Abstract
Radioisotopic assays involve expense, multistep protocols, equipment, and radioactivity safety requirements which are problematic in high-throughput drug testing. This study reports an alternative, simple, robust, inexpensive, one-step fluorescence assay for use in antimalarial drug screening. Parasite growth is determined by using SYBR Green I, a dye with marked fluorescence enhancement upon contact with Plasmodium DNA. A side-by-side comparison of this fluorescence assay and a standard radioisotopic method was performed by testing known antimalarial agents against Plasmodium falciparum strain D6. Both assay methods were used to determine the effective concentration of drug that resulted in a 50% reduction in the observed counts (EC50) after 48 h of parasite growth in the presence of each drug. The EC50s of chloroquine, quinine, mefloquine, artemisinin, and 3,6-bis-ɛ-(N,N-diethylamino)-amyloxyxanthone were similar or identical by both techniques. The results obtained with this new fluorescence assay suggest that it may be an ideal method for high-throughput antimalarial drug screening.
The global scope of malaria and the spread of drug-resistant Plasmodium falciparum make the need for improved therapy undeniable (4). Assessment of both existing drugs and new antimalarials, alone or in combination, requires reliable methods for high-throughput testing. For decades, antimalarial drug effects have been measured in vitro by quantifying parasite uptake of radioactive substrates as a measure of growth and viability in the presence of the test drug (2, 3). [3H]hypoxanthine is the most widely used radiolabel, but because it requires purine starvation prior to the assay, the [3H]ethanolamine assay is favored by our laboratory. While these methods are accurate and reliable, they rely on relatively expensive radioisotopes and multistep procedures that become increasingly problematic and impractical as the volume of testing increases. We report on the development of an alternative, fluorescence-based in vitro assay for use for the high-throughput screening of the activities of drugs against P. falciparum. The goals for the assay included simplicity, cost savings, robust performance, applicability to automated analysis, and speed.
The principle behind the assay is the contrast between host erythrocytes, which lack DNA and RNA, and the malaria parasites, which do not and which are thus readily stained with dyes that show enhanced fluorescence in the presence of nucleic acids (8). Fluorescence-based in vitro antimalarial assays have been developed previously (1, 12) but have required complex, multistep protocols or additional equipment not amenable to rapid, high-throughput use. This report establishes and validates a facile, one-step method that uses the same principle.
MATERIALS AND METHODS
Chloroquine diphosphate, quinine sulfate, artemisinin, and saponin were purchased from Sigma Aldrich Chemical Company (St. Louis, Mo.); mefloquine was obtained from Walter Reed Army Institute for Research (Silver Spring, Md.); and 3,6-bis-ɛ-(N,N-diethylamino)-amyloxyxanthone (C5) (6) was obtained from Interlab Corporation (Lake Oswego, Oreg.). SYBR Green I nucleic acid staining dye (10,000× stock concentration) was purchased from Molecular Probes, Inc. (Eugene, Oreg.), stored frozen at −20°C, and freshly thawed before use. Lysis buffer consisted of Tris (20 mM; pH 7.5), EDTA (5 mM), saponin (0.008%; wt/vol), and Triton X-100 (0.08%; vol/vol), which was prepared in advance and stored at room temperature. [3H]ethanolamine was purchased from American Radiolabeled Chemicals (St. Louis, Mo.). P. falciparum strain D6 was obtained from Dennis E. Kyle of the Walter Reed Army Institute for Research. Human erythrocytes and frozen serum were obtained from Lampire Biological Laboratories (Pipersville, Pa.).
Cultivation of P. falciparum and plate setup.
