Abstract
Purpose: A transportation technique for cell sheets is necessary to standardize regenerative medicine. The aim of this article is to develop and evaluate a new transportation technique for cell sheets.
Material and Methods: We developed a transportation container with three basic functions: the maintenance of interior temperature, air pressure, and sterility. The interior temperature and air pressure were monitored by a recorder. Human oral mucosal epithelial cells obtained from two healthy volunteers were cultured on temperature-responsive culture dishes. The epithelial cell sheets were transported via an airplane between the Osaka University and Tohoku University using the developed cell transportation container. Histological and immunohistochemical analyses and flow cytometric analyses for cell viability and cell purity were performed for the cell sheets before and 12 h after transportation to assess the influence of transportation on the cell sheets. Sterility tests and screening for endotoxin and mycoplasma in the cell sheets were performed before and after transportation.
Results: During transportation via an airplane, the temperature inside the container was maintained above 32°C, and the changes in air pressure remained within 10 hPa. The cell sheets were well stratified and successfully harvested before and after transportation. The expression patterns of keratin 3/76, p63, and MUC16 were equivalent before and after transportation. However, the expression of ZO-1 in the cell sheet after transportation was slightly weaker than that before transportation. The cell viability was 72.0% before transportation and 77.3% after transportation. The epithelial purity was 94.6% before transportation and 87.9% after transportation. Sterility tests and screening for endotoxin and mycoplasma were negative for all cell sheets.
Conclusion: The newly developed transportation technique for air travel is essential technology for regenerative medicine and promotes the standardization and spread of regenerative therapies.
Introduction
Limbal autograft can be used as a treatment method for patients with unilateral limbal stem cell deficiency.1 However, this procedure requires a large limbal graft from the healthy eye (incurring a risk of limbal stem cell deficiency in the healthy eye2) and cannot be applied for bilaterally affected patients.3 Limbal allograft transplantation can be performed in patients with unilateral or bilateral deficiencies,4 but the procedure requires long-term immunosuppression, which involves high risks of serious eye and systemic complications, including infection and liver and kidney dysfunction. Moreover, in patients with Stevens–Johnson syndrome or ocular pemphigoid, graft failure is common even with immunosuppression due to serious preoperative conditions, such as persistent inflammation of the ocular surface, abnormal epithelial differentiation of the ocular surface, severe dry eye conditions, and lid-related abnormalities.5–7 To address these problems, tissue-engineered oral mucosal epithelial cell sheets have been successfully used to reconstruct eyes affected with severe ocular surface disorders.8,9
The cell-processing center (CPC) is a clean room that serves as an essential area for aseptic culturing or processing of human cells for regenerative medicine. Human cells are manipulated in a biohazard cabinet of class 100, which indicates that less than 100 particles larger than 0.5 μm are present in each cubic foot of air space. All chemicals and samples are managed using a barcode system, and all manufacturing procedures are delivered and recorded by this procedure control system together with such environmental monitoring data as air particles, temperature, humidity, and air pressure. Additionally, workers in the CPC are required to wear disposable dust-free garments to avoid contamination. Although many hospitals require tissue-engineered epithelial cell sheets for treatment, it is impossible for all hospitals to cover the cost due to the high expense of a CPC. Therefore, many hospitals should share one CPC to standardize and spread regenerative therapy using tissue-engineered oral mucosal epithelial cell sheets. In this work, we address the need for the development of a cell transportation technique for bridging many hospitals.
To the best of our knowledge, no previous reports exist on a technique for cell transportation by means of an airplane for clinical use. In this study, we developed a cell transportation technique for clinical study using tissue-engineered human oral mucosal epithelial cell sheets.
Materials and Methods
Evaluation of the cell transportation container
We set three basic functions of transportation container for clinical study: maintenance of temperature, air pressure, and sterility. We believe that the three basic functions are sufficient conditions, not necessary conditions. We actually developed the cell transportation container with the three basic functions. And then, the interior temperature, pressure, and sterility of the cell transportation container were evaluated under a mimicked transportation environment.
We measured the temperature maintenance over time between 24°C and 26°C assuming both an ambient room temperature between 3°C and 5°C and typical transportation conditions in winter in Japan. The container was placed in an air-conditioned room (23°C to 25°C) and a cold room (3°C to 5°C), and the temperature maintenance over time was evaluated.
We investigated whether the interior pressure could be maintained under an outside air pressure of 650 and 700 hPa. To evaluate the interior pressure in the mimicked transportation environment, the sealing apparatus was exposed to low pressure, between 650 and 700 hPa, for 24 h.
We assessed whether the packaging chamber could maintain sterility. To evaluate the sterility of the inner packaging chamber, a liquid that included bacteria (Bacillus subtilis ATCC6633: 1.2×105 CFU/mL; Eiken Chemical Co., Ltd.) was spread onto the external sides of the outer packaging chamber. The bacteria were attached to the external side of the outer packaging chamber by a piece of paper with the liquid containing bacteria, and culture dishes within the packaging chamber were cultured for 1 day at 37°C. After the chamber was opened through aseptic operation in a biological safety cabinet, the outer and inner packaging chamber and culture dish were soaked and cultured in the soybean casein digest medium (SCD) to culture the attached Bacillus subtilis for 1 day. After culture, the existence of bacteria in each apparatus was determined according to whether the SCD medium was contaminated.
Culture and transportation of a rabbit oral mucosal epithelial cell sheet
A New Zealand white rabbit (2.0 kg) was sacrificed with an overdose of anesthetic agent (pentobarbital), and buccal mucosal epithelial tissue was harvested. Oral mucosal epithelial cells were collected by removing all epithelial layers after treatment with dispase II (2.4 U/mL, Invitrogen) at 4°C for 4 h. The separated epithelial layers were treated with trypsin-EDTA (Invitrogen), and the resuspended cells were plated on temperature-responsive 3.5-cm culture dishes (CellSeed) at an initial cell density of 4.0×105 cells/dish with feeder cells.1 For the feeder layers, 3T3J2 cells were lethally irradiated with 60 Gly and subsequently seeded onto tissue culture dishes at a density of 2.7×104 cells/cm2. The cells were cultured for 14 days.
