Abstract
New human cytomegalovirus (HCMV) therapies with novel mechanisms of action are needed to treat drug-resistant HCMV that arises during therapy with currently approved agents. 2-Bromo-5,6-dichloro-1-β-d-ribofuranosyl-1H-benzimidazole (BDCRB) is an effective anti-HCMV agent with a novel mechanism of action, but problems with in vivo stability preclude clinical development. A d-ribopyranosyl derivative of BDCRB, GW275175X, displays similar antiviral activity without the in vivo stability problems. We present an initial description of the activity of GW275175X against HCMV, other herpesviruses, and selected nonherpesviruses. In addition, we show that it acts as a DNA maturation inhibitor like the parent compound, BDCRB, rather than via the mechanisms of action of 1263W94 or any anti-HCMV drugs approved for marketing. GW275175X is a promising candidate for clinical development as an anti-HCMV agent.
Human cytomegalovirus (HCMV) infection is widespread in the general population, being present in from 40 to 100% of different populations in the United States (for a review, see Sia and Patel [21]). Primary infection is generally inconsequential, resulting occasionally in mononucleosis-like symptoms before persistent infection is established (8). Longer-term HCMV infection generally persists without obvious viral replication and viral shedding, with occasional recurrences of viral replication accompanied by virus release but without symptomatic disease. However, in some individuals—e.g., a fetus in utero, a patient undergoing transplant operations, or a patient with an immune-disabling disease such as AIDS—HCMV primary infection, reactivation of persistent infection, or reinfection can cause morbidity or mortality through disease in a number of organ systems.
Current HCMV disease therapies include ganciclovir (GCV) and its orally bioavailable prodrug valganciclovir, foscarnet (PFA), and cidofovir (CDV). Significant toxicities are associated with prolonged exposure to these HCMV therapies, including bone marrow toxicity (GCV) and nephrotoxicity (CDV and PFA) (13). The antisense drug fomivirsen is an oligonucleotide that targets the HCMV mRNA encoding the major immediate-early region 2 proteins. Fomivirsen is approved for treatment of HCMV retinitis by intravitreal injection. Fomivirsen treatment may result in various ocular adverse events, such as intraocular inflammation (27).
Therapy for HCMV infections can result in the emergence of drug resistance mutations in the UL54 open reading frame (ORF) encoding the viral DNA polymerase (PFA, GCV, and CDV resistance) or in the UL97 ORF encoding the UL97 protein kinase (GCV resistance). Although resistance can arise via different molecular targets, most of the currently available HCMV drugs share a common mechanism of action, ultimately targeting the HCMV DNA polymerase (7). Resistant strains selected by one drug may have cross-resistance to other HCMV drugs. For example, Chou et al. (4) recently described a clinical isolate resistant to GCV, PFA, and CDV in which the multidrug resistance was mapped to a single deletion in the conserved region of the HCMV DNA polymerase gene. Compounds that do not target the HCMV polymerase should be active against such resistant strains.
2-Bromo-5,6-dichloro-1-β-d-ribofuranosyl-1H-benzimidazole (BDCRB) and its 2-chloro homolog, 2,5,6-trichloro-1-β-d-ribofuranosyl-1H-benzimidazole (TCRB), are nucleoside analogs active against HCMV that were originally synthesized by Townsend et al. (23). Unlike most currently marketed anti-HCMV agents, BDCRB and TCRB do not inhibit viral DNA synthesis, even at concentrations that completely prevent generation of infectious virus (23), but instead exert antiviral activity by inhibition of HCMV DNA maturation (26). Genetic mapping experiments showed that inhibition of viral DNA maturation is mediated by interactions involving the products of the HCMV UL89 (26) and UL56 (17) ORFs. However, clinical development was not pursued after preclinical pharmacokinetic studies demonstrated that both BDCRB and TCRB are cleaved in vivo to produce the less active but more cytotoxic aglycones (6).
Synthetic chemistry efforts were then directed towards improving the in vivo stability of BDCRB (25). Approaches utilized included the synthesis of carbocyclic analogs of benzimidazole nucleosides (24), fluoro-sugar analogs (10), C-nucleoside analogs (9, 11), l-ribofuranosyl sugar analogs (6), and d-ribopyranosyl sugar analogs. These efforts provided two classes of compounds that were pursued as clinical candidates for the treatment of HCMV diseases: (i) compounds such as the l-sugar analogue 5,6-dichloro-2-(isopropylamino)-1-β-l-ribofuranosyl-1H-benzimidazole (1263W94, maribavir), an agent that inhibits HCMV DNA synthesis (2) and nuclear egress (15, 29), and (ii) compounds including d-sugar analogs, represented here by GW275175X (2-bromo-5,6-dichloro-1-β-d-ribopyranosyl-1H-benzimidazole), which also inhibits HCMV activity in vitro. Previous reports on 1263W94 have described in vitro antiviral activity and mechanism of action (2), preclinical toxicology (14), and results of initial phase I and II clinical trials with subjects with HCMV infection (6).
