Abstract
Human parainfluenza viruses are important respiratory tract pathogens, especially of children. However, no vaccines or specific therapies for infections caused by these viruses are currently available. In the present study we characterized the efficacy of the novel parainfluenza virus inhibitors BCX 2798 and BCX 2855, which were designed based on the three-dimensional structure of the hemagglutinin-neuraminidase (HN) protein. The compounds were highly effective in inhibiting hemagglutinin (HA) and neuraminidase (NA) activities and the growth of hPIV-1, hPIV-2, and hPIV-3 in LLC-MK2 cells. The concentrations required to reduce the activity to 50% of that of a control ranged from 0.1 to 6.0 μM in HA inhibition assays and from 0.02 to 20 μM in NA inhibition assays. The concentrations required to inhibit virus replication to 50% of the level of the control ranged from 0.7 to 11.5 μM. BCX 2798 and BCX 2855 were inactive against influenza virus HA and NA and bacterial NA. In mice infected with a recombinant Sendai virus whose HN gene was replaced with that of hPIV-1 [rSV(hHN)], intranasal administration of BCX 2798 (10 mg/kg per day) and of BCX 2855 (50 mg/kg per day) 4 h before the start of infection resulted in a significant reduction in titers of virus in the lungs and protection from death. Treatment beginning 24 h after the start of infection did not prevent death. Together, our results indicate that BCX 2798 and BCX 2855 are effective inhibitors of parainfluenza virus HN and may limit parainfluenza virus infections in humans.
The human parainfluenza viruses (hPIVs), which are members of the Paramyxoviridae family, are important respiratory tract pathogens of infants, children, and young adults. Four different types of hPIVs have been identified, all of which cause a spectrum of illnesses of the upper and lower respiratory tracts of children (20, 23, 28). Annual epidemics of parainfluenza virus infections continue to occur, and the resources required to deal with these infections cost millions of dollars annually (14).
At this time there are no effective vaccines or specific therapies to control parainfluenza virus infections.
Parainfluenza virus infection requires the hemagglutinin-neuraminidase (HN) protein, a major surface glycoprotein that has functional sites for cell attachment and neuraminidase (NA) activity (5). HN recognizes sialic acid-containing receptors on the cell surface, and this recognition allows the virus to bind to target cells (22). HN also acts as an NA, removing sialic acid from virus particles and thus preventing self-aggregation of virus and promoting efficient spread of virus (21). In addition, HN promotes the activity of the fusion (F) protein, thereby allowing the virus to penetrate the cell surface (32, 43). The F protein is another major glycoprotein that is located on the surface of the virion and plays an important role in parainfluenza virus replication. Early in infection, the F glycoprotein mediates penetration of the host cell by fusion of the viral envelope to the plasma membrane. At a late stage of infection, the protein mediates fusion of the infected cells with contiguous uninfected cells, leading to the formation of a syncytium and the spread of infection in the local area. Many studies have shown that a type-specific functional interaction between HN and F is required for efficient membrane fusion (15, 16). The binding of HN protein to its receptor induces the conformational change of residues near the hydrophobic surface of the HN protein and, probably, this change triggers the activation of the F protein, which initiates membrane fusion (34).
The efficient inhibition of the HN protein has to block cell attachment, fusion promotion, and NA activities, thereby preventing both infection by virus and virus spreading. Because of the key role of HN in the infectivity of parainfluenza virus, attention was concentrated on the development of selective inhibitors for the prophylaxis and treatment of hPIV infections. The extensive crystallographic and biochemical studies of the HN protein of Newcastle disease virus (NDV) (8, 33) yielded a high level of structural information for the design of new drug candidates. The resolution of the three-dimensional (3D) structure of the HN of NDV showed that the amino acid residues around the receptor-binding/NA active site are highly conserved and common to all parainfluenza viruses. These findings allowed us to use NDV HN as a model in the structure-based design of potential inhibitors of hPIVs. Previously, this approach was successfully put into practice for the rational design of the highly potent and selective inhibitors of influenza virus NA, zanamivir and oseltamivir (19, 39). Both compounds were synthesized by using computer-aided design techniques on the basis of the 3D structure of influenza virus NA (41, 36).
Because of its role in releasing newly formed virions from infected cells, its location on the surface of the virion, and its enzymatic structure, influenza virus NA has been a target for which potential antiviral agents have been developed in recent years. As predicted, zanamivir and oseltamivir, were shown to be high-affinity inhibitors of influenza virus sialidase in vitro and in vivo (25, 31, 41). Clinical studies have demonstrated the efficacy of zanamivir and oseltamivir against both influenza A and B viruses (12, 13). Because parainfluenza viruses possess NA activity, too, influenza virus NA inhibitors have been evaluated as possible inhibitors of hPIV HN (41, 42). However, these agents showed no significant anti-parainfluenza virus activity (concentrations required to reduce the activity to 50% of that of a control [IC50] values exceeded 1,000 μM). These data indicated the importance of development of new antiviral compounds by using the rational design of protein structure as we have done in developing new inhibitors of parainfluenza virus HN.
The present study is the first to describe the development and the efficacy of novel and potent parainfluenza virus HN inhibitors BCX 2798 and BCX 2855, which were designed on the basis of the 3D structure of the HN protein. We evaluated the potency of novel agents in vitro and then investigated the efficacy of BCX 2798 and BCX 2855 against lethal parainfluenza virus infection in a mouse model by using rSV(hHN). The hPIVs infect experimental animals poorly (6, 24, 26, 35). Sendai virus (SV) belongs to the Paramyxoviridae family and causes fatal pneumonia in mice, its natural host (1, 9, 44). To evaluate the efficacy of both compounds in vivo against hPIVs, we established a mouse model using a recombinant virus. This construct showed biological compatibility of the HN protein of hPIV-1 virus with the F protein of SV in vitro as observed previously (3) and induced strong parainfluenza virus infection in mice. Our results showed that the compounds inhibited the binding and NA activities and growth of hPIVs in vitro and prevented the growth of rSV(hHN) in the lungs of infected mice.
MATERIALS AND METHODS
Compounds.
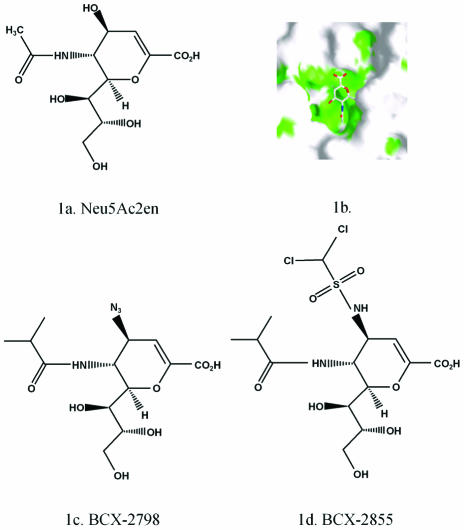
The compounds BCX 2798 and BCX 2855 were developed by BioCryst Pharmaceuticals, Inc. (Birmingham, Ala.), through structure-based drug design based on the structure of the lead compound, Neu5Ac2en (2-deoxy-2,3-dehydro-N-acetyl neuraminic acid) (Fig. 1a), bound to the active site of NDV HN. Neu5Ac2en inhibits most NAs or sialidases. The structure of the Neu5Ac2en-HN complex revealed that a large cavity around the O4 position of the ligand is lined with amino acids that are largely conserved among all paramyxoviruses (Fig. 1b). BCX 2798 and BCX 2855 are derivatives of Neu5Ac2en in which the O4 hydroxyl group has been replaced by bulky groups designed to fill the cavity. In BCX 2798 (4-azido-5-isobutyrylamino-2,3-didehydro-2,3,4,5-tetradeoxy-d-glycero-d-galacto-2-nonulopyranosic acid), O4 has been replaced with an azido group, and in BCX 2855 (4-dichloromethanesulfonylamino-5-isobutyryl-amino-2,3-didehydro-2,3,4,5-tetradeoxy-d-glycero-d-galacto-2-nonulopyranosicacid), O4 has been replaced with a dichloromethanesulfonylamino group (Fig. 1c and d). Analysis of the Neu5Ac2en-HN complex also suggested that a bulkier hydrophobic group could be accommodated in place of the methyl group of the acetamido moiety at C-5. Both compounds therefore have an isopropyl group in place of the methyl group of acetamido moiety at C-5.
