Abstract
Purpose
We sought to examine if there are race/ethnic disparities in lower urinary tract symptoms (LUTS) in men.
Materials and Methods
Race/ethnic disparities were examined using the American Urological Association Symptom Index (AUASI) in two large cohorts, the California Men's Health Study (CMHS) and Research Program in Genes, Environment and Health (RPGEH). The prevalence and incidence were calculated within each age and race/ethnicity strata and multivariable analyses conducted to assess the association between race/ethnicity and LUTS.
Results
The prevalence of LUTS increased with age (p<0.05) within each race/ethnic category. The mean age-adjusted AUASI score for Black, Hispanic, Other/mixed, non-Hispanic white, and Asian men was 9.57 (standard deviation (sd) 5.83), 9.35 (sd 6.30), 9.32 (sd 6.22), 8.99 (sd 5.89), and 8.41 (sd 5.59), respectively.
In multivariable models, Hispanic and Black men were at increased risk of moderate LUTS compared to white men, while only the Hispanic men were at higher risk of severe LUTS. Asian men were a lower risk of moderate or severe LUTS compared to white men. Rates of incident LUTS increased with increasing baseline age for nearly all race/ethnicity groups and varied from 32 to 56% of men. Asian and Hispanic men had lower risk of incident LUTS compared to white men, even after adjusting for sociodemographic factors, health-related behaviors and comorbidity.
Conclusions
There are race/ethnic disparities in LUTS that persist after accounting for putative and established risk factors.
Introduction
The prevalence of lower urinary tract symptoms (LUTS) rises with age and may be caused by factors such as benign prostatic hyperplasia (BPH), obesity, and diabetes 1-5. However, our knowledge of the epidemiology of LUTS is surprisingly limited. While recent studies have shed light on other putative factors that may increase or decrease the risk of LUTS 6, aspects of the basic descriptive epidemiology are missing or incomplete. One facet missing from our knowledge of basic LUTS epidemiology is whether race/ethnic disparities exist.
Prior studies have been limited or inconclusive on this topic, primarily due to the small number of minority men who have been included in such studies. Moreover, even fewer studies have included sufficient numbers of men from all four major race/ethnicity groups (e.g., whites, Blacks, Hispanics and Asians) from a single population. Finally, nearly all of these studies have used different definitions of LUTS.
We therefore sought to examine if there are disparities by race/ethnicity in the occurrence of LUTS and, if any were observed, whether factors associated with LUTS could explain it. We also sought to estimate the incidence of new LUTS by race/ethnicity. To address these research objectives we examined data from two large multiethnic cohorts that ascertained LUTS using a standardized instrument and included a large number of putative risk factors.
Materials and Methods
Male participants of the California Men's Health Study (CMHS) and Research Program on Genes, Environment and Health (RPGEH) cohorts formed the study population for this study. Both cohorts were originally based in the membership of Kaiser Permanente in California.
Details of the CMHS have been previously published 7. Briefly, CMHS baseline data were collected between 2001-2002 on 84,170 men, aged 45 to 69 years as of 1/1/2001, who were Kaiser Permanente in California members. A second or follow-up questionnaire that included the AUASI was administered to the CMHS men in 2008-2009. The RPGEH includes a cohort with baseline data obtained in 2007-2008 on 140,139 men who were adult members of Kaiser Permanente Northern California for at least two years as of 12/3/2006.
For both cohorts, data on socioeconomic status, comorbidity, and lifestyle factors were ascertained at the baseline questionnaire. In addition, data on LUTS were obtained using the standardized American Urological Association Symptom Index (AUASI).
Data for the prevalence analyses included 78,273 CMHS and 106,373 RPGEH men after exclusions for prevalent prostate cancer or missing data. For the analysis related to new onset or incident LUTS, only the 63,245 CMHS men who completed a second assessment and did not have prostate cancer at baseline or follow-up were included.
