A (ages 13–17) and NCS‐R (ages 18+) samples were combined without weighting to adjust for the higher probability of selection of adolescents than adults in the two surveys.
The ratios of 12‐month to lifetime prevalence vary substantially in sub‐samples defined by age. The highest ratios are consistently found among adolescents and the lowest among the elderly, presumably reflecting the joint effects of associations between age and time‐since‐onset and between time‐since‐onset and persistence. The only two disorders for which this pattern does not hold are GAD and specific phobia. These discrepancies might reflect the fact that GAD has a chronic‐episodic course reflected in intermediate prevalence ratios for all three age groups (roughly 35–50% of lifetime cases in episode in the 12 months before interview) while specific phobia has a persistent course reflected in high prevalence ratios for all three age groups (roughly 75–80% of lifetime cases in episode in the 12 months before interview). The ratios also vary substantially across disorders, presumably reflecting the joint effects of differences in time‐since‐onset and persistence. Among adolescents, where the ratios are generally quite high, the most striking discrepancy other than the one noted earlier in this paragraph for GAD is the very low ratio for SAD (only about 20% of lifetime cases still in episode in the 12 months before interview). This reflects the more rapid resolution of child‐adolescent SAD than the other disorders considered here. Among adults in the age range 18–64, in addition to a similarly low ratio for SAD, there is a comparatively low ratio for single‐episode MDE/MDD (roughly 15–45% of lifetime cases in episode in the 12 months before interview). The ratios are consistently low among elderly respondents with the notable exceptions of specific phobia (where roughly 75% of lifetime cases continue to have the disorder in the 12 months before interview) and OCD.
Discussion
The data presented here represent the first comprehensive overview of epidemiological information on the prevalence and LMR of anxiety and mood disorders in the US general population in the format called for by the DSM‐5. We focus on prevalence estimates using DSM‐IV criteria due to the fact that DSM‐5 criteria have not yet been finalized. We will not know if similar patterns hold for DSM‐5 disorders until the new criteria are finalized and epidemiological studies using these criteria are carried out. Earlier reports of prevalence estimates from these surveys focused either on adolescents (Kessler et al., 2012) or on all adults aged 18+ without distinguishing between those 18–64 and 65+ (Kessler et al., 2005a, 2005b). The more complete data presented here that combines results for adolescents and adults to estimate prevalence, AOO, and LMR should help inform the scientific community with more comprehensive estimates for the United States. As noted earlier, these data are intended for use in the text description of the upcoming DSM revision (DSM‐5) in order to ensure consistency of reporting standards and conventions.
The broad sweep of results reconfirms findings from many previous epidemiological studies in several respects. First, we show that major depression, specific phobia, and social phobia are the most common anxiety‐mood disorders, and agoraphobia and OCD are the least such common disorders in the United States. This is consistent with previous data from the United States (Kessler et al., 1994; Regier et al., 1998) and elsewhere in the world (Demyttenaere et al., 2004; WHO International Consortium in Psychiatric Epidemiology, 2000). Second, we show that phobias and SAD have earlier ages‐of‐onset than other anxiety‐mood disorders, while GAD, MDE, and PD have the latest ages‐of‐onset among these classes of disorders. Again, these findings are consistent with previous epidemiological studies (Christie et al., 1988; Kessler et al., 2007a; McGorry et al., 2011). Third, we show that LMR is considerably higher than lifetime prevalence for most anxiety‐mood disorders, although the magnitude of this difference is much higher for disorders with later rather than earlier ages‐of‐onset. Similar patterns have been found in previous World Mental Health reports (Kessler et al., 2005a, 2007b), but we are unaware of other epidemiological studies that have examined this issue. Fourth, we show that while 12‐month prevalence of anxiety‐mood disorders is considerably lower than lifetime prevalence, the ratio of 12‐month to lifetime prevalence varies meaningfully in ways consistent with independent evidence about differential persistence of these disorders (Kessler et al., 2010; Kessler and Wang, 2009).