FIG. 1.
Chemical structures of BCX 2798 and BCX 2855 compounds. (A) Neu5Ac2en (2-deoxy-2,3-dehydro-N-acetyl neuraminic acid); (B) interaction of amino acids of the active site of HN NDV with Neu5Ac2en; (C) BCX 2798 (4-azido-5-isobutyrylamino-2,3-didehydro-2,3,4,5-tetradeoxy-d-glycero-d-galacto-2-nonulopyranosic acid); (D) BCX 2855 (4-dichloromethanesulfonylamino-2,3-didehydro-2,3,4,5-tetradeoxy-d-glycero-d-galacto-2-nonulopyranosic acid).
The compounds were provided as lyophilized powder and stored at 4°C. They were solubilized in tissue culture medium for in vitro experiments and in distilled water for in vivo studies.
Cells and viruses.
LLC-MK2 cells were obtained from the American Type Culture Collection (Manassas, Va.) and were grown in Eagle minimal essential medium containing 5% fetal bovine serum in a humidified atmosphere of 5% CO2. The 293T (human kidney epithelial) cells (10) used for the rescue of rSV(hHN) were cultured in Dulbecco modified Eagle medium supplemented with 10% fetal bovine serum.
The viruses hPIV-1, hPIV-2, hPIV-3, and SV were obtained from the American Type Culture Collection. The rSV(hHN) virus was rescued by using a reverse-genetics system according to the procedure described below. Influenza viruses A/New Caledonia/20/99 (H1N1) and B/Yamanashi/166/98 were kindly provided by R. G. Webster (St. Jude Children's Research Hospital, Memphis, Tenn.).
The hPIVs were grown in LLC-MK2 cells in Dulbecco modified Eagle medium containing 0.1% bovine serum albumin and 1 μg of acetylated trypsin/ml. SV and rSV(hHN) were propagated in the allantoic cavity of 10-day-old embryonated chicken eggs. Virus stocks were divided into aliquots and kept frozen at −70°C until use. Before the viruses were used in analyses of the putative HN inhibitors, their infectivity titers were determined by endpoint dilution assay (determine the amount of virus in 1 ml that infects 50% of the cells in culture [TCID50/ml]). Parainfluenza viruses used in HA inhibition (HI) and NA inhibition (NI) assays were concentrated and purified through a gradient of 30 to 50% sucrose in phosphate-buffered saline (PBS) as described previously (38).
Rescue of recombinant SV carrying the HN gene of hPIV-1.
SV (strain E) was rescued from the full-length SV cDNA genome pSeV(+) (18). Reverse-genetics methods were used to rescue rSV(hHN), which contains the hPIV-1 HN gene (hHN) instead of the SV HN gene. The full-length cDNA clone of SV was mutated to include an NotI site upstream and an AscI site downstream of the HN gene; thus, the pSV(+)AN plasmid was created. These restriction sites were also added to the hHN cDNA and used to exchange the HN gene. For the rescue of the recombinant virus, 293T cells were infected with the vaccinia virus vTF7-3, which expresses T7 RNA polymerase, and transfected with the full-length rSV(hHN) genome, as well as the NP, P, and L genes in expression vectors (4). Two days after transfection, the infected and transfected cells were subjected to three cycles of freezing and thawing and injected into the allantoic cavity of 10-day-old embryonated chicken eggs to amplify the virus. The rescued virus was plaque-purified on LLC-MK2 cells and amplified in embryonated chicken eggs. The sequence of the HN gene of egg-grown rSV(hHN) did not differ from that of wild-type hPIV-1.
HI assay.
BCX 2798 and BCX 2855 were serially diluted (ratio, 1:2), and the dilutions were preincubated with a standard dose of virus (four hemagglutination units) for 1 h at room temperature. Chicken red blood cells (0.5%) were added to mixtures containing hPIV-1 or hPIV-2; turkey red blood cells (0.5%) were added to mixtures containing hPIV-3. The 50% hemagglutination end point was read after incubation for 45 min at 4°C. The concentration of the compound that shows 50% agglutination was considered the IC50. The results presented are the mean values (± the standard deviation [SD]) from at least three independent experiments.
NI assay.
Before the NI assays were conducted, the activity of each viral NA and bacterial NA (Clostridium perfringens NA; New England Biolabs, Beverly, Mass.) was measured by a standard fluorometric assay with 2′-(4-methylumbelliferyl)-α-d-N-acetylneuraminic acid (MUN; Sigma Chemical Co., St. Louis, Mo.) as the substrate (27). Briefly, virus was serially diluted (ratio, 1:2) in enzyme buffer consisting of 32.5 mM 2-(N-morpholino)ethanesulfonic acid (pH 6.5) and 4 mM CaCl2, and 50 μl of each dilution was mixed with 50 μl of substrate whose final concentration was 150 μM. After 1 h of incubation at 37°C, the reaction was stopped by the addition of 100 μl of 0.1 M glycine-NaOH (pH 10.7) in 25% ethanol. The fluorescence of the cleavage product was quantified in a Fluoroskan II spectrophotometer (Labsystems, Helsinki, Finland; excitation wavelength, 355 nm; emission wavelength, 460 nm).
The extent of NI was defined as the concentration of compound required to reduce the NA activity of the treated virus to 50% of that of the control virus. Each compound was diluted (ratio, 1:4), and 25 μl of each dilution was incubated for 30 min at room temperature with 25 μl of diluted virus whose NA activity was equal to 100 to 150 relative fluorescence units. The reaction was started by the addition of substrate and stopped after 1 h of incubation at 37°C. The IC50 values were calculated by plotting the percentage of fluorescence inhibition relative to the control versus the log concentrations of the compounds. The results presented are the mean values (± the SD) from at least three independent experiments.
Inhibition of virus growth in LLC-MK2 cells.
LLC-MK2 cells in 24-well plates were infected with parainfluenza viruses at a low multiplicity of infection (range, 0.0001 to 0.001 TCID50/cell). BCX 2798 and BCX 2855 (range, of final concentrations, 0.1 to 100 μM) were added to cells 1 h before infection. The presence of virus in cell culture was determined by hemagglutination test 72 h after infection. The concentrations required to inhibit virus replication to 50% of the level of the control (EC50) values for each compound were expressed as the concentrations that inhibited virus growth in half of the quadruplicate test cultures. The results are reported as the mean values (± the SD) of three to five independent experiments.