Questionnaire data included race/ethnicity (White, African-American/Black, Asian, Hispanic, Other), marital status (never, married/living with partner, widowed or separated), place of birth (native born versus other), height, weight, diabetes (no/yes), cardiovascular disease (no/yes), hypertension (no/yes)), hyperlipidemia (no/yes), smoking (never, former, current), alcohol use (no, moderate, heavy) and physical activity. Physical activity was categorized as minimal, moderate or strenuous based on type of activity and frequency, and consistent with recommendations in a NIH consensus statement on physical activity 8. Body mass index (BMI) was calculated as weight in kilograms/height in meters2 and included in the analyses as an indicator variable with three categories (i.e., <25, 25-<30, 30+). Phosphodiesterase inhibitor use (no/yes) data were derived from either self-report or clinical records.
LUTS were measured in both cohorts using the AUASI, which is scored on a 0-35 scale based on seven questions. These data were categorized as no or mild (AUASI score of 0-7); moderate (AUASI 8-19); or severe LUTS (AUASI ≥20). Incident LUTS was examined only among men with no prostate cancer at baseline or in the follow-up period, who did not undergo BPH treatment before baseline and had an AUASI in the no or mild category. Men were classified as having incident LUTS if they met any of the following criteria; AUASI score increased from ≤7 to 8 or more at the second assessment or treatment for BPH. The latter criteria included selected drugs (e.g., α-blockers or 5-α-reductase inhibitors), surgery (e.g., a transurethral prostatectomy) or minimally invasive procedures (e.g., transurethral microwave thermotherapy or transurethral needle ablation).
Statistical Analysis
Prevalence estimates were calculated using the number affected divided by the number of men within each age and race/ethnic stratum.
Incidence estimates were limited to the CMHS cohort as a second assessment of LUTS was not available for the RPGEH cohort. Age-specific rates were calculated in a manner similar to that of prevalence data. Race-ethnic specific age adjusted mean AUASI scores were obtained via linear regression.
Because the conditions that are known or suspected to be associated with LUTS are also known to vary by age and race/ethnicity, we then analyzed the data using logistic regression models to obtain odds ratios for LUTS associated with race/ethnicity adjusted for covariates that may confound that relationship. Separate regression models were run for LUTS dichotomized as moderate vs. no/mild and as severe vs. no/mild. Our base model adjusted for age only, and the full models adjusted for age, marital status, birth place (native born versus other), physical activity, smoking, alcohol use, general health, BMI, hypertension, diabetes, hyperlipidemia, cardiovascular disease, and phosphodiesterase inhibitor use. In the combined cohort analyses we also included an indicator variable for cohort. Statistical tests of the regression coefficients were based on the likelihood ratio test and Wald 95% confidence intervals were calculated for each estimated odds ratio.
The study was reviewed and approved by the Institutional Review Boards of Kaiser Permanente Northern and Southern California.
Results
Over 78,000 participants of the California Men's Health Study (CMHS) and 106,000 from the Research Program in Genes, Environment and Health (RPGEH) were included in this study. In these two cohorts over 63,000 men (34.4%) reported being in one or more traditional minority groups. Cohorts were similar in the crude distributions of LUTS, socioeconomic factors, health-related behaviors and medical conditions after accounting for the broader age range included in the RPGEH cohort.
The age- and race/ethnicity-specific LUTS prevalence by cohort is presented in Figure 1. For all men the prevalence increased with age. Within nearly all age categories, the highest prevalence was among Hispanic men, and then followed by Black, white and Asian men. After age-adjustment Black, Hispanic, Other/mixed, non-Hispanic white, and Asian men had a mean AUASI score of 9.57 (standard deviation (sd) 5.83), 9.35 (sd 6.30), 9.32 (sd 6.22), 8.99 (sd 5.89), and 8.41 (sd 5.59), respectively.
Figure 1. The prevalence of lower urinary tract symptoms by age, race/ethnicity and cohort, CMHS and RPGEH Cohorts, Kaiser Permanente.
