Abstract
Two antifungal phenyl-phenalenone phytoalexins isolated from the banana plant (Musa acuminata) elicited with the fungus Fusarium oxysporum, together with a methoxy derivative of one of them and two epoxide precursors of their chemical synthesis, were tested for leishmanicidal activity on Leishmania donovani promastigotes and L. infantum amastigotes. Drugs inhibited proliferation of both forms of the parasite with a 50% lethal concentration range between 10.3 and 68.7 μg/ml. Their lethal mechanism was found linked to the respiratory chain by a systematic approach, including electron microscopy, measurement of the oxygen consumption rate on digitonin-permeabilized promastigotes, and enzymatic assays on a mitochondrial enriched fraction. Whereas the whole set of compounds inhibited the activity of fumarate reductase in the mitochondrial fraction (50% effective concentration [EC50] between 33.3 and 78.8 μg/ml) and on purified enzyme (EC50 = 53.3 to 115 μg/ml), inhibition for succinate dehydrogenase was only observed for the two phytoalexins with the highest leishmanicidal activity: anigorufone and its natural analogue 2-methoxy-9-phenyl-phenalen-1-one (EC50 = 33.5 and 59.6 μg/ml, respectively). These results provided a new structural motif, phenyl-phenalenone, as a new lead for leishmanicidal activity, and support the use of plant extracts enriched in antifungal phytoalexins, synthesized under fungal challenge, as a more rational and effective strategy to screen for new plant leishmanicidal drugs.
The human protozoan parasite Leishmania is the causative agent of leishmaniasis, a disease with a wide variety of clinical manifestations, ranging from self-healing cutaneous lesions (mostly species from Leishmania tropica and L. mexicana complexes) to life-threatening visceral infections caused by different species of the donovani complex (L. donovani, L. infantum, and L. chagasi). Leishmania threatens 350 million people worldwide with an annual incidence of 2 million cases and more than 12 million people infected (http://www.who.int/emc/diseases/leish/leisdis1.html). Due to the lack of a reliable human vaccine, together with the daunting control of parasite vectors and reservoirs, treatment relies exclusively on chemotherapy, with organic pentavalent antimonials as the first-line drugs (17). Nevertheless, their efficacy is impaired by the growing incidence of parasite resistance and their frequent and severe side effects (19). Alternative treatments, based on amphotericin B, paramomycin, allopurinol, and more recently, miltefosine, are also available (17), although most of these treatments have secondary effects (10). Thus, there is a pressing need for new leishmanicidal drugs.
One of the main sources for new leishmanicidal reagents is the isolation of secondary metabolites from plants (8, 15, 21). The biosynthesis of these molecules is carried out either in a constitutive, pathogen-independent manner (phytoanticipins) or is induced as a part of the plant defensive response against infection by bacteria, fungi, or nematodes (phytoalexins) (16). As expected from this functional classification, the structural diversity for both groups is extremely large, and structures such as flavanones, isoflavones, aurones, stilbenes, or phenalenones are gathered under the common name of phytoalexins (13, 16, 18, 25). A survey of the literature addressing the microbicidal activity of phytoalexins on human pathogens revealed an unexpectedly scarce number of works; these reports mainly focused on in vitro assays for bactericidal and fungicidal activities (7) and, to our knowledge, none of these studies examined the use of phytoalexins as antiprotozoal agents.
Anigorufone is an antifungal phenyl-phenalenone phytoalexin, isolated from the banana plant (Musa acuminata). Its synthesis is triggered by infection with the fungus Fusarium oxysporum, a saprophytic pathogenic fungus that causes Panama's disease in the banana plant (25). We tested anigorufone and reference 20 (REF20), another phytoalexin from the same origin, and reference 5 (REF5), a methoxy derivative of anigorufone, together with epoxide 5 (EP5) and epoxide 6 (EP6), two precursors of their chemical synthesis, on L. donovani promastigotes and L. infantum axenic amastigotes. All of these compounds demonstrated leishmanicidal activity. In a further step, definition of their targets was undertaken. Mitochondrial respiratory chain, the essential source for ATP production in Leishmania spp. (1, 38), was found to be one of the main targets for these compounds.
MATERIALS AND METHODS
Reagents.
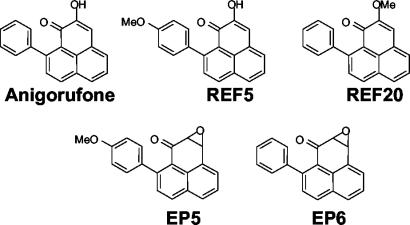
Anigorufone and 2-methoxy-9-phenyl-phenalen-1-one (REF20) were isolated from rhizomes of M. acuminata (AAA) infected with the fungus F. oxysporum (25). 2-Hydroxy-9-(p-methoxyphenyl)phenalen-1-one (REF5), was synthesized as previously reported (24). 2,3-Epoxy-9-(p-methoxyphenyl)phenalen-1-one (EP5) (24) and 2,3-epoxy-9-phenyl-phenalen-1-one (EP6) (25) were prepared as part of a general synthetic route to phenyl-phenalenone-type phytoalexins. Their structures and molecular weight are shown in Fig. 1.
FIG. 1.
Chemical structures of the compounds assayed for leishmanicidal activity: anigorufone, 2-hydroxy-9-phenyl-phenalen-1-one (molecular weight = 272.31); REF5, 2-hydroxy-9-(p-methoxyphenyl)- phenalen-1-one (molecular weight = 302.33); REF20, 2-methoxy-9-phenyl-phenalen-1-one (molecular weight = 286.33); EP5, 2,3-epoxy-9-(p-methoxyphenyl)phenalen-1-one (molecular weight = 302.33); and EP6, 2,3-epoxy-9-phenyl-phenalen-1-one (molecular weight = 272.31).
MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide] was purchased from Sigma Chemical Co. (St. Louis, Mo.). SYTOX Green and DMNPE-luciferin [d-luciferin, 1-(4,5-dimethoxy-2-nitrophenyl)ethyl ester] were obtained from Molecular Probes (Leiden, The Netherlands).
Parasites.
Promastigotes from the L. donovani strain MHOM/SD/00/1S-2D were grown in RPMI 1640 medium (Gibco, Paisley, United Kingdom), supplemented with 10% heat-inactivated fetal calf serum (HIFCS), 24 mM NaHCO3, 25 mM HEPES, 2 mM l-glutamine, 100 U of uniciline/ml, and 48 μg of gentamicin/ml at pH 7.2 (RPMI+HIFCS) at 25°C. Its derived 3-Luc L. donovani strain was obtained by transfection with the expression vector pX63NEO-3Luc, which encodes for a Photinus pyralis luciferase form mutated in its C-terminal tripeptide as described previously (27). Parasites were grown under identical conditions in the medium described above but supplemented with 30 μg of Geneticin/ml (G418; Gibco). L. infantum axenic amastigotes (MCAN/ES/89/IPZ229/1/89) were grown at 37°C as described previously (2).
Cell proliferation measurements.
Parasites were harvested at late exponential phase, washed twice in Hanks buffer supplemented with 10 mM d-glucose (pH 7.2; Hanks+Glc) at 4°C, and resuspended in the same buffer at 2 × 107 cells/ml. Unless stated otherwise, these conditions were maintained for the rest of the experiments. Parasites (20 μl) were incubated with the drugs for 2 h at 25 or 37°C for promastigotes and amastigotes, respectively, washed with 1 ml of Hanks+Glc in order to remove unbound reagent, and resuspended in 100 μl of their respective culture medium devoid of phenol red. Parasites were then transferred into a 96-well microplate for a 48-h proliferation period according to their respective growth conditions. Finally, 100 μl of a 1-mg/ml MTT solution in Hanks+Glc was added, and substrate reduction was allowed to proceed for 2 h. The resulting formazan was solubilized by addition of 100 μl of 10% (wt/vol) sodium dodecyl sulfate solution and read in a 450 Bio-Rad microplate enzyme-linked immunosorbent assay (ELISA) reader, equipped with a 600-nm filter (23).
Assay for cytotoxic activity against mammalian cells.
Murine macrophages of the J-774 (ATCC TIB-67) cell line were grown in RPMI+HIFCS and maintained at 37°C in 5% CO2 and 90% humidity. Macrophages were resuspended in growth medium at a final concentration of 105 cells/ml, placed in a 96-well culture microplate (100 μl/well), and incubated with the test drugs for 48 h. Cytotoxicity was assessed by using the colorimetric MTT reduction assay (6) and expressed as percentage of MTT reduction relative to control cells.
Bioluminescence assays.
The substrate for luciferase, DMNPE-luciferin, was prepared as a 5 mM stock solution in dimethyl sulfoxide. It was added to the 3-Luc promastigote suspension (2 × 107 cells/ml) in Hanks+Glc at a final concentration of 25 μM, and aliquots of this suspension were immediately distributed into a 96-well microplate (100 μl/well). Drugs were added once the luminescence reached a plateau. This point was considered as time zero, and its luminescence was taken as 100%. Changes in luminescence were recorded in a Polarstar Galaxy microplate reader (BMG Labotechnologies, Offenburg, Germany) fitted with luminescence optics, with measurements averaged every 4 s. Inhibition of in vitro luminescence with purified luciferase (Sigma) was carried out as described previously (27).
Promastigote membrane permeabilization.
The procedure described by Thevissen et al. (34) was adapted to Leishmania promastigotes. Briefly, parasites (2 × 107 cells/ml) were incubated with SYTOX green in Hanks+Glc (1 μM, final concentration) for 5 min in darkness, and 100-μl aliquots from this suspension were transferred into a 96-well microplate. After fluorescence stabilization, drugs were added at their respective 50% lethal concentration (LC50). Maximal permeabilization was considered as that achieved by 0.1% Triton X-100. The fluorescence increase, due to binding of the dye to intracellular nucleic acids, was measured in a Polarstar Galaxy microplate reader equipped with 485- and 520-nm filters for excitation and emission wavelengths, respectively.
Electron microscopy.
After incubation with drugs for 1 h, promastigotes were washed twice in phosphate-buffered saline (PBS), fixed in 5% (wt/vol) glutaraldehyde in PBS, included with 2.5% (wt/vol) OsO4 for 1 h, gradually dehydrated in ethanol (30, 50, 70, 90, and 100% [vol/vol]; 30 min each) and propylene oxide (1 h), embedded in Epon 812 resin, and observed in a JEOL-1230 electron microscope.
Oxygen consumption rates.
Oxygen consumption rates were measured in a Clark oxygen electrode (Hansatech, KingsLynn, United Kingdom) at 25°C by using 1 ml of a parasite suspension (108 cells/ml) permeabilized with 60 μM digitonin and supplemented with 100 μM ADP as described previously (1). After drug addition and once a steady rate was reached, a selective set of substrates and inhibitors of the respiratory chain was assayed at final concentrations of 6.7 mM α-glycerophosphate, 0.1 mM tetramethyl-p-phenylenediamine plus 1.7 mM ascorbate (TMPD-ascorbate), 2 mM malonate, 1.9 μM antimycin A, and 10 mM KCN.