In this report, we present an initial description of the activity of GW275175X against HCMV, other herpesviruses, and selected nonherpesviruses and show that the mechanism of action is like that of the original parent compound, BDCRB, rather than 1263W94 or any other anti-HCMV drug approved for marketing.
MATERIALS AND METHODS
Chemicals and reagents.
The benzimidazole compounds GW275175X, BDCRB, TCRB, and 1263W94 were synthesized at GlaxoWellcome (Research Triangle Park, N.C., now GlaxoSmithKline) and at the University of Michigan (Ann Arbor, Mich.). Phosphonoformic acid (PFA) was obtained from Fluka-AG (Buchs, Switzerland), and CDV was kindly provided by John Martin of Gilead Pharmaceuticals (Foster City, Calif.). GCV and acyclovir (ACV) were synthesized at Burroughs Wellcome (Research Triangle Park, N.C., now GlaxoSmithKline).
Growth media and reagents were obtained from Gibco/Invitrogen Corp. (Carlsbad, Calif.) except as noted. SeaPlaque agarose for plaque reduction assays was from FMC Bioproducts (Rockland, Maine). Radiolabeling of DNA hybridization probes was performed by using [α-32P]dATP from Amersham (Piscataway, N.J.).
HCMV strains and host cell lines.
HCMV laboratory strains used for antiviral assays and mechanism of action studies were the AD169 strain, obtained from the American Type Culture Collection (Rockville, Md.), and the Towne strain, kindly provided by M. F. Stinski of the University of Iowa. The following drug-resistant isolates were obtained from the referenced sources: GDGP53 (22), XbaF (2), 1117r (derived by passage of AD169 with 9-(3-hydroxy-2-phosphonyl-methoxypropyl)-adenine; K. Biron, unpublished data), 2916rA (2), 4760rec (1), ACVrB (derived by passage of AD169 with ACV; Biron, unpublished), and 1038rB (26). Ten clinical isolates of HCMV were obtained from immunocompromised patients from geographically diverse locations who had not yet received treatment for HCMV infection (2).
Human diploid fibroblasts (MRC-5 cells and human foreskin fibroblast [HFF] cells) were obtained from BioWhittaker (Walkersville, Md.) or were isolated from foreskins obtained at circumcision. HCMV strains were grown on MRC-5 monolayers in Eagle's minimal essential medium (MEM) containing 2 mM l-glutamine, 50 U of penicillin G/ml, and 50 μg of streptomycin sulfate/ml, supplemented with 8% fetal bovine serum (FBS) (HyClone Laboratories, Logan, Utah). HFF cells were propagated in MEM with 10% FBS, glutamine, and penicillin-gentamicin.
HCMV antiviral assays. (i) Plaque reduction assays.
Monolayer cultures of MRC-5 cells in 12-well plates were infected in triplicate with 100 PFU of virus per well. Cell culture overlay was made with 1× MEM, 4% FCS, and 0.4% agarose containing compound serial dilutions. Infection proceeded at 37°C for 8 to 9 days until plaques were visible, and the plaques were counted for determination of the 50% infective concentration (IC50) by using Probit (SAS), unless otherwise noted.
(ii) DNA hybridization assays.
MRC-5 cells were seeded in 96-well plates at a concentration of approximately 7.5 × 103 cells/well. DNA hybridization assays were performed as previously described (26). Briefly, HCMV-infected MRC-5 monolayers were lysed and treated with proteinase K in a sodium dodecyl sulfate-containing buffer, extracted with phenol-chloroform, and blotted onto supported nitrocellulose membranes. HCMV DNA was measured by hybridizing the filters with α-32P-labeled HCMV cosmid DNAs, followed by quantitation with a Molecular Dynamics PhosphorImager.
Mechanism of action assays. (i) Compound time of addition assay.