The cytotoxicity of the compounds was evaluated by the trypan blue vital staining procedure (37) before growth inhibition of the virus was examined. Briefly, BCX 2798 and BCX 2855 were added to LLC-MK2 cells at concentrations of 0.01 to 100 μM. After incubation for 5 days, the cell suspension was mixed with 0.4% of trypan blue, and then the stained (dead) cells and the unstained (living) cells were counted separately in a hemocytometer to determine the percentage of viable cells per milliliter. The concentration of the compound was assumed to be nontoxic if the percentage of viable cells in an experimental suspension (treated cells) was equal to that in a control suspension (untreated cells).
Evaluation of antiviral activity of compounds in a mouse model.
Eight-week-old female 129x1/SvJ mice (weight, 18 to 20 g; Jackson Laboratories, Bar Harbor, Maine) were anesthetized by inhalation of isoflurane (2.5%; Baxter Healthcare Corp., Deerfield, Ill.) and inoculated intranasally with 50 μl of rSV(hHN). The viral dose used in this study was equivalent to 106.5 TCID50 per mouse and killed approximately 90% of the infected mice. Test compounds were administered intranasally (in a volume of 50 μl) twice daily for 5 days; administration began 4 h before inoculation with virus or 24 h after inoculation. BCX 2798 and BCX 2855 (1, 5, 10, 25, or 50 mg/kg per day) were administered to groups of 5 to 10 mice. Control animals were infected but were treated only with PBS. Mice were observed daily for 21 days to detect signs of infection and to determine the number that died and the date of death. The compounds were evaluated on the basis of the prevention of weight loss and death and the length of survival time. The change in weight during infection is shown as the percentage of the mouse's weight on the day before viral infection.
To assess toxicity, we administered each compound (1, 10, or 50 mg/kg per day) to mice intranasally (in a volume of 50 μl) twice daily for 5 days. Animals were observed daily for 21 days to evaluate changes in weight and to determine the number of mice that died.
Animal studies were performed in a Biosafety Level 3 facility at St. Jude Children's Research Hospital. All experimental procedures were approved by the institution's Animal Care and Use Committee.
Titration of virus from mouse lungs.
At 6, 12, and 18 h or at 1, 3, 5, 7, or 9 days after infection with rSV(hHN), three mice from each group were euthanized. Lungs were removed under sterile conditions, washed three times with PBS, ground, and suspended in PBS (total volume, 1 ml). The suspensions were centrifuged at 2,000 × g for 10 min to clear from cellular debris. Virus titers (TCID50/milliliter) were determined by adding 0.1 ml of each suspension, which had been serially diluted (1:10), to LLC-MK2 cells in 24-well plates. The mean titers ± the standard error of the mean (SEM) are presented.
Pathological studies.
Lungs were removed at day 9 after infection with rSV(hHN) and fixed with 10% neutrally buffered formalin for 24 h. After the lungs were embedded in paraffin, they were cut into 5-μm sections. The sections were stained with hematoxylin and eosin and then examined microscopically to detect any histopathologic changes.
Statistical analysis.
The Kaplan-Meier method (7, 17) was used to estimate and compare survival curves (survival probabilities) of mice in groups that received different treatments (BCX 2798, BCX 2855, or control [PBS]), that received treatment whose administration began at different times (4 h before inoculation and 24 h after inoculation), and that received treatment at different doses. Survival rates among the groups were compared by using the Fisher exact test. The univariate log-rank test (40) was used to compare the survival curves of control and treatment groups during the first 21 days after the start of infection. The mean day to death was estimated as the number of days that the mice survived after viral infection. If no death occurred during the observation period, the mean day to death was considered to be 21 days. Repeated-measures analysis of variance was used to estimate and compare the effects of compounds on weight changes of infected and uninfected mice and titers of virus from lungs of infected animals. Statistical significance was indicated if P values were <0.05. The analyses were performed by using SAS software (version 8; Cary, N.C.) (30).
RESULTS
Inhibition of HI and NA activity and virus replication by BCX 2798 and BCX 2855.
We first evaluated the ability of BCX 2798 and BCX 2855 to inhibit attachment (HA activity), release (NA activity), and replication of the parainfluenza viruses hPIV-1, hPIV-2, hPIV-3, and rSV(hHN) in vitro. The results of the HI assays showed that both compounds inhibited the binding of the three hPIVs, although some differences in anti-HA binding activity were observed between the two agents (Table 1). The IC50 values for BCX 2798 in HI assays with hPIV-1, hPIV-2, or hPIV-3 were within a wide range: 0.1 to 4.8 μM. BCX 2798 was ca. 20 times more effective in inhibiting the binding of hPIV-1 and rSV(hHN) than that of hPIV-2 and hPIV-3. The IC50 values for BCX 2855 (2.0 to 6.0 μM) varied less than those for BCX 2798. Although both compounds were equally effective against hPIV-2 and hPIV-3 (range of IC50 values, 2.0 to 4.8 μM), BCX 2798 was 50 times more potent in inhibiting the binding of hPIV-1 and rSV(hHN) than was BCX 2855. BCX 2798 and BCX 2855 compounds were highly specific for parainfluenza viruses: both agents failed to inhibit the HA activity of influenza viruses A/New Caledonia/20/99 and B/Yamanashi/166/98, even when the concentrations of the compounds were as high as 1000 μM (data not shown).
TABLE 1.
Inhibitory effects of BCX 2798 and BCX 2855 in vitro
| Virus | Inhibitiona (mean concn [μM])
|
|||||
|---|---|---|---|---|---|---|
| HA activityb (mean IC50 ± SD)
|
NA activityc (mean IC50 ± SD)
|
Growth in LLC-MK2 cellsd (mean EC50 ± SD)
|
||||
| BCX 2798 | BCX 2855 | BCX 2798 | BCX 2855 | BCX 2798 | BCX 2855 | |
| hPIV-1 | 0.1 ± 0.0 | 6.0 ± 1.9 | 0.04 ± 0.0 | 1.2 ± 0.2 | 1.1 ± 0.3 | 11.5 ± 1.0 |
| hPIV-2 | 2.2 ± 0.6 | 2.7 ± 0.6 | 1.6 ± 0.4 | 1.9 ± 0.2 | 7.0 ± 0.0 | 1.8 ± 0.9 |
| hPIV-3 | 4.8 ± 0.1 | 2.0 ± 0.5 | 20.0 ± 1.7 | 4.3 ± 0.2 | 11.3 ± 1.1 | 2.4 ± 0.7 |
| rSV (hHN) | 0.1 ± 0.0 | 4.8 ± 0.1 | 0.02 ± 0.0 | 1.8 ± 0.3 | 0.7 ± 0.1 | 8.6 ± 1.3 |
| Total (range) | 0.1-4.8 | 2.0-6.0 | 0.02-20.0 | 1.2-4.3 | 0.7-11.3 | 1.8-11.5 |
Each mean concentration was calculated from values obtained from at least three independent experiments.
Determined in HI assays. IC50 values are the mean concentrations (except as noted) of the compound that caused 50% agglutination.
Determined in NI assays. IC50 values are the mean concentrations (except as noted) of the compound required to reduce the NA activity to 50% of that of untreated controls. MUN (final concentration, 150 μM) was the substrate.