Prior to the experiments, the parasites were cultivated by the method of Trager and Jensen (13), with minor modifications. Cultures were maintained in fresh group A-positive human erythrocytes suspended at 2% hematocrit in RPMI 1640 containing 10% human serum, 3 g of glucose per liter, 45 μg of hypoxanthine per liter, and 50 μg of gentamicin per liter. Flasks were incubated at 37°C under a gas mixture of 5% O2, 5% CO2, and 90% N2. Every 3 to 4 days, infected erythrocytes were transferred into fresh complete medium with uninfected erythrocytes. The stock culture was synchronized with 5% sorbitol, as described previously (7), and then approximately 96 h later, the level of parasitemia was determined by light microscopy by counting of a minimum of 500 erythrocytes on a Giemsa-stained thin blood smear. Parasites were noted to be late-ring and early trophozoites, with no evident schizonts. The stock culture was then diluted with complete medium and normal human erythrocytes to a starting 4% hematocrit and 0.5% parasitemia. For each test drug, plates for both assay methods were prepared in parallel with the same cells and medium.
For the [3H]ethanolamine assay, 180 μl of the cell suspension was dispensed into each test well of a 96-well plate. For the fluorescence assay, 90 μl of the cell suspension was put into each test well; in addition, several wells containing nonparasitized erythrocytes at 4% hematocrit served as reference controls.
Stock solutions of the test drugs were prepared at a concentration of 10 mM (chloroquine in water, all other drugs in ethanol), serially diluted in complete medium, and dispensed into triplicate test wells to yield final concentrations ranging from 0 to 10−5 M. Final well volumes were 200 and 100 μl for the [3H]ethanolamine and fluorescence assays, respectively. The plates were then incubated as described above.
Determination of EC50.
For the [3H]ethanolamine assay, after 24 h of growth, 1 μCi (1 μl) of [3H]ethanolamine with 19 μl of complete medium was added to each well. After an additional 24 h (a total of 48 h of growth), the contents of the plates used for the [3H]ethanolamine assay were harvested by collecting the cells and placing them onto glass-fiber filters with a semiautomated 96-well harvester (Tomac, Orange, Conn.). Radiolabel uptake was quantified by scintillation counting of the filters with a Wallac (Gaithersburg, Md.) 1205 Betaplate counter.
For the fluorescence assay, after 48 h of growth, 100 μl of SYBR Green I in lysis buffer (0.2 μl of SYBR Green I/ml of lysis buffer) was added to each well, and the contents were mixed until no visible erythrocyte sediment remained. After 1 h of incubation in the dark at room temperature, fluorescence was measured with a Cytofluor II fluorescence multiwell plate reader from PerSeptive Biosystems (Framingham, Mass.) with excitation and emission wavelength bands centered at 485 and 530 nm, respectively, and a gain setting equal to 50. By using the accompanying Cytofluor software, the background reading for an empty well was subtracted to yield fluorescence counts for analysis.
Analysis of the counts obtained by both assay methods was performed with Graphpad Prism (San Diego, Calif.) software. The counts were plotted against the logarithm of the drug concentration and curve fitting by nonlinear regression (sigmoidal dose-response/variable slope equation) to yield the drug concentration that produced 50% of the observed decline from the maximum counts in the drug-free control wells (EC50).
Freeze-thaw effect.
After the fluorescence measurements described above were obtained, to determine whether a freeze-thaw cycle would substantively change the results, the fluorescence plates were placed in the dark at −20°C overnight and then left in the dark at room temperature for 3 h, after which the fluorescence was again measured and analyzed as described above.
Assessment of fluorescence linearity.
In a separate experiment, a stock culture of P. falciparum strain D6 was synchronized by the sorbitol method and judged to be free of schizonts by microscopic examination of a Giemsa-stained slide. The culture was then diluted serially with nonparasitized erythrocytes and complete medium to yield a hematocrit of 4% and parasitemia levels ranging from 0 to 13.5%. Triplicate wells containing 100 μl of each dilution were prepared in a 96-well plate, immediately followed by the addition of 100 μl of SYBR Green I in lysis buffer (0.2 μl of SYBR Green I/ml of lysis buffer), mixing, and 1 h of subsequent incubation in the darkness at room temperature. Fluorescence was determined as described above. The background fluorescence for the empty well and the nonparasitized erythrocytes was subtracted, and the counts were then plotted and analyzed by linear regression.