HFF cells were seeded at 12,500 cells per well in 96-well cluster plates and infected 24 h later with HCMV (Towne strain) at a multiplicity of infection (MOI) of 0.5. At infection and every 8 h thereafter, the medium was replaced with either fresh medium or media containing selected virus-inhibitory but nontoxic drug concentrations in duplicate plus 5% FBS. After being incubated at 37°C for 96 h in an atmosphere of 5% CO2, the plates were removed and placed at −80°C for subsequent titer determination. Fresh 96-well plates were seeded with 12,500 HFF cells/well and incubated as above for 24 h. The experimental plates were thawed, the contents were mixed by repeated pipetting, and the virus titer of each suspension was determined by serial dilution on the fresh plates with medium containing 0.5% methylcellulose and 5% FBS. The plaques in the well in each serial dilution that contained from 2 to 10 plaques were counted, and the titer was determined from this result and the amount of dilution.
(ii) Compound time-of-removal assay.
MRC5 cell monolayers were infected with HCMV strain AD169 at an MOI of 0.1 and incubated in the presence of 5 μM BDCRB, 5 μM GW275175X, 10 μM GCV, or 1 μM 1263W94. The supernatants were collected at 48 h postinfection and frozen at −80°C for baseline titers. At 72 h postinfection, the monolayer cultures were washed once with growth medium without antiviral compounds and once with phosphate-buffered saline. Growth medium without antiviral compounds was added. Cells were incubated for 1 h to allow the antiviral compounds to equilibrate from the cells into the medium. The growth medium was replaced with fresh medium and the supernatants were collected at 18, 26, 42, and 52 h postwashout and frozen at −80°C. The titers of the collected supernatants were determined on MRC5 cell monolayers on 96-well plates.
(iii) CHEF electrophoresis.
The cell monolayers were pretreated with the known DNA maturation inhibitor BDCRB to cause precursor high-molecular-weight (HMW) viral DNA to accumulate. The washout of BDRCB and its replacement with DNA synthesis inhibitors allow the generation of genome length viral DNA from the precursor DNA, while replacement with DNA maturation inhibitors continues to block the generation of genome length DNA. MRC5 monolayers were infected at an MOI of 1 in 12-well plates. BDCRB was added to a final concentration of 20 μM, and the cells were incubated at 37°C. After 4 days, BDCRB was removed by replacing the medium, and serially diluted test compound (GW275175X or BDCRB) was added. After 1 day at 37°C, the infected monolayers were collected, embedded in agarose, and lysed in the presence of proteinase K. Viral genomic DNA was separated by clamped homogenous electric field (CHEF) electrophoresis by using a CHEF Mapper apparatus (Bio-Rad) as previously described (26).
RESULTS
Antiviral activity of GW275175X.
The antiviral efficacy of GW275175X was compared to that of GCV and 1263W94 in DNA hybridization assays and plaque reduction assays using the AD169 laboratory strain. In DNA hybridization assays, the mean IC50 of GW275175X was 0.7 ± 0.1 μM (based on 23 assays), compared to previously published mean IC50 values of 0.53 ± 0.04 μΜ for GCV and 0.12 ± 0.01 μΜ for 1263W94 (2). The mean IC50 of GW275175X determined from plaque reduction assays was 1.4 ± 0.1 μΜ (based on four assays), compared to 7.3 ± 1.1 μΜ for GCV and 0.54 ± 0.05 μΜ for 1263W94 (2). Thus, depending on the assay, GW275175X was as active as, or more active than, GCV and was from three- to sevenfold less active than 1263W94.
To determine whether GW275175X would also be effective against clinically relevant viruses, the activity of GW275175X against 10 clinically relevant HCMV isolates (described in reference 2) was measured by using plaque reduction assays. For 1263W94 (data from Biron et al. [2]), the IC50 ranged from 0.12 to 0.56 μΜ, with a median of 0.275 μΜ and a mean of 0.32 ± 0.16 μΜ. For GW275175X, the IC50 ranged from 0.16 to 2.21 μΜ, with a median of 1.31 μΜ and a mean of 1.38 ± 0.60 μΜ. Overall, GW275175X was less active than 1263W94 based on the data used for comparison, with a mean IC50 approximately fourfold greater than that of 1263W94.
The selectivity of GW275175X against other herpesviruses and nonherpesviruses was assessed by assays. Plaque reduction assays found little or no activity against herpes simplex virus types 1 and 2 (HSV-1 and HSV-2) and varicella zoster virus (data not shown). Perhaps the lack of BDCRB activity against other herpesviruses may be associated with the lack within their corresponding ORFs of the acidic residue found at amino acid 344 of the HCMV UL89 ORF (as discussed in reference 26). GW275175X was also not active against bovine viral diarrhea virus, hepatitis B virus, influenza virus, respiratory syncytial virus, SV40, and yellow fever virus (data not shown).