We transported the tissue-engineered rabbit oral mucosal epithelial cell sheets between the Osaka University and Tohoku University via an airplane, a distance of ∼650 km. The cell sheets were carried by airplane, train, and walking. In the airplane, the container was brought into the cabin and tied down in a seat. An application was submitted to the airline company to allow the container on board. X-ray inspection was avoided, and the inside of the container was checked by visual examination at the airport. The duration of transportation was 5 h.
Before transportation, a heat storage material was prewarmed in an incubator at 37°C and was held for 3 days in the incubator to stabilize its temperature. The cell container was prewarmed with spare heat storage material for 5 h before examination. Then, the heat storage material for prewarming was substituted with new material, and the temperature change was measured with a temperature/pressure recorder (T&D Corporation). The recorder measured temperature and pressure at 1-min intervals.
Four kinds of transportation liquid were checked for rabbit cell sheet: keratinocyte culture medium (KCM),10 KCM minus fetal bovine serum (FBS), and epidermal growth factor (EGF), DMEM/F12 (3:1), and Hanks' Balanced Salt Solutions (HBSS; Gibco). The dishes were placed in the packaging chamber in a safety cabinet under aseptic conditions. Four packaging containers each containing four culture dishes and a temperature/pressure recorder were rapidly packed into a prewarmed sealing apparatus and a cell transportation container. Leakage of medium was also evaluated.
Culture and transportation of a human oral mucosal epithelial cell sheet
Human oral mucosal epithelial tissues were obtained from healthy volunteers, and a tissue-engineered human oral mucosal epithelial cell sheet was fabricated. pH measurement and real-time PCR were conducted using cell sheets from one volunteer. Other evaluations were performed using cell sheets from two independent volunteers. The cells were cultured for 14 days.
The tissue-engineered human oral mucosal epithelial cell sheets were transported between the Osaka University and Tohoku University via an airplane in the same manner as the rabbit oral mucosal epithelial cells. HBSS (Gibco) was used as the transportation liquid, and the duration of transportation was 12 h. pH of transportation liquid was checked using pH meter (Horiba).
Validation of the cell sheet before and after transportation
Validation of the cell sheet was performed as reported.11 The cell morphology, cell recovery, viability, and purity were evaluated for the rabbit oral mucosal epithelial cell sheets. All of the following items were also evaluated for the human cell sheets using the identical methods for clinical study.
Cell morphology
Cultured epithelial cells were observed under a phase-contrast microscope, and microphotographs were taken at 100-fold magnification (Axiovert40; Carl Zeiss) to examine the cell morphological aberrations and deficits.
Sheet recovery test
After examination with phase-contrast microscopy, the cultured epithelial cells were subjected to incubation at 20°C for 30 min. Next, a donut-shaped support membrane (18-mm outer diameter, 10-mm inner diameter, polyvinylidene difluoride; Millipore) was placed on the epithelial cells. Finally, the cells were challenged through harvesting in the presence of support membranes. The harvested epithelial cell sheets were divided into two equal groups. One group was subjected to flow cytometry, and the other group was subjected to histological analyses.
Cell viability and epithelial cell purity
Cell viability was evaluated with a dye exclusion test. An aliquot of cell suspension was incubated in the DMEM with 7-aminoactinomycin D (7′AAD; BD Biosciences) staining at room temperature for 10 min and subjected to flow cytometry (FACS Calibur, BD).
After trypsin-EDTA treatment, an aliquot of the cell suspension was centrifuged, fixed, and permeabilized with the Cytofix/Cytoperm kit (BD Biosciences) according to the manufacturer's protocol. Next, the cell suspension was split into two tubes and incubated with either a FITC-conjugated anti-pancytokeratin IgG2a antibody (clone Pan1–8; Progen) or a FITC-conjugated mouse control IgG2a antibody (Santa Cruz Biotechnology) at room temperature for 60 min. After washing twice with PBS, the nuclei were stained with 7′AAD and the cells were examined by flow cytometry.
Hematoxylin and eosin staining and immunofluorescence analyses
The portion of cell sheets for histological analyses was divided into two quadrants. One quadrant was fixed with formalin and embedded in paraffin. Hematoxylin and eosin staining was performed to observe the morphology and degree of stratification of the cultured epithelial cells. Microphotographs were taken with a light microscope (BZ-9000; Keyence).
The other quadrant of cell sheets was embedded in Tissue-Tek® O.C.T.™ compound (Sakura Seiki), and processed into 10-μm-thick frozen sections. Cryosections from the cell sheets were immunostained with monoclonal antibodies against keratin 3/76 (K3/76, AE5; Progen), p63 (4A4; Santa Cruz Biotechnology), ZO-1 (1A12; Zymed), and MUC16 (Ov185; Abcam) followed by incubation with Alexa488-labeled secondary antibodies (Molecular Probes). The nuclei were costained with Hoechst 33342 (Sigma), and the cell sheets were mounted with PermaFluor (Beckman Coulter). Slides were observed using a confocal laser scanning microscope (LSM-710; Carl Zeiss). The same concentration of a corresponding normal nonspecific IgG was used as a negative control.
Quantitative reverse transcription-polymerase chain reaction
Total RNA was extracted from three cell sheets before and after transportation cells using ISOGEN reagent (Nippon Gene). Complementary DNAs were synthesized using a SuperScript III first-strand synthesis system (Invitrogen) according to the manufacturer's protocol. Primers and TaqMan probe mixtures were purchased from Life Technologies. Quantitative PCR was carried out using a 7500 Fast Real-Time PCR System (Life Technologies). Data were normalized to glyceraldehyde 3-phosphate dehydrogenase expression.
Sterility test and screening for endotoxin and mycoplasma in the cell sheet before and 12 h after transportation
Sterility testing and screening for endotoxin and mycoplasma contaminants were performed on the human oral mucosal epithelial cell sheets. The tests were performed on the media used in the epithelial cell cultures before transportation and the transportation media were used for the cell sheets after transportation.
Sterility testing was performed using two methods. First, a commercial colorimetric assay system was applied, namely, the BacT/ALERT 3D system from bioMerieux. This method used cultured media and a 7-day incubation at 37°C for a short testing period. The other method used direct inoculation using two types of media for the detection of bacterial and fungal contaminants. A tryptone soya broth and the thioglycollate medium were incubated at 32°C and 25°C, respectively, for 14 days.