As noted earlier, we conclude that two measures of particular value in characterizing the descriptive epidemiology of anxiety‐mood disorders are LMR and 12‐month prevalence. The measure of LMR, especially when considered in combination with information on AOO, is useful in making researchers aware of the high risk of mental disorders over the lifespan, although it is also important to recognize that the accuracy of LMR estimates depends on stability of AOO distributions over generations as well as on accuracy of estimates of AOO in the cohorts studies with available data. If accepted as accurate, this information can be used for planning cohort studies and designing targeted research to identify studies of vulnerability and risk factors for disorder onset most efficiently. This information can also be useful to the public and patients in increasing awareness about the true size and burden of specific mental disorders and, it might be hoped, by diminishing stigma by virtue of making it clear that these disorders are commonly occurring and widely distributed in the general population.
Estimates of 12‐month prevalence, in comparison, are compromise measures with regard to policy issues that balance the problem of recall bias associated with measures having longer recall intervals with the problem of low significance of measures having shorter recall intervals. With regard to the issue of shorter intervals, it is important to remember that anxiety and mood disorders have an episodic course or a persisting, though frequently fluctuating, course. When we speak here of fluctuations we refer to the fact that symptom severity fluctuates around the diagnostic threshold set by the diagnostic criteria, resulting in symptom reports having the two‐week or four‐week time frames preferred by some epidemiologists showing much more instability than reports over longer recall periods. This can lead to dramatic errors in estimating the true size and burden of disorders for resource policy planning purposes. An added problem with the use of prevalence estimates based on short recall periods is that a number of common anxiety‐mood disorders require that symptoms persist for much longer periods of time in order to qualify for diagnoses (e.g. GAD, dysthymic disorder), making it technically awkward to assign diagnoses in shorter time windows. This can be an especially important issue in making differential diagnoses. An additional issue with using short‐time frames concerns the assessment of treatment. Because it very often takes patients a long time to seek treatment for mental disorders, it is more valuable to determine whether or not treatment was sought over a longer time period than to focus only on a very short‐time frame. Although a 12‐month recall period is to some degree arbitrary, it is appealing in that health policy planning is typically designed for a 12 month period. In interpreting the earlier results, it is important to remember that estimates of LMR are based on a projection model that uses information about lifetime prevalence and AOO reported by older respondents to make estimates about future disorder onsets among younger respondents. It is also important to recall that lifetime prevalence estimates are so low among older respondents that it raises concerns about either sample bias, reporting bias related to old age, or the relevance of DSM diagnostic criteria to the mental disorders of elderly people. As noted in the introduction, we also have good reason to believe that recall bias is pervasive even for short recall periods and across the entire age range. To the extent that those biases exist, estimates of both lifetime prevalence and LMR in the total sample will be downwardly biased, while estimates of 12‐month to lifetime prevalence ratios will likely be upwardly biased. These same biases could, of course, also exist among younger respondents, but would presumably be less extreme.
Acknowledgements
Author Contributions: Kessler had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Financial Disclosure: Dr Kessler has been a consultant for AstraZeneca, Analysis Group, Bristol‐Myers Squibb, Cerner‐Galt Associates, Eli Lilly & Company, GlaxoSmithKline Inc., HealthCore Inc., Health Dialog, Hoffman‐LaRoche, Inc., Integrated Benefits Institute, John Snow Inc., Kaiser Permanente, Matria Inc., Mensante, Merck & Co, Inc., Ortho‐McNeil Janssen Scientific Affairs, Pfizer Inc., Primary Care Network, Research Triangle Institute, Sanofi‐Aventis Groupe, Shire US Inc., SRA International, Inc., Takeda Global Research & Development, Transcept Pharmaceuticals Inc., and Wyeth‐Ayerst. Dr. Kessler has served on advisory boards for Appliance Computing II, Eli Lilly & Company, Mindsite, Ortho‐McNeil Janssen Scientific Affairs, Johnson & Johnson, Plus One Health Management and Wyeth‐Ayerst.