RESULTS
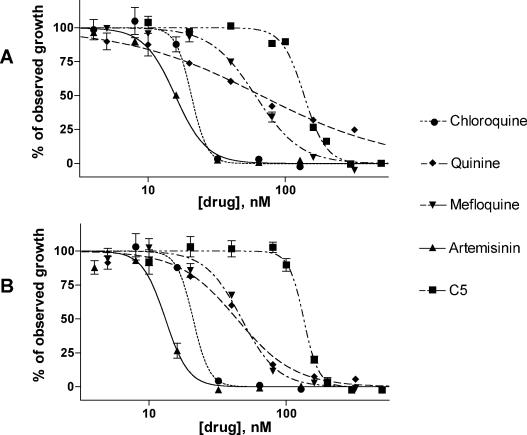
The EC50s of the drugs tested by the fluorescence and the radioisotopic assay methods were similar or identical (Table 1). Figure 1 shows the similarities between the dose-response curves for both methods and that the variability in the results between triplicate fluorescence wells was low, with no aberrant results evident over the range of drug concentrations tested. After a single freeze-thaw cycle, the numbers of fluorescence units increased nearly 10-fold, but without substantive changes in the EC50s. Although the results were not identical in each case, the results of the qualitative drug comparisons were the same by the radioisotopic or the fluorescence assay, with or without a freeze-thaw step.
TABLE 1.
Comparison of the EC50s of the test drugs by fluorescence and radioisotopic assays
| Test drug | EC50 (nM)a
|
||
|---|---|---|---|
| Fluorescence method
|
[3H]ethanolamine method | ||
| At 1 h | After freezing-thawing | ||
| Chloroquine | 21 ± 1.7 | 20 ± 3.0 | 21 ± 1.7 |
| Quinine | 62 ± 12.9 | 41 ± 3.7 | 44 ± 3.1 |
| Mefloquine | 61 ± 3.0 | 32 ± 5.0 | 47 ± 3.0 |
| Artemisinin | 16 ± 0.4 | 15 ± 1.8 | 13 ± 0.8 |
| C5 | 137 ± 3.6 | 118 ± 4.9 | 134 ± 5.8 |
Values are means ± standard errors of the means of a single parallel determination run in triplicate.
FIG. 1.
Comparison of test drug dose-response curves determined by the fluorescence (A) and radioisotope (B) assays. Values are normalized by use of the upper and the lower plateaus of the best-fit curve as 100 and 0% responses, respectively, and are plotted as the means ± standard errors of the means for triplicate wells.
Figure 2 illustrates the relationship between the levels of parasitemia and the numbers of fluorescence units determined by the study assay method. A well-correlated, linear relationship (r2 = 0.9763) is evident through and beyond the range of parasitemia likely to be encountered by use of the assay starting conditions.
FIG. 2.
Relationship between parasitemia and measured fluorescence. Values are plotted as the means ± standard errors of the means for triplicate wells after subtraction of the background fluorescence for nonparasitized erythrocytes.
DISCUSSION
This report clearly demonstrates the feasibility of a fluorescence-based assay for high-throughput screening of antimalarials. The results of this malaria SYBR Green I-based fluorescence (MSF) assay were in agreement with those of the [3H]ethanoloamine assay, and the level of fluorescence was shown to have a direct, linear correlation with the level of parasitemia. The drugs tested in this study are varied in their structures, mechanistic actions, and potencies; and the range of parasitemia tested for fluorescence linearity includes the entire realistic range encountered in a 48-h assay under our starting conditions. On the basis of this side-by-side comparison, these observations suggest that the assay will be broadly applicable, and that has been our experience. In fact, in our laboratory the MSF assay has replaced assays that use radioisotopes and has been used to perform several hundred assays involving a wide range of experimental conditions.
