Abstract
Aims: The goal of this study was to use two manganese (Mn)-based superoxide dismutase (SOD) mimics to test the hypothesis that reactive oxygen species contribute to both acute and long-term outcomes in a galactose-1P uridylyltransferase (GALT)-null Drosophila melanogaster model of classic galactosemia. Results: We tested the impact of each of two Mn porphyrin SOD mimics, MnTnBuOE-2-PyP5+, and MnTE-2-PyP5+, (i) on survival of GALT-null Drosophila larvae reared in the presence versus absence of dietary galactose and (ii) on the severity of a long-term movement defect in GALT-null adult flies. Both SOD mimics conferred a significant survival benefit to GALT-null larvae exposed to galactose but not to controls or to GALT-null larvae reared in the absence of galactose. One mimic, MnTE-2-PyP5+, also largely rescued a galactose-independent long-term movement defect otherwise seen in adult GALT-null flies. The survival benefit of both SOD mimics occurred despite continued accumulation of elevated galactose-1P in the treated animals, and studies of thiolated proteins demonstrated that in both the presence and absence of dietary galactose MnTE-2-PyP5+ largely prevented the elevated protein oxidative damage otherwise seen in GALT-null animals relative to controls. Innovation and Conclusions: Our results confirm oxidative stress as a mediator of acute galactose sensitivity in GALT-null Drosophila larvae and demonstrate for the first time that oxidative stress may also contribute to galactose-independent adult outcomes in GALT deficiency. Finally, our results demonstrate for the first time that both MnTnBuOE-2-PyP5+ and MnTE-2-PyP5+ are bioavailable and effective when administered through an oral route in a D. melanogaster model of classic galactosemia. Antioxid. Redox Signal. 20, 2361–2371.
Introduction
Classic galactosemia is a potentially lethal, autosomal recessive disorder that results from profound deficiency of galactose-1-phosphate uridylyltransferase (GALT), the middle enzyme in the Leloir pathway of galactose metabolism (Fig. 1) (16). Most infants with classic galactosemia are born apparently healthy, but after exposure to breast milk or a milk-based formula, which contains large amounts of galactose, suffer a rapid and devastating demise. Early diagnosis by population newborn screening, coupled with immediate and rigorous dietary restriction of galactose, prevents or resolves the acute sequelae of classic galactosemia; however, significant complications appear later in childhood for many patients. These include speech and/or cognitive disabilities, behavioral issues, difficulties with movement, and ovarian insufficiency, among other problems. Although profound GALT deficiency has been the recognized biochemical basis of classic galactosemia for more than 50 years (22), the mechanisms that underlie the acute and long-term sequelae of this disorder remain unknown.
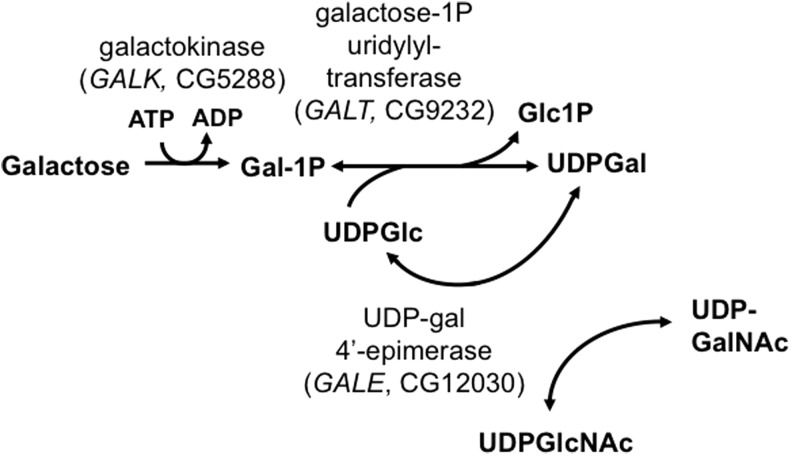
FIG. 1.
The Leloir pathway of galactose metabolism. Galactose 1-phosphate uridylyltransferase (GALT), the middle enzyme in the pathway, is profoundly impaired in patients with classic galactosemia. The three enzymes of the Leloir pathway, galactokinase (GALK), GALT, and UDP-galactose 4′-epimerase (GALE), are indicated together with the names of their encoding genes in Drosophila melanogaster.
Innovation.
The results reported here are significant in terms of their contribution to explaining the molecular pathogenesis of galactose sensitivity and long-term outcome in a Drosophila melanogaster model of classic galactosemia. Our findings also extend what is known about the impact of manganese porphyrin superoxide dismutase mimics provided via an oral route in Drosophila. Finally, our results highlight the potential role of protein-bound thiols as candidate markers of disease in galactose-1P uridylyltransferase deficiency.
Recently, we created a GALT-null Drosophila melanogaster model of classic galactosemia that, like patients, dies in development after exposure to high levels of galactose but is rescued by dietary galactose restriction (27). These animals also demonstrate a long-term movement defect that, like long-term complications in patients, appears to be independent of galactose exposure during development (41). Of note, both the acute and long-term defects observed in GALT-null Drosophila are rescued by expression of a human GALT transgene. This GALT-null fruit fly is the only whole animal genetic model reported to date that mimics outcomes reminiscent of classic galactosemia.
Previous studies from other groups have demonstrated that galactose exposure of mammalian cells in culture increases oxygen consumption and reliance on mitochondrial function (32), while galactose exposure of genetically wild-type animals, including fruit flies, rodents, and dogs, results in heightened oxidative stress and negative cognitive and physiological outcomes (9,14,43). Motivated by this literature trail, we previously tested whether oxidative stress might also contribute to the galactose sensitivity of GALT-null Drosophila (25). In brief, we exposed GALT-null and control Drosophila larvae to foods that either did or did not contain galactose, and also that either did or did not contain varying levels of known oxidants and antioxidants. We found that each of two oxidants tested, paraquat (1,1′-dimethyl-4-4′-bipyridinium dichloride) (6) and dimethyl sulfoxide (20), heightened the galactose sensitivity of GALT-null larvae at concentrations that showed no impact on controls, while each of two antioxidants tested, vitamin C (13,39) and α-mangostin (8,29), decreased the galactose sensitivity of GALT-null larvae at concentrations that showed no impact on controls. Biochemical studies, including measurement of oxidized and reduced glutathione (GSSG and GSH) and cysteine/cystine, demonstrated that galactose exposure caused heightened oxidative stress, especially in GALT-null animals (25), and quantitative real-time polymerase chain reaction studies of cDNA demonstrated a dramatic induction of transcript levels for two glutathione S-transferase genes (GSTD6 and GSTE7) (25) involved in oxidative signaling and response (1,31). These data illustrated the connection between galactose exposure and oxidative stress in Drosophila, and raised the intriguing possibility that GALT deficiency might magnify that connection. However, this prior work did not address the potential role of oxidative stress as a candidate mediator of the galactose-independent long-term complications experienced by galactosemia patients or GALT-null Drosophila.
Here we have extended from our previous work, testing the role of oxidative stress as a candidate mediator of both acute and long-term outcomes in GALT-null fruit flies. Specifically, we tested the impact of oral exposure of GALT-null larvae to each of two small molecule superoxide dismutase (SOD) mimics: manganese(III) meso-tetrakis(N-butoxyethylpyridinium-2-yl)porphyrin (MnTnBuOE-2-PyP5+, BMX-001) and manganese(III)meso-tetrakis(N-ethylpyridinium-2-yl)porphyrin (MnTE-2-PyP5+, AEOL10113). These Mn porphyrins act in a variety of mammalian cells and whole animal contexts as potent SOD mimics that (i) catalyze superoxide dismutation as seen with endogenous SODs, (ii) are widely bioavailable, and (iii) are relatively nontoxic (2). The cationic properties of Mn porphyrins favor their accumulation in critical intracellular compartments, including mitochondria, and promote binding to electron rich anionic reactive species enabling them to scavenge superoxide, peroxynitrite, and other free radicals (2).
In our experiments described here, oral treatment with MnTnBuOE-2-PyP5+ and MnTE-2-PyP5+ each provided significant survival benefit to GALT-null Drosophila larvae exposed to dietary galactose during development. This survival benefit was seen independent of changes in the accumulation of galactose-1-phosphate (gal-1P) but in parallel with changes in the levels of protein-bound glutathione and cysteine, two markers of protein oxidative damage in cells (12). Of note, 10 μM MnTE-2-PyP5+ also significantly rescued a movement defect otherwise seen in adult GALT-null Drosophila and, like the movement defect itself, this rescue was independent of exposure to dietary galactose. Combined, these results confirm oxidative stress as a mediator of the acute galactose sensitivity of GALT-null Drosophila larvae and for the first time also implicate oxidative stress as a modifier of the long-term, galactose-independent complications associated with GALT deficiency.