Obtention of mitochondrial fraction.
The protocol described previously by Chen et al. was used (8). Promastigotes were washed twice in Hanks buffer and resuspended in hypo-osmotic 5 mM Tris-HCl (pH 7.4) for 10 min at 25°C. This suspension was homogenized in a Potter-Elvehjem homogenizer on ice and then centrifuged at 1,000 × g for 10 min to remove cellular debris. The supernatant was next centrifuged for 20 min at 13,000 × g. The pellet, containing the mitochondrial fraction, was resuspended in isotonic phosphate saline buffer (50 mM sodium phosphate [pH 7.2], 90 mM NaCl, 5 mM KCl) at a protein concentration of 0.5 mg/ml. Aliquots (100 μl/well) of this mitochondrial fraction were distributed into a 96-well microplate and incubated for 1 h at 25°C with the respective drugs. These conditions were maintained for the rest of the enzymatic assays with the mitochondrial fractions.
SDH activity.
The succinate dehydrogenase (SDH) activity was measured spectrophotometrically at 600 nm in a 450 Bio-Rad microplate ELISA reader in the presence of 3 mM succinate, 0.5 mM 2,6-dichlorophenol indophenol (DCPIP), and 0.1 mM phenazine methosulfate (8).
Succinate- and NADH-cytochrome c reductase activities.
Succinate- and NADH-cytochrome c reductase activities were measured as changes in 550-nm absorption of a solution containing 20 μM cytochrome c and 5 mM succinate or 0.2 mM NADH, respectively, in a 450 Bio-Rad microplate ELISA reader with a 550-nm filter (8).
FRD activity.
Fumarate reductase (FRD) activity was assessed as the rate of NADH oxidation (100 μM) upon 1 mM fumarate addition. The decrease of NADH fluorescence was monitored in a Polarstar Galaxy microplate reader equipped with 340- and 470-nm filters for excitation and emission wavelengths, respectively.
Purification of NADH-FRD.
FRD activity was purified according to the protocol described by Chen et al. (8). The promastigote mitochondrial fraction was resuspended in a high-ionic-strength buffer (50 mM sodium phosphate [pH 7.2], 90 mM NaCl, and 150 mM KCl), incubated for 30 min in ice, and spun down (100,000 × g, 40 min, 4°C). The FRD activity, present in the supernatant, was purified by an initial concentration on HiTrap QFF column (Amersham Biosciences, Barcelona, Spain), followed by size exclusion chromatography on a HiPrep 16/60 Sephacryl S-200HR column (Amersham Biosciences), and finally fractionated on an HPLC Mono-Q HR 5/5 (Pharmacia, Uppsala, Sweden) through a 0.05 to 1 M NaCl gradient, with a purification factor between 90 and 120, similar to that previously reported (8).
Statistical analysis.
Data represent the mean of triplicates ± the standard deviation. Experiments were repeated at least twice. The 50% effective concentration (EC50) and the LC50 values were calculated by the Litchfield and Wilcoxon procedure, and their 95% confidence interval is included in parentheses in Table 1.
TABLE 1.
Comparison of the inhibitory effect caused by phenyl-phenalenones on parasite proliferation, FRD and SDH from the mitochondrial fraction, and purified FRDa
| Drug | LC50 (μg/ml)
|
EC50 (μg/ml)
|
|||
|---|---|---|---|---|---|
| Promastigotes | Amastigotes | Mitochondrial fraction
|
Purified FRD | ||
| SDH | FRD | ||||
| Anigorufone | 12.0 (10.8-13.0) | 13.3 (7.3-24.2) | 33.5 (17.1-65.7) | 53.1 (33.2-84.7) | 89.0 (57.5-138.0) |
| REF5 | 24.2 (21.0-27.8) | 23.9 (15.1-37.9) | >80 | 78.8 (65.0-95.3) | 115 (72.6-182.2) |
| REF20 | 10.3 (7.3-14.5) | 10.5 (7.4-14.8) | 59.6 (51.8-68.6) | 47.8 (41.0-55.6) | 77.2 (49.8-119.6) |
| EP5 | 38.4 (28.4-51.9) | 15.6 (13.3-18.2) | >80 | 61.8 (46.3-82.5) | 100.0 (77.6-128.8) |
| EP6 | 68.7 (60.8-77.7) | 17.2 (10.9-26.6) | >100 | 33.3 (29.9-41.9) | 53.3 (33.6-84.5) |
LC50 and EC50 values were estimated by the procedure of Litchfield and Wilcoxon. The 95% confidence interval values are given in parentheses. Assays were carried out as described in Materials and Methods.
RESULTS
Leishmanicidal activity of phenyl-phenalenones.
Promastigote proliferation was inhibited by the five compounds assayed (Table 1). Nevertheless, substantial differences were observed among them; whereas anigorufone and REF20 showed the highest activity (LC50 = 12.0 and 10.3 μg/ml, respectively), REF5, EP5, and EP6 required a concentration two to six times higher (Table 1). The LC50s for anigorufone, REF5, and REF20 on L. infantum axenic amastigotes (Table 1) were very similar to those obtained on promastigotes, whereas EP5 and EP6 were two to four times more active on amastigotes (LC50 = 15.6 and 17.2 μg/ml, respectively).
None of the phenyl-phenalenones caused a decrease in J774 cell proliferation beyond 10% at a concentration fivefold their respective LC50 for Leishmania amastigotes.
Inhibition of luminescence.