Determined by endpoint dilution assays (TCID50). EC50 values are the mean concentrations (except as noted) of the compound required to inhibit virus replication in treated LLC-MK2 cells to 50% of that in untreated controls.
The ability of BCX 2798 and BCX 2855 to inhibit NAs of hPIVs was evaluated in NI assays (Table 1). Both compounds effectively inhibited the NA activity of the test viruses: the IC50 values for BCX 2798 ranged from 0.02 to 20 μM, and those for BCX 2855 ranged from 1.2 to 4.3 μM. Similar to the results obtained in HI assays with hPIVs, the IC50 values determined in NI assays were more constant for BCX 2855 than for BCX 2798. BCX 2798 was 80 to 1,000 times more effective against of the NA activity of hPIV-1 and rSV(hHN) than against that of hPIV-2 and hPIV-3. The lowest anti-NA activity was observed when BCX 2798 was tested against hPIV-3 (IC50 value, 20 μM). BCX 2855 demonstrated equal effectiveness against the NA activity of hPIV-1 and hPIV-2 but was slightly less effective against the NA activity of hPIV-3. As observed in the HI assays, both compounds were equally effective in inhibiting the NA activity of hPIV-2. BCX 2855 was about five times more active against hPIV-3 than was BCX 2798, but BCX 2798 was 30 to 90 times more effective in the NI assay against hPIV-1 and rSV(hHN) than was BCX 2855. Neither compound effectively inhibited the NAs of the influenza viruses A/New Caledonia/20/99 and B/Yamanashi/166/98 or the NA of the bacterium Clostridium perfringens, even when concentrations of the compounds as high as 1,000 μM were used (data not shown).
Because the results of HI and NI assays indicated that BCX 2798 and BCX 2855 inhibited the ability of the parainfluenza viruses to bind to and be released from the cell, we hypothesized that the life cycle of the viruses was disrupted by the inhibition of these crucial activities. To test this hypothesis, we evaluated the abilities of BCX 2798 and BCX 2855 to inhibit the growth of parainfluenza viruses in LLC-MK2 cells. The trypan blue vital staining of uninfected cells treated with either compound showed that neither BCX 2798 nor BCX 2855 were cytotoxic at concentrations as high as 100 μM (data not shown).
Both agents were effective in inhibiting the growth of parainfluenza viruses in cell culture: the EC50 values for BCX 2798 ranged from 0.7 to 11.3 μM, and those for BCX 2855 ranged from 1.8 to 11.5 μM (Table 1). BCX 2798 was 6 to 16 times more effective in inhibiting the growth of hPIV-1 and rSV(hHN) than that of hPIV-2 and hPIV-3. BCX 2855 was more active against hPIV-2 and hPIV-3 than against hPIV-1 and rSV(hHN). BCX 2798 was at least 10 times more effective in inhibiting the growth of hPIV-1 and rSV(hHN) than was BCX 2855, but BCX 2855 was better than BCX 2798 at inhibiting the growth of hPIV-2 and hPIV-3 (1.8 to 2.4 μM compared to 7.0 to 11.3 μM). In general, the results of the growth inhibition study were consistent with those of the HI and NI assays and indicated a high sensitivity of tested viruses to BCX 2798 and BCX 2855 compounds.
Pathogenicity of rSV(hHN) virus in mice.
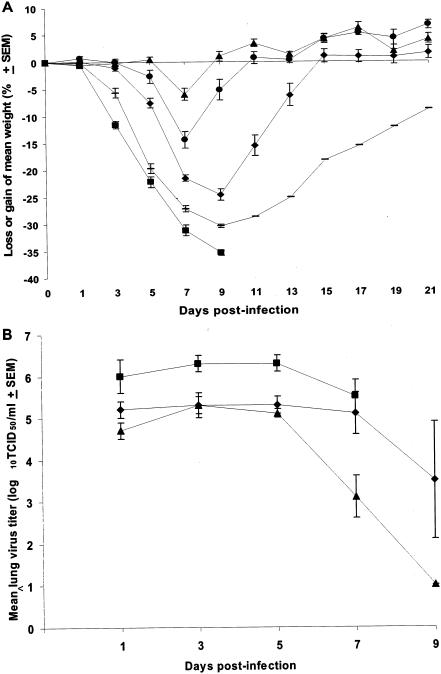
The hPIVs are strict pathogens of humans and cause no disease in mice. To establish an animal model that can be used for the evaluation of the efficacy of BCX 2798 and BCX 2855 in vivo, we rescued rSV(hHN), in which the HN gene of SV was replaced with that of hPIV-1. Before we tested the compounds in vivo, we determined whether rSV(hHN) is pathogenic in 129x1/SvJ mice. Animals were infected with doses of rSV(hHN) ranging from 105 to 107 TCID50 per mouse, and the number of mice that survived (Table 2) and the changes in body weight were determined (Fig. 2A). All mice infected with the highest dose of virus (107 TCID50 per mouse) lost >35% of their initial weight and died within 9 days after the start of infection. Only 1 of 15 mice survived infection when an inoculation dose of 106.5 TCID50 per mouse was used. In contrast, 80% of the mice infected with 106 TCID50 of rSV(hHN) survived. Infection with no more than 105.5 TCID50 killed no mice, although weight loss (≤15% of initial weight) and signs of infection were observed.
TABLE 2.
Infectivity of rSV(hHN) in micea
| Virus dose (TCID50/mouse) | No. of survivors/total | Survival (%) | Mean day to death ± SD |
|---|---|---|---|
| 105 | 15/15 | 100.0 | NA |
| 105.5 | 15/15 | 100.0 | NA |
| 106 | 12/15 | 80.0 | 18.5 ± 4.9 |
| 106.5 | 1/15b | 6.7 | 9.1 ± 1.2 |
| 107 | 0/5b | 0.0 | 7.8 ± 1.3 |
Mice infected with different virus doses were monitored for 21 days to determine the the number of mice that died and the mean day to death.
The number of mice who survived infection differed significantly from that of mock- infected mice (P < 0.05). NA, not applicable.
FIG. 2.
Pathogenicity of rSV(hHN) in mice. (A) Mice were infected with rSV(hHN) at doses of 105 (▴), 105.5 (•), 106 (⧫), 106.5 (-), or 107 (▪) TCID50 per mouse. Weight changes were calculated through day 21 as a percentage of the mouse's weight on day 0 (before infection). Values are the averages for each group, plotted with error bars indicating the SEM. (B) Mice were infected with rSV(hHN) at doses of 105 (▴), 106 (⧫), or 107 (▪) TCID50 per mouse. Lungs were collected 1, 3, 5, 7, and 9 days after infection. Values are mean titers of virus from three animals, plotted with error bars indicating the SEM. All mice infected with 107 TCID50 died by day 9.
To further examine the pathogenicity of rSV(hHN) in mice, we determined the growth of the recombinant virus in lungs of mice infected with different doses (105, 106, and 107 TCID50; Fig. 2B). Titers of virus from lungs collected on days 1, 3, 5, 7, and 9 were determined. The virus titers and the clearance of virus from mouse lungs were dependent on the dose of virus. The highest virus titer (∼6 log10 TCID50/ml) was recovered on days 1, 3, and 5 from mice infected with 107 TCID50. Titers of virus from lungs of mice infected with 105 or 106 TCID50 were ca. 10 times lower than those from the lungs of mice infected with 107 TCID50 at the same time points. The virus titers decreased in all groups after day 7, but the decrease was more rapid and significant in groups of mice infected with the lower doses than in those infected with the higher doses. Thus, no virus was detected at day 9 in mice infected with 105 TCID50, whereas detectable levels of infectious virus (3.5-log10 TCID50/ml) were present in the lungs of mice infected with 106 TCID50. Despite a reduction on day 7 in the titers of virus from lungs of animals infected with 107 TCID50, all mice in this group died by day 9.