Dr Kessler has had research support for his epidemiological studies from Analysis Group Inc., Bristol‐Myers Squibb, Eli Lilly & Company, EPI‐Q, GlaxoSmithKline, Johnson & Johnson Pharmaceuticals, Ortho‐McNeil Janssen Scientific Affairs., Pfizer Inc., Sanofi‐Aventis Groupe, Shire US, Inc., and Walgreens Co. Dr Kessler owns 25% share in DataStat, Inc., Dr Wittchen serves as a DSM‐5 Work Group member. The remaining authors report no financial relationships with commercial interests.
Funding/Support: The National Comorbidity Survey Replication and its adolescent supplement are supported by the National Institute of Mental Health (NIMH; U01‐MH60220, R01‐MH66627 and U01MH060220‐09S1) with supplemental support from the National Institute on Drug Abuse (NIDA), the Substance Abuse and Mental Health Services Administration (SAMHSA), the Robert Wood Johnson Foundation (RWJF; Grant 044780), and the John W. Alden Trust. A complete list of NCS‐R and NCS‐A publications can be found at: http://www.hcp.med.harvard.edu/ncs. The NCS‐R and NCS‐A are carried out in conjunction with the World Health Organization World Mental Health (WMH) Survey Initiative. We thank the staff of the WMH Data Collection and Data Analysis Coordination Centers for assistance with instrumentation, fieldwork, and consultation on data analysis. The WMH Data Coordination Centers have received support from NIMH (R01‐MH070884, R13‐MH066849, R01‐MH069864, R01‐MH077883), NIDA (R01‐DA016558), the Fogarty International Center of the National Institutes of Health (FIRCA R03‐TW006481), the John D. and Catherine T. MacArthur Foundation, the Pfizer Foundation, and the Pan American Health Organization. The WMH Data Coordination Centers have also received unrestricted educational grants from Astra Zeneca, BristolMyersSquibb, Eli Lilly and Company, GlaxoSmithKline, Ortho‐McNeil, Pfizer, Sanofi‐Aventis, and Wyeth. A complete list of WMH publications can be found at http://www.hcp.med.harvard.edu/wmh/.
Role of the Sponsors: The sponsors had no role in the design and conduct of the study, the collection, management, analysis, or interpretation of the data, or the preparation, review, or approval of the manuscript.
References
- Adams P.F., Hendershot G.E., Marano M.A. (1999) Current estimates from the National Health Interview Survey, 1996. Vital and Health Statistics, 10(200), 1–203. [PubMed] [Google Scholar]
- Andrade L.H., Wang Y.P., Andreoni S., Silveira C.M., Alexandrino‐Silva C., Siu E.R., Nishimura R., Anthony J.C., Gattaz W.F., Kessler R.C., Viana M.C. (2012) Mental disorders in megacities: findings from the Sao Paulo megacity mental health survey, Brazil. PLoS One, 7(2), e31879 DOI: 10.1371/journal.pone.0031879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braaten E.B., Biederman J., DiMauro A., Mick E., Monuteaux M.C., Muehl K., Faraone S.V. (2001) Methodological complexities in the diagnosis of major depression in youth: an analysis of mother and youth self‐reports. Journal of Child and Adolescent Psychopharmacology, 11(4), 395–407. DOI: 10.1089/104454601317261573 [DOI] [PubMed] [Google Scholar]
- Carra G., Johnson S., Bebbington P., Angermeyer M.C., Heider D., Brugha T., Azorin J.M., Toumi M. (2012) The lifetime and past‐year prevalence of dual diagnosis in people with schizophrenia across Europe: findings from the European Schizophrenia Cohort (EuroSC). European Archives of Psychiatry and Clinical Neurosciences. DOI: 10.1007/s00406-012-0305-z [DOI] [PubMed] [Google Scholar]