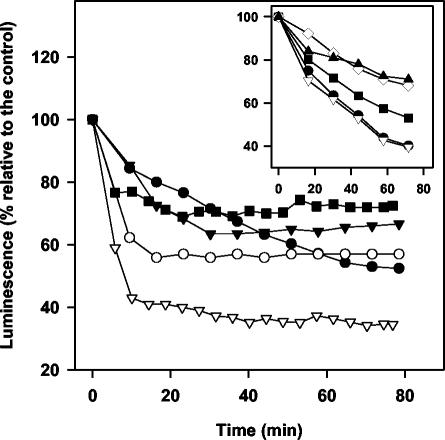
In vivo real-time monitoring of intracellular ATP levels can be easily performed with the 3-Luc L. donovani strain (27). In order to bypass the poor membrane permeability of d-luciferin at neutral pH, it was substituted by its free membrane-permeable caged analogue DMNPE-luciferin. The luminescence was inhibited in a dose-dependent manner for the whole set of compounds, as illustrated for anigorufone (Fig. 2, inset). To compare the inhibition of promastigote luminescence for the five compounds, they were assayed at their corresponding LC50s. The results are represented in Fig. 2. Except for EP6, all of them caused 40 to 60% inhibition relative to the initial value. Anigorufone displayed a steady decline extended for 60 min, whereas the pattern for the rest of compounds followed an exponential decay with a fast initial step lasting for 10 min. None of the drugs inhibited in vitro purified luciferase activity at their highest concentration beyond 5% (data not shown).
FIG. 2.
In vivo variation of the luminescence of 3-Luc L. donovani parasites after drug addition at their respective LC50. Parasites (2 × 107 cells/ml) were incubated with 25 μM DMNPE-luciferin. When luminescence reached a plateau, drug was added (t = 0) and luminescence was monitored as described in Materials and Methods. Drugs were administered as follows: 12 μg of anigorufone/ml (•), 24 μg of REF5/ml (▾), 10 μg of REF20/ml (○), 38 μg of EP5/ml (▿), and 69 μg of EP6/ml (▪). The inset shows the dose-dependent variation of luminescence with anigorufone at 2.5 μg/ml (▴), 5 μg/ml (⋄), 10 μg/ml (▪), 15 μg/ml (▿), and 20 μg/ml (•). The results were normalized relative to the luminescence variation in control parasites.
Membrane permeabilization.
To account for the drop in intracellular ATP levels by phenyl-phenalenones, we sought two of its more likely origins, membrane permeabilization and inhibition of ATP production. To test the first hypothesis, membrane permeabilization was assessed by the entrance into the promastigote of SYTOX green, a vital dye excluded from the cell with an intact membrane. Once in the cytoplasm, its fluorescence increases as it binds to intracellular nucleic acids. As none of the drugs produced an increase of fluorescence at a fivefold LC50 concentration (data not shown), this alternative was ruled out.
Electron microscopy.
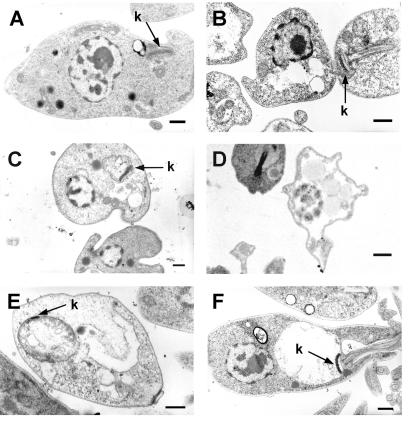
In order to gain insight into the damage caused to the parasite by the drugs, electron microscopy of treated parasites was carried out. Figure 3 shows the promastigotes treated with the different drugs at their LC50s. Swelling of mitochondria was observed in more than 50% of the parasites for the whole set of phenyl-phenalenones and was particularly evident for EP5 and EP6 (Fig. 3E and F), whereas the plasma membrane integrity appeared unharmed. Furthermore, a more dramatic effect was observed for anigorufone and REF20 since they caused extensive intracellular damage with strong disorganization of cytoplasmic organelles (Fig. 3B and D). At lower concentrations the extent and percentage of injured cells was lower (data not shown).
FIG. 3.
Electron microscopy of L. donovani promastigotes treated with phenyl-phenalenones at their respective LC50s. (A) Control parasites; (B) anigorufone; (C) REF5; (D) REF20; (E) EP5. (F) EP6. k, kinetoplast. Bar, 0.5 μm.
Inhibition of oxygen consumption rate.
The results from electron microscopy supported the hypothesis that the decrease of luminescence, hence of ATP levels, may likely be due to a faulty function of the oxidative phosphorylation as the main source of ATP for this parasite (1, 39). In order to test this suggestion, we measured the oxygen consumption rate of digitonin-permeabilized promastigotes after drug addition at their LC50. Control parasites permeabilized with digitonin showed an oxygen consumption rate of 10.2 nmol min−1 × 10−8 promastigotes, with succinate as substrate, in agreement with previous reports (1). The drug effectiveness, ranked according to the inhibition relative to the rate of control parasites was as follows: anigorufone (83% inhibition) > REF20 (68%) > EP5 (60%) ≅ EP6 (58%) > REF5 (39%). In drug-treated promastigotes, addition of TMPD-ascorbate, an electron donor to cytochrome c (28), increased the oxygen consumption rates by 3.2 to 3.6 nmol min−1 × 10−8 promastigotes, in other words a 35% recovery of the rate before phenyl-phenalenone inhibition. This restricts the potential target upstream cytochrome c. After inhibition of the oxygen uptake either by phenyl-phenalenones or malonate, a 30% reinstatement of the respiratory activity was achieved upon addition of 6.7 mM α-glycerophosphate, similar to that reported in L. tropica (28). This supports complex II as the more likely target for these drugs, as in Leishmania spp., oxidation of α-glycerophosphate is carried out through the respiratory chain and linked to NAD (28). Malonate, antimycin A, and KCN caused a complete inhibition and were used as controls for the respiratory chain.