The determination of EC50s by the MSF assay only requires a comparison of the relative nucleic acid content in an experiment, without the multistep technique that would be required for true DNA quantitation or the tight controls that would be needed for comparison of the levels of absolute growth between different experiments. Thus, the curve in Fig. 2 should not be treated as a standard curve, since even subtle differences in experimental conditions or the detection device used would change the slope and intercept of the relationship line. Fortunately, due to the method by which EC50 is determined, such changes alone do not alter the EC50, although they may shift the growth curves up or down or change the difference between the “no-effect” upper plateau of the curve and the “full-effect” lower plateau of the curve. This evidently accounts for the similarities of the EC50s with and without a freeze-thaw cycle. The 10-fold increase in fluorescence observed after a freeze-thaw cycle, which we speculate is related to additional cell lysis, shifts the entire growth curve but does not change EC50 substantively. Similarly, although we have selected 1 h as a standard delay prior to reading of the plate, we find that the increasing fluorescence which also develops over time at room temperature results in similar EC50s whether the EC50s are determined immediately or at 1, 2, 6, or 24 h after dye addition.
It is expected, and it is certainly our experience, that even subtle changes in starting hematocrit levels, parasitemia levels, parasite developmental stage, and other variables will change the assessment of absolute growth by use of fluorescence readings; but this is also the case with any method of P. falciparum growth measurement. Side-by-side comparisons of the EC50s of the drugs eliminate this concern and are as important with the MSF assay as they would be with any other method. The conditions described in this report are those that we predict will be broadly applicable, with consistently acceptable signal-to-noise results in a one-step 48-h assay under a variety of conditions, including assays with different P. falciparum strains at different developmental stages and assays with variations in culture conditions. Even though this report describes specific conditions, our experience has shown that the assay is adaptable to a wide range of conditions. During development of the MSF assay, successful assays were performed with starting parasitemia levels ranging from 0.2 to 2%, hematocrit levels ranging from 1 to 4%, asynchronous and synchronized cultures, growth durations of 24 h (schizont maturation assay) to 72 h, and plate reading at a variety of intervals after addition of the dye. We have used the MSF assay to study the effects of single drugs and drug combinations against both the chloroquine-sensitive D6 strain and the chloroquine-resistant W2 strain. Additional study will be needed to establish whether improved dyes, lysis buffers, starting parasitemia or hematocrit levels, or other adjustments can further optimize the method; but this study and our experience clearly validate the approach.
SYBR Green I is one of the most sensitive stains available for the detection of double-stranded DNA (9). Single-stranded DNA and RNA are also detected, although at approximately 10-fold lower sensitivities (14). It is typically used in gel electrophoresis methods and is 50 to 100 times more sensitive than ethidium bromide as a nucleic acid-specific fluorochrome. Its sensitivity for nucleic acid detection results both from a remarkable affinity for DNA and from the marked fluorescence enhancement caused by the SYBR Green I-nucleic acid interaction (11). When SYBR Green I is bound to DNA, its maximal fluorescence excitation peak is at 497 nm (with minor peaks at 290 and 380 nm), with emission centered at 520 nm (MP 07567, SYBR Green I nucleic acid gel stain product information, 2001; Molecular Probes, Inc.).