- Christie K.A., Burke J.D., Jr , Regier D.A., Rae D.S., Boyd J.H., Locke B.Z. (1988) Epidemiologic evidence for early onset of mental disorders and higher risk of drug abuse in young adults. The American Journal of Psychiatry, 145(8), 971–975. [DOI] [PubMed] [Google Scholar]
- Cisler J.M., Begle A.M., Amstadter A.B., Resnick H.S., Danielson C.K., Saunders B.E., Kilpatrick D.G. (2012) Exposure to interpersonal violence and risk for PTSD, depression, delinquency, and binge drinking among adolescents: data from the NSA‐R. Journal of Traumatic Stress, 25(1), 33–40. DOI: 10.1002/jts.21672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costello E.J., Mustillo S., Erkanli A., Keeler G., Angold A. (2003) Prevalence and development of psychiatric disorders in childhood and adolescence. Archives of General Psychiatry, 60(8), 837–844. DOI: 10.1001/archpsyc.60.8.837 [DOI] [PubMed] [Google Scholar]
- Demyttenaere K., Bruffaerts R., Posada‐Villa J., Gasquet I., Kovess V., Lepine J.P., Angermeyer M.C., Bernert S., de Girolamo G., Morosini P., Polidori G., Kikkawa T., Kawakami N., Ono Y., Takeshima T., Uda H., Karam E.G., Fayyad J.A., Karam A.N., Mneimneh Z.N., Medina‐Mora M.E., Borges G., Lara C., de Graaf R., Ormel J., Gureje O., Shen Y., Huang Y., Zhang M., Alonso J., Haro J.M., Vilagut G., Bromet E.J., Gluzman S., Webb C., Kessler R.C., Merikangas K.R., Anthony J.C., Von Korff M.R., Wang P.S., Brugha T.S., Aguilar‐Gaxiola S., Lee S., Heeringa S., Pennell B.E., Zaslavsky A.M., Ustun T.B., Chatterji S. (2004) Prevalence, severity, and unmet need for treatment of mental disorders in the World Health Organization World Mental Health Surveys. Journal of the American Medical Association, 291(21), 2581–2590. DOI: 10.1001/jama.291.21.2581 [DOI] [PubMed] [Google Scholar]
- Eaton W.W., Alexandre P., Kessler R.C., Martins S.S., Mortensen P.B., Rebok G.W., Storr C., Roth K. (2012) The population dynamics of mental disorders In Eaton W.W. (ed.) Public Mental Health, Oxford, Oxford University Press; pp. 125–150. [Google Scholar]
- Eisenhower D., Mathiowetz N.A., Morganstein D. (2004) Recall error: sources and bias reduction In Biemer P.P., Groves R.M., Lyberg L.E., Mathiowetz N.A., Sudman S. (eds) Measurement Error in Surveys (2nd ed.), pp. 127–144, Chichester, John Wiley & Sons. [Google Scholar]
- Fairbank J.A., Fairbank D.W. (2009) Epidemiology of child traumatic stress. Current Psychiatry Reports, 11(4), 289–295. DOI: 10.1007/s11920-009-0042-9 [DOI] [PubMed] [Google Scholar]
- First M.B., Spitzer R.L., Gibbon M., Williams J.B.W. (2002) Structured Clinical Interview for DSM‐IV Axis I Disorders, Research Version, Non‐patient Edition (SCID‐I/NP), New York, Biometrics Research, New York State Psychiatric Institute. [Google Scholar]
- Fuchs C., Steinberg D.M., Poyurovsky M. (2010) On the estimation and comparison of lifetime morbid risks. Journal of Data Science, 8(4), 645–664. [Google Scholar]
- Futterman L.A., Rapkin A.J. (2006) Diagnosis of premenstrual disorders. The Journal of Reproductive Medicine, 51(4 Suppl), 349–358. [PubMed] [Google Scholar]
- Grossardt B.R., Bower J.H., Geda Y.E., Colligan R.C., Rocca W.A. (2009) Pessimistic, anxious, and depressive personality traits predict all‐cause mortality: the Mayo Clinic cohort study of personality and aging. Psychosomatic Medicine, 71(5), 491–500. DOI: 10.1097/PSY.0b013e31819e67db [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halli S.S., Rao K.V., Halli S.S. (1992) Advanced Techniques of Population Analysis, New York, Plenum Press. [Google Scholar]