Results
Small molecule Mn-based mimics of SOD improve survival of GALT-null Drosophila larvae exposed to galactose during development
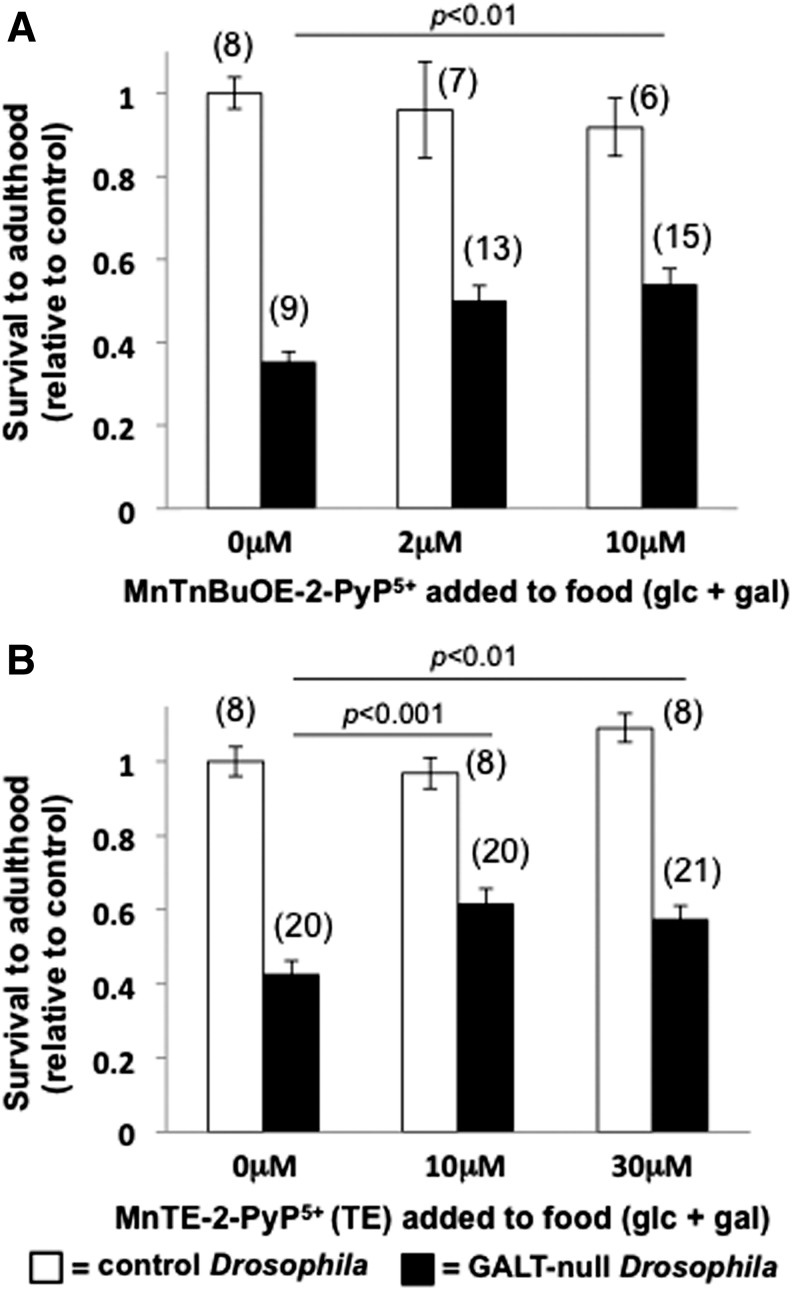
We tested the impact of each of two small molecule SOD mimics, MnTnBuOE-2-PyP5+ and MnTE-2-PyP5+ (2), on the survival rates of GALT-null and control Drosophila larvae reared in the presence versus absence of dietary galactose. In foods containing 555 mM glucose plus 200 mM galactose, both MnTnBuOE-2-PyP5+ and MnTE-2-PyP5+ increased the survival rates of GALT-null larvae to adulthood by 30% to 50% (p<0.01, Fig. 2 shaded bars); there was no significant impact of either SOD mimic on the survival rates of control larvae reared under comparable conditions (Fig. 2, open bars). Similarly, there was no significant impact of either SOD mimic on GALT-null or control larvae reared in the absence of dietary galactose (Supplementary Fig. S1; Supplementary Data are available online at www.liebertpub.com/ars).
FIG. 2.
Impact of small molecule superoxide dismutase mimics MnTnBuOE-2-PyP5+ and MnTE-2-PyP5+ on survival of control and GALT-null Drosophila larvae exposed to galactose. Relative survival of control (open bars) and GALT-null (shaded bars) Drosophila larvae reared on foods that contained both 555 mM glucose and 200 mM galactose in the absence versus presence of the indicated levels of MnTnBuOE-2-PyP5+ (A) and MnTE-2-PyP5+ (B). Values plotted represent average±standard error of the mean. The number of cohort replicates for each experimental condition is indicated in parenthesis above the corresponding bar. Relative survival of each cohort was calculated as the ratio of the number of larvae surviving to adulthood from that cohort over the number of control (GALT+/+) larvae surviving to adulthood in the same food lacking additive. Significant differences are indicated. Corresponding data for larvae raised in the absence of galactose are presented in Supplementary Figure S1.
A small molecule Mn-SOD mimic largely rescues a galactose-independent movement defect in adult GALT-null Drosophila
Previously, we demonstrated that adult GALT-null Drosophila exhibit a movement abnormality that is independent of dietary exposure to galactose (41). That this long-term defect in GALT-null flies occurs despite complete and life-long dietary restriction of galactose mirrors what is seen with long-term complications in patients with classic galactosemia (16). That these phenotypes are galactose independent also raises the important question of whether the mechanisms that underlie the long-term complications of GALT deficiency might be different from those that underlie the acute galactose sensitivity of both human infants with classic galactosemia and GALT-null Drosophila larvae.
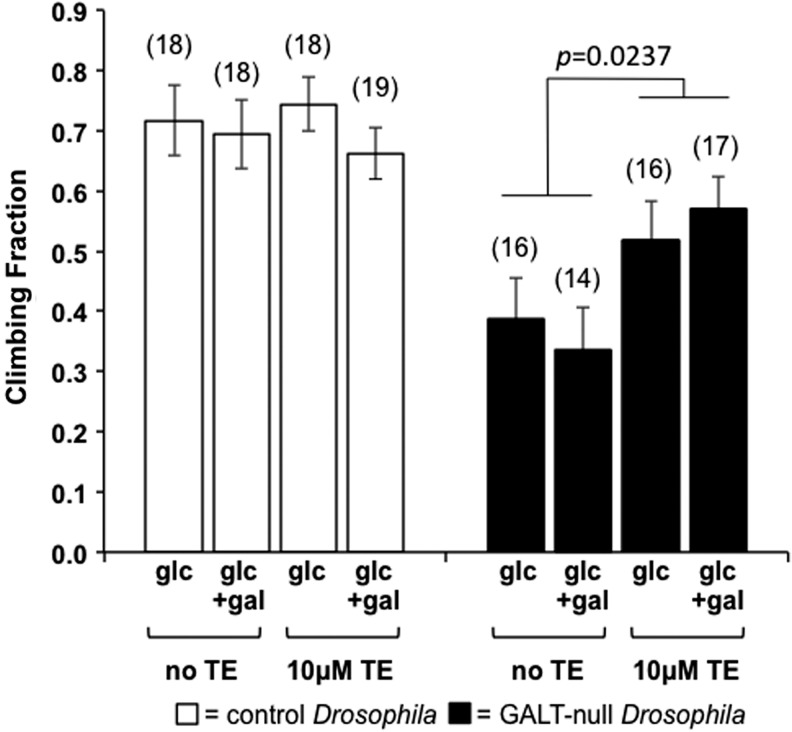
To test this possibility, we explored the impact of dietary MnTE-2-PyP5+ (TE) exposure on a movement defect in GALT-null flies using a modified simple climbing assay (see “Materials and Methods” section). To accentuate the difference in climbing ability between control and GALT-null animals, in this assay we incubated all flies at 39°C for 25 min immediately before testing (see “Materials and Methods” section). In the absence of TE the fraction of GALT-null flies that climbed above a designated mark in the allotted time was approximately half that seen for control animals; as expected (41) this same result was observed regardless of whether or not the animals were exposed to sublethal levels of dietary galactose in development (Fig. 3, shaded bars). However, the addition of 10 μM TE to the fly food significantly increased the fraction of GALT-null flies that climbed past the designated mark in the assay (p=0.0237); again, this result was independent of sublethal galactose exposure. Of note, the same level of TE exerted no significant impact on the movement phenotype of control animals (Fig. 3, open bars).