The lysis buffer was included and formulated in an attempt to achieve the broadest possible utility and the greatest speed of the assay. Specifically, we sought to allow discrimination of small differences in samples with very low signal-to-background fluorescence values (i.e., low levels of parasitemia) by use of a widely available technology and without the need for a freeze-thaw cycle. We found that newer fluorometers, which allow very precise control of excitation and emission wavelengths and which have great sensitivities, were able to detect fluorescence without the use of lysis buffer. Since many laboratories, particularly in less affluent centers, would be expected to lack such equipment, we developed the assay using commonly available equipment and settings. The resulting lysis buffer cocktail uses saponin and dilute detergent to facilitate an adequate dye-nucleic acid interaction and the detection of fluorescence. During lysis buffer development, the use of higher concentrations of saponin resulted in unacceptably high background fluorescence levels and the use of higher concentrations of Triton X-100 slightly diminished the level of fluorescence detected, perhaps due to increased micelle formation. The addition of sodium deoxycholate detergent in an experimental trial caused unacceptable background fluorescence, whereas the addition of sodium dodecyl sulfate substantially reduced all fluorescence detection. Interestingly, we have noted by fluorescence microscopy that erythrocyte lysis and parasite lysis are not complete at 1 h under our experimental conditions, yet parasite fluorescence is evident in all morphological stages. Although the increase in fluorescence after a freeze-thaw step further indicates that the fluorescence at 1 h is far from maximal, the addition of this step does not appear to change the EC50s significantly.
The advantages of the MSF assay are evident. Simplicity is improved by elimination of the radioisotope-loading step, harvesting of the contents of the plate onto a glass-fiber filter mat, and preparation of the isotope filter mat for scintillation counting. The special safe-handling, record-keeping, and disposal requirements for radioisotopes are eliminated. When only the cost of [3H]ethanolamine is compared with the cost of the MSF assay reagents, the cost to process the 96-well plate decreases from $60.00 to $0.60 per plate. Additional savings result from decreased technician time and the elimination of harvesting filters, filter bags, and scintillation fluid. Since reagent addition, mixing, and incubation can be done in a single step without any specimen transfer, the method seems well suited for high-throughput, semiautomated or automated techniques.
The recent development of P. falciparum isolates expressing green fluorescent protein (GFP) also offers the possibility of very simple growth assay methods (5), but this method requires the induction and monitoring of stable GFP expression in each clone studied, prior to drug evaluation. Given the enormous number of known and unknown parasite strains, the technical limitations of many laboratories, and the irrelevance of the GFP method for the study of patient-derived strains in the field, there is clearly a role for a more broadly applicable method, such as the MSF assay.
One possible limitation of this method is drug interference with fluorescence excitation and emission over a range of fluorescence excitation and emission similar to that of SYBR Green I. Anecdotally, the MSF assay proved successful with the one such drug that we have tested (results not shown), but for this reason, the possibility remains that the method may not be applicable to some drugs. A second theoretical limitation involves drugs which interact with nucleic acids and directly compete with SYBR Green I binding, although this possibility seems remote, given the extraordinary affinity of SYBR Green I for nucleic acids.
Special safeguards are advisable when the MSF assay or any assay involving dyes which bind to DNA is used. SYBR Green I appears to be significantly less mutagenic than ethidium bromide (10), but it is prudent to wear disposable gloves at all times when the dye reagent is handled and to incinerate all assay plates. In addition, when excess dye reagent is disposed, extra safety can be accomplished by filtration of the reagent through activated charcoal, followed by incineration of the charcoal. One gram of activated charcoal will adsorb the amount of dye used in more than 100 full 96-well plates.
In conclusion, this report demonstrates the feasibility of a simple, rapid, inexpensive fluorescence-based assay for use in high-throughput antimalarial drug screening. Determination of the ultimate role for this technique in antimalarial testing will require additional study. Further optimization and realization of unforeseen limitations will determine whether fluorescence-based assays will be an adjunct to or a replacement for radioisotope-based assays and whether fluorescence-based assays can be adapted to field settings where radioisotope use is not practical.
ADDENDUM IN PROOF
Subsequent to acceptance of this manuscript, an alternative fluorescence-based assay was reported (Y. Corbett et al., Am. J. Trop. Med. Hyg. 70:119-124, 2004) which utilizes PicoGreen as the fluorophore and a 2% Triton-X detergent lysis agent. Our preliminary comparison indicates that the MSF assay is more sensitive and less expensive. In addition, PicoGreen, like ethidium bromide, is an intercalating dye and its safety will need to be compared to that of SYBR Green I.