Endotoxin detection was determined through the Limulus ES-II Single Test WAKO on a Toxinometer ET-301 (WAKO Chemical Co.). Mycoplasma detection was evaluated by a two-stage nested PCR assay with first- and second-step primers. The DNA was extracted with the QIAamp DNA Mini Kit (QIAGEN) according to the manufacturer's instructions. The PCR reaction ran at 94°C for 30 s for denaturing, at 55°C for 2 min for annealing, and at 72°C for 1 min for extension for 30 cycles on a TGeneAmp PCR system 9700 (Applied Biosystems).
This study was conducted with the approval of the institutional review board of Osaka University Graduate School of Medicine and Tohoku University School of Medicine.
Results
Specifications of the cell transportation container
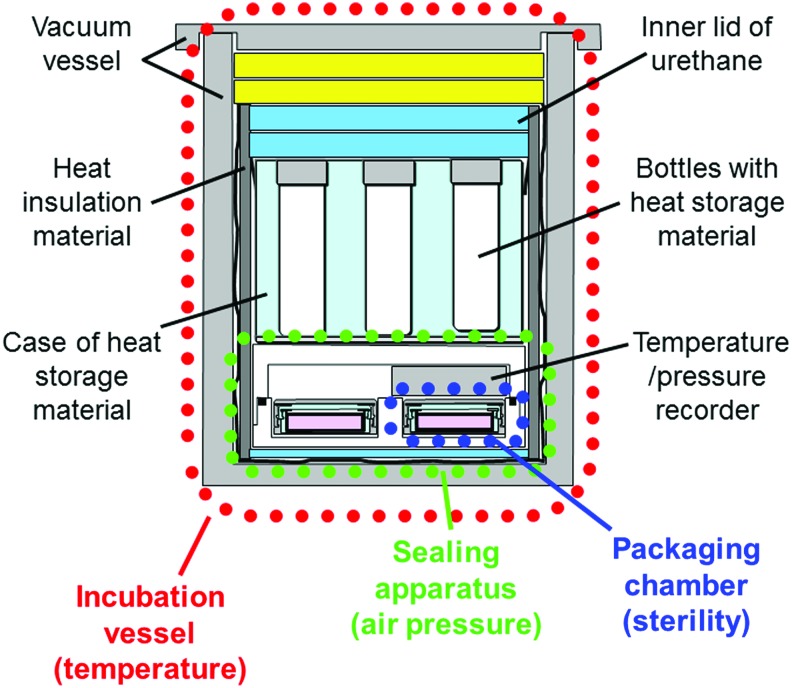
We developed a cell transportation container that consists of three parts: an incubation vessel for temperature, a sealing apparatus for air pressure, and packaging chambers for sterility (Fig. 1). The incubation vessel was composed of a vacuum vessel and heat storage materials (Fig. 2). The vacuum vessel functions to maintain the stability of the inside temperature, and the heat storage material maintains the temperature near the melting point. The sealing apparatus can accommodate four packaging chambers with culture dishes and a temperature/air pressure recorder (Fig. 3). The packaging chamber is designed to maintain the inside sterility, and the culture dish is doubly packed in the inner and outer packaging chambers (Fig. 4). The bottom of a 3.5-cm dish without a lid can be sealed tightly using silicon rubber on the lid of the inner packaging chamber. It was assumed that the packing of the 3.5-cm dish was performed in a biological safety cabinet in the CPC. Therefore, the packaging chamber was constructed of material that can be sterilized by ethylene oxide gas.
FIG. 1.
Cross-sectional view of a cell transportation container for cell sheets consisting of an incubation vessel for temperature, a sealing apparatus for air pressure, and four packaging chambers for sterility. Bottles with heat storage material are set inside the incubation vessel. Color images available online at www.liebertpub.com/tec
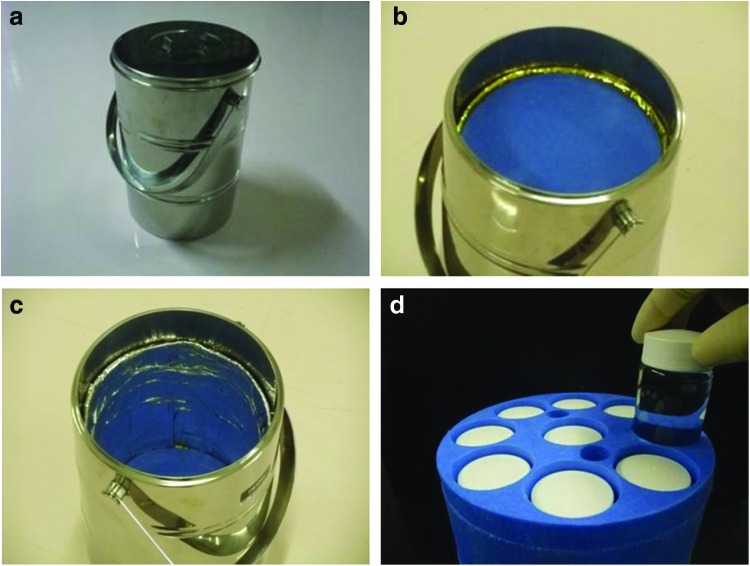
FIG. 2.
Incubation container vessel for maintenance of the inner temperature at 35°C (20 cm in diameter at the top, 30 cm in height, 8 kg in weight): (a) appearance, (b) inner lid of incubator vessel, (c) interior portion of incubator vessel, (d) case of heat storage material; nine bottles with heat storage material are placed within the container. Color images available online at www.liebertpub.com/tec
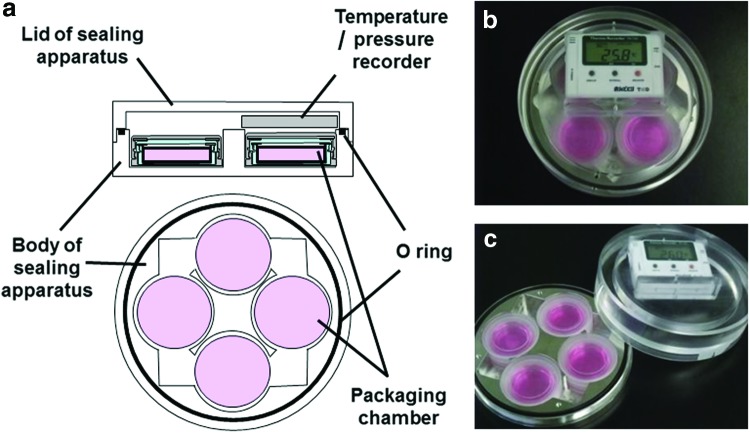
FIG. 3.