FIG. 3.
Small molecule superoxide dismutase mimic, MnTE-2-PyP5+, partially rescues a long-term movement defect in adult GALT-null Drosophila independent of galactose exposure. Control (open bars) and GALT-null (shaded bars) Drosophila were reared on foods containing either zero or 10 μM MnTE-2-PyP5+ (TE) in either the presence or absence of 50 mM galactose, and adult animals were tested for climbing ability at 2 days posteclosion after 25 min at 39°C immediately before testing (see “Materials and Methods” section). Among control flies close to 70% of each cohort climbed above a designated mark regardless of the presence or absence of TE. In contrast, only between 30% and 40% of each cohort of GALT-null flies climbed above the designated mark in the absence of TE, while close to 60% climbed above the mark in the presence of TE. This difference was significant and occurred independent of the presence or absence of 50 mM galactose in the food. Values plotted represent average±standard error of the mean, n≥14. The number of cohort replicates for each experimental condition is indicated in parenthesis above the corresponding bar.
We also conducted a limited test of the developmental window of TE's influence on long-term outcome in GALT-null Drosophila. In brief, instead of exposing GALT-null animals to TE as larvae and also as adults, as was the case above, we reared all of the larvae in the absence of TE, and then exposed a subset of newly eclosed male adults to food containing TE for 2 days before testing; the remaining animals continued to eat food lacking TE. Considering the nature of the outcome defect (impaired climbing ability) and the potential roles of both development and short-term energy metabolism as candidate modifiers of that outcome, it was not immediately obvious which time period, larval versus adult, might be the more susceptible to the apparent rescue. Climbing assays performed on both cohorts clearly demonstrated that short-term exposure to TE in adulthood was insufficient to rescue the climbing defect in the GALT-null flies (data not shown). While this result is consistent with a larval window of TE impact, other interpretations are not excluded. For example, larvae consume much larger quantities of food per body weight than do adults, and the effect of the SOD mimic might be cumulative over time. More detailed future studies will be required to define the developmental window when TE has its greatest impact on long-term outcome in GALT-null Drosophila.
MnTnBuOE-2-PyP5+ and MnTE-2-PyP5+ accumulate in both GALT-null and control Drosophila larvae
To assess how much MnTnBuOE-2-PyP5+ and MnTE-2-PyP5+ accumulated in the bodies of GALT-null and control Drosophila larvae under conditions where a survival and/or long-term benefit was observed, we raised parallel cohorts of animals in foods with glucose+galactose either with or without 10 μM MnTE-2-PyP5+ or 10 μM MnTnBuOE-2-PyP5+. Third-instar (L3) larvae were collected as they emerged from the food; these animals were processed as described in “Materials and Methods” section. As expected, larvae reared on foods lacking the SOD mimics showed no accumulation of these compounds, while larvae reared in the presence of these compounds showed clear accumulation. For GALT-null larvae the level of MnTE-2-PyP5+ detected was 7.3±1.6 pmol/mg protein and for MnTnBuOE-2-PyP5+ the level detected was 3.3±2.3 pmol/mg protein. For control larvae the corresponding levels were 3.3±0.5 and 1.3±0.2 pmol/mg protein, respectively. The implications of these levels and apparent differences are discussed below.
MnTnBuOE-2-PyP5+ and MnTE-2-PyP5+ show no impact on accumulation of gal-1P in GALT-null Drosophila larvae exposed to galactose
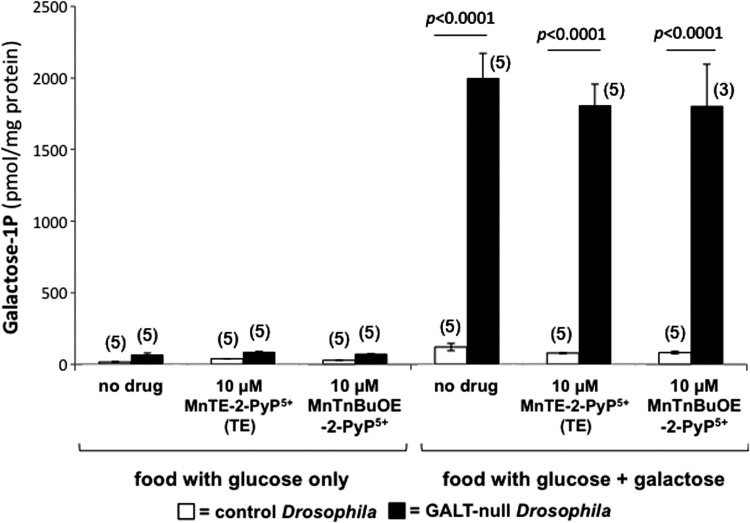
Gal-1P is a Leloir pathway metabolite that accumulates to very high levels in the tissues of both galactosemic infants [reviewed in (16)] and GALT-null Drosophila larvae exposed to galactose (27); however, the connection between gal-1P accumulation and acute or long-term outcomes remains unclear [reviewed in (16)]. Recently, we reported that a small collection of oxidants and antioxidants that modified survival of GALT-null Drosophila larvae exposed to galactose did so without impacting the apparent gal-1P levels in those animals (25). To test whether this was also true for SOD mimics we extracted and quantified gal-1P from GALT-null and control L3 larvae exposed to foods containing glucose versus glucose+galactose, either with or without 10 μM MnTnBuOE-2-PyP5+ or 10 μM MnTE-2-PyP5+ (see “Materials and Methods” section).
In the absence of galactose (Fig. 4, left side), control animals, represented by open bars, accumulated negligible gal-1P regardless of the presence or absence of the SOD mimics. Under these same conditions, GALT-null larvae, represented by shaded bars, accumulated slightly higher levels of gal-1P, perhaps reflecting the compromised ability of these animals to metabolize even endogenously synthesized galactose (5). In food containing galactose, however (Fig. 4, right side), the GALT-null larvae accumulated 30-fold increased gal-1P, and this accumulation was not significantly affected by the presence or absence of 10 μM MnTnBuOE-2-PyP5+ or MnTE-2-PyP5+. The positive impact of the SOD mimics on survival of GALT-null larvae exposed to galactose, and on the climbing defect of GALT-null adult flies independent of galactose exposure; therefore, cannot be explained simply by changes in the accumulation of gal-1P.
FIG. 4.
Impact of MnTnBuOE-2-PyP5+ and MnTE-2-PyP5+ on the accumulation of gal-1P in control and GALT-null Drosophila larvae reared in the presence versus absence of dietary galactose. Gal-1P was extracted from control (open bars) and GALT-null (shaded bars) late-stage larvae reared on foods either with or without 200 mM added galactose, and spiked with the indicated levels of MnTE-2-PyP5+ or MnTnBuOE-2-PyP5+. Significant differences are indicated. Values plotted represent average±standard error of the mean, n≥3, as indicated. The number of cohort replicates for each experimental condition is indicated in parenthesis above the corresponding bar.