- Haro J.M., Arbabzadeh‐Bouchez S., Brugha T.S., de Girolamo G., Guyer M.E., Jin R., Lepine J.P., Mazzi F., Reneses B., Vilagut G., Sampson N.A., Kessler R.C. (2006) Concordance of the Composite International Diagnostic Interview Version 3.0 (CIDI 3.0) with standardized clinical assessments in the WHO World Mental Health surveys. International Journal of Methods in Psychiatric Research, 15(4), 167–180. DOI: 10.1002/mpr.196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins R., Bebbington P., Brugha T., Farrell M., Gill B., Lewis G., Meltzer H., Petticrew M. (1997a) The National Psychiatric Morbidity surveys of Great Britain – strategy and methods. Psychological Medicine, 27(4), 765–774. DOI: 10.1017/S003329179700531X [DOI] [PubMed] [Google Scholar]
- Jenkins R., Lewis G., Bebbington P., Brugha T., Farrell M., Gill B., Meltzer H. (1997b) The National Psychiatric Morbidity surveys of Great Britain – initial findings from the household survey. Psychological Medicine, 27(4), 775–789. DOI: 10.1017/S0033291797005308 [DOI] [PubMed] [Google Scholar]
- Jenkins R., Lewis G., Bebbington P., Brugha T., Farrell M., Gill B., Meltzer H. (2003) The National Psychiatric Morbidity Surveys of Great Britain – initial findings from the household survey. International Review of Psychiatry, 15(1–2), 29–42. DOI: 10.1080/0954026021000045921 [DOI] [PubMed] [Google Scholar]
- Kaplan E.L., Meier P. (1958) Nonparametric estimation from incomplete observations. Journal of the American Statistical Association, 53(282), 457–481. [Google Scholar]
- Kaufman J., Birmaher B., Brent D., Rao U., Flynn C., Moreci P., Williamson D., Ryan N. (1997) Schedule for Affective Disorders and Schizophrenia for School‐Age Children‐Present and Lifetime Version (K‐SADS‐PL): initial reliability and validity data. Journal of the American Academy of Child and Adolescent Psychiatry, 36(7), 980–988. DOI: 10.1097/00004583-199707000-00021 [DOI] [PubMed] [Google Scholar]
- Kessler R.C., Amminger G.P., Aguilar‐Gaxiola S., Alonso J., Lee S., Ustun T.B. (2007a) Age of onset of mental disorders: a review of recent literature. Current Opinion in Psychiatry, 20(4), 359–364. DOI: 10.1097/YCO.0b013e32816ebc8c [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler R.C., Angermeyer M., Anthony J.C., Demyttenaere K., Gasquet I., Gluzman S., Gureje O., Haro J.M., Kawakami N., Karam A., Levinson D., Medina Mora M.E., Oakley Browne M.A., Posada‐Villa J., Stein D.J., Adley Tsang C.H., Aguilar‐Gaxiola S., Alonso J., Lee S., Heeringa S., Pennell B.E., Berglund P., Gruber M.J., Petukhova M., Chatterji S., Üstün T.B. (2007b) Lifetime prevalence and age‐of‐onset distributions of mental disorders in the World Health Organization's World Mental Health Survey Initiative. World Psychiatry, 6(3), 168–176. [PMC free article] [PubMed] [Google Scholar]
- Kessler R.C., Avenevoli S., Costello E.J., Georgiades K., Green J.G., Gruber M.J., He J.P., Koretz D., McLaughlin K.A., Petukhova M., Sampson N.A., Zaslavsky A.M., Merikangas K.R. (2012) Prevalence, persistence, and sociodemographic correlates of DSM‐IV disorders in the National Comorbidity Survey Replication Adolescent Supplement. Archives of General Psychiatry, 69(4), 372–380. DOI: 10.1001/archgenpsychiatry.2011.160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler R.C., Avenevoli S., Costello E.J., Green J.G., Gruber M.J., Heeringa S., Merikangas