Inhibition of mitochondrial enzymatic activities.
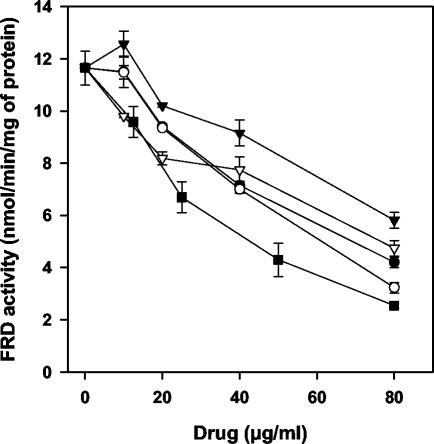
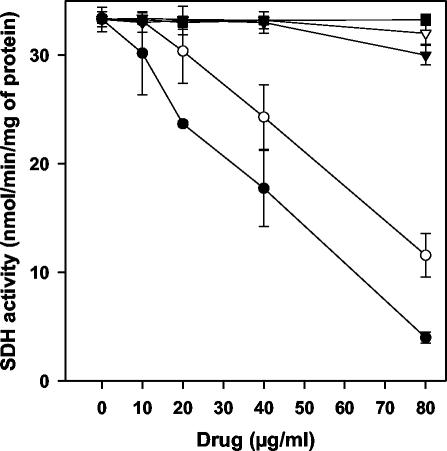
We next set out to further define the Leishmania target for phenyl-phenalenones. We proceeded by testing in the promastigote mitochondrial fraction of the inhibitory activity on the enzymatic activities associated with complex II. At their LC50 for promastigotes, none of the drugs inhibited more than 3% succinate- or NADH-cytochrome c reductase activities (data not shown), which are involved in the electron transfers from complex II to III and from complex I to III, respectively. FRD activity was inhibited by the whole set of drugs in a dose-dependent manner (Fig. 4). EP6 caused the highest inhibition (EC50 = 33.3 μg/ml), whereas REF5 showed the lowest activity (EC50 = 78.8 μg/ml) (Table 1). In contrast, SDH activity (32.9 nmol/min/mg of protein) was only inhibited by anigorufone and REF20 and at concentrations higher than their LC50 values (Fig. 5). When compared on purified FRD, all of the drugs inhibited the enzyme (Table 1), although with EC50s consistently higher (∼2-fold) relative to those obtained on mitochondrial fraction; a similar situation was previously reported for licochalcone A and was attributed to changes in the physiological conditions (8).
FIG. 4.
Inhibition of FRD activity in the mitochondrial fraction of L. donovani promastigotes, with different phenyl-phenalenones. The results for anigorufone (•), REF5 (▾), REF20 (○), EP5 (▿), and EP6 (▪) are given. The data represent the average of at least two different experiments.
FIG. 5.
Inhibition of SDH activity in the mitochondrial fraction of L. donovani promastigotes. SDH was assayed by determining the reduction of DCPIP as described in Materials and Methods. The results for anigorufone (•), REF5 (▾), REF20 (○), EP5 (▿), and EP6 (▪) are shown. Maximal inhibition (100%) was considered to be that caused by 12 mM malonate.
DISCUSSION
Phytoalexins fulfill two major criteria: their synthesis under pathogen challenge and a certain cidal activity that frequently is not exclusively directed against the eliciting agent but against a broader pathogen spectrum, including fungi (13). Chemotherapy for Leishmania spp. has frequently profited by some natural antifungal drugs, such as amphotericin B, azole derivatives, paramomycin, saponins, and naphthoquinones (3, 10, 11, 30), since similar pharmacological targets are present in these two groups of organisms. In fact, recent successful approaches to search for new leishmanicidal drugs profit from development programs for antifungal drugs. To test this strategy in phytoalexins, we assayed the leishmanicidal activity of two natural products, anigorufone and REF20 (25), and three related phenyl-phenalenone structures. These phytoalexins are synthesized under fungal challenge and showed antibiotic activity against the eliciting fungus, albeit their mechanism of action is completely unknown at present (25). The whole set of drugs caused the inhibition of both promastigote and amastigote proliferation with similar efficacy; moreover, the inhibition of amastigote proliferation was even better for EP5 and EP6. To unravel their mechanism of action, we proceeded with a systematic search for their targets in Leishmania. All of these compounds caused a decrease in intracellular ATP levels, monitored by the real-time luminescence decay in living parasites. Two major scenarios were considered to account for it: either a drug-induced waste or leakage of intracellular ATP to an extent that its cellular synthesis cannot cope with this demand or an inhibition of ATP synthesis. Plasma membrane permeabilization is a plausible explanation for the first hypothesis and in fact, the plasma membrane is the target for other leishmanicidal plant products (31). Nevertheless, for phenyl-phenalenones this option was discarded because (i) the kinetics of ATP decrease were much smoother than the steep drop caused by other paradigmatic membrane-permeabilizing reagents, such as peptides derived from cecropin A-melittin (9, 12, 26) or amphotericin B (26), and (ii) in electron micrographs, the plasma membrane of the promastigotes remained intact. These observations, combined with the negligible SYTOX green permeation into the cytoplasm, suggest that the functionality of the plasma membrane as a permeability barrier was kept intact after phenyl-phenalenone incubation.