Impact of MnTE-2-PyP5+ on the accumulation of thiolated proteins in GALT-null Drosophila larvae
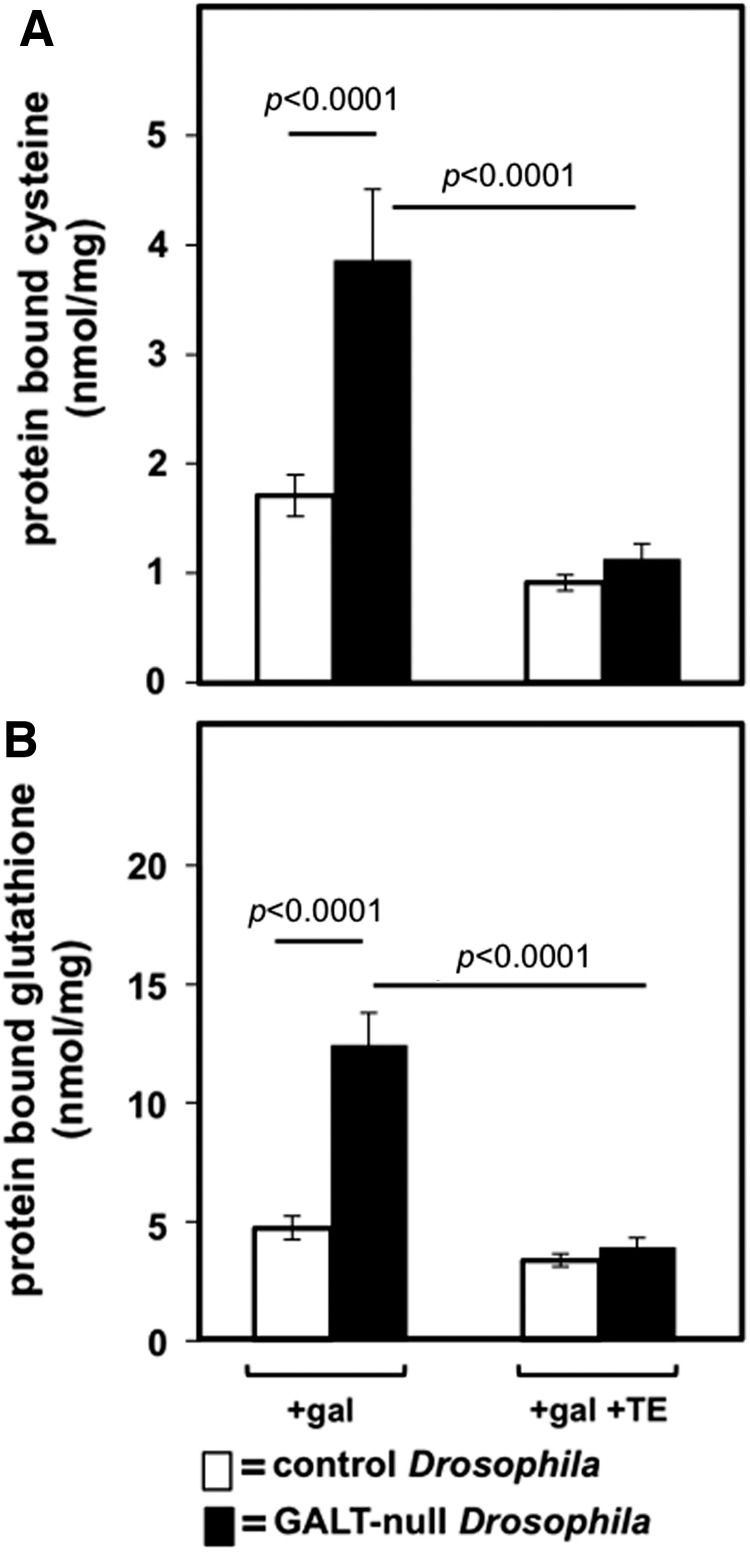
As a test of the impact of SOD mimics on oxidative stress in GALT-null and control Drosophila larvae we quantified the levels of protein-bound cysteine and glutathione in animals maintained in the presence of 200 mM dietary galactose (in addition to 555 mM glucose) and either in the presence or absence of 10 μM MnTE-2-PyP5+ (TE). Protein thiolation (e.g., protein bound to cystine or GSSG through disulfide bonds) is a recognized marker of oxidative damage in cells (12) and animals (37). In the presence of dietary galactose, GALT-null larvae exhibited close to twice the level of protein-bound cysteine and glutathione as was seen in control larvae (Fig. 5). When 10 μM TE was added to the food, however, the levels of protein-bound cysteine and glutathione in the GALT-null and control larvae were indistinguishable (Fig. 5). These results imply that in the presence of dietary galactose GALT-null Drosophila larvae experience increased protein oxidative damage relative to their GALT-normal counterparts, and exposure to the Mn-SOD mimic TE prevents or reverses that damage.
FIG. 5.
Impact of MnTE-2-PyP5+ on the abundance of protein-bound glutathione and cysteine in control and GALT-null Drosophila early larvae (L2) reared in the presence of dietary galactose. Protein thiolation is a marker of oxidative stress in biological systems. Samples tested were from control (open bars) and GALT-null (shaded bars) second instar (L2) larvae harvested from foods containing 200 mM galactose (in addition to 555 mM glucose) that did versus did not contain 10 μM MnTE-2-PyP5+, as indicated. Values plotted represent average±standard error of the mean, n=6. (A) Levels of protein-bound cysteine, and (B) levels of protein-bound glutathione, are plotted. Significant differences are indicated.
We also tested the levels of protein-bound cysteine and glutathione in GALT-null and control Drosophila larvae maintained in foods lacking galactose (Supplementary Fig. S2). In the absence of TE, GALT-null larvae again exhibited close to twice the level of protein-bound cysteine and glutathione as was seen in control larvae under parallel conditions, and again exposure to TE negated this difference. However, unlike in the presence of galactose, where TE clearly lowered the levels of protein-bound cysteine and glutathione in the GALT-null animals until they matched those in the controls (Fig. 5), in the absence of galactose the picture was more complex. In brief, in the absence of galactose TE appeared to equalize the protein-bound cysteine and glutathione in the GALT-null and control animals through a combination of both lowering the levels in the mutants and raising the levels in the controls, though individual changes did not always reach statistical significance (Supplementary Fig. S2). The explanation for this observation remains unclear, and is discussed below.
Impact of MnTnBuOE-2-PyP5+ and MnTE-2-PyP5+ on the levels of soluble GSSG and GSH and cysteine/cystine in GALT-null Drosophila larvae
We also monitored the effects of galactose and SOD mimics on the levels of soluble GSH and GSSG and cysteine/cystine in lysates from GALT-null and control second instar (L2) and L3 larvae (Supplementary Figs. S3 and S4). While some statistically significant differences were evident, overall we saw few patterns that explained the apparent rescue of acute or long-term outcomes by the SOD mimics.
For example, galactose exposure alone caused a significant rise in the level of GSSG in GALT-null L3s, but not in L2s (Supplementary Fig. S3) or in controls, and the L3 effect was independent of MnTnBuOE-2-PyP5+ or MnTE-2-PyP5+ treatment (Supplementary Fig. S4B). While we had observed the impact of galactose on GSSG levels previously (25), in our earlier study it was dwarfed by the impact of galactose combined with paraquat, and so was not highlighted. Intracellular redox state (Eh) in L3s calculated from the ratio of GSSG to GSH was also impacted by galactose, and minimally by MnTE-2-PyP5+ and MnTnBuOE-2-PyP5+, but only in GALT-null L3s and not in controls (Supplementary Fig. S4E, shaded bars), and not in L2s (Supplementary Fig. S3). In contrast, the levels of reduced cysteine (Cys) and oxidized cystine (CySS) were impacted by galactose exposure and in some cases also by MnTnBuOE-2-PyP5+ and MnTE-2-PyP5+ in both GALT-null and control L3s (Supplementary Fig. S4C, D) so that some small changes also were evident in the estimated extracellular redox status calculated from the ratio of CySS/Cys for these animals (Supplementary Fig. S4F). However, these differences were not observed in the L2s (Supplementary Fig. S3). Combined, there were no clear trends that explained the partial rescue of galactose sensitivity of GALT-null larvae by both MnTnBuOE-2-PyP5+ and MnTE-2-PyP5+, or the partial rescue of the galactose-independent movement defect in GALT-null adult flies by MnTE-2-PyP5+.
Discussion
Here we tested the ability of Mn-based SOD mimics, MnTnBuOE-2-PyP5+ and MnTE-2-PyP5+, to protect GALT-null Drosophila larvae against acute galactose toxicity and GALT-null adult flies against a galactose-independent long-term movement abnormality. We found that both mimics were effective at rescuing larval survival in the presence of galactose and one (MnTE-2-PyP5+) also showed significant rescue of the galactose-independent defect. These results support the hypothesis that reactive oxygen species (ROS), ostensibly scavenged by these SOD mimics, contribute to both acute galactose toxicity and also to galactose-independent long-term complications in GALT-null adult Drosophila. The results presented here further demonstrate the first clear evidence that MnTnBuOE-2-PyP5+ and MnTE-2-PyP5+ remain stable, bioavailable, and effective when introduced into a Drosophila model of classic galactosemia via an oral route.