Sealing apparatus for maintaining constant inner pressure. (15 cm in diameter at the top, 5 cm in height, 780 g in weight): (a) cross-sectional view of sealing apparatus, (b) appearance, (c) unpacked state of the apparatus. Color images available online at www.liebertpub.com/tec
FIG. 4.
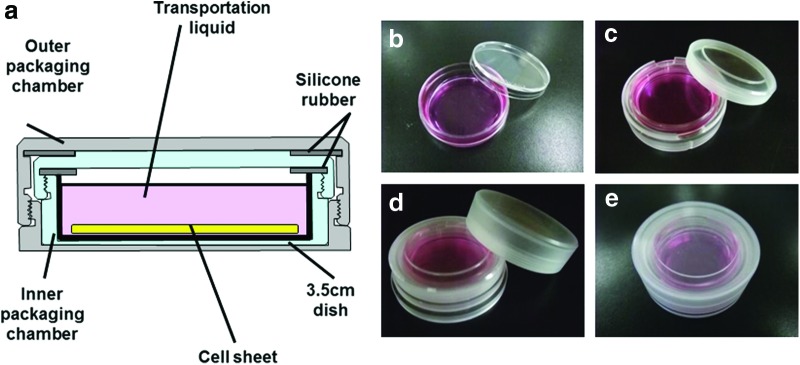
Packaging chamber for maintenance of inner sterility. (5.2 cm in diameter at the top, 1.8 cm in height, 27 g in weight): (a) cross-sectional view of packaging chamber; the culture dish is doubly packed in the inner and outer packaging chambers, (b) 3.5-cm dish, (c) bottom of the 3.5-cm dish placed in the inner packaging chamber, (d) inner packaging chamber placed in the outer packaging chamber, (e) appearance of a packaging chamber containing a culture dish and cell sheet. Color images available online at www.liebertpub.com/tec
Although X-ray examination is essential for all cargo carried by airplane, X-ray exposure must be avoided in this experiment because the cell chromosomes can be damaged by exposure to X-rays. In the airports, unveiling of the cell transportation container and displaying the contents to an examiner was carried out instead of X-ray examination. For this purpose, we used transparent packaging chambers and a transparent lid for the packing chamber.
For temperature maintenance, this container was designed to carry tissue-engineered oral mucosal epithelial cell sheets on temperature-responsive culture surfaces. Cell sheets attached to the surfaces are spontaneously detached by reducing the temperature to below 32°C due to the phase transition change of the temperature-responsive polymer from a hydrophobic to hydrophilic state.12,13 When the cell sheets are transported at temperatures under 32°C, they detach from the surface and float in the culture medium. Floating cell sheets can be damaged during air transportation14 because the container can be shaken and tilted during flight. However, temperatures higher than 37°C (e.g., 42°C) can cause apoptosis due to heat shock. Therefore, we chose an inner temperature for transportation between 32°C and 37°C.
For the heat storage material in this container, we chose N-eicosane, a pure hydrocarbon material with a melting temperature of 36.4°C. Because the heat storage material was prewarmed at 37°C before use, the heat storage material in the liquid state decreased its temperature gradually and was held at 36.4°C. Using this characteristic of the heat storage material, the inner temperature was maintained near 35°C during transportation.
The outside pressure can be reduced to ∼800 hPa in the airplane cabin. Thus, the sealing apparatus acts to maintain the inner air pressure as a constant. An O-ring is installed between the lid and bottom of the container. If the lid and bottom are tightly pinched, the inner air pressure is maintained independently of the outer air pressure. Additionally, the recorder inside the sealing apparatus can monitor the temperature and air pressure during transportation. For sterility, we chose to pack the culture dish with an inner and outer packaging chamber as a double structure.
Evaluation of the cell transportation container
The cell transportation container's ability to maintain the inner temperature was evaluated. When the container was placed in an air-conditioned room (23°C to 26°C), the inner temperature declined to below 34.0°C after 61.3±0.2 h (n=3). There was little change in the inner temperature with the change in the outer temperature. In the cold room (3–5°C), the inner temperature was maintained at over 35°C for 24.1±0.1 h (n=3).
The inside pressure change in the sealing apparatus was also measured. When the sealing apparatus was exposed to low pressure (between 650 and 700 hPa), the inner pressure of the sealing apparatus was maintained between 950 and 1050 hPa over 24 h (n=4), and the culture medium in the culture dishes did not leak.
For sterility, the culture dishes in the packaging chamber were cultured for 1 day in an incubator at 37°C after attachment of the bacteria, and each component was cultured in the SCD medium for 1 day. Biological contamination was observed in the outer packaging chambers in all experiments (n=9). However, no biological contamination was observed in the inner packaging chambers and culture dishes (n=9). As a control, each component was also evaluated directly after ethylene oxide gas sterilization, and no biological contamination was observed in the SCD medium (n=3). The SCD medium injected with bacterial liquid showed biological contamination in all experiments (n=3). Therefore, it was concluded that the packaging chambers are able to maintain the sterility of the culture dishes.