Because the virus titers reached their peak in the lungs within 24 h after infection, we examined whether replication or the dose of virus contribute to the peak titers on day 1. Mice were infected with 106.5 TCID50 of rSV(hHN), and the titers of virus in the lungs were determined 6, 12, 18, and 24 h after infection. The virus titer 6 h after infection was ca. 100 times lower (104 TCID50/ml) than the administered dose and increased steadily every 6 h until a peak titer was reached 24 h after the start of infection (data not shown).
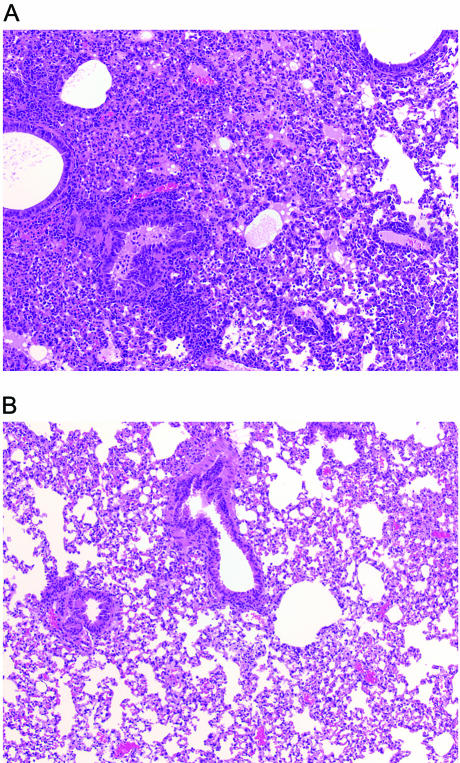
We also examined the lungs of mice for histopathologic changes caused by rSV(hHN) at day 9 after infection (Fig. 3A). Animals infected with a sublethal dose of virus (105.5 TCID50) (Table 2) experienced pathological changes in their airways and interstitium. Alveolar spaces were filled with moderate inflammatory infiltrates of lymphocytes, macrophages, and neutrophils, and alveolar edema was present. Fibrin deposition and alveolar necrosis were also observed. The mucosal epithelium in bronchi was hyperplastic, and there were focal areas of mucosal necrosis with sloughing of degenerate cells into the bronchial lumen. Mononuclear cuffing of vessels was prominent. No signs of lung inflammation were seen in mice in the control group that received only PBS (Fig. 3B).
FIG. 3.
Histopathologic changes in the lungs of mice infected with rSV(hHN). (A) Mice (three per group) were infected with a dose of 105.5 TCID50. Lungs were removed 9 days after infection, fixed, and cut into 5-μm sections that were later stained with hematoxylin and eosin. Low-power view (×10) of the stained section is shown. (B) Control mice (three per group) were given PBS instead of virus. The lungs were prepared and examined as described in panel A.
The results described in this section indicated that the severe illness characterized by weight loss and death reflected the replication of the rSV(hHN) and pathological changes in mouse lungs. We have therefore established a mouse model for the evaluation of the antiviral activity of BCX 2798 and BCX 2855, as well as that of other potential antiviral compounds.
Efficacy of BCX 2798 and BCX 2855 in mice infected with a lethal dose of rSV(hHN).
To determine the efficacy of BCX 2798 and BCX 2855 in a mouse model, we administered the compounds intranasally to mice at dosages of 1.0 to 50 mg/kg per day twice daily for 5 consecutive days. Administration began 4 h before or 24 h after lethal challenge with rSV(hHN) (106.5 TCID50). At a dosage as high as 50 mg/kg per day, neither compound showed toxicity in uninfected mice in terms of weight change and survival during the observation period (data not shown).
The duration of survival of infected animals and changes in weight were assessed in our evaluation of the effectiveness of the two compounds in vivo. Both parameters were monitored for 21 days after infection. Changes in body weight on days 5, 7, and 9 after infection underwent statistical analysis. Table 3 shows the results of our analysis of the survival duration and weight changes for groups of mice pretreated with different dosages of either compound 4 h before virus infection.
TABLE 3.
Efficacy of pretreatment with BCX 2798 or BCX 2855 on rSV(hHN) infection in micea
| Compound | Dosage (mg/kg per day) | Loss or gain (% ± SD)b of mean wt on postinfection day:
|
No. of survivors/total no. | Survival rate (%) | Mean day to death ± SDc | ||
|---|---|---|---|---|---|---|---|
| 5 | 7 | 9 | |||||
| BCX 2798 | 10 | −8.2 ± 5.8 | −15.9 ± 6.2 | −13.4 ± 9.7 | 12/12d | 100 | |
| 5 | −16.5 ± 4.8 | −22.8 ± 6.8 | −18.8 ± 9.4 | 4/6d | 66.7 | 17.0 ± 6.2 | |
| 1 | −15.7 ± 4.9 | −23.3 ± 4.6 | −27.4 ± 5.2 | 2/12 | 16.7 | 12.3 ± 4.2 | |
| BCX 2855 | 50 | −10.3 ± 6.4 | −17.3 ± 9.3 | −13.9 ± 8.9 | 10/12d | 83.3 | 19.3 ± 3.9 |
| 25 | −15.0 ± 6.7 | −24.2 ± 4.0 | −25.4 ± 9.4 | 2/6 | 33.3 | 14.2 ± 5.3 | |
| 10 | −13.0 ± 4.9 | −20.6 ± 4.0 | −24.5 ± 7.5 | 2/11 | 18.2 | 12.2 ± 4.5 | |
| PBS | 0 | −17.1 ± 4.9 | −24.9 ± 4.5 | −28.6 ± 5.3 | 3/21 | 14.2 | 11.3 ± 4.3 |
BCX 2798 or 2855 were administered intranasally to 129×1/SvJ mice for 5 days beginning 4 h before viral infection with 106.5 TCID50. Control mice were infected but were treated only with sterile PBS on the same schedule.
Differences in weight loss between the group of treated mice and the group of control mice were evaluated by using repeating measures analysis of variations.
The mean day to death was the number of days of survival after the lethal challenge with rSV(hHN). Survival curves were estimated by the Kaplan-Meier method.
The number of survivors differed significantly from that in the control group (P < 0.05).