A long literature trail documents the therapeutic potential of Mn porphyrins in a wide variety of pathological conditions [reviewed in (2)]. Our findings extend that list, confirming the therapeutic potential of these compounds in the face of galactose-mediated toxicity and a galactose-independent long-term complication in GALT-null flies. These acute and long-term outcome benefits occurred independent of changes in the accumulation of gal-1P and in parallel with resolution of the excessive protein oxidative damage (thiolation) that was otherwise seen in the GALT-null animals.
Galactose-1P
That the SOD mimics tested here improved both acute and long-term outcome in our GALT-null Drosophila without significantly impacting the accumulation of gal-1P in these animals is not surprising. This is the same result observed in our prior study testing the impact of both oxidants and antioxidants on acute outcome in GALT-null larvae (25). This result is notable, however, because it adds to the weight of evidence challenging the commonly held assumption that gal-1P accumulation underlies the acute and long-term complications experienced by patients with classic galactosemia.
Gal-1P accumulation is one of the most widely accepted biochemical markers of galactosemia used for diagnosis and follow-up, and gal-1P was long a prime suspect to underlie the acute if not the long-term sequelae of classic galactosemia [e.g., (7)]; indeed, small molecule inhibition of GALK to limit gal-1P synthesis has been proposed as a potential novel intervention for galactosemia [e.g., (7,45)]. However, a substantial body of published data also challenge the idea that gal-1P underlies the long-term sequelae of classic galactosemia. For example, infants with Duarte (DG) galactosemia exposed to milk accumulate levels of gal-1P that can rival those seen in infants with classic galactosemia (15), yet DG patients demonstrate none of the acute or severe long-term sequelae seen in classic galactosemics—whether or not they are taken off milk. Further, numerous studies of patients with classic galactosemia on treatment have failed to show a reproducible correlation between gal-1P levels and long-term outcome severity (19,42,48). Finally, a mouse GALT knock-out created by Dr. Nancy Leslie in the 1990s accumulated very high levels of gal-1P when exposed to dietary galactose, yet this mouse also failed to demonstrate any of the acute or long-term sequelae of classic galactosemia (30,34). Whether gal-1P is a disease marker versus modifier of outcome in GALT deficiency therefore, remains an open question.
Protein-bound cysteine and glutathione
It is interesting to note that in the presence of dietary galactose TE exposure lowered the levels of protein-bound cysteine and glutathione in GALT-null Drosophila to “control levels” (Fig. 5), while in the absence of galactose TE showed little if any effect on thiolated protein in GALT-null larvae but raised the levels in controls (Supplementary Fig. S2). This difference might reflect something about the different physiological states of the GALT-null and control larvae in the presence versus absence of dietary galactose, or might reflect potentially confounding effects of the experimental design that are not well understood at this time. One possible contributing factor derives from the reality that in the presence of 200 mM galactose, while the control larvae would be generally healthy despite the presence or absence of TE, the GALT-null larvae would not; in the absence of TE most of these larvae would be days from death, while in the presence of TE many more would be destined to survive.
It is also interesting to note that the differences in protein thiolation observed between cohorts of GALT-null versus control Drosophila larvae described here paralleled outcome but were not reflected in clear or consistent patterns of the soluble glutathione and cysteine/cystine redox pairs quantified in either L2 or L3 larvae from those cohorts. One possible explanation is that multiple or changing factors might have influenced the soluble redox pairs and thiolated proteins in these larvae concurrently, and the soluble and protein-bound thiols might have responded with different kinetics to the changing conditions leading to apparent disparities. Protein-bound glutathione has been described as an early response to mild oxidative stress (10,11), which may account for the changes we observed in protein-bound thiols in L2 larvae when no parallel changes were seen in soluble thiols at the same developmental stage. Of course, protein thiolation also plays a role in signal transduction (21). This additional role may account for the levels of protein-bound glutathione and protein-bound cysteine we recorded under glucose-only conditions as a consequence of TE supplementation, particularly in the control animals.
MnTnBuOE-2-PyP5+ and MnTE-2-PyP5+
Exposure of GALT-null Drosophila larvae to 10 μM MnTnBuOE-2-PyP5+ or 10 μM MnTE-2-PyP5+ in the presence of 200 mM galactose resulted in comparable degrees of larval rescue (Fig. 2), yet MnTE-2-PyP5+ accumulated to almost twice the level of MnTnBuOE-2-PyP5+ in those larvae (see “Results” section). Both cationic Mn porphyrins are equally redox active, with similar kcat for the catalysis of O2.− dismutation, and both are among the most potent known SOD mimics and peroxynitrite scavengers (2–4). Further, both are able to reduce levels of other reactive species, such as CO3.− radical, lipid radicals, and both also affect activation of redox-sensitive transcription factors impacting cellular inflammatory and immune responses (2–4,33). MnTE-2-PyP5+ is a hydrophilic molecule of molecular weight 965 Da, whereas MnTnBuOE-2-PyP5+, which has longer side-chains, has a molecular weight of 1254 Da and is close to 4 orders of magnitude more lipophilic as judged by octanol/water partition (36).
Despite these different properties, MnTnBuOE-2-PyP5+ and MnTE-2-PyP5+ exhibit very similar plasma oral bioavailability in mouse (22% and 23%, respectively) although in mouse heart MnTnBuOE-2-PyP5+ preferentially accumulates in mitochondria relative to cytosol by about 2.0-fold more than does MnTE-2-PyP5+ (Weitner et al., unpublished) (33,44,49). If this differential subcellular distribution also occurs in GALT-null Drosophila it might explain why both MnTE-2-PyP5+ and MnTnBuOE-2-PyP5+ provided similar levels of protection against galactose toxicity despite their observed differential levels of accumulation. Further, the twofold difference in the levels of MnTnBuOE-2-PyP5+ and MnTE-2-PyP5+ that accumulated in whole GALT-null larvae compared with control larvae raised under parallel conditions might reflect anything from differential body size and composition of the mutant and control animals, which could potentially influence the ability of the Mn porphyrins to enter or remain within cells or tissues, to differences in the amounts of food, and therefore, the amounts of SOD mimic, consumed by the mutant and control animals. Food was available to all larvae ad libitum in all experiments, and we did not measure the amount of food consumed by individual larvae.
It is also possible that the apparent differential accumulation of both SOD mimics in GALT-null versus control larvae reflected a combination of individual variation and ascertainment bias of the samples tested. In short, because a significant proportion of the GALT-null larvae would have died during the course of the experiment, but we only harvested living L3s for biochemical analysis, it is possible that those GALT-null larvae that consumed or accumulated more SOD mimic than their peers were more likely to survive, and therefore, more likely to be harvested and analyzed. Because the control larvae were unlikely to die regardless of how much SOD mimic they consumed or accumulated, they would not have been subject to this same selection bias. Future studies will be required to distinguish between these possibilities.
That both MnTnBuOE-2-PyP5+ and MnTE-2-PyP5+ provided similar survival benefits to GALT-null Drosophila exposed to galactose despite their chemical and other differences is notable. It is also striking that MnTE-2-PyP5+ largely rescued a long-term movement defect in adult GALT-null flies independent of galactose exposure. The simplest assumption regarding mechanism may be that the SOD mimics provided an antioxidant effect, in the food and in the larvae, perhaps in or near the mitochondria, reducing the accumulation of ROS and the oxidative damage, such as protein thiolation, that might otherwise follow. Of course, oxidative stress is not simply an imbalance of oxidants and antioxidants causing macromolecular damage but rather a disruption of redox signaling and control mechanisms [reviewed in (23,46)]. Hydrogen peroxide is recognized as a signaling molecule (17,18,47) and superoxide could also have signaling functions. Application of systems biology approaches with gene expression profiling, proteomics and other information-rich methods may be required to dissect the role of oxidative stress in acute and long-term outcomes in GALT deficiency. Such an approach will be the subject of future investigation.
Materials and Methods
Drosophila stocks and maintenance
We used two excision alleles of the D. melanogaster gene encoding GALT, dGALTΔAP2 and dGALTC2, each generated by mobilizing an existing P insertion in the 5′-untranslated region of the CG9232 locus (KG00049), as previously described (27). dGALTΔAP2 carries a 1647 bp deletion that removes most of the dGALT gene and encodes no detectable GALT enzymatic activity, while dGALTC2 carries a precise excision of the P element and encodes wild-type GALT activity. We have demonstrated previously that flies homozygous for the dGALTΔAP2 allele die in development when raised on fly food containing both glucose and galactose (27); before death these animals also accumulate substantial levels of the metabolite gal-1P. Drosophila homozygous for the control allele remain healthy despite the presence of galactose in their food, and these animals accumulate only low levels of gal-1P. In the absence of dietary galactose both control and GALT-null Drosophila larvae survive at comparable rates.