Mechanism-of-action studies. (i) Activity against drug-resistant HCMV strains.
Plaque reduction assays were used to test the activity of GW275175X and other anti-HCMV compounds against a panel of viruses with drug resistance mutations. GW275175X was active against HCMV strains resistant to GCV, CDV, PFA, ACV, and 1263W94 but was not active against a strain resistant to BDCRB (Table 1). In addition, HCMV with a UL56 plus a UL89 resistance mutation (HCMV C4; see reference 17) had ca. twofold-increased resistance versus that of HCMV which had only the UL89-associated resistance mutation (HCMV D10; see reference 17) (data not shown). Altogether, these results suggest that the mechanism of action of GW275175X differs from that of other clinically relevant HCMV agents but is similar to that of the parent compound, BDCRB.
TABLE 1.
Activity of GW275175X against drug-resistant HCMV strains
| HCMV strain | Site of mutation | Amino acid substitution or deletion | Drug resistance profile | Mean IC50 ± SE (no. of experiments) | Fold resistancea |
|---|---|---|---|---|---|
| AD169 | None | None | None | 1.4 ± 0.1 (4) | 1 |
| GDGP53 | DNA polymerase | A987G | GCV | 1.3 ± 0.1 (3) | 0.9 |
| XbaF | UL97 | Δ (591-593) | GCV | 0.9 ± 0.1 (3) | 0.6 |
| 1117r | DNA polymerase | K513N | CDV | 1.2 ± 0.1 (3) | 0.9 |
| 4760rec | DNA polymerase | V715M | PFA | 1.3 ± 0.1 (2) | 0.9 |
| 1038rB | UL89 | D344E A355T | BDCRB | NDb | >20 |
| 2916rA | UL97 | L397R | 1263W94 | 1.7 ± 0.04 (1) | 1.2 |
| ACVrB | DNA polymerase | L802M | ACV | 1.4 ± 0.1 (1) | 1 |
Relative to wild-type AD169.
No inhibition at 5 μM; ∼30% inhibition at 30 μM.
(ii) Time-of-addition studies.
Replication of herpesviruses requires the synthesis of a HMW head-to-tail concatemeric DNA precursor, which is then matured to unit length genomes and packaged into capsids (18). Antiviral agents that inhibit DNA synthesis should be effective only before production of the HCMV DNA precursors; once formed, precursor DNA matures into unit lengths and is packaged into capsids to generate infectious virus. An antiviral agent that inhibited DNA maturation could prevent the later DNA maturation and packaging steps, acting after DNA synthesis but before viral packaging.
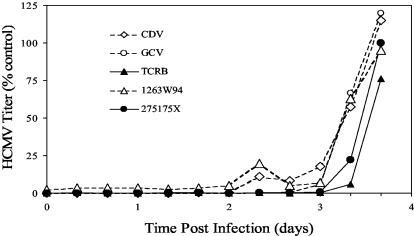
Previous work has established that both BDCRB (26) and the close analog TCRB (16) exert activity against HCMV by preventing maturation of the concatemeric HCMV DNA precursors. Figure 1 shows the amount of infectious HCMV generated in cell culture following the addition of GW275175X, TCRB, or a DNA synthesis inhibitor (GCV, CDV, or 1263W94) at various times after infection. Both GW275175X and TCRB prevented infectious virus generation when added later, unlike the DNA synthesis inhibitors GCV, CDV, or 1263W94. This result suggests that GW275175X targets a process occurring later than DNA synthesis and further supports the hypothesis suggested by the cross-resistance data (Table 1) that the mechanism of action of GW275175X differs from that of GCV, CDV, and 1263W94.
FIG. 1.
Time-of-addition study with benzimidazole nucleosides and control compounds. At time zero, cells in a 96-well plate were infected with HCMV. Antiviral compounds were added once to separate wells at time zero or at 8-h intervals until 88 h postinfection. Incubation was stopped at 96 h, and virus titers were determined by plaque assay. The time of compound addition is shown on the x axis, and the HCMV titer at 96 h is shown on the y axis.
(iii) Time-of-removal studies.