Comparison of the survival curves of rSV(hHN)-infected mice treated or untreated with novel compounds showed that treatment with either BCX 2798 (5 mg/kg per day) or BCX 2855 (50 mg/kg per day) was protective against lethal infection (P < 0.05). BCX 2798 at a dosage of 5 mg/kg per day protected 66.7% of infected mice from death at day 21 after viral infection, whereas 86% of the mice in the control group (infected but treated with PBS only) died. The mean day to death for treated mice was 17 days. In contrast, the mean day to death of mice in the control group was 11.3 days. Complete protection against lethal challenge was observed when mice were treated with 10 mg/kg per day of BCX 2798. Unlike mice in the control group, which lost a maximum of 28.6% of their initial weight by day 9, mice treated with 5 or 10 mg/kg per day of BCX 2798 lost a maximum of 22.8 or 15.9%, respectively, of their initial weight by day 7 and began to regain weight by day 9. Analysis of survival curves of mice treated with different dosages of BCX 2798 and BCX 2855 showed that BCX 2855 was less effective than BCX 2798 in protecting mice against lethal infection by rSV(hHN) (P < 0.05). Only treatment with 50 mg of BCX 2855/kg per day was sufficient to protect 83.3% of infected mice from death 21 days after infection by a lethal dose of virus and increased the mean day to death to 19.3 days. Mice from this group lost a maximum of 17.3% of initial weight.
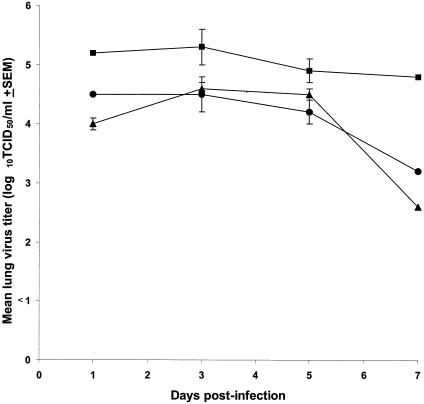
To determine the effect of the test compounds on virus replication in lungs, we assayed the virus titers from the lungs of infected mice given protective concentrations of either BCX 2798 (10 mg/kg per day) or BCX 2855 (50 mg/kg per day). Treatment began 4 h before inoculation and continued twice daily for 5 days (Fig. 4). The virus titers in lungs of animals treated with either agent were significantly lower than those of the control mice (infected but treated with PBS only) (P < 0.05). The virus titers in the lungs of control mice on days 1, 3, and 7 were ca. 10 times greater than those of treated mice on the same days. On day 7, the titers of virus in the lungs of treated mice were more than 50 times less than they were on day 5. Our results indicated that the antiviral effects of both compounds were associated with the inhibition of virus replication in lungs.
FIG. 4.
Effect of pretreatment with BCX 2798 or BCX 2855 on virus titers from lungs of mice infected with rSV(hHN). BCX 2798 (10 mg/kg per day [▴]) and BCX 2855 (50 mg/kg per day [•]) were intranasally administered to 129x1/SvJ mice for 5 days; administration began 4 h before viral infection. Three mice per group were infected with rSV(hHN) (106.5 TCID50 per mouse). Control infected mice were treated only with PBS (▪). The averages for each group are plotted with error bars indicating the SEM.
To assess the potential therapeutic usefulness of BCX 2798 and BCX 2855 against parainfluenza virus infection, we examined the effectiveness of the agents when they were given 24 h after inoculation. The number of survivors and weight changes in the treated groups did not differ from those of the control group (mice that were infected but treated with only PBS) even when the highest tested dosage as 50 mg/kg per day of either compound was used (data not shown). Mice infected with a lethal dose of rSV(hHN) and treated with either compound consistently lost weight, and all died 7 to 10 days after inoculation.
Thus, the results of our in vivo experiments indicated that BCX 2798 and BCX 2855 were effective for prophylactic, but not for therapeutic, purposes.
DISCUSSION
Because of the wide geographic distribution of parainfluenza viruses and the frequency of parainfluenza virus reinfection throughout life, prevention of parainfluenza virus infection continues to be an important public health concern. Our results indicate that the novel compounds BCX 2798 and BCX 2855, whose designs were based on the crystal structure of the HN of the NDV, are specific and potent inhibitors of hPIVs. These inhibitory effects were seen in vitro over a wide range of concentrations of the compounds. The HA activity of parainfluenza viruses was inhibited by either agent at concentrations ranging from 0.1 to 6.0 μM; the NA activity was inhibited by concentrations ranging from 0.04 to 20 μM, and the growth of virus in LLC-MK2 cells was inhibited by concentrations ranging from 0.7 to 11.5 μM. In general, BCX 2798 demonstrated greater antiviral activity against the HN of hPIV-1 in all in vitro tests. BCX 2855 was slightly more effective in inhibiting the growth of hPIV-2 and hPIV-3 in cell culture. The differences in the inhibitory activity of BCX 2798 and BCX 2855 against various parainfluenza viruses may reflect differences in the active site of the HN protein of the different genera or types. Moreover, the differences between the inhibitory effect of BCX 2798 and that of BCX 2855 against a specific virus reflect the differences in the structures of the two compounds. Insight into these possibilities will come from ongoing structural studies, which will demonstrate in detail the interaction of each compound with the active site of the HN molecule of a specific parainfluenza virus. We speculate that only the azido group at O4 of BCX 2798 was successfully accommodated in the active site of the HN of hPIV-1. The dichloromethanesulfonylamino group at O4 of BCX 2855 seems to be too bulky or too hydrophobic for the compound to efficiently block the HN of tested hPIVs.
One chief aim of the present study was to evaluate the effectiveness of BCX 2798 and BCX 2855 against parainfluenza virus infection in vivo. The absence of significant disease in animals (other than nonhuman primates) infected with hPIVs was overcome by the use of a recombinant SV in which the HN gene of SV was replaced with that of hPIV-1. Our results showed that rSV(hHN), like SV, infect mice causing pathological changes in lungs and as result weight loss and death.
Using the mouse model, we determined whether BCX 2798 or BCX 2855 protected mice against lethal rSV(hHN) infection. When mice were treated with either compound 4 h before inoculation with virus, a dose response (change in weight and number of survivors) was observed. BCX 2798 was more effective in protecting mice against lethal rSV(hHN) infection than was BCX 2855. This finding was consistent with our in vitro experiments, i.e., the antiviral activity of BCX 2798 against hPIV-1 and rSV(hHN) was superior to that of BCX 2855 against the same viruses.
Titration of the virus from the lungs showed that the protection of mice from lethal challenge with rSV(hHN) was due to the inhibition of virus replication in the lungs. Daily treatment of infected mice with 10 mg of BCX 2798 and 50 mg of BCX 2855/kg per day starting 4 h before viral infection significantly reduced the titer of virus in mouse lungs compared to the level of virus titers in lungs of infected animals treated only with PBS (P < 0.05).
Similar types of inhibitors that block NA activity have been developed against influenza viruses (19, 39). Two of them, zanamivir and oseltamivir, have been approved by the Food and Drug Administration (FDA) for the treatment of influenza virus infection. In preclinical studies, zanamivir, like our novel anti-parainfluenza virus agents, was administered intranasally to mice. Depending on the strain of influenza virus used, the range of dosages of zanamivir that prevented death in 100% of mice whose treatment began 4 h before inoculation with virus was 1 to 10 mg/kg per day (11, 29, 31). The similar level of in vivo efficacy for BCX 2798 when tested against rSV(hHN) in the 4-h pretreatment model indicated the potency of BCX 2798 as an inhibitor of hPIV-1. Like zanamivir and oseltamivir, BCX 2798 and BCX 2855 reduced the level of pathogenic virus in the mouse lungs. This reduction suggests that the inhibition of parainfluenza virus HN is essential to prevent lethal infection.