Unless otherwise indicated, the fly stocks used in these experiments were maintained at 25°C on molasses-based food that contained 43.5 g/L cornmeal, 17.5 g/L yeast extract, 8.75 g/L agar, 54.7 ml/L molasses, 10 ml/L propionic acid, and 14.4 ml/L tegosept mold inhibitor (10% w/v in ethanol). For experiments that measured galactose sensitivity, animals were reared under non-overcrowding conditions on a glucose-based diet that consisted of 555 mM glucose (Fisher Scientific Co.), 5.5 g/L agar, 40 g/L yeast, 90 g/L cornmeal, 10 ml/L propionic acid, 14 ml/L tegosept mold inhibitor (10% w/v in ethanol), and the indicated amount of D-(+)-galactose (Sigma-Aldrich Corp.) added from a 20% w/v galactose solution. Other compounds to be tested also were added to the desired final concentration, while the food was cooling but still in a liquid state to ensure homogeneous distribution.
Survival of Drosophila larvae to adulthood
To test the impact of varying dietary exposures on survival of our Drosophila larvae we followed our previously described protocol (25). Both dGALTΔAP2 and dGALTC2 homozygotes were raised under non-overcrowding conditions in parallel vials on glucose-only (555 mM) or glucose (555 mM) plus galactose (200 mM) food, which either did or did not include each additive. The galactose concentration for these experiments (200 mM) was selected as previously described (25) because it enabled us to identify additives that both increased and decreased survival rates. To control for larval density, parents of the desired genotypes were allowed to mate and deposit embryos for 24 h on grape juice/agar plates to generate embryo cohorts. Twenty-four hours later, groups of 20 first-instar larvae were collected and transferred to 12×75 mm2 polystyrene vials each containing 2 ml of the appropriate fly food. Each vial was plugged with cotton and maintained under conditions of controlled temperature (25°C) and humidity (60%) and monitored for 19 days. Over the course of this time, the number of adults eclosing in each vial was recorded. Ten to 20 replicate vials were monitored for each genotype and condition; statistical analyses were performed as described below. Values plotted were normalized to the survival rate of larvae in the corresponding control lacking additive.
Modified climbing assay for adult Drosophila flies
Drosophila homozygous for dGALTΔAP2 or dGALTC2 were raised on 555 mM glucose-only (555 mM) or glucose (555 mM) plus galactose (50 mM) food, which either did or did not include 10 μM MnTE-2-PyP5+. Newly eclosed adult males were collected into fresh vials containing the same food and maintained at 25°C for 36–48 h before testing in cohorts of 10. On the day of the experiment animals were incubated at 39°C for 25 min, as described previously (38), and immediately tested in a simple climbing assay. The numbers of flies in each cohort capable of climbing 2 cm in 5 s were recorded.
Compounds tested
We tested the impact of two small molecule mimics of SOD: manganese(III) meso-tetrakis(N-(2′-butoxyethyl)pyridinium-2-yl) porphyrin (MnTnBuOE-2-PyP5+) and manganese(III)meso-tetrakis(N-ethylpyridinium-2-yl)porphyrin (MnTE-2-PyP5+), both kindly provided with permission by BioMimetix Pharmaceutical, Inc. Stock solutions of MnTnBuOE-2-PyP5+ and MnTE-2-PyP5+ were supplied already suspended in phosphate buffered saline (PBS). The same volumes of solvent and/or additive were added to each batch of food. We were careful to avoid exposing supplements to high temperatures or excessive light, as recommended by the suppliers. The doses of the SOD mimics were selected based on the understanding that MnTnBuOE-2-PyP5+ is well-tolerated by yeast in a range of 5–30 μM (36) and that for MnTE-2-PyP5+ at least 5 μM is required for mitochondrial protection (2).
Galactose-1P extraction and analysis
Cohorts of 20 L3 wandering larvae were collected from vials housing the indicated genotype of Drosophila reared in fly food containing 555 mM glucose either with or without 200 mM galactose and/or 10 μM MnTnBuOE-2-PyP5+ or 10 μM MnTE-2-PyP5+. Each cohort of larvae was placed into 125 μl of ice-cold high performance liquid chromatography (HPLC) grade water and ground for 15 s using a Teflon micropestle and handheld motor (Kimble Chase Life Science and Research Products LLC). A sample was taken from each lysate for protein quantification using the BioRad DC Assay with bovine serum albumin (BSA) as the standard. Galactose-1P was extracted from the remaining lysate as previously described (35,40). The aqueous phase was dried under vacuum with no heat (Eppendorf Vacufuge). All samples were normalized for protein concentration by dilution with HPLC-grade water, and then centrifuged through 0.22-μm Costar Spin-X centrifuge tube filters (Corning Inc.) at 4000g for 4 min to remove any particulates. Finally, the soluble phase from each sample was transferred to a glass HPLC vial and analyzed using a Dionex ICS-2500 instrument fitted with a CarboPac PA10 4×250 mm2 analytical column; metabolites were separated and gal-1P was quantified as previously described (40).
Accumulation of MnTnBuOE-2-PyP5+ and MnTE-2-PyP5+ in Drosophila larvae
MnTnBuOE-2-PyP5+ and MnTE-2-PyP5+ were measured in three to five replicate soluble lysates each prepared from 20 wandering L3 larvae collected from vials containing fly food with 555 mM glucose+200 mM galactose with either no SOD mimic added, or with 10 μM MnTnBuOE-2-PyP5+ or 10 μM MnTE-2-PyP5+. Each cohort of larvae was placed into 125 μl of ice-cold lysis buffer [one complete mini protease inhibitor cocktail pellet, ethylenediaminetetraacetic acid free (Roche) dissolved in 10 ml of 100 mM glycine, pH 8.7] and ground for 15 s using a Teflon micropestle and handheld motor (Kimble Chase Life Science and Research Products LLC). A sample was taken from each lysate for protein quantification (using the BioRad DC Assay with BSA as a standard).
Liquid chromatography-tandem mass spectroscopy (LC-MS/MS) analysis of MnTE-2-PyP5+ and MnTnBuOE-2-PyP5+ was performed on an Agilent 1200 series HPLC (LC) - Applied Biosystems MDS Scie4000 tandem-mass spectrometer (MS/MS) at the Pharmacology Laboratory (Shared Resource PK/PD and Small Molecule Analysis Core) of the Duke Cancer Institute, as described previously for MnTE-2-PyP5+ and MnTnHex-2-PyP5+ (49). In brief, the analytes were extracted from the lysate and proteins precipitated by addition of a two-fold volume of 1% acetic acid in methanol; the supernatant was dried under nitrogen, the residue reconstituted in initial composition of LC elution gradient, and 40 μl was injected into the LC-MS/MS system. The method was modified to include measurement of MnTnBuOE-2-PyP5+ at m/z 857.3/643.4. MnTnHep-2-PyP5+ was used as an internal standard at m/z 853.5/639.5. Mobile phase A: 1% acetonitrile and 0.05% heptafluorobutyric acid in LC/MS grade water. Mobile phase B: 50% acetonitrile and 50% methanol. Elution gradient: 0–0.2 min, 20% B; 0.2–0.21 min, 20%–95% B; 0.21–0.7 min, 95% B; 0.7–0.71 min, 95%–20% B; 0.71–3 min, 20% B. Assay quantification range: 0.5 (lower limit of quantitation)–10 pmol/mg.
Measurements of protein-bound glutathione and cysteine in Drosophila larvae
Cohorts of L2 larvae were collected by floatation in a 20% glucose solution, rinsed with PBS and collected on ice in microcentrifuge tubes containing 500 μl ice-cold 100 g/L trichloroacetic acid solution. Larvae were homogenized for 15 s using a Teflon micropestle and handheld motor (Kimble Chase Life Science and Research Products) and the homogenate centrifuged at 15,700g for 5 min at 4°C. Pellets were washed with acetone, and then resuspended in 50 μl of 0.1 M sodium hydroxide. Samples were treated sequentially with 2 mM dithiothreitol and 50 g/L perchloric acid. Aliquots of 300 μl of the supernatant were transferred to fresh tubes for further analysis. The remaining supernatant fluid was discarded and the protein pellets and supernatant were stored at −80°C until analysis. Pellets were resuspended in 300 μl of 1 N sodium hydroxide overnight to measure the amount of acid-insoluble protein using the BioRad DC Assay with BSA as a standard. Supernatant was prepared as previously described (25).