Transportation of rabbit tissue-engineered oral mucosal epithelial cell sheet using airplane
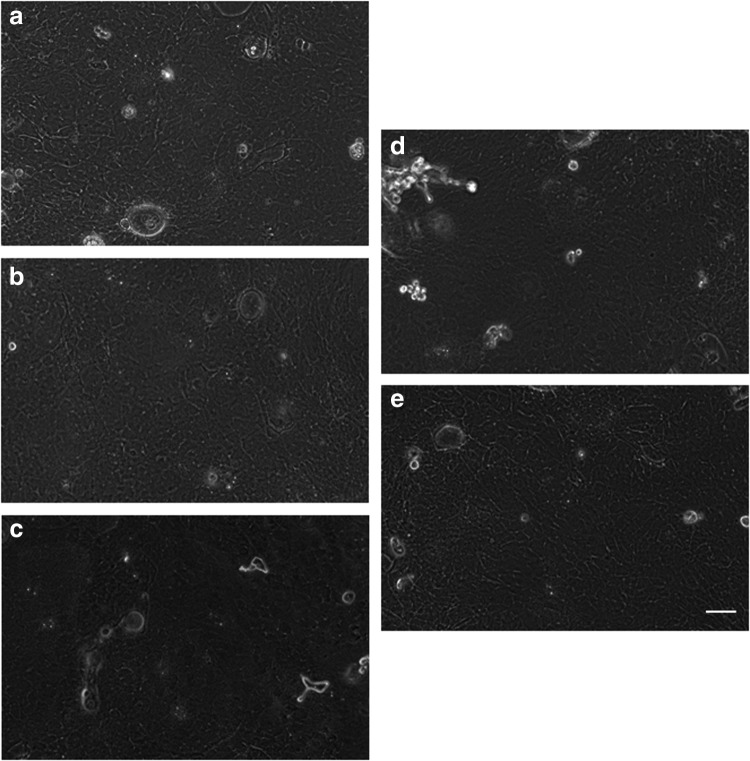
Rabbit oral mucosal epithelial cell sheets were successfully fabricated, and the cell morphology is shown in Figure 5. Leakage of the transportation liquid was not observed. The cell morphologies of the cell sheets after transportation with four types of transportation media were similar to the cell morphologies before transportation. In other words, small basal cells with a high nucleus/cytoplasm ratio were observed under a phase-contrast microscope. All the cell sheets were harvested through temperature reduction before and after transportation. The cell viability was 82.3% before transportation, and after transportation, the cell viability was 89.9% with KCM, 93.8% with KCM minus FBS and EGF, 87.1% with DMEM/F12, and 90.7% with HBSS. The epithelial cell purity was 95.8% before transportation, and after transportation, the epithelial cell purity was 97.7% with KCM, 98.0% with KCM minus FBS and EGF, 96.2% with DMEM/F12, and 97.2% with HBSS.
FIG. 5.
Tissue-engineered rabbit oral mucosal epithelial cell sheets before and 5 h after transportation. Examination of cell morphology was performed using phase-contrast microscopy: (a) cell sheet before transportation, (b) cell sheet after transportation with KCM, (c) cell sheet after transportation with KCM minus fetal bovine serum and epidermal growth factor, (d) cell sheet after transportation with DMEM/F12, (e) cell sheet after transportation with HBSS. Scale bars: 100 μm. HBSS, Hanks' balanced salt solution; KCM, keratinocyte culture medium.
Transportation of human tissue-engineered oral mucosal epithelial cell sheet using airplane
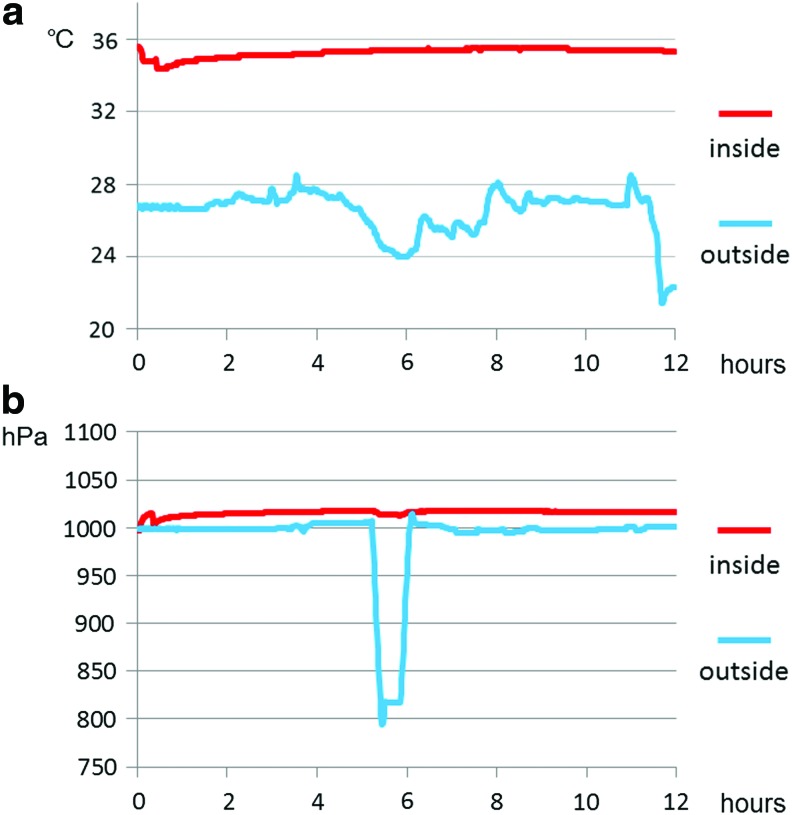
The changes in temperature and air pressure during transportation are presented in Figure 6. Although the outside temperature changed, the inside temperature was stable and remained above 32°C. Additionally, the outside air pressure fell below 800 hPa in the cabin, but the inside pressure was constant. The air pressure changes were all within 10 hPa. The pH of transportation liquid was 7.61 before transportation and 7.18 after transportation.
FIG. 6.
Temperature and air pressure changes in the inner container during transportation using airplane: (a) temperature inside (red line) and outside (blue line) the sealing apparatus, (b) air pressure inside (red line) and outside (blue line) the sealing apparatus. Color images available online at www.liebertpub.com/tec
The results of validation for the tissue-engineered human oral mucosal epithelial dell sheets are summarized in Table 1. Human oral mucosal epithelial cell sheets were successfully cultured, and the cell morphologies after transportation were equivalent to those before transportation (Fig. 7a, b). Additionally, all of the cell sheets were successfully harvested before and after transportation by reducing the temperature to 20°C for 30 min. Therefore, all of the cell sheets passed the recovery test. The harvested cell sheets before and 12 h after transportation were composed of two to four layers of small basal cells, flattened middle cells, and polygonal flattened superficial cells (Fig. 7c, d).