K.R., Pennell B.E., Sampson N.A., Zaslavsky A.M. (2009a) Design and field procedures in the US National Comorbidity Survey Replication Adolescent Supplement (NCS‐A). International Journal of Methods in Psychiatric Research, 18(2), 69–83. DOI: 10.1002/mpr.279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler R.C., Avenevoli S., Costello E.J., Green J.G., Gruber M.J., Heeringa S., Merikangas K.R., Pennell B.E., Sampson N.A., Zaslavsky A.M. (2009b) National comorbidity survey replication adolescent supplement (NCS‐A): II. Overview and design. Journal of the American Academy of Child and Adolescent Psychiatry, 48(4), 380–385. DOI: 10.1097/CHI.0b013e3181999705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler R.C., Avenevoli S., Green J., Gruber M.J., Guyer M., He Y., Jin R., Kaufman J., Sampson N.A., Zaslavsky A.M. (2009c) National comorbidity survey replication adolescent supplement (NCS‐A): III. Concordance of DSM‐IV/CIDI diagnoses with clinical reassessments. Journal of the American Academy of Child and Adolescent Psychiatry, 48(4), 386–399. DOI: 10.1097/CHI.0b013e31819a1cbc [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler R.C., Berglund P., Chiu W.T., Demler O., Heeringa S., Hiripi E., Jin R., Pennell B.E., Walters E.E., Zaslavsky A., Zheng H. (2004) The US National Comorbidity Survey Replication (NCS‐R): design and field procedures. International Journal of Methods in Psychiatric Research, 13(2), 69–92. DOI: 10.1002/mpr.167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler R.C., Berglund P., Demler O., Jin R., Merikangas K.R., Walters E.E. (2005a) Lifetime prevalence and age‐of‐onset distributions of DSM‐IV disorders in the National Comorbidity Survey Replication. Archives of General Psychiatry, 62(6), 593–602. DOI: 10.1001/archpsyc.62.6.593 [DOI] [PubMed] [Google Scholar]
- Kessler R.C., Chiu W.T., Demler O., Merikangas K.R., Walters E.E. (2005b) Prevalence, severity, and comorbidity of 12‐month DSM‐IV disorders in the National Comorbidity Survey Replication. Archives of General Psychiatry, 62(6), 617–627. DOI: 10.1001/archpsyc.62.6.617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler R.C., McGonagle K.A., Zhao S., Nelson C.B., Hughes M., Eshleman S., Wittchen H.U., Kendler K.S. (1994) Lifetime and 12‐month prevalence of DSM‐III‐R psychiatric disorders in the United States. Results from the National Comorbidity Survey. Archives of General Psychiatry, 51(1), 8–19. [DOI] [PubMed] [Google Scholar]
- Kessler R.C., Merikangas K.R. (2004) The National Comorbidity Survey Replication (NCS‐R): background and aims. International Journal of Methods in Psychiatric Research, 13(2), 60–68. DOI: 10.1002/mpr.166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler R.C., Ruscio A.M., Shear K., Wittchen H.U. (2010) Epidemiology of anxiety disorders. In Stein M.B. & Steckler T. (Eds.), Behavioral Neurobiology of Anxiety and its Treatment (pp. 21–35). Heidelberg, Germany: Springer Publishers. [Google Scholar]
- Kessler R.C., Üstün T.B. (2004) The World Mental Health (WHM) Survey Initiative Version of the World Health Organization (WHO) Composite International Diagnostic Interview (CIDI). International Journal of Methods in Psychiatric Research, 13(2), 93–121. DOI: 10.1002/mpr.168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler R.C., Üstün T.B. (2008) The WHO World Mental Health Surveys: Global Perspectives on the Epidemiology of Mental Disorders, Cambridge, Cambridge University Press. [Google Scholar]