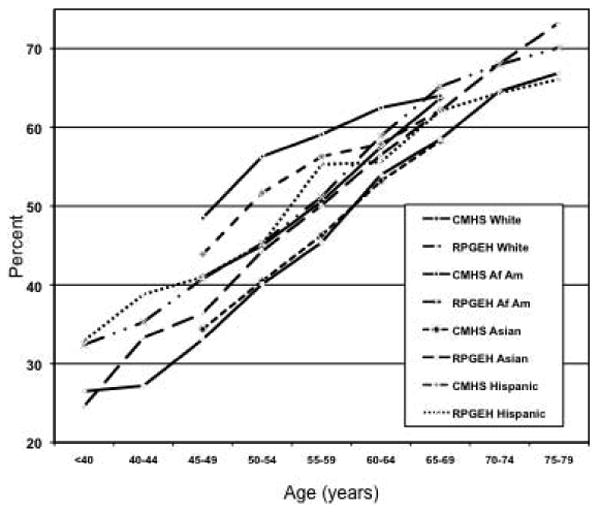
When age-adjusted and fully-adjusted odds ratios by race/ethnicity are compared for each cohort the risk estimates are generally attenuated in the fully-adjusted models. However, race/ethnic differences remained. In general, Hispanic participants were more likely to report moderate LUTS and Asian participants less likely compared to white men. Blacks and men of Other race/ethnicity in the CMHS were more likely to report LUTS compared to white men, while there was no association in RPGEH cohort for Black men and an inverse association for Other men. Hispanic men were also more likely to report severe LUTS, while Asian men less likely compared to white men. Other men were more likely to report severe LUTS only in the CMHS cohort. In a combined cohort analysis, Hispanic (OR=1.07; 95% CI 1.04-1.11) and Black (OR=1.09; 95% CI 1.06-1.13) men were at increase risk of moderate LUTS compared to white men, while only the Hispanic men were at higher risk of severe LUTS (OR=1.16; 95% CI 1.09-1.24). Asian men had a lower risk of both moderate and severe LUTS compared to white men (OR=0.85; 95% CI 0.82-0.87 and OR=0.82; 95% CI 0.75-0.88, respectively).
Figure 2 shows the age- and race/ethnicity-specific rate of incident LUTS by baseline age. The cumulative rates over the approximately seven years of follow-up varied from about 32% (Hispanic men aged 45-49 years at baseline) to over 54% (white men aged 65-69 years at baseline). The rate increased with age for each race/ethnicity except for Black men, where there was a peak in the 55-59 year old category.
Figure 2. Percent with incident LUTS by race/ethnicity and age (at baseline), CMHS Cohort, Kaiser Permanente.
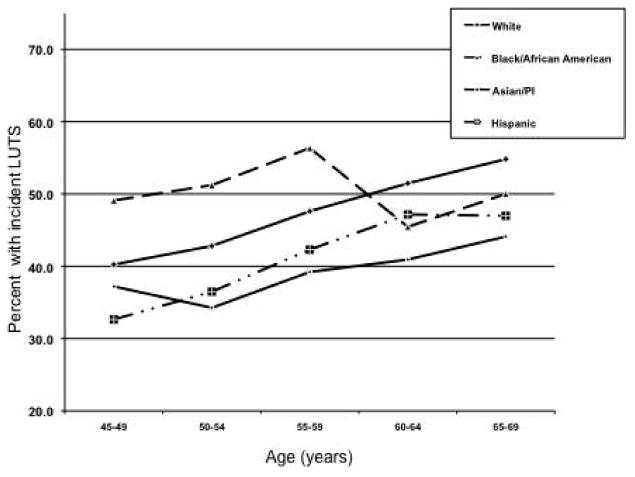
In general, the pattern of risk for incident LUTS by race/ethnicity after adjusting for socioeconomic factors, health-related behaviors and comorbidity was somewhat different from that of the prevalence data in that Hispanic (OR=0.76; 95% CI 0.66-0.87) and Asian men (OR=0.75; 95% CI 0.66-0.85) at lower risk of incident LUTS compared to white men, while no association was observed for Black men (OR=1.10; 95% CI 0.92-1.32).
Discussion
In two large and diverse cohorts we found that LUTS increased with age within each race/ethnic group, as expected. The estimates from our study are consistent with other studies that have used the AUASI to assess LUTS.