Table 1.
Results of Validation for Tissue-Engineered Human Oral Mucosal Epithelial Cell Sheets
| Transportation | Phase contrast | Cell sheet recovery | Viability | Purity | Stratification | K3/76 | p63 | ZO-1 | MUC16 | |
|---|---|---|---|---|---|---|---|---|---|---|
| Sheet1 | Before | Normal | Possible | 76.6 | 93.7 | Normal 2–4 layers | + | + | + | + |
| After | Normal | Possible | 75.3 | 93.1 | Normal 2–4 layers | + | + | + | + | |
| Sheet2 | Before | Normal | Possible | 67.3 | 95.5 | Normal 2–4 layers | + | + | + | + |
| After | Normal | Possible | 79.2 | 82.7 | Normal 2–4 layers | + | + | + | + |
FIG. 7.
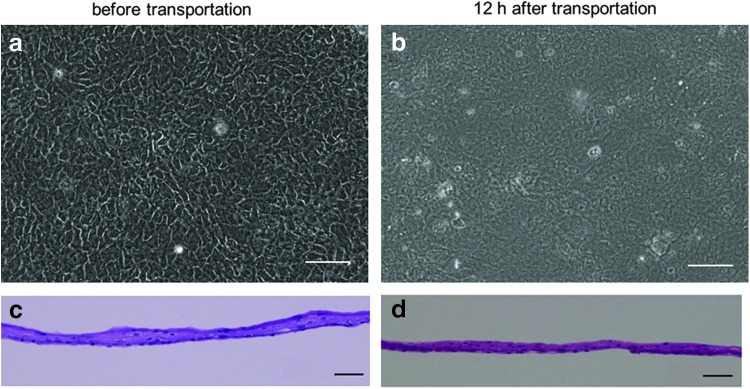
Tissue-engineered human oral mucosal epithelial cell sheets before and 12 h after transportation. Examination of cell morphology was performed using phase-contrast microscopy (a, b) and hematoxylin and eosin staining (c, d). Scale bars: 100 μm (a, b), 50 μm (c, d).
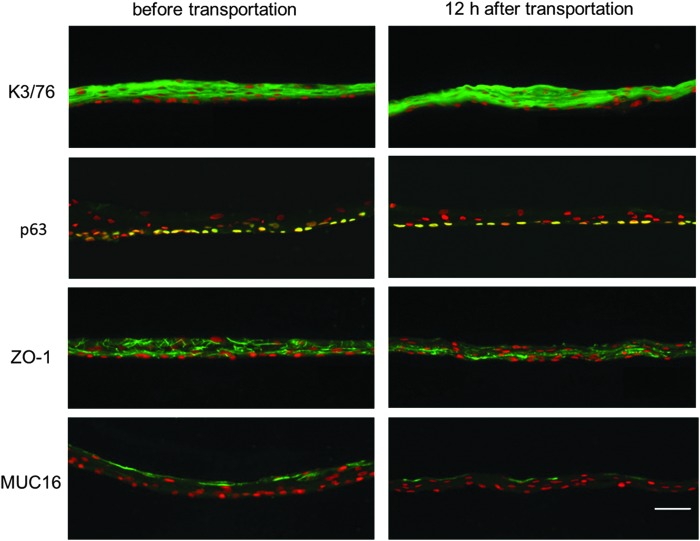
The immunofluorescence analyses revealed that the cell sheets displayed similar marker expression patterns before and after transportation (Fig. 8). A marker for corneal and oral mucosal differentiated epithelial cells, K3/76,15 was positive in both sheets, and p63, which has been proposed as a corneal epithelial stem/progenitor cell marker,16 was expressed in the basal cells of both sheets. Additionally, ZO-1, a marker of tight junctions and MUC 16, a membrane-associated mucin specific to ocular surfaces, were expressed in both sheets. However, the expression of ZO-1 in the cell sheet after transportation was slightly weaker than that before transportation.
FIG. 8.
Immunohistochemical analyses of tissue-engineered human oral mucosal epithelial cell sheets before and 12 h after transportation. Human oral mucosal epithelial cell sheets were stained with anti-keratin 3/76 (K3/76), anti-p63 (K12), anti-ZO-1 (ZO-1), and anti-musin16 (MUC-16) antibodies. Nuclei were costained with Hoechst 33342. Scale bars: 50 μm.
The cell viability was 72.0% before transportation and 77.3% after transportation. The epithelial purity was 94.6% before transportation and 87.9% after transportation.
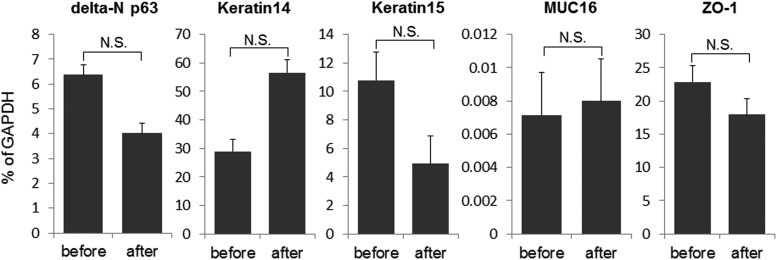
Real-time RT-PCR confirmed that there was no significant effect on delta-N p63 expression by transportation of cell sheets (Fig. 9).
FIG. 9.
Gene expression analyses of tissue-engineered human oral mucosal epithelial cell sheets before and 12 h after transportation. Real-time RT-PCR showed that the difference of the expression of delta-N p63, Keratin14, Keratin15, MUC16, and ZO-1 before and after transportation was not statistically significant. The graph showed the mean±S.E. of three samples, respectively. N.S., not significant. (paired t-test).
All cell sheets were free from growth of microbial contaminants under a phase-contrast microscope, and the sterility tests and screening for endotoxin and mycoplasma of the cell sheet before and 12 h after transportation were negative.
Discussion
We have previously developed a portable homothermal container designed for transportation through a land route for cell sheets fabricated on temperature-responsive culture surfaces.14 With this container, we demonstrated that rat fibroblast cell sheets cultured on temperature-responsive surfaces could be transported by car over a period of 8 h. Although maintenance of sterility is a crucial function for transportation containers intended for clinical studies, the previously developed container did not contain a function that provided for the sealing of culture dishes. Thus, the sealing of the culture dishes was incomplete, and culture medium leaked easily during transportation. Although air transportation is the fastest mode of travel and can be used for long-distance transportation (including international travel), the previous container could be not used for transportation using the airplane. Therefore, the previously developed container was not suitable for transportation of products for regenerative medicine.