The second option, inhibition of ATP synthesis, was supported by the consistent observation in treated parasites of swollen mitochondria, only identified by the presence of the electron-dense kinetoplast. In parasites treated with REF5, EP5, or EP6, this was the main structural observation; a similar effect was reported for licochalcone A, a leishmanicidal drug targeting the mitochondria (39). In contrast, in promastigotes treated with anigorufone or REF20, a more extensive intracellular damage, not limited to mitochondria, was evidenced. However, alteration of plasma membrane was not observed for any drug.
We addressed the aforementioned possibility by searching the phenyl-phenalenone targets on the respiratory chain, since oxidative phosphorylation is the major source for energy generation in Leishmania (1, 38). In digitonin-permeabilized parasites, phenyl-phenalenones inhibited the oxygen consumption rate by using succinate as the substrate. Since this was partially restored by addition of TMPD-ascorbate, which donates electrons to cytochrome c, the putative phytoalexins targets were restricted upstream cytochrome c. The addition of α-glycerophosphate partially restored oxygen consumption; thus, complex II was considered to be the more likely target for phenyl-phenalenones (28). In a step beyond, it was found that SDH and FRD, associated with the mitochondrial fraction, were inhibited by anigorufone and REF20 or by the whole set of phenyl-phenalenones, respectively. An unspecific effect was ruled out, since neither succinate- nor NADH-cytochrome c reductase was inhibited by any phenyl-phenalenone, but all of them inhibited the purified FRD and preserved the same pattern of inhibitory activity obtained in the mitochondrial fraction. Nevertheless, at a given concentration of any of these drugs, the inhibition of FRD was consistently lower in the purified enzyme than in the mitochondrial fraction. This effect was also reported for licochalcone A, another inhibitor of FRD in Leishmania (8). This is most likely due to the loss of modulation of the FRD-soluble catalytic portion by its membrane hydrophobic domain, which is absent in the purified enzyme. In fact, for other organisms, it has been reported that the hydrophobic portion is involved in electron transfer to the catalytic subunits (37). Accordingly, from a physiological perspective, the results obtained for mitochondrial fraction are more relevant than those obtained for the purified enzyme, as noted when the EC50s for both forms of the enzyme (membrane-bound and purified) were compared to their respective LC50 values.
Despite some controversies concerning the bioenergetic metabolism of Leishmania (4, 5, 8, 33, 38), FRD is likely an important target for the leishmanicidal activity of phenyl-phenalenones since (i) it is the only enzymatic activity from the panel assayed that is inhibited by the five compounds, (ii) inhibition of this enzyme is enough to account for the leishmanicidal effect of new drugs such as licochalcone A, aurones, or 2-mercaptopyridine-N-oxide (8, 22, 36), and (iii) FRD is an enzyme absent in mammalian cells (8, 37).
The large divergences inherent in the three experimental approaches used to assess phenyl-phenalenone activity (the use of live parasites, an enriched mitochondrial fraction, and purified FRD) may account for the quantitative differences among the inhibition of these parameters by phenyl-phenalenones. Furthermore, at present we cannot discard additional alternative targets for these drugs, as exemplified by anigorufone and REF20, the two compounds with the highest leishmanicidal effect, since they inhibited FRD and SDH activities simultaneously. Likewise, other sites of action, apart from the respiratory chain, cannot be excluded.
Natural plant products such as licochalcone A, plumbagin, and 2-substituted quinolones are new promising leishmanicidal drugs and have provided important leads for chemotherapy (8, 10). Unexpectedly, medical applications for phytoalexins, rather than to their antimicrobial activities (7), were related to cardiovascular or cancer prevention as reported for resveratrol (20, 32, 35), genistein (14), or brassinin (29). To the best of our knowledge, leishmanicidal activity has not yet been described for any compound explicitly defined as phytoalexin. Nevertheless, given that pathogen elicitation in plants is continuous under natural conditions, the odds for the unsuspected presence of phytoalexins in extracts or compounds with previously reported leishmanicidal activity are quite high.
In the present study we have demonstrated the leishmanicidal activity of five molecules based on a phenyl-phenalenone skeleton, a new lead structure for future development of leishmanicidal drugs. Their inhibition of SDH and FRD activities pinpoints these enzymes as their more likely targets. Two of them were originally isolated from the banana plant (M. acuminata) elicited with the fungus F. oxysporum (25). Thus, a new approach to define leishmanicidal agents will profit from this higher probability of finding new leishmanicidal compounds in plants previously challenged with fungi. This elicitation will induce antifungal phytoalexins that, in the best scenario, will share some of their antifungal targets with Leishmania. In a step beyond, this rationale might be further optimized by plant infection with a much closer evolutionary organism such as Phytomonas, a plant trypanosomatid. The feasibility of this new experimental approach will require additional investigation.
Acknowledgments
This study was supported by grants from C.A.M. (08.2/0054/2001.02) and the Plan Estratégico de Grupos en Biotecnología (EU grants QLK2-CT-2001-01404 and CICyT BIO2003-09056-CO2-02) to L.R. C.I.B. belongs to the Red Española de Investigación en Patología Infecciosa, supported by the Fondo de Investigaciones Sanitarias (grant C03/14). Work at the Instituto de Bio-Orgánica A. G. González was funded by the Spanish Ministry of Science and Technology (grant BQU2001-3721). L.R.I. thanks the Spanish Ministry of Science and Technology for a doctoral fellowship. The Departamento de Parasitología was supported by grant 1802380102 from La Laguna University.