Hispanic men had the highest prevalence of lower urinary tract symptoms followed by Black, white and then Asian men. These findings were essentially the same in both cohorts we studied. However, the relative differences in the mean AUASI by race/ethnicity are within the range that some have argued to be clinically significant (e.g., four points on the AUASI) 9. In multivariable models that account for known or suspected risk factors for LUTS, Hispanic and Black men were at higher risk and Asian men at lower risk of LUTS compared to white men.
Placing our results in the context of past studies is difficult because the definitions of what ‘counts’ as lower urinary tract symptoms has varied across the studies. Some studies that examined race/ethnic disparities in LUTS included surgery as an endpoint 10,11. However, surgery is likely to be a poor endpoint for assessing LUTS (or BPH) because strong selection factors by race/ethnicity may affect the likelihood of undergoing surgery. Most prior studies have used a mixed definition of BPH that included BPH surgery, symptoms or self-reported results of a digital rectal exam 12-15. A study within the Health Professionals Follow-up Study reported no race/ethnic differences for BPH surgery, moderate or severe symptoms, or self-reported ‘enlarged prostate’ 16. Unfortunately, small numbers in the non-white participants in this study (<5%) precludes definitive conclusions. A study nested within the Massachusetts Male Aging Study found non-significant increase in risk of “clinical BPH for ‘non-white’ men compared to white men that included only a small number of minority men 17. A study using National Health and Nutrition Examination Survey (NHANES) data reported that Mexican Americans were more likely to report 3 or more lower urinary tract symptoms than non-Hispanic Whites among men 60 years and older and no difference between non-Hispanic Black and white men 18. Unfortunately NHANES data do not include a standardized assessment such as the AUASI/IPSS and had few men in each minority group. Another study using a modified and abbreviated AUASI based within the Piedmont Survey of the Elderly noted that African-American men reported lower LUTS than white men 19. The abbreviated nature of the LUTS assessment and the small numbers of participants in each race category (<257) limit inferences that can be made from this study.
Only three prior studies examining race/ethnic differences have used the full AUASI. Sarma and colleagues found that black men (n=369) in the Flint Men's Health Study reported a higher prevalence of LUTS (AUASI ≥7) compared to white men in the Olmsted County Study of Urinary Symptoms and Health Status (n=2,111) (41% vs. 34%, respectively, for men aged 40-79 years) 20. Interpreting this difference is somewhat difficult as these data were based on two separate studies, diverse locations and time points, despite being designed to be complementary studies. In addition, observed differences were not adjusted for other likely important conditions (e.g., diabetes and obesity). A second study, nested in the placebo arm of the Prostate Cancer Prevention Trial, found no race/ethnic differences for ‘total’ BPH, but did find Black men to be at elevated risk of severe BPH compared to white men 21. While the study was limited by the small number of minority participants (<430 in all minority groups combined) and subject to the selection factors of the recruitment process of a clinical trial, it did examine how race/ethnicity was related to incident LUTS (BPH). A Boston-area study found no significant differences by race/ethnicity in the prevalence of LUTS (defined as AUASI ≥8) in a sample that included men and women22. The prevalence estimates by age for men were considerably lower than what we observed and no data were reported for Asian men.
In contrast, our study included over 63,000 minority men who were all assessed with the same standardized instrument. We also determined that the disparities in LUTS could not be explained by differences in the prevalence of suspected or known risk factors for LUTS. There are several possible explanations for these disparities. First, the regulation or levels of hormones or growth factors may be due to race/ethnic differences in environmental or genetic factors which may in turn explain the observed results. Exposures or factors that occur over the life span that increase inflammation in the prostate or alter may explain the disparities. Cultural differences in the interpretation of the AUASI may also be responsible for our results. However, access to care is unlikely to influence these results since all men include had essentially equal access to care and our primary outcome measure was administered to all men in a uniform way. While these findings may not have a direct clinical implication, we do believe understanding the factors that underlie the disparities can help direct interventions for prevention and possibly treatment.