Release of infectious virus grown in the presence of a viral DNA synthesis inhibitor or a DNA maturation inhibitor from HCMV-infected cell culture should occur at different times after removal of the antiviral compound, depending on the stage in the viral replication cycle targeted by the antiviral compound. Removal of a DNA maturation inhibitor should result in more-rapid release of infectious virus compared to that for a viral DNA synthesis inhibitor.
HCMV-infected MRC5 cell monolayers were treated for 48 h with a DNA synthesis inhibitor (1263W94 or GCV), a known DNA maturation inhibitor (BDCRB), or GW275175X. The compounds were then washed from the cultures, and the amount of infectious virus released was measured at various times after compound removal. As expected, the control culture incubated in the absence of compound showed the most rapid appearance of infectious virus, followed by cultures treated with either BDCRB or GW275175X. BDCRB and GW275175X appeared to have the same effect, with both compounds showing virus titers of 10 to 15% of the untreated control titers at 26 h postwashout.
Treatment with DNA synthesis inhibitors resulted in a longer delay before the appearance of infectious virus. In cultures treated with 1263W94, the amount of infectious HCMV measured at 42 h after compound removal was ∼9% of that seen in the untreated control. The longest delay in appearance of infectious virus occurred in cells treated with GCV, with infectious virus produced at only ∼2% of the level measured in the untreated control at 52 h following compound removal. Throughout the experimental time course, cultures treated with BDCRB or GW275175X produced higher titers than cultures treated with 1263W94 or GCV. This more rapid appearance of infectious virus following BDCRB and GW275175X removal suggests that DNA maturation is a mechanism of action. In the presence of either of these compounds, the precursor polygenomic DNA could still be synthesized and would thus be available for processing and packaging into capsids after compound removal.
(iv) HCMV DNA maturation analysis.
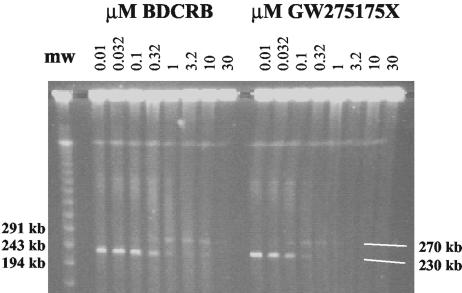
HCMV polygenomic DNA is too large to enter an agarose gel during electrophoresis, even under pulsed-field conditions, although unit length genomes can be effectively separated (26). Figure 2 shows an ethidium bromide-stained CHEF gel upon which was separated intact viral DNA released by treating infected cells embedded in agarose blocks with mild detergent and protease K. Cellular and HMW viral DNA remained at the top of the gel, with a band representing monomeric viral DNA released from the HMW precursor migrating slightly below the 243-kb lambda molecular weight marker. The DNA at about 270 kb is an as yet uncharacterized potential precursor seen during treatment with the benzimidazole DNA maturation inhibitors (26). Both BDCRB and GW275175X inhibited viral DNA maturation in a dose-dependent fashion (Fig. 2). When 1263W94 or GCV was used instead of BDCRB, viral DNA matured to unit-genome length in apparently normal fashion, albeit in greatly reduced amounts (data not shown; see Biron et al. [2]).
FIG. 2.
Analysis of HCMV DNA maturation using CHEF electrophoresis. Lambda concatemeric molecular weight markers are shown in the far left lane. The remaining lanes show HCMV DNA from infected cells incubated in the presence of increasing concentrations of BDCRB or GW275175X. The white lines in the lower right corner indicate the location of monomeric viral DNA of ∼230 kb near the bottom of the gel and of a ∼270-kb viral form that appears during treatment with partially inhibitory concentrations of BDCRB.
DISCUSSION
HCMV antiviral therapy currently involves the use of drugs with less than ideal toxicity profiles and dosing characteristics (13); furthermore, prolonged HCMV treatment can result in therapeutic failure due to drug resistance. There remains a significant, unmet need for safe and effective therapies to treat patients with HCMV disease. We describe here the antiviral efficacy and mechanism of action of the novel HCMV agent GW275175X, an analog of benzimidazole derivatives BDCRB and 1263W94. GW275175X is a direct analog of BDCRB that differs only by having the sugar in the pyran configuration rather than the furan configuration.