Measurements of soluble GSH and GSSG and cysteine/cystine
Lysates were prepared and analyzed from cohorts of 30 wandering L3 larvae as described previously (25). Cohorts of L2 larvae (wet mass about 50 mg) were collected from plates of fly food by floatation in a 20% glucose solution and rinsed with PBS before lysis, as described (25). Concentrations of thiols and disulfides were determined by integration relative to an internal standard (24). Redox potential (Eh) was calculated from the GSH and GSSG, or Cys and CySS, concentrations by the Nernst equation as described previously (26).
Statistical analyses
Experiments testing the survival rates of GALT-null and control larvae reared under different conditions were performed in parallel by two experimenters who each loaded a comparable number of replicate vials per genotype and treatment group. We then analyzed survival rates for each diet (i.e., with and without galactose) separately. Values plotted were normalized to the survival rate of larvae in the corresponding control lacking additive. For each SOD-mimic tested, two-way analysis of covariance (with genotype and treatment as independent variables) with experimenter as covariate was used to compare significant differences in survival to adulthood for both genotypes. A linear regression with genotype, galactose, and additive as independent variables and with all four possible interaction terms between those variables was used to compare differences in long-term climbing outcome, metabolite accumulation, soluble GSSG and GSH and cysteine levels, and protein-bound glutathione and cysteine levels. Post-hoc tests were performed on the least-square means to determine significant differences between groups. The criterion for statistical significance was p<0.05 but p-values were adjusted for multiple comparisons when applicable using a simple Bonferroni correction. Statistical analyses were performed using either SAS (Version 9.2; SAS Institute Cary) or JMP-SAS software (Version 10.0).
Supplementary Material
Abbreviations Used
- BSA
bovine serum albumin
- Cys
cysteine (reduced state)
- CySS
cystine (oxidized state)
- Da
Dalton
- Gal-1P
galactose-1-phosphate
- GALE
UDP-galactose 4′-epimerase
- GALK
galactokinase
- GALT
galactose-1-phosphate uridylyltransferase
- GSH
glutathione (reduced state)
- GSSG
glutathione (oxidized state)
- HPLC
high performance liquid chromatography
- L2
second instar
- L3
third instar
- LC-MS/MS
liquid chromatography-tandem mass spectroscopy
- Mn
manganese
- MnTE-2-PyP5+
manganese(III) meso-tetrakis(N-ethylpyridinium-2-yl)porphyrin
- MnTnBuOE-2-PyP5+
manganese(III) meso-tetrakis(N-butoxyethylpyridinium-2-yl)porphyrin
- PBS
phosphate buffered saline
- ROS
reactive oxygen species
- SOD
superoxide dismutase
Acknowledgments
We thank our colleagues in the Fridovich-Keil, Jones, and Sanyal laboratories at Emory University and also Dr. Douglas Moellering at the University of Alabama at Birmingham for many helpful discussions. We are especially grateful to Dr. Young-Mi Go Kang and Mr. Michael Orr in the Jones lab for their advice and help. We also thank Dr. James Crapo and colleagues at BioMimetix Pharmaceutical, Inc., for allowing us to use MnTnBuOE-2-PyP5+ and MnTE-2-PyP5+ in these studies. This work was supported in part by funds from the National Institutes of Health (R01DK046403 to J.L.F.K.). H.M.B. was supported in part by funds from the Howard Hughes Medical Institute (grant 52005873) in support of the Summer Undergraduate Research Program at Emory (SURE).
Author Disclosure Statement
Both Drs. Batinic-Haberle and Spasojevic are consultants for BioMimetix Pharmaceutical, Inc., Duke University and Drs. Batinic-Haberle and Spasojevic also have patent rights and have licensed technology to BioMimetix Pharmaceutical, Inc., related to this technology.
All other coauthors have nothing to disclose.
References
- 1.Alias Z. and Clark AG. Studies on the glutathione S-transferase proteome of adult Drosophila melanogaster: responsiveness to chemical challenge. Proteomics 7: 3618–3628, 2007 [DOI] [PubMed] [Google Scholar]
- 2.Batinic-Haberle I, Rajic Z, Tovmasyan A, Reboucas J, Ye X, Leong K, Dewhirst M, Vujaskovic Z, Benov L, and Spasojevic I. Diverse functions of cationic Mn(III) N-substituted pyridylporphyrins, recognized as SOD mimics. Free Radic Biol Med 51: 1035–1053, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Batinic-Haberle I, Reboucas J, and Spasojevic I. Superoxide dismutase mimics: chemistry, pharmacology, and therapeutic potential. Antioxid Redox Signal 13: 877–918, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Batinic-Haberle I, Spasojevic I, Tse H, Tovmasyan A, Rajic Z, St Clair D, Vujaskovic Z, Dewhirst M, and Piganelli J. Design of Mn porphyrins for treating oxidative stress injuries and their redox-based regulation of cellular transcriptional activities. Amino Acids 42: 95–113, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Berry GT, Nissim I, Lin Z, Mazur AT, Gibson JB, and Segal S. Endogenous synthesis of galactose in normal men and patients with hereditary galactosemia. Lancet 346: 1073–1074, 1995 [DOI] [PubMed] [Google Scholar]
- 6.Bonilla E, Medina-Leendertz S, Villalobos V, Molero L, and Bohórquez A. Paraquat-induced oxidative stress in Drosophila melanogaster: effects of melatonin, glutathione, serotonin, minocycline, lipoic acid and ascorbic acid. Neurochem Res 31: 1425–1432, 2006 [DOI] [PubMed] [Google Scholar]
- 7.Bosch AM. Classical galactosaemia revisited. J Inherit Metab Dis 29: 516–525, 2006 [DOI] [PubMed] [Google Scholar]
- 8.Bumrungpert A, Kalpravidh RW, Chuang C-C, Overman A, Martinez K, Kennedy A, and McIntosh M. Xanthones from mangosteen inhibit inflammation in human macrophages and in human adipocytes exposed to macrophage-conditioned media. J Nutr 140: 842–847, 2010 [DOI] [PubMed] [Google Scholar]
- 9.Cui X, Wang L, Zuo P, Han Z, Fang Z, Li W, and Liu J. D-Galactose-caused life shortening in Drosophila melanogaster and Musca domestica is associated with oxidative stress. Biogerontology 5: 317–325, 2004 [DOI] [PubMed] [Google Scholar]
- 10.Dalle-Donne I, Rossi R, Giustarini D, Colombo R, and Milzani A. Is there an answer? IUBMB Life 57: 189–192, 2005 [DOI] [PubMed] [Google Scholar]
- 11.Dalle-Donne I, Rossi R, Giustarini D, Colombo R, and Milzani A. S-glutathionylation in protein redox regulation. Free Radic Biol Med 43: 883–898, 2007 [DOI] [PubMed] [Google Scholar]
- 12.Di Simplicio P, Franconi F, Frosalí S, and Di Giuseppe D. Thiolation and nitrosation of cysteines in biological fluids and cells. Amino Acids 25: 323–339, 2003 [DOI] [PubMed] [Google Scholar]
- 13.Duarte TL, and Lunec J. Review part of the series: from dietary antioxidants to regulators in cellular signalling and gene expression review: when is an antioxidant not an antioxidant? A review of novel actions and reactions of vitamin C. Free Radic Res 39: 671–686, 2005 [DOI] [PubMed] [Google Scholar]
- 14.Engerman RL, and Kern TS. Experimental galactosemia produces diabetic-like retinopathy. Diabetes 33: 97–100, 1984 [DOI] [PubMed] [Google Scholar]
- 15.Ficicioglu C, Thomas N, Yager C, Gallagher PR, Hussa C, Mattie A, Day-Salvatore DL, and Forbes BJ. Duarte (DG) galactosemia: a pilot study of biochemical and neurodevelopmental assessment in children detected by newborn screening. Mol Genet Metab 95: 206–212, 2008 [DOI] [PubMed] [Google Scholar]
- 16.Fridovich-Keil JL, and Walter JH. Galactosemia. In: The Online Metabolic and Molecular Bases of Inherited Disease, edited by Valle D, Beaudet A, Vogelstein B, Kinzler K, Antonarakis S, and Ballabio A. New York, NY: McGraw Hill, 2008. www.ommbid.com/ [Google Scholar]