The current study has features that are important to bear in mind when interpreting these results. The underlying causes of LUTS, such as obstruction secondary to an enlarged prostate, or vascular or neurogenic bladder in origin, are unmeasured in this study. In addition, other known but relatively uncommon factors associated with LUTS (e.g., infections, neurodegenerative diseases, etc.) were not considered in our analyses. However, these conditions overall have relatively low prevalence and are unlikely to greatly affect our estimates. The absolute prevalence measures are also conditional on the individuals who have agreed to participate in these two cohort studies. As such, they may not represent the complete universe of men. Nonetheless, we believe they provide reasonable estimates due to the large sample size and uniform assessment. In addition, there is internal validity since our multivariate analyses were conducted wholly within the respondents to the two cohort studies.
Conclusions
We observed race/ethnic disparities in the prevalence and incidence of LUTS and these associations were attenuated, but not eliminated, with control for a wide range of likely confounders. These differences may be due to un- or incompletely measured confounding, or factors yet to be identified, including host cause. If the differences observed here are true, additional research should be directed at identifying the underlying causes. Once identified, preventive strategies or treatment directed at these causes may be of benefit to all men.
Supplementary Material
Acknowledgments
We thank the men who have generously participated in California Men's Health Study and the Research Program in Genes, Environment and Health.
Funding: Original cohort funding obtained from the California Cancer Research Program, The Wayne and Gladys Valley Foundation, The Robert Woods Johnson Foundation, and the Kaiser Permanente Community Benefits Program. Study funding from the National Institute of Diabetes, Digestive Diseases and Kidney through the Urologic Diseases in America Project at UCLA.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kristal AR, Arnold KB, Schenk JM, et al. Race/ethnicity, obesity, health related behaviors and the risk of symptomatic benign prostatic hyperplasia: results from the prostate cancer prevention trial. J Urol. 2007;177:1395. doi: 10.1016/j.juro.2006.11.065. [DOI] [PubMed] [Google Scholar]
- 2.Giovannucci E, Rimm EB, Chute CG, et al. Obesity and benign prostatic hyperplasia. Am J Epidemiol. 1994;140:989. doi: 10.1093/oxfordjournals.aje.a117206. [DOI] [PubMed] [Google Scholar]
- 3.Meigs JB, Mohr B, Barry MJ, et al. Risk factors for clinical benign prostatic hyperplasia in a community-based population of healthy aging men. J Clin Epidemiol. 2001;54:935. doi: 10.1016/s0895-4356(01)00351-1. [DOI] [PubMed] [Google Scholar]
- 4.Sarma AV, Kellogg PJ. Diabetes and benign prostatic hyperplasia: emerging clinical connections. Curr Urol Rep. 2009;10:267. doi: 10.1007/s11934-009-0044-5. [DOI] [PubMed] [Google Scholar]
- 5.Rohrmann S, Smit E, Giovannucci E, et al. Associations of obesity with lower urinary tract symptoms and noncancer prostate surgery in the Third National Health and Nutrition Examination Survey. Am J Epidemiol. 2004;159:390. doi: 10.1093/aje/kwh060. [DOI] [PubMed] [Google Scholar]
- 6.Parsons JK. Modifiable risk factors for benign prostatic hyperplasia and lower urinary tract symptoms: new approaches to old problems. J Urol. 2007;178:395. doi: 10.1016/j.juro.2007.03.103. [DOI] [PubMed] [Google Scholar]
- 7.Enger SM, Van Den Eeden SK, Sternfeld B, et al. California Men's Health Study (CMHS): a multiethnic cohort in a managed care setting. BMC Public Health. 2006;6:172. doi: 10.1186/1471-2458-6-172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Physical activity and cardiovascular health. NIH Consensus Development Panel on Physical Activity and Cardiovascular Health. JAMA. 1996;276:241. [PubMed] [Google Scholar]