The antiviral testing results presented herein are in accord with those of our collaboration with Kern and collaborators. In that study, described in Williams et al. (28), GW275175X was not cytotoxic at concentrations well above those at which it was active against HCMV. Additionally, GW275175X was approximately fourfold less active against HCMV than its close analog BDCRB and had little or no activity against HSV-1, HSV-2, and VZV. One notable difference is that 1263W94 showed lower activity against HCMV in the study by Kern and collaborators than we have reported here, and consequently, the anti-HCMV activity of GW275175X in their study was equal to or greater than that of 1263W94. Results from our two laboratories were very similar for GW275175X, but we have no explanation for the differing results with 1263W94.
Replication of herpesviruses (including HCMV) involves the synthesis of requisite HMW genomic concatemers that are matured and packaged into preformed capsids as unit length genomes with specific termini (18). We have shown previously that BDCRB exerts activity against HCMV by inhibiting the maturation of viral DNA concatemers to unit length genomes (26). Although the precise mechanism of action by which BDCRB inhibits DNA maturation is unknown, data from genetic mapping (17, 26) and biochemical analyses (12, 19, 20) are consistent with inhibition of the viral cleavage and packaging machinery.
Several lines of evidence suggest that the mechanism of action of GW275175X is the same as that of the parent compound, BDCRB. First, the HCMV strain 1038rB, a BDCRB-resistant strain derived by passage of AD169 through increasing concentrations of BDCRB (26), showed cross-resistance to GW275175X but was not cross-resistant to 1263W94 or any of the clinically available anti-HCMV agents. Lack of cross-resistance to strains resistant to antiviral agents other than BDCRB is consistent with a mechanism of action unlike that of the DNA synthesis inhibitors such as GCV and 1263W94.
Time-of-addition and time-of-removal assays provide further support for the proposed mechanism of action. The time-of-addition studies showed that both TCRB (a close analog of BDRCB) and GW275175X were able to inhibit HCMV when added later in infection than the DNA synthesis inhibitors. This result is consistent with these compounds acting to inhibit a process later in the replication pathway. Once the polygenomic precursor DNA is made, addition of DNA synthesis inhibitors such as GCV or 1263W94 should not prevent the maturation and packaging of unit length genomes from precursor DNA; however, addition of a DNA maturation inhibitor should block these steps and thereby prevent the generation of infectious virus.
The time-of-removal studies demonstrated the relative length of time it takes to generate infectious HCMV following removal of the inhibitors in cell culture. The time delay before generation of infectious virus should be greater following removal of a DNA synthesis inhibitor than following removal of a DNA maturation inhibitor, due to the need to synthesize DNA genomes de novo in the former case. Synthesis of polygenomic precursors may still occur in the presence of a maturation inhibitor, with the potential for precursors to be matured and packaged to generate infectious virus upon removal of maturation inhibitor. In the experiments described, both the known DNA maturation inhibitor BDCRB and the posited DNA maturation inhibitor GW275175X allowed infectious virus release relatively soon, following that seen for untreated control cultures. Conversely, both 1263W94 and GCV showed a more delayed release of infectious virus, with GCV being significantly more delayed than 1263W94. The longer delay following the removal of GCV could perhaps be ascribed to intracellular sequestration of GCV due to its intracellular phosphorylation status (3).
Additional evidence that GW275175X is a DNA maturation inhibitor comes from CHEF analysis of the generation of unit length HCMV genomes. This approach allows direct measurement of large DNA molecules up to and including such large molecules as yeast genomes (5). The HCMV AD169 genome, approximately 230 kb in size, was previously analyzed using CHEF in a study of the mechanism of action of BDCRB (26). Our experiments demonstrated that GW275175X, like the parent compound BDCRB, inhibited HCMV genome maturation in a dose-dependent fashion.
In summary, GW275175X has demonstrated good activity against laboratory HCMV strains, clinical isolates, and HCMV strains resistant to clinically relevant anti-HCMV agents. In addition, GW275175X exerts its antiviral effect by inhibition of viral DNA maturation, unlike currently available anti-HCMV agents. Since the formation and packaging of DNA concatemers lacks a known analogous function in mammalian cells, inhibition of viral DNA maturation and packaging might be expected to provide a selective and less toxic antiviral effect than DNA synthesis inhibitors. Moreover, GW275175X has shown promising results in extensive pharmacokinetic and toxicity analyses, including a phase I trial in healthy volunteers (unpublished data). With in vitro antiviral activity equal to or greater than that of GCV and with a unique mechanism of action, GW275175X is a strong candidate for development as a new anti-HCMV agent for clinical use.
Acknowledgments
We thank Roger G. Ptak for expert performance of the time-of-addition studies.