L. infantum axenic amastigotes (MCAN/ES/89/IPZ229/1/89) were kindly provided by María Colmenares (C.I.B.-C.S.I.C). The technical assistance of María Dolores Guirao Rey (C.I.B.-C.S.I.C) with the electron microscopy and critical reading of the manuscript by Victoria Herrán Coombs (Universidade Da Coruña) are greatly appreciated.
REFERENCES
- 1.Álvarez-Fortes, E., L. M. Ruiz-Pérez, F. Bouillaud, E. Rial, and L. Rivas. 1998. Expression and regulation of mitochondrial uncoupling protein 1 from brown adipose tissue in Leishmania major promastigotes. Mol. Biochem. Parasitol. 93:191-202. [DOI] [PubMed] [Google Scholar]
- 2.Armson, A., S. W. Kamau, F. Grimm, J. A. Reynoldson, W. M. Best, L. M. MacDonald, and R. C. Thompson. 1999. A comparison of the effects of a benzimidazole and the dinitroanilines against Leishmania infantum. Acta Trop. 73:303-311. [DOI] [PubMed] [Google Scholar]
- 3.Balaña-Fouce, R., R. M. Reguera, J. C. Cubría, and D. Ordóñez. 1998. The pharmacology of leishmaniasis. Gen. Pharmacol. 30:435-443. [DOI] [PubMed] [Google Scholar]
- 4.Bermúdez, R., F. Dagger, J. A. D'Aquino, G. Benaim, and K. Dawidowicz. 1997. Characterization of mitochondrial electron-transfer in Leishmania mexicana. Mol. Biochem. Parasitol. 90:43-54. [DOI] [PubMed] [Google Scholar]
- 5.Blum, J. J. 1993. Intermediary metabolism of Leishmania. Parasitol. Today 9:118-122. [DOI] [PubMed] [Google Scholar]
- 6.Carmichael, J., W. G. DeGraff, A. F. Gazdar, J. D. Minna, and J. B. Mitchell. 1987. Evaluation of a tetrazolium-based semiautomated colorimetric assay: assessment of chemosensitivity testing. Cancer Res. 47:936-942. [PubMed] [Google Scholar]
- 7.Chan, M. M. 2002. Antimicrobial effect of resveratrol on dermatophytes and bacterial pathogens of the skin. Biochem. Pharmacol. 63:99-104. [DOI] [PubMed] [Google Scholar]
- 8.Chen, M., L. Zhai, S. B. Christensen, T. G. Theander, and A. Kharazmi. 2001. Inhibition of fumarate reductase in Leishmania major and L. donovani by chalcones. Antimicrob. Agents Chemother. 45:2023-2029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chicharro, C., C. Granata, R. Lozano, D. Andreu, and L. Rivas. 2001. N-terminal fatty acid substitution increases the leishmanicidal activity of CA(1-7)M(2-9), a cecropin-melittin hybrid peptide. Antimicrob. Agents Chemother. 45:2441-2449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Croft, S. L., and V. Yardley. 2002. Chemotherapy of leishmaniasis. Curr. Pharm. Des. 8:319-342. [DOI] [PubMed] [Google Scholar]
- 11.Curreli, N., F. Sollai, L. Massa, O. Comandini, A. Rufo, E. Sanjust, A. Rinaldi, and A. C. Rinaldi. 2001. Effects of plant-derived naphthoquinones on the growth of Pleurotus sajor-caju and degradation of the compounds by fungal cultures. J. Basic Microbiol. 41:253-259. [DOI] [PubMed] [Google Scholar]
- 12.Díaz-Achirica, P., A. Guinea, J. Ubach, D. Andreu, and L. Rivas. 1998. The plasma membrane from Leishmania donovani promastigotes is the main target for CA(1-8)M(1-18), a synthetic cecropin A-melittin hybrid. Biochem. J. 330:453-460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dixon, R. A. 2001. Natural products and plant disease resistance. Nature 411:843-847. [DOI] [PubMed] [Google Scholar]
- 14.Dixon, R. A., and D. Ferreira. 2002. Genistein. Phytochemistry 60:205-211. [DOI] [PubMed] [Google Scholar]
- 15.Fournet, A., and V. Munoz. 2002. Natural products as trypanocidal, antileishmanial, and antimalarial drugs. Curr. Top. Med. Chem. 2:1215-1237. [DOI] [PubMed] [Google Scholar]
- 16.Grayer, R. J., and T. Kokubun. 2001. Plant-fungal interactions: the search for phytoalexins and other antifungal compounds from higher plants. Phytochemistry 56:253-263. [DOI] [PubMed] [Google Scholar]
- 17.Guerin, P. J., P. Olliaro, S. Sundar, M. Boelaert, S. L. Croft, P. Desjeux, M. K. Wasunna, and A. D. Bryceson. 2002. Visceral leishmaniasis: current status of control, diagnosis, and treatment, and a proposed research and development agenda. Lancet Infect. Dis. 2:494-501. [DOI] [PubMed] [Google Scholar]
- 18.Heil, M., and R. M. Bostock. 2002. Induced systemic resistance (ISR) against pathogens in the context of induced plant defenses. Ann. Bot. 89:503-512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Herwaldt, B. L. 1999. Leishmaniasis. Lancet 354:1191-1199. [DOI] [PubMed] [Google Scholar]
- 20.Jang, M., L. Cai, G. O. Udeani, K. V. Slowing, C. F. Thomas, C. W. Beecher, H. H. Fong, N. R. Farnsworth, A. D. Kinghorn, R. G. Mehta, R. C. Moon, and J. M. Pezzuto. 1997. Cancer chemopreventive activity of resveratrol, a natural product derived from grapes. Science 275:218-220. [DOI] [PubMed] [Google Scholar]