Containers for transportation of blood or corneas have been already reported.17,18 These vessels can be sealed tightly by a screw structure in the lids and bodies of the containers or sealed in plastic bags. Therefore, the medium did not leak due to perturbation or decreased pressure during transportation. Our newly developed transportation container contains a sealing apparatus that can maintain the inside air seal even under decreased air pressure. Moreover, the arrangement in which the culture dishes were placed in packaging chambers eliminated the influence of perturbation during transportation. Thus, medium leakage was avoided due to the sealing apparatus and packaging chambers. For maintenance of sterility, the culture dishes were doubly wrapped in the packaging chambers. Contamination did not occur during transportation, which suggested that sterility was maintained during transportation.
A common problem of the present container is that it lacks the function of supplying O2 and CO2. Oxygen was not supplied for over 12 h of transportation, but the cell sheets did not display any damage. In addition, change of pH was within 0.5. However, the container design should be improved for other types of cells that require more oxygen.
It is believed that the transport liquid is also important in successful transportation techniques. The tissue-engineered human oral mucosal epithelial cell sheet will be classified as a medical device under the Pharmaceutical Affairs Act in the near future. From this perspective, a transport liquid without serum or growth factor is ideal because the changes in the cell sheet after shipment from the CPC should be minimized. Because experiments with rabbit cell sheets proved that HBSS is sufficient for use as the transportation liquid, we used HBSS for the human cell sheets. HBSS is a relatively simple buffered solution that provides cells with water and certain bulk inorganic ions as well as the carbohydrates and glucose essential for cell metabolism. HBSS does not contain any growth factors. Therefore, we believe that HBSS is sufficient for use as the transport liquid base in our current study.
We also succeeded in transporting tissue-engineered oral mucosal epithelial cell sheets using airplane. The inner temperature and air pressure of the container were kept stable during transportation. We evaluated the cell sheets using our validation method11 and obtained positive results. The sterility tests and screening for endotoxin and mycoplasma of the cell sheet inside the container were all negative even after transportation. Thus, it was strongly suggested that the oral mucosal epithelial cell sheets could be successfully used for ocular reconstruction in a clinical study 12 h after transportation. These results also indicate that we can carry cell sheets to locations far from the CPC, that is, other countries, and can also treat a large number of patients.
The organ-cultured cornea is a well-documented product concerning the microbiological safety and quality of the tissue.19,20 The stage of donor corneas in organ culture is up to 4–5 weeks, and the cornea can be transported to the hospital that requires the cornea. However, a preservation technique for cell sheets has not been established as yet. Therefore, immediate transportation after fabrication of cell sheets is required for clinical application. Tauber et al. reported precise temperature control of donor cornea tissue with a reusable thermal container.21 This newly developed device maintained steady acceptable air temperatures compared to conventional polystyrene containers with wet ice. Although this is a move forward in transportation methods for the donor cornea, only temperature stability was evaluated in the article. On the other hand, we not only developed a transportation container, but also proved that cell sheets can be safely transported using it.
Wright et al. recently reported an efficient transport/storage system for corneal epithelial cells using a structurally modified calcium alginate hydrogel.22 They demonstrated that controlling the alginate gel shape and pore size together provides a more practical and economical alternative to established corneal tissue/cell storage methods. However, they demonstrated that the suspended corneal epithelial cells could be stored or transported, whereas our method indicates that the final product for treatment, that is, tissue-engineered cell sheets, can be safely transported. If suspended cells are carried to the institute for surgery, the institute still requires a CPC to produce the final product for treatment.
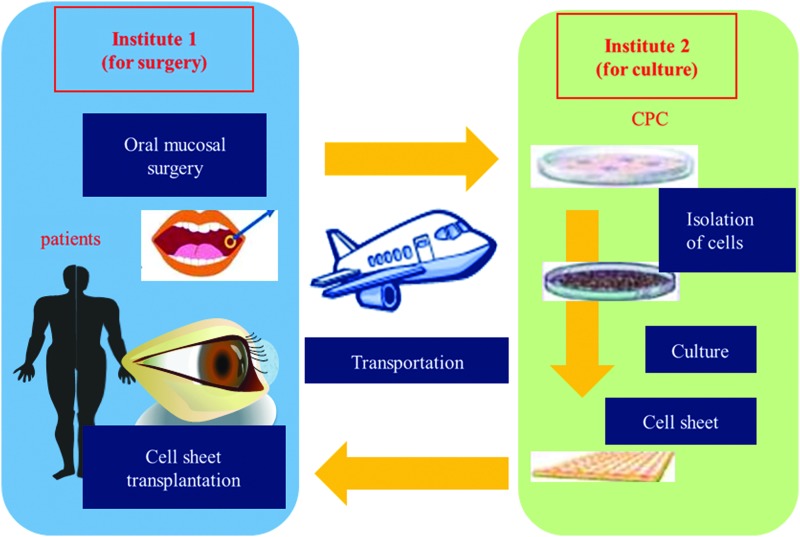
We are conducting a multicenter clinical study using the transportation technique described in this article (Fig. 10). In this clinical study, we will take oral mucosal epithelial tissues from patients in institute 1 and transport them to institute 2. At institute 2, a cell sheet will be fabricated in the CPC. Next, the cell sheet will be sent back to institute 1 for transplantation. We will culture oral mucosal epithelial cells from two other institutes in the Osaka University. Culturing at a single CPC enables better control of the quality of the tissue-engineered human oral mucosal epithelial cell sheets. If this effort is successful, we will be able to treat many patients in many hospitals all over the world without the need for a CPC.
FIG. 10.