REFERENCES
- 1.Baldanti, F., M. Underwood, S. Stanat, K. Biron, S. Chou, A. Sarasini, E. Silini, and G. Gerna. 1996. Single amino acid changes in the DNA polymerase confer foscarnet resistance and slow-growth phenotype, while mutations in the UL97-encoded phosphotransferase confer ganciclovir resistance in three double-resistant human cytomegalovirus strains recovered from patients with AIDS. J. Virol. 70:1390-1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Biron, K., S. Chamberlain, R. Harvey, S. Good, A. Smith, M. Davis, C. Talarico, W. Miller, R. Ferris, R. Dornsife, S. Stanat, J. Drach, L. Townsend, and G. Koszalka. 2002. Potent and selective inhibition of human cytomegalovirus replication by 1263W94, a benzimidazole l-riboside with a unique mode of action. Antimicrob. Agents Chemother. 46:2365-2372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Biron, K., S. Stanat, J. Sorrell, J. Fyfe, P. Keller, C. Lambe, and D. Nelson. 1985. Metabolic activation of the nucleoside analog 9-{[2-hydroxy-1-(hydroxymethyl)ethoxy]methyl}guanine in human diploid fibroblasts infected with human cytomegalovirus. Proc. Natl. Acad. Sci. USA 82:2473-2477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chou, S., N. S. Lurain, K. D. Thompson, R. C. Miner, and W. L. Drew. 2003. Viral DNA polymerase mutations associated with drug resistance in human cytomegalovirus. J. Infect. Dis. 188:32-39. [DOI] [PubMed] [Google Scholar]
- 5.Chu, G., D. Vollrath, and R. Davis. 1986. Separation of large DNA molecules by contour-clamped homogeneous electric fields. Science 234:1582-1585. [DOI] [PubMed] [Google Scholar]
- 6.Chulay, J., K. Biron, L. Want, M. Underwood, S. Chamberlain, L. Frick, S. Good, M. Davis, R. Harvey, L. Townsend, J. Drach, and G. Koszalka. 1999. Development of novel benzimidazole riboside compounds for treatment of cytomegalovirus disease, p. 129-134. In J. Mills, P. Volberding, and L. Corey (ed.), Antiviral therapy 5: new directions for clinical applications and research. Kluwer Academic/Plenum Publishers, New York, N.Y. [DOI] [PubMed]
- 7.Drew, W., C. Paya, and V. Emery. 2001. Cytomegalovirus (CMV) resistance to antivirals. Am. J. Transplant. 1:307-312. [PubMed] [Google Scholar]
- 8.Field, A. 1999. Human cytomegalovirus: challenges, opportunities, and new drug development. Antivir. Chem. Chemother. 10:219-232. [DOI] [PubMed] [Google Scholar]
- 9.Gudmundsson, K., J. Drach, and L. Townsend. 1998. Synthesis of the first C3 ribosylated imidazo[1,2-a]pyridine C-nucleoside by enantioselective construction of the ribose moiety. J. Org. Chem. 63:984-989. [Google Scholar]
- 10.Gudmundsson, K., G. Freeman, J. Drach, and L. Townsend. 2000. Synthesis of fluorosugar analogues of 2,5,6-trichloro-1-(β-d-ribofuranosyl)benzimidazole as antivirals with potentially increased glycosidic bond stability. J. Med. Chem. 43:2473-2478. [DOI] [PubMed] [Google Scholar]
- 11.Gudmundsson, K., J. Williams, J. Drach, and L. Townsend. 2003. Synthesis and antiviral activity of novel erythrofuranosyl imidazo[1,2-a]pyridine C-nucleosides constructed via palladium coupling of iodoimidazo[1,2-a]pyridines and dihydrofuran. J. Med. Chem. 46:1449-1455. [DOI] [PubMed] [Google Scholar]
- 12.Hwang, J., and E. Bogner. 2002. ATPase activity of the terminase subunit pUL56 of human cytomegalovirus. J. Biol. Chem. 277:6943-6948. [DOI] [PubMed] [Google Scholar]
- 13.Khare, M., and M. Sharland. 2001. Cytomegalovirus treatment options in immunocompromised patients. Expert Opin. Pharmacother. 2:1247-1257. [DOI] [PubMed] [Google Scholar]