- Kessler R.C., Wang P.S. (2009) The epidemiology of depression In Gotlib I.H., Hammen C.L. (eds) Handbook of Depression (2nd ed.), New York, Guilford Press; pp. 5–22. [Google Scholar]
- Kessler R.C., Wittchen H.‐U., Abelson J.M., McGonagle K.A., Schwarz N., Kendler K.S., Knäuper B., Zhao S. (1998) Methodological studies of the Composite International Diagnostic Interview (CIDI) in the US National Comorbidity Survey. International Journal of Methods in Psychiatric Research, 7(1), 33–55. DOI: 10.1002/mpr.33 [DOI] [Google Scholar]
- Kish L., Frankel M.R. (1974) Inferences from complex samples. Journal of the Royal Statistical Society, 36(1), 1–37. [Google Scholar]
- Knäuper B., Cannell C.F., Schwarz N., Bruce M.L., Kessler R.C. (1999) Improving the accuracy of major depression age of onset reports in the US National Comorbidity Survey. International Journal of Methods in Psychiatric Research, 8(1), 39–48. DOI: 10.1002/mpr.55 [DOI] [Google Scholar]
- Lefèvre T., Singh‐Manoux A., Stringhini S., Dugravot A., Lemogne C., Consoli S.M., Goldberg M., Zins M., Nabi H. (2011) Usefulness of a single‐item measure of depression to predict mortality: the GAZEL prospective cohort study. European Journal of Public Health. DOI: 10.1093/eurpub/ckr103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcks B.A., Weisberg R.B., Dyck I., Keller M.B. (2011) Longitudinal course of obsessive‐compulsive disorder in patients with anxiety disorders: a 15‐year prospective follow‐up study. Comprehensive Psychiatry, 52(6), 670–677. DOI: 10.1016/j.comppsych.2011.01.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEvoy P.M., Grove R., Slade T. (2011) Epidemiology of anxiety disorders in the Australian general population: findings of the 2007 Australian National Survey of Mental Health and Wellbeing. The Australian and New Zealand Journal of Psychiatry, 45(11), 957–967. DOI: 10.3109/00048674.2011.624083 [DOI] [PubMed] [Google Scholar]
- McGorry P.D., Purcell R., Goldstone S., Amminger G.P. (2011) Age of onset and timing of treatment for mental and substance use disorders: implications for preventive intervention strategies and models of care. Current Opinion in Psychiatry, 24(4), 301–306. DOI: 10.1097/YCO.0b013e3283477a09 [DOI] [PubMed] [Google Scholar]
- Merikangas K., Avenevoli S., Costello J., Koretz D., Kessler R.C. (2009) National comorbidity survey replication adolescent supplement (NCS‐A): I. Background and measures. Journal of the American Academy of Child and Adolescent Psychiatry, 48(4), 367–369. DOI: 10.1097/CHI.0b013e31819996f1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oakley Browne M.A., Wells J.E., Scott K.M., McGee M.A. (2006) Lifetime prevalence and projected lifetime risk of DSM‐IV disorders in Te Rau Hinengaro: the New Zealand Mental Health Survey. The Australian and New Zealand Journal of Psychiatry, 40(10), 865–874. DOI: 10.1111/j.1440-1614.2006.01905.x [DOI] [PubMed] [Google Scholar]
- Park S., Cho M.J., Chang S.M., Jeon H.J., Cho S.J., Kim B.S., Bae J.N., Wang H.R., Ahn J.H., Hong J.P. (2011) Prevalence, correlates, and comorbidities of adult ADHD symptoms in Korea: results of the Korean epidemiologic catchment area study. Psychiatry Research, 186(2–3), 378–383. DOI: 10.1016/j.psychres.2010.07.047 [DOI] [PubMed] [Google Scholar]