REFERENCES
- 1.Davis, W., C. Wyatt, M. Hamilton, and W. Goff. 1992. A rapid, reliable method of evaluating growth and viability of intraerythrocytic protozoan hemoparasites using fluorescence flow cytometry. Mem. Inst. Oswaldo Cruz 87:235-239. [DOI] [PubMed] [Google Scholar]
- 2.Desjardins, R., G. Canfield, J. Haynes, and J. Chulay. 1979. Quantitative assessment of antimicrobial activity in vitro by a semiautomated microdilution technique. Antimicrob. Agents Chemother. 16:710-718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Elabbadi, N., M. Ancelin, and H. Vial. 1992. Use of radioactive ethanolamine incorporation into phospholipids to assess in vitro antimalarial activity by the semiautomated microdilution technique. Antimicrob. Agents Chemother. 36:50-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guerin, P., P. Olliaro, P. Druilhe, R. Laxminarayan, F. Binka, W. Kilama, N. Ford, and N. White. 2002. Malaria: current status of control, diagnosis, treatment, and a proposed agenda for research and development. Lancet Infect. Dis. 2:564-573. [DOI] [PubMed] [Google Scholar]
- 5.Kadekoppala, M., K. Kline, T. Akompong, and K. Haldar. 2000. Stable expression of a new chimeric fluorescent reporter in the human malaria parasite Plasmodium falciparum. Infect. Immun. 68:2328-2332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kelly, J., R. Winter, D. Peyton, D. Hinrichs, and M. Riscoe. 2002. Optimization of xanthones for antimalarial activity: the 3,6-bis-ω-diethylaminoalkoxyxanthone series. Antimicrob. Agents Chemother. 46:144-150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lambros, C., and J. Vanderberg. 1979. Synchronization of Plasmodium falciparum erythrocytic stages in culture. J. Parasitol. 65:418-420. [PubMed] [Google Scholar]
- 8.Makler, M., L. Ries, J. Ries, R. Horton, and D. Hinrichs. 1991. Detection of Plasmodium falciparum infection with the fluorescent dye, benzothiocarboxypurine. Am. J. Trop. Med. Hyg. 44:11-16. [DOI] [PubMed] [Google Scholar]
- 9.Rengarajan, K., S. Cristol, M. Mehta, and J. Nickerson. 2002. Quantifying DNA concentrations using fluorometry: a comparison of fluorophores. Mol. Vis. 8:416-421. [PubMed] [Google Scholar]
- 10.Singer, V., T. Lawlor, and S. Yue. 1999. Comparison of SYBR Green I nucleic acid gel stain mutagenicity and ethidium bromide mutagenicity in the Salmonella/mammalian microsome reverse mutation assay (Ames test). Mutat. Res. 439:37-47. [DOI] [PubMed] [Google Scholar]
- 11.Skeidsvall, J., and P. Veland. 1995. Analysis of double-stranded DNA capillary electrophoresis laser-induced fluorescence detection using the monomeric dye SYBR Green I. Anal. Biochem. 231:359-365. [DOI] [PubMed] [Google Scholar]
- 12.Smeijsters, L., N. Zijlstra, F. Franssen, and J. Overdulve. 1996. Simple, fast, and accurate fluorometric method to determine drug susceptibility of Plasmodium falciparum in 24-well suspension cultures. Antimicrob. Agents Chemother. 40:835-838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Trager, W., and J. Jensen. 1976. Human malaria parasites in continuous culture. Science 193:673-675. [DOI] [PubMed] [Google Scholar]
- 14.Vitzthum, F., G. Geiger, H. Bisswanger, H. Brunner, and J. Bernhagen. 1999. A quantitative fluorescence-based microplate assay for the determination of double-stranded DNA using SYBR Green I and standard ultraviolet transilluminator gel imaging system. Anal. Biochem. 276:59-64. [DOI] [PubMed] [Google Scholar]