- 14.Koszalka, G., N. Johnson, D. Perkins, S. Good, L. Boyd, S. Chamberlain, and K. Biron. 2002. Preclinical and toxicology studies of 1263W94, a potent and selective inhibitor of human cytomegalovirus replication. Antimicrob. Agents Chemother. 46:2373-2380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Krosky, P., M. Baek, and D. Coen. 2003. The human cytomegalovirus UL97 protein kinase, an antiviral drug target, is required at the stage of nuclear egress. J. Virol. 77:905-914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Krosky, P., K. Borysko, M. Nassiri, R. Devivar, R. Ptak, M. Davis, K. Biron, L. Townsend, and J. Drach. 2002. Phosphorylation of β-d-ribosylbenzimidazoles is not required for activity against human cytomegalovirus. Antimicrob. Agents Chemother. 46:478-486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Krosky, P., M. Underwood, S. Turk, K. Feng, R. Jain, R. Ptak, A. Westerman, K. Biron, L. Townsend, and J. Drach. 1998. Resistance of human cytomegalovirus to benzimidazole ribonucleosides maps to two open reading frames: UL89 and UL56. J. Virol. 72:4721-4758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Roizman, B., and A. Sears. 1996. Herpes simplex viruses and their replication, p. 2231-2295. In B. Fields, D. Knipe, P. Howley, (ed.), Fields virology, 3rd ed., vol. 2. Lippincott-Raven Publishers, Philadelphia, Pa. [Google Scholar]
- 19.Scheffczik, H., C. Savva, A. Holzenburg, L. Kolesnikova, and E. Bogner. 2002. The terminase subunits pUL56 and pUL89 of human cytomegalovirus are DNA-metabolizing proteins with toroidal structure. Nucleic Acids Res. 30:1695-1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Scholz, B., S. Rechter, J. Drach, L. Townsend, and E. Bogner. 2003. Identification of the ATP-binding site in the terminase subunit pUL56 of human cytomegalovirus. Nucleic Acids Res. 31:1426-1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sia, I., and R. Patel. 2000. New strategies for prevention and therapy of cytomegalovirus infection and disease in solid-organ transplant recipients. Clin. Microbiol. Rev. 13:83-121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sullivan, V., K. Biron, C. Talarico, S. Stanat, M. Davis, L. Pozzi, and D. Coen. 1993. A point mutation in the human cytomegalovirus DNA polymerase gene confers resistance to ganciclovir and phosphonylmethoxyalkyl derivatives. Antimicrob. Agents Chemother. 37:19-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Townsend, L., R. Devivar, S. Turk, M. Nassiri, and J. Drach. 1995. Design, synthesis, and antiviral activity of certain 2,5,6-trihalo-1-(beta-d-ribofuranosyl)benzimidazoles. J. Med. Chem. 38:4098-4105. [DOI] [PubMed] [Google Scholar]
- 24.Townsend, L., J. Drach, S. Good, S. DaLuge, and M. Martin. July. 1996. Therapeutic nucleosides. U.S. patent 5,534,535.
- 25.Townsend, L., K. Gudmundsson, S. Daluge, J. Chen, Z. Zhu, G. Koszalka, L. Boyd, S. Chamberlain, G. Freeman, K. Biron, and J. Drach. 1999. Studies designed to increase the stability and antiviral activity (HCMV) of the active benzimidazole nucleoside, TCRB. Nucleosides Nucleotides 18:509-519. [DOI] [PubMed] [Google Scholar]
- 26.Underwood, M., R. Harvey, S. Stanat, M. Hemphill, T. Miller, J. Drach, L. Townsend, and K. Biron. 1998. Inhibition of human cytomegalovirus DNA maturation by a benzimidazole ribonucleoside is mediated through the UL89 gene product. J. Virol. 72:717-725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vitravene Study Group. 2002. Safety of intravitreous fomivirsen for treatment of cytomegalovirus retinitis in patients with AIDS. Am. J. Ophthalmol. 133:484-498. [DOI] [PubMed] [Google Scholar]
- 28.Williams, S., C. Hartline, N. Kushner, E. Harden, D. Bidanset, J. Drach, L. Townsend, M. Underwood, K. Biron, and E. Kern. 2003. In vitro activities of benzimidazole d- and l-ribonucleosides against herpesviruses. Antimicrob. Agents Chemother. 47:2186-2192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wolf, D., C. Courcelle, M. Prichard, and E. Mocarski. 2001. Distinct and separate roles for herpesvirus-conserved UL97 kinase in cytomegalovirus DNA synthesis and encapsidation. Proc. Natl. Acad. Sci. USA 98:1895-1900. [DOI] [PMC free article] [PubMed] [Google Scholar]