BCX 2798 and BCX 2855 were active only when they were administered intranasally prior to parainfluenza virus infection. None of the mice survived the lethal challenge with rSV(hHN) virus when the administration of these inhibitors began 24 h after infection. Our finding is similar to the earlier observations that the quantity of the viral challenge dose and the timing of treatment with inhibitors of influenza virus NA are extremely critical in determining the effectiveness of the inhibitors in preventing death (2, 29, 31). The effectiveness of the influenza virus NA inhibitors was significantly reduced when drugs were administered 48 h after lethal virus challenge. The ineffectiveness of the parainfluenza virus HN inhibitors in the delayed (24-h) treatment model could be attributed to the facts that the titers of rSV(hHN) in the lungs reach their peak at this time point and that the virus has spread throughout the lungs before the beginning of therapy. The load of rSV(hHN) in mouse lungs 24 h after infection might have been too high to allow BCX 2798 or BCX 2855 to effectively control the infection.
Overall, the results of our in vivo experiments provide strong evidence of the efficacy of the HN inhibitors BCX 2798 and BCX 2855 in limiting parainfluenza virus infections.
Acknowledgments
This study was supported by research grant 345501 from BioCryst Pharmaceuticals, by research grant 38956 from National Institute of Allergy and Infectious Diseases, by a Cancer Center Support Grant (CA 21765), and by the American Lebanese Syrian Associated Charities.
We thank Ruth Ann Scroggs and Amy Martin for technical assistance and Julia Cay Jones for editing the manuscript.
REFERENCES
- 1.Appell, L. H., R. M. Kovatch, J. M. Reddecliff, and P. J. Gerone. 1971. Pathogenesis of Sendai virus infection in mice. Am. J. Vet. Res. 32:1835-1841. [PubMed] [Google Scholar]
- 2.Bianta, S., C. D. Parker, S. L. Ananth, L. L. Horn, K. Andries, P. Chand, P. L. Kotian, A. Denghani, Y. El-Kattan, T. Lin, T. L. Hutchison, J. A. Montogomery, D. L. Kellog, and Y. S. Babu. 2001. Comparison of the anti-influenza activity of RWJ-270201 with those of oseltamivir and zanamivir. Antimicrob. Agents Chemother. 45:1162-1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bousse, T., T. Takimoto, W. L. Gorman, T. Takahashi, and A. Portner. 1994. Regions on the hemagglutinin-neuraminidase proteins of human parainfluenza virus type-1 and Sendai virus important for membrane fusion. Virology 204:506-514. [DOI] [PubMed] [Google Scholar]
- 4.Bousse, T., T. Takimoto, T. Matrosovich, and A. Portner. 2001. Two regions of the P protein are required to be active with the L protein for human parainfluenza virus type 1 RNA polymerase activity. Virology 283:306-314. [DOI] [PubMed] [Google Scholar]
- 5.Chanock, R. M., B. R. Murphy, and P. L. Collins. 2001. Parainfluenza viruses, p. 1341-1379. In D. M. Knipe and P. M. Howley (ed.), Fields virology, 4th ed. Lippincott/The Williams & Wilkins Co., Philadelphia, Pa.
- 6.Collier, A. M., and W. A. Clyde, Jr. 1977. Model systems for studying the pathogenesis of infections causing bronchiolitis in man. Pediatr. Res. 11:243-246. [PubMed] [Google Scholar]
- 7.Cox, D. R., and D. Oakes. 1984. Analysis of survival data. Chapman & Hall, London, United Kingdom.
- 8.Crennell, S., T. Takimoto, A. Portner, and G. Taylor. 2000. Crystal structure of the multifunctional paramyxovirus hemagglutinin-neuraminidase. Nat. Struct. Biol. 7:1068-1074. [DOI] [PubMed] [Google Scholar]
- 9.Degre, M., and H. Rollag, Jr. 1980. Pathogenesis of Sendai virus infection in mice: on the possible role of interferon on the development of disease. Acta Pathol. Microbiol. Scand. 88:177-181. [PubMed] [Google Scholar]
- 10.Du Bridge, R. Tang, H. C. Hsia, P. M. Leong, J. H. Miller, and M. P. Calos. 1987. Analysis of mutation in human cells by using an Epstein-Barr virus shuttle system. Mol. Cell. Biol. 6:379-387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gubareva, L. V., C. R. Penn, and R. G. Webster. 1995. Inhibition of replication of avian influenza viruses by neuraminidase inhibitor 4-guanidino-2,4-dideoxy-2,3-dehydro-N-acetylneuraminic acid. Virology 212:323-330. [DOI] [PubMed] [Google Scholar]
- 12.Hayden, F. G., A. D. Osterhaus, J. J. Treanor, D. M. Fleming, F. Y. Aoki, K. G. Nicholson, A. M. Bohnen, H. M. Hirst, O. Keene, K. Wightman, et al. 1977. Efficacy and safety of the neuraminidase inhibitor zanamovir in the treatment of influenza virus infections. N. Engl. J. Med. 337:874-880. [DOI] [PubMed] [Google Scholar]
- 13.Hayden, F. G., J. J. Treanor, R. S. Fritz, M. Lobo, R. F. Betts, M. Miller, N. Kinnersley, R. G. Mills, P. Ward, and S. E. Straus. 1999. Use of the oral neuraminidase inhibitor oseltamivir in experimental human influenza: randomized controlled trials for prevention and treatment. JAMA 282:1240-1246. [DOI] [PubMed] [Google Scholar]
- 14.Henrickson, K. J., S. M. Kuhn, and L. L. Savatski. 1994. Epidemiology and cost of infection with human parainfluenza virus types 1 and 2 in young children. Clin. Infect. Dis. 18:770-779. [DOI] [PubMed] [Google Scholar]
- 15.Horvath, C. M., R. G. Paterson, M. A. Shaughnessy, R. Wood, and R. A. Lamb. 1992. Biological activity of paramyxovirus fusion protein: factors influencing formation of syncytia. J. Virol. 66:4564-4569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hu, X., R. Ray, and R. W. Compans. 1992. Functional interaction between the fusion protein and hemagglutinin-neuraminidase of human parainfluenza viruses. J. Virol. 66:1528-1534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kaplan, E. L., and P. Meier. 1958. Nonparametric estimation from incomplete observations. J. Am. Stat. Assoc. 53:457-481. [Google Scholar]
- 18.Kato, A., Y. Sakai, T. Shioba, T. Kondo, M. Nakanishi, and Y. Nagai. 1996. Initiation of Sendai virus multiplication from transfected cDNA or RNA with negative or positive sense. Genes Cells 1:569-579. [DOI] [PubMed] [Google Scholar]
- 19.Kim, C. U., W. Lew, M. A. Williams, H. Wu, L. Zhang, X. Chen, P. A. Escarpe, D. B. Mendel, W. C. Laver, and R. C. Stevens. 1998. Structure-activity relationship studies of novel carbocyclic influenza neuraminidase inhibitors. J. Med. Chem. 41:2451-2460. [DOI] [PubMed] [Google Scholar]
- 20.Knott, A. M., C. E. Long, and C. B. Hall. 1994. Parainfluenza viral infections in pediatric outpatients: seasonal patterns and clinical characteristics. Pediatr. Infect. Dis. J. 13:269-273. [DOI] [PubMed] [Google Scholar]
- 21.Lamb, R. A., and D. Kolakofsky. 1996. The paramyxoviruses, p. 577-604. In B. N. Fields, D. M. Knipe, and P. M. Howley (ed.), Fields virology, 3rd ed. Lippincott-Raven Publishers, Philadelphia, Pa.