- 21.Kayser, O., A. F. Kiderlen, U. Folkens, and H. Kolodziej. 1999. In vitro leishmanicidal activity of aurones. Planta Med. 65:316-319. [DOI] [PubMed] [Google Scholar]
- 22.Kayser, O., M. Chen, A. Kharazmi, and A. F. Kiderlen. 2002. Aurones interfere with Leishmania major mitochondrial fumarate reductase. Z. Naturforsch. 57:717-720. [DOI] [PubMed] [Google Scholar]
- 23.Kiderlen, A. F., and P. M. Kaye. 1990. A modified colorimetric assay of macrophage activation for intracellular cytotoxicity against Leishmania parasites. J. Immunol. Methods 127:11-18. [DOI] [PubMed] [Google Scholar]
- 24.Luis, J. G., W. Q. Fletcher, F. Echeverri, T. A. Grillo, A. Perales, and J. A. González. 1995. Intermediates with biosynthetic implications in de novo production of phenyl-phenalenone-type phytoalexins by Musa acuminata. Revised structure of emenolone. Tetrahedron 51:4117-4130. [Google Scholar]
- 25.Luis, J. G., W. Q. Fletcher, F. Echeverri, T. Abad, M. P. Kishi, and A. Perales. 1995. New phenalenone-type phytoalexins from Musa acuminata (COLLA AAA) gran nain. Nat. Prod. Lett. 6:23-30. [Google Scholar]
- 26.Luque-Ortega, J. R., J. M. Saugar, C. Chiva, D. Andreu, and L. Rivas. 2003. Identification of new leishmanicidal peptide lead structures by automated real-time monitoring of changes in intracellular ATP. Biochem. J. 375:221-230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Luque-Ortega, J. R., O. M. Rivero-Lezcano, S. L. Croft, and L. Rivas. 2001. In vivo monitoring of intracellular ATP levels in Leishmania donovani promastigotes as a rapid method to screen drugs targeting bionergetic metabolism. Antimicrob. Agents Chemother. 45:1121-1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Martin, E., and A. J. Mukkada. 1979. Identification of the terminal respiratory chain in kinetoplast-mitochondrial complexes of Leishmania tropica promastigotes. J. Biol. Chem. 254:12192-12198. [PubMed] [Google Scholar]
- 29.Mehta, R. G., J. Liu, A. Constantinou, C. F. Thomas, M. Hawthorne, M. You, C. Gerhuser, J. M. Pezzuto, R. C. Moon, and R. M. Moriarty. 1995. Cancer chemopreventive activity of brassinin, a phytoalexin from cabbage. Carcinogenesis. 16:399-404. [DOI] [PubMed] [Google Scholar]
- 30.Okunji, C. O., M. M. Iwu, J. E. Jackson, and J. D. Tally. 1996. Biological activity of saponins from two Dracaena species. Adv. Exp. Med. Biol. 404:415-428. [DOI] [PubMed] [Google Scholar]
- 31.Plock, A., W. Sokolowska-Köhler, and W. Presber. 2001. Application of flow cytometry and microscopical methods to characterize the effect of herbal drugs on Leishmania spp. Exp. Parasitol. 97:141-153. [DOI] [PubMed] [Google Scholar]
- 32.Roemer, K., and M. Mahyar-Roemer. 2002. The basis for the chemopreventive action of resveratrol. Drugs Today 38:571-580. [DOI] [PubMed] [Google Scholar]
- 33.Santhamma, K. R., and Bhaduri, A. 1995. Characterization of the respiratory chain of Leishmania donovani promastigotes. Mol. Biochem. Parasitol. 75:43-53. [DOI] [PubMed] [Google Scholar]
- 34.Thevissen, K., F. R. Terras, and W. F. Broekaert. 1999. Permeabilization of fungal membranes by plant defensins inhibits fungal growth. Appl. Environ. Microbiol. 65:5451-5458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tsan, M. F., J. E. White, J. G. Maheshwari, and G. Chikkappa. 2002. Anti-leukemia effect of resveratrol. Leuk. Lymphoma 43:983-987. [DOI] [PubMed] [Google Scholar]
- 36.Turrens, J. F., C. L. Newton, L. Zhong, F. R. Hernández, J. Whitfield, and R. Docampo. 1999. Mercaptopyridine-N-oxide, an NADH-fumarate reductase inhibitor, blocks Trypanosoma cruzi growth in culture and in infected myoblasts. FEMS Microbiol. Lett. 175:217-221. [DOI] [PubMed] [Google Scholar]
- 37.Van Hellemond, J. J., and A. G. M. Tielens. 1994. Expression and functional properties of fumarate reductase. Biochem. J. 304:321-331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Van Hellemond, J. J., P. Van der Meer, and A. G. M. Tielens. 1997. Leishmania infantum promastigotes have a poor capacity for anaerobic functioning and depend mainly on respiration for their energy generation. Parasitology 114:351-360. [Google Scholar]
- 39.Zhai, L., J. Blom, M. Chen, S. B. Christensen, and A. Kharazmi. 1995. The antileishmanial agent licochalcone A interferes with the function of parasite mitochondria. Antimicrob. Agents Chemother. 39:2742-2748. [DOI] [PMC free article] [PubMed] [Google Scholar]