A multicenter clinical study with the cell sheet transportation system: oral surgery for mucosal tissue and cell sheet transplantation are performed at institute 1; cell sheets are cultured in the CPC at institute 2. CPC, cell-processing center. Color images available online at www.liebertpub.com/tec
In conclusion, we developed a cell container that can maintain the inner temperature and pressure without the risk of biological contamination. Regenerative medicine for the corneal epithelium is one of the fields in which clinical application has progressed rapidly and has led to the need for and development of transportation techniques. We believe that the newly developed transportation technique for air travel is an essential technology for regenerative medicine and promotes the standardization and spread of regenerative therapies.
Acknowledgments
We thank Dr. Rika Homma (Kawagoe-Nishi eye clinic) for the valuable comments. This work was supported, in part, by the Advanced Medical Care Development Zone (Super Zone) program from the Cabinet Office, the Ministry of Economy, Trade and Industry, the Ministry of Health, Labor and Welfare, and the Ministry of Education in Japan. This study was additionally supported by a Health and Labor Sciences Research Grant (H16-Jitsuyouka-Shitei-001) from the Ministry of Health, Labor, and Welfare.
Disclosure Statement
No competing financial interests exist.
References
- 1.Kenyon K.R., and Tseng S.C.Limbal autograft transplantation for ocular surface disorders. Ophthalmology 96,709, 1989 [DOI] [PubMed] [Google Scholar]
- 2.Chen J.J., and Tseng S.C.Corneal epithelial wound healing in partial limbal deficiency. Invest Ophthalmol Vis Sci 31,1301, 1990 [PubMed] [Google Scholar]
- 3.Dua H.S., and Azuara-Blanco A.Autologous limbal transplantation in patients with unilateral corneal stem cell deficiency. Br J Ophthalmol 84,273, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tsubota K., Satake Y., Kaido M., et al. Treatment of severe ocular-surface disorders with corneal epithelial stem-cell transplantation. N Engl J Med 340,1697, 1999 [DOI] [PubMed] [Google Scholar]
- 5.Samson C.M., Nduaguba C., Baltatzis S., and Foster C.S.Limbal stem cell transplantation in chronic inflammatory eye disease. Ophthalmology 109,862, 2002 [DOI] [PubMed] [Google Scholar]
- 6.Ilari L., and Daya S.M.Long-term outcomes of keratolimbal allograft for the treatment of severe ocular surface disorders. Ophthalmology 109,1278, 2002 [DOI] [PubMed] [Google Scholar]
- 7.Shimazaki J., Shimmura S., Fujishima H., and Tsubota K.Association of preoperative tear function with surgical outcome in severe Stevens-Johnson syndrome. Ophthalmology 107,1518, 2000 [DOI] [PubMed] [Google Scholar]
- 8.Nishida K., Yamato M., Hayashida Y., et al. Corneal reconstruction with tissue-engineered cell sheets composed of autologous oral mucosal epithelium. N Engl J Med 351,1187, 2004 [DOI] [PubMed] [Google Scholar]
- 9.Nakamura T., Inatomi T., Sotozono C., et al. Transplantation of cultivated autologous oral mucosal epithelial cells in patients with severe ocular surface disorders. Br J Ophthalmol 88,1280, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Oie Y., Hayashi R., Takagi R., et al. A novel method of culturing human oral mucosal epithelial cell sheet using post-mitotic human dermal fibroblast feeder cells and modified keratinocyte culture medium for ocular surface reconstruction. Br J Ophthalmol 94,1244, 2010 [DOI] [PubMed] [Google Scholar]
- 11.Hayashi R., Yamato M., Takayanagi H., et al. Validation system of tissue engineered epithelial cell sheets for corneal regenerative medicine. Tissue Eng Part C Methods 16,553, 2010 [DOI] [PubMed] [Google Scholar]
- 12.Kushida A., Yamato M., Kikuchi A., and Okano T.Two-dimensional manipulation of differentiated Madin-Darby canine kidney (MDCK) cell sheets: the noninvasive harvest from temperature-responsive culture dishes and transfer to other surfaces. J Biomed Mater Res 54,37, 2001 [DOI] [PubMed] [Google Scholar]
- 13.Kushida A., Yamato M., Konno C., Kikuchi A., Sakurai Y., and Okano T.Decrease in culture temperature releases monolayer endothelial cell sheets together with deposited fibronectin matrix from temperature-responsive culture surfaces. J Biomed Mater Res. 15,355, 1999 [DOI] [PubMed] [Google Scholar]
- 14.Nozaki T., Yamato M., Inuma T., Nishida K., and Okano T.Transportation of transplantable cell sheets fabricated with temperature-responsive culture surfaces for regenerative medicine. J Tissue Eng Regen Med 2,190, 2008 [DOI] [PubMed] [Google Scholar]
- 15.Schermer A., Galvin S, and Sun T.T.Differentiation-related expression of a major 64K corneal keratin in vivo and in culture suggests limbal location of corneal epithelial stem cells. J Cell Biol 103,49, 1986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pellegrini G., Dellambra E., Golisano O., et al. p63 identifies keratinocyte stem cells. Proc Natl Acad Sci U S A 98,3156, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Net M., Trias E., Navarro A., Ruiz A., Diaz P., Fontenla J.R., and Manyalich M.Cold chain monitoring during cold transportation of human corneas for transplantation. Transplant Proc 35,2036, 2003 [DOI] [PubMed] [Google Scholar]
- 18.Macnab A.J., Pattman B., and Wadsworth L.D.Potentially fatal hemolysis of cross-matched blood during interfacility transport: standards of practice for safe transport of stored blood products. Air Med J 15,69, 1996 [DOI] [PubMed] [Google Scholar]
- 19.Pels E., and Rijneveld W.J.Organ culture preservation for corneal tissue. Technical and quality aspects. Dev Ophthalmol 43,31, 2009 [DOI] [PubMed] [Google Scholar]
- 20.Pels L.Organ culture: the method of choice for preservation of human donor corneas. Br J Ophthalmol 81,523, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tauber S., Bowman J., Bango J., and Fuerst R.Precise temperature control of donor cornea tissue with reusable passive thermal container. Cornea 30,977, 2011 [DOI] [PubMed] [Google Scholar]
- 22.Wright B., Cave R.A., Cook J.P., et al. Enhanced viability of corneal epithelial cells for efficient transport/strage using a structurally modified calcium alginate hydrogel. Regen Med 7,295, 2012 [DOI] [PubMed] [Google Scholar]