- 9.Bautista OM, Kusek JW, Nyberg LM, et al. Study design of the Medical Therapy of Prostatic Symptoms (MTOPS) trial. Control Clin Trials. 2003;24:224. doi: 10.1016/s0197-2456(02)00263-5. [DOI] [PubMed] [Google Scholar]
- 10.Sidney S, Quesenberry CJ, Sadler MC, et al. Risk factors for surgically treated benign prostatic hyperplasia in a prepaid health care plan. Urology. 1991;38:13. doi: 10.1016/0090-4295(91)80193-b. [DOI] [PubMed] [Google Scholar]
- 11.Platz EA, Smit E, Curhan GC, et al. Prevalence of and racial/ethnic variation in lower urinary tract symptoms and noncancer prostate surgery in U.S. men. Urology. 2002;59:877. doi: 10.1016/s0090-4295(01)01673-9. [DOI] [PubMed] [Google Scholar]
- 12.Meigs JB, Mohr B, Barry MJ, et al. Risk factors for clinical benign prostatic hyperplasia in a community-based population of healthy aging men. J Clin Epidemiol. 2001;54:935. doi: 10.1016/s0895-4356(01)00351-1. [DOI] [PubMed] [Google Scholar]
- 13.Platz EA, Kawachi I, Rimm EB, et al. Race, ethnicity and benign prostatic hyperplasia in the health professionals follow-up study. J Urol. 2000;163:490. [PubMed] [Google Scholar]
- 14.Platz EA, Smit E, Curhan GC, et al. Prevalence of and racial/ethnic variation in lower urinary tract symptoms and noncancer prostate surgery in U.S. men. Urology. 2002;59:877. doi: 10.1016/s0090-4295(01)01673-9. [DOI] [PubMed] [Google Scholar]
- 15.Kristal AR, Arnold KB, Schenk JM, et al. Race/ethnicity, obesity, health related behaviors and the risk of symptomatic benign prostatic hyperplasia: results from the prostate cancer prevention trial. J Urol. 2007;177:1395. doi: 10.1016/j.juro.2006.11.065. [DOI] [PubMed] [Google Scholar]
- 16.Platz EA, Kawachi I, Rimm EB, et al. Race, ethnicity and benign prostatic hyperplasia in the health professionals follow-up study. J Urol. 2000;163:490. [PubMed] [Google Scholar]
- 17.Meigs JB, Mohr B, Barry MJ, et al. Risk factors for clinical benign prostatic hyperplasia in a community-based population of healthy aging men. J Clin Epidemiol. 2001;54:935. doi: 10.1016/s0895-4356(01)00351-1. [DOI] [PubMed] [Google Scholar]
- 18.Platz EA, Smit E, Curhan GC, et al. Prevalence of and racial/ethnic variation in lower urinary tract symptoms and noncancer prostate surgery in U.S. men. Urology. 2002;59:877. doi: 10.1016/s0090-4295(01)01673-9. [DOI] [PubMed] [Google Scholar]
- 19.Howard DL, Taylor YJ, Ross LE. Differences in lower urinary tract symptoms, treatment and mortality among African-American and white elderly men. J Natl Med Assoc. 2008;100:1146. doi: 10.1016/s0027-9684(15)31480-2. [DOI] [PubMed] [Google Scholar]
- 20.Sarma AV, Wei JT, Jacobson DJ, et al. Comparison of lower urinary tract symptom severity and associated bother between community-dwelling black and white men: the Olmsted County Study of Urinary Symptoms and Health Status and the Flint Men's Health Study. Urology. 2003;61:1086. doi: 10.1016/s0090-4295(03)00154-7. [DOI] [PubMed] [Google Scholar]
- 21.Kristal AR, Arnold KB, Schenk JM, et al. Race/ethnicity, obesity, health related behaviors and the risk of symptomatic benign prostatic hyperplasia: results from the prostate cancer prevention trial. J Urol. 2007;177:1395. doi: 10.1016/j.juro.2006.11.065. [DOI] [PubMed] [Google Scholar]
- 22.Kupelian V, Wei JT, O'Leary MP, et al. Prevalence of lower urinary tract symptoms and effect on quality of life in a racially and ethnically diverse random sample: the Boston Area Community Health (BACH) Survey. Arch Intern Med. 2006;166:2381. doi: 10.1001/archinte.166.21.2381. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