- Pietrzak R.H., Goldstein R.B., Southwick S.M., Grant B.F. (2011) Prevalence and Axis I comorbidity of full and partial posttraumatic stress disorder in the United States: results from Wave 2 of the National Epidemiologic Survey on Alcohol and Related Conditions. Journal of Anxiety Disorders, 25(3), 456–465. DOI: 10.1016/j.janxdis.2010.11.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radloff L.S. (1977) The CES‐D scale: a self‐report depression scale for research in the general population. Applied Psychological Measurement, 1(3), 385–401. DOI: 10.1177/014662167700100306 [DOI] [Google Scholar]
- Regier D.A., Kaelber C.T., Rae D.S., Farmer M.E., Knauper B., Kessler R.C., Norquist G.S. (1998) Limitations of diagnostic criteria and assessment instruments for mental disorders. Implications for research and policy. Archives of General Psychiatry, 55(2), 109–115. [DOI] [PubMed] [Google Scholar]
- SAS Institute Inc . (2001) SAS/STAT® Software, Version 9.1 for Unix, Cary, NC, SAS Institute Inc. [Google Scholar]
- Scott K.M., Bruffaerts R., Tsang A., Ormel J., Alonso J., Angermeyer M.C., Benjet C., Bromet E., de Girolamo G., de Graaf R., Gasquet I., Gureje O., Haro J.M., He Y., Kessler R.C., Levinson D., Mneimneh Z.N., Oakley Browne M.A., Posada‐Villa J., Stein D.J., Takeshima T., Von Korff M. (2007) Depression‐anxiety relationships with chronic physical conditions: results from the World Mental Health Surveys. Journal of Affective Disorders, 103(1–3), 113–120. DOI: 10.1016/j.jad.2007.01.015 [DOI] [PubMed] [Google Scholar]
- Scott K.M., Von Korff M., Alonso J., Angermeyer M.C., Bromet E., Fayyad J., de Girolamo G., Demyttenaere K., Gasquet I., Gureje O., Haro J.M., He Y., Kessler R.C., Levinson D., Medina Mora M.E., Oakley Browne M., Ormel J., Posada‐Villa J., Watanabe M., Williams D. (2009) Mental‐physical co‐morbidity and its relationship with disability: results from the World Mental Health Surveys. Psychological Medicine, 39(1), 33–43. DOI: 10.1017/S0033291708003188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiffman S., Stone A.A., Hufford M.R. (2008) Ecological momentary assessment. Annual Review of Clinical Psychology, 4, 1–32. DOI: 10.1146/annurev.clinpsy.3.022806.091415 [DOI] [PubMed] [Google Scholar]
- Wittchen H.U., Jacobi F. (2005) Size and burden of mental disorders in Europe – a critical review and appraisal of 27 studies. European Neuropsychopharmacology, 15(4), 357–376. DOI: 10.1016/j.euroneuro.2005.04.012 [DOI] [PubMed] [Google Scholar]
- Wittchen H.U., Jacobi F., Rehm J., Gustavsson A., Svensson M., Jonsson B., Olesen J., Allgulander C., Alonso J., Faravelli C., Fratiglioni L., Jennum P., Lieb R., Maercker A., van Os J., Preisig M., Salvador‐Carulla L., Simon R., Steinhausen H.C. (2011) The size and burden of mental disorders and other disorders of the brain in Europe 2010. European Neuropsychopharmacology, 21(9), 655–679. DOI: 10.1016/j.euroneuro.2011.07.018 [DOI] [PubMed] [Google Scholar]
- World Health Organization (WHO) International Consortium in Psychiatric Epidemiology . (2000) Cross‐national comparisons of the prevalences and correlates of mental disorders. Bulletin of the World Health Organization, 78(4), 413–426. [PMC free article] [PubMed] [Google Scholar]
- Youngstrom E.A., Birmaher B., Findling R.L. (2008) Pediatric bipolar disorder: validity, phenomenology, and recommendations for diagnosis. Bipolar Disorder, 10(1 Pt 2), 194–214. DOI: 10.1111/j.1399-5618.2007.00563.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanni G.R. (2007) Patient diaries: charting the course. The Consultant Pharmacist, 22(6), 472–476, 479–482. [DOI] [PubMed] [Google Scholar]