- 22.Markwell, M. A. K. 1991. New frontiers opened by the exploration of host cell receptors, p. 407-425. In D. W. Kingsbury (ed.), The paramyxoviruses. Plenum Press, Inc., New York, N.Y.
- 23.Marx, A., T. J. Torok, R. C. Holman, M. J. Clarke, and L. J. Anderson. 1997. Pediatric hospitalization for croup (laryngotracheobronchitis): biennial increases associated with human parainfluenza virus 1 epidemics. J. Infect. Dis. 176:1423-1427. [DOI] [PubMed] [Google Scholar]
- 24.Mascoli, C. C., D. P. Metzgar, E. J. Larson, A. A. Fuscaldo, and T. A. Gower. 1975. An animal model for studying infection and immunity to and attenuation of human parainfluenza viruses. Dev. Biol. Stand. 28:414-421. [PubMed] [Google Scholar]
- 25.Mendel, D. B., C. Y. Tai, P. A. Escarpe, W. Li, R. W. Sidwell, J. H. Huffman, C. Sweet, K. J. Jakeman, S. Merson, S. A. Lacy, N. Lew, M. A. Williams, L. Zhang, M. S. Chen, N. Bischofberger, and C. U. Kim. 1998. Oral administration of a prodrug of the influenza virus neuraminidase inhibitor GS 4071 protects mice and ferrets against influenza infection. Antimicrob. Agents Chemother. 42:640-646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Murphy, T. F., E. J. Dubovi, and W. A. Clyde, Jr. 1981. The cotton rat as an experimental model of human parainfluenza virus 3 disease. Exp. Lung Res. 2:97-109. [DOI] [PubMed] [Google Scholar]
- 27.Potier, M., L. Mameli, M. Belishem, L. Dallaire, and S. B. Melancon. 1979. Fluorometric assay of neuraminidase with a sodium (4-methylumbelliferyl-α-d-N-acetylneuraminate) substrate. Anal. Biochem. 94:287-296. [DOI] [PubMed] [Google Scholar]
- 28.Reed, G., P. H. Jewett, J. Thompson, S. Tollefson, and P. F. Wright. 1997. Epidemiology and clinical impact of parainfluenza virus infections in otherwise healthy infants and young children <5 years old. J. Infect. Dis. 175:807-813. [DOI] [PubMed] [Google Scholar]
- 29.Ryan, D. M., J. Ticehurst, M. H. Dempsey, and C. R. Penn. 1994. Inhibition of influenza virus replication in mice by GG167 (4-guanidino-2,4-dideoxy-2,3-dehydro-N-acetylneuraminic acid) is consistent with extracellular activity of viral neuraminidase (sialidase). Antimicrob. Agents Chemother. 38:2270-2275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.SAS Institute. 1999. SAS/STAT user's guide, version 8, vol. 2. SAS Institute, Inc., Cary, N.C.
- 31.Sidwell, R. W., J. H. Huffman, D. L. Barnard, K. W. Bailey, M.-H. Wong, A. Morrison, T. Synergaard, and C. U. Kim. 1998. Inhibition of influenza virus infection in mice by GS4104, an orally effective influenza virus neuraminidase inhibitor. Antivir. Res. 37:107-120. [DOI] [PubMed] [Google Scholar]
- 32.Stone-Hulslander, J., and T. G. Morrison. 1997. Detection of an interaction between the HN and F proteins in Newcastle disease virus-infected cells. J. Virol. 71:6287-6295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Takimoto, T., G. L. Taylor, S. L. Crennell, R. A. Scroggs, and A. Portner. 2000. Crystallization of Newcastle disease virus hemagglutinin-neuraminidase glycoprotein. Virology 270:208-214. [DOI] [PubMed] [Google Scholar]
- 34.Takimoto, T., G. L. Taylor, H. C. Connaris, S. J. Crennell, and A. Portner. 2002. Role of the hemagglutinin-neuraminidase protein in the mechanism of paramyxovirus-cell membrane fusion. J. Virol. 76:13028-13033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tao, T., F. Davoodi, C. J. Cho, M. H. Skiadopoulos, A. P. Durbin, P. L. Collins, and B. R. Murphy. 2000. A live attenuated recombinant chimeric parainfluenza virus (PIV) candidate vaccine containing the hemagglutinin-neuraminidase and fusion glycoproteins of PIV1 and the remaining proteins from PIV3 induces resistance to PIV1 even in animals immune to PIV3. Vaccine 18:1359-1366. [DOI] [PubMed] [Google Scholar]
- 36.Taylor, N. R., and M. von Itzstein. 1994. Molecular modeling studies on ligand binding to sialidase from influenza virus and the mechanism of catalysis. J. Med. Chem. 37:616-624. [DOI] [PubMed] [Google Scholar]
- 37.Tennant, J. R. 1964. Evaluation of the trypan blue technique for determination of cell viability. Transplantation 2:685-694. [DOI] [PubMed] [Google Scholar]
- 38.Thompson, S. D., W. G. Laver, K. G., Murti, and A. Portner. 1988. Isolation of a biologically active soluble form of the hemagglutinin-neuraminidase protein of Sendai virus. J. Virol. 62:4653-4660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Varghese, J. N., W. G. Laver, and P. M. Colman. 1983. Structure of the influenza virus glycoprotein antigen neuraminidase at 2.9 Å resolution. Nature 303:35-40. [DOI] [PubMed] [Google Scholar]
- 40.Venables, W. N., and B. D. Ripley. 1997. Modern applied statistics, p. 223-242. Springer-Verlag, New York, N.Y.
- 41.von Itzstein, M., W.-Y. Wu, G. B. Kok, M. S. Pegg, J. C. Dyason, B. Jin, T. van Plan, M. L. Smythe, H. F. White, S. W. Oliver, P. M. Colman, J. N. Varghese, D. M. Ryan, J. M. Woods, R. C. Bethell, V. J. Hothman, J. M. Cameron, and C. R. Penn. 1993. Rational design of potent sialidase-based inhibitors of influenza virus replication. Nature 363:418-423. [DOI] [PubMed] [Google Scholar]
- 42.Woods, J. M., R. C. Bethell, J. A. V. Coates, N. Healy, S. A. Hiscox, B. A. Pearson, D. M. Ryan, J. Ticehurst, J. Tilling, S. M. Walcott, and C. R. Renn. 1993. 4-Guanidino-2,4-dideoxy-2,3-dehydro-N-acetylneuraminic acid is a highly effective inhibitor both of the sialidase (neuraminidase) and growth of a wide range of influenza A and B viruses in vitro. Antimicrob. Agents Chemother. 37:1473-1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yao, Q., X. Hu, and R. W. Compans. 1997. Association of the parainfluenza virus fusion and hemagglutinin-neuraminidase glycoproteins on cell surfaces. J. Virol. 71:650-656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zurcher, C., J. D. Burek, M. C. Van Nunen, and S. P. Meihuizen. 1977. A naturally occurring epizootic caused by Sendai virus in breeding and aging rodent colonies. I. Infection in the mouse. Lab. Anim. Sci. 27:955-962. [PubMed] [Google Scholar]