- 17.Go Y, and Jones D. Redox control systems in the nucleus: mechanisms and functions. Antioxid Redox Signal 13: 489–509, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Go Y, and Jones D. Thiol/disulfide redox states in signaling and sensing. Crit Rev Biochem Mol Biol 48: 173–181, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hughes J, Ryan S, Lambert D, Geoghegan O, Clark A, Rogers Y, Hendroff U, Monavari A, Twomey E, and Treacy E. Outcomes of siblings with classical galactosemia. J Pediatr 154: 721–726, 2009 [DOI] [PubMed] [Google Scholar]
- 20.Hyland K, and Auclair C. The formation of superoxide radical anions by a reaction between O2, OH- and dimethyl sulfoxide. Biochem Biophys Res Commun 102: 531–537, 1981 [DOI] [PubMed] [Google Scholar]
- 21.Iciek M, Chwatko G, Lorenc-Koci E, Bald E, and Włodek L. Plasma levels of total, free and protein bound thiols as well as sulfane sulfur in different age groups of rats. Acta Biochim Pol 51: 815–824, 2004 [PubMed] [Google Scholar]
- 22.Isselbacher KJ, Anderson EP, Kurahashi K, and Kalckar HM. Congenital galactosemia, a single enzyme block in galactose metabolism. Science 123: 635–636, 1956 [DOI] [PubMed] [Google Scholar]
- 23.Jones D. Redefining oxidative stress. Antioxid Redox Signal 8: 1865–1879, 2006 [DOI] [PubMed] [Google Scholar]
- 24.Jones DP, Carlson JL, Mody VC, Cai J, Lynn MJ, and Sternberg P. Redox state of glutathione in human plasma. Free Radic Biol Med 28: 625–635, 2000 [DOI] [PubMed] [Google Scholar]
- 25.Jumbo-Lucioni P, Hopson M, Hang D, Liang Y, Jones D, and Fridovich-Keil J. Oxidative stress contributes to outcome severity in a Drosophila melanogaster model of classic galactosemia. Dis Model Mech 6: 84–94, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kirlin WG, Cai J, Thompson SA, Diaz D, Kavanagh TJ, and Jones DP. Glutathione redox potential in response to differentiation and enzyme inducers. Free Radic Biol Med 27: 1208–1218, 1999 [DOI] [PubMed] [Google Scholar]
- 27.Kushner RF, Ryan EL, Sefton JM, Sanders RD, Lucioni PJ, Moberg KH, and Fridovich-Keil JL. A Drosophila melanogaster model of classic galactosemia. Dis Model Mech 3: 618–627, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.This reference has been deleted.
- 29.Larson RT, Lorch JM, Pridgeon JW, Becnel JJ, Clark GG, and Lan Q. The biological activity of alpha-mangostin, a larvicidal botanic mosquito sterol carrier protein-2 inhibitor. J Med Entomol 47: 249–257, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Leslie ND, Yager KL, McNamara PD, and Segal S. A mouse model of galactose-1-phosphate uridyl transferase deficiency. Biochem Mol Med 59: 7–12, 1996 [DOI] [PubMed] [Google Scholar]
- 31.Li HM, Buczkowski G, Mittapalli O, Xie J, Wu J, Westerman R, Schemerhorn BJ, Murdock LL, and Pittendrigh BR. Transcriptomic profiles of Drosophila melanogaster third instar larval midgut and responses to oxidative stress. Insect Mol Biol 17: 325–339, 2008 [DOI] [PubMed] [Google Scholar]
- 32.Marroquin L, Hynes J, Dykens J, Jamieson J, and Will Y. Circumventing the crabtree effect: replacing media glucose with galactose increases susceptibility of HepG2 cells to mitochondrial toxicants. Toxicol Sci 97: 539–547, 2007 [DOI] [PubMed] [Google Scholar]
- 33.Miriyala S, Spasojevic I, Tovmasyan A, Salvemini D, Vujaskovic Z, St Clair D, and Batinic-Haberle I. Manganese superoxide dismutase, MnSOD and its mimics. Biochim Biophys Acta 1822: 794–814, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ning C, Reynolds R, Chen J, Yager C, Berry GT, McNamara PD, Leslie N, and Segal S. Galactose metabolism by the mouse with galactose-1-phosphate uridyltransferase deficiency. Pediatr Res 48: 211–217, 2000 [DOI] [PubMed] [Google Scholar]
- 35.Openo K, Schulz J, Vargas C, Orton C, Epstein M, Schnur R, Scaglia F, Berry G, Gottesman G, Ficicioglu C, Slonim A, Shroer R, Yu C, Rangel V, Kenan J, Lamance K, and Fridovich-Keil J. Epimerase-deficiency galactosemia is not a binary condition. Am J Hum Genet 78: 89–102, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rajic Z, Tovmasyan A, Spasojevic I, Sheng H, Lu M, Li A, Gralla E, Warner D, Benov L, and Batinic-Haberle I. A new SOD mimic, Mn(III) ortho N-butoxyethylpyridylporphyrin, combines superb potency and lipophilicity with low toxicity. Free Radic Biol Med 52: 1828–1834, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rebrin I, Bayne A, Mockett R, Orr W, and Sohal R. Free aminothiols, glutathione redox state and protein mixed disulphides in aging Drosophila melanogaster. Biochem J 382: 131–136, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Repnikova E, Koles K, Nakamura M, Pitts J, Li H, Ambavane A, Zoran M, and Panin V. Sialyltransferase regulates nervous system function in Drosophila. J Neurosci 30: 6466–6476, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rose RC, and Bode AM. Biology of free radical scavengers: an evaluation of ascorbate. FASEB J: official publication of the Federation of American Societies for Experimental Biology 7: 1135–1142, 1993 [PubMed] [Google Scholar]
- 40.Ross KL, Davis CN, and Fridovich-Keil JL. Differential roles of the Leloir pathway enzymes and metabolites in defining galactose sensitivity in yeast. Mol Genet Metab 83: 103–116, 2004 [DOI] [PubMed] [Google Scholar]
- 41.Ryan E, Duboff B, Feany M, and Fridovich-Keil J. Mediators of a long-term movement abnormality in a Drosophila melanogaster model of classic galactosemia. Dis Model Mech 5: 796–803, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schweitzer S, Shin Y, Jakobs C, and Brodehl J. Long-term outcome in 134 patients with galactosemia. Eur J Pediatr 152: 36–43, 1993 [DOI] [PubMed] [Google Scholar]
- 43.Shen Y, Xu S, Wei W, Sun X, Yang J, Liu L, and Dong C. Melatonin reduces memory changes and neural oxidative damage in mice treated with D-galactose. J Pineal Res 32: 173–178, 2002 [DOI] [PubMed] [Google Scholar]
- 44.Spasojevic I, Miryala S, Tovmasyan A, Salvemini D, Vujaskovic Z, Batinic-Haberle I, and St Clair D. Lipophilicity of Mn(III) N-alkylpyridylporphyrins dominates their accumulation within mitochondria and therefore in vivo efficacy. A mouse study. Free Radic Biol Med 51: S98, 2011 [Google Scholar]
- 45.Tang M, Odejinmi S, Vankayalapati H, Wierenga K, and Lai K. Innovative therapy for classic galactosemia: tale of two HTS. Mol Genet Metab October2011[Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Thannickal V, and Fanburg B. Reactive oxygen species in cell signaling. Am J Physiol Lung Cell Mol Physiol 279: L1005–L1028, 2000 [DOI] [PubMed] [Google Scholar]
- 47.Truong T, and Carroll K. Redox regulation of protein kinases. Crit Rev Biochem Mol Biol May3, 2013[Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Walter JH, Collins JE, Leonard JV, Chiswick M, and Marcovitch H. Recommendations for the management of galactosaemia: commentary. Arch Dis Child 80: 93–96, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Weitner T, Sheng H, Miriyala S, Leu D, Tovmasyan A, Kos I, Reboucas J, Fan P, Vujaskovic Z, Batinic-Haberle I, Huang T, St Clair D, Warner D, and Spasojevic I. Comprehensive pharmocokinetic studies and biodistribution of two cationic Mn porphyrins-based catalysts, MnTE-2-PyP5+ and MnTnHex-2-PyP5+: plasma and organ oral availability, mitochondrial, cytosolic, whole brain, hippocampus and cortex distribution. Free Radic Biol Med 53: S118, 2012 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.