Abstract
The central Blood Pressure (CBP) has been established as a relevant indicator of cardiovascular disease. Despite its significance, CBP remains particularly challenging to measure in standard clinical practice. The objective of this study is to introduce Pulse Wave-based Ultrasound Manometry (PWUM) as a simple-touse, non-invasive ultrasound-based method for quantitative measurement of the central pulse pressure. Arterial wall displacements are estimated using radiofrequency (RF) ultrasound signals acquired at high frame rates and the pulse pressure waveform is estimated using both the distension waveform and the local Pulse Wave Velocity (PWV). The method was tested on the abdominal aorta of 11 healthy subjects (age 35.7± 16 y.o.). PWUM pulse pressure measurements were compared to those obtained by radial applanation tonometry using a commercial system. The average intra-subject variability of the pulse pressure amplitude was found to be equal to 4.2 mmHg, demonstrating good reproducibility of the method. Excellent correlation was found between the waveforms obtained by PWUM and those obtained by tonometry in all subjects (0.94 <r < 0.98). A significant bias of 4.7 mmHg was found between PWUM and tonometry. PWUM is a highly translational method that can be easily integrated in clinical ultrasound imaging systems. It provides an estimate of the pulse pressure waveform at the imaged location, and may offer therefore the possibility to estimate the pulse pressure at different arterial sites. Future developments include the validation of the method against invasive estimates on patients, as well as its application to other large arteries.
1. Introduction
Measuring Blood Pressure (BP) by sphygmomanometry at the brachial artery is a well-established technique and widely used at the point of care for the prediction of cardiovascular disease and mortality (Goldberg et al 1996, WHO Guidelines 1999). The arterial pressure waveform varies throughout the entire arterial tree, as it is the resultant of the forward pulse wave and the reflected waves that occur at multiple arterial sites (Nichols and O’Rourke 2005). Thus, the pulse pressure measured at the brachial artery differs significantly from the central pulse pressure, particularly in young subjects where pulse pressure amplification is most important (Nichols and O’Rourke 2005). Blood pressure measured at the peripheral arteries is therefore significantly different from the central blood pressure that most major organs such as the brain, heart and kidney experience. The importance of the central pressure waveform is particularly true in the case of the left ventricle: Stiffening of the central arteries will result in changes in both the wave reflection patterns and arrival times of the reflected waves at the left ventricle. This can be either burdensome, if the reflected wave adds to the main systolic pressure peak, hence increasing left-ventricular workload, or beneficial, if the reflected wave arrives at late systole or early diastole, resulting in an increased coronary perfusion. For these reasons, central pressure appears to be a better predictor than peripheral pressure for cardiovascular risk (Avolio et al 2009, Roman et al 2007, Safar et al 2002).
Hypertension management is also an important application where differences between central and peripheral BP should be taken into account. In clinical practice, the effect of anti-hypertensive drugs is commonly monitored using the brachial BP. However, it has been highlighted that different BP-lowering pharmacological treatments have different effects on the central BP reduction while maintaining similar brachial BP reduction (London et al 2004, Williams et al 2006: Conduit Artery Function Evaluation -CAFE- study). In the CAFE study, a strong association was found between central BP reduction and clinical outcome, which highlighted the importance of targeting specifically the central BP in the treatment of hypertension. Finally, it is important to emphasize that the clinical relevance of the central BP is not only limited to the standard diastolic and systolic values. As illustrated by the example of the effect of the reflected wave on the left ventricle, the full pulse pressure waveform contains information on the timing, shape and amplitude of the incident and reflected waves3. Hence, the clinical significance of measuring not only the central pulse pressure amplitude, but also its temporal waveform, is very high.
Interest in methods that allow to perform such measurements non-invasively has increased over the last 20 years. The most commonly used non-invasive method is applanation tonometry. It relies on the measurement of the pulse pressure waveform at a peripheral site, typically, the radial artery, through a transcutaneous tonometer equipped with a pressure sensor that compresses slightly the artery and acquires the pressure waveform exerted on the sensor. Brachial diastolic and systolic BP are measured by sphygmomanometry, and the central aortic pressure waveform is estimated through the use of generalized transfer functions that have been derived from patients undergoing catheterization (Chen et al 1997, Fetics et al 1999, Hope et al 2002, Hope et al 2003, Karamanoglu et al 1993). However, the validity of such transfer functions to the general population is still subject to debate (Lehmann et al 2000, Hope et al 2008), particularly due to the lack of prospective studies aiming at validating the method versus invasive reference methods on the general population. In addition to the debate regarding the general applicability of the transfer function, these methods suffer from limitations that are related to the practice of the tonometry in general. In particular, in order to obtain reliable measurements through tonometry, the artery has to be correctly flattened between the tonometer and a harder structure, which can be particularly challenging to perform in obese subjects (Van Bortel et al 2001).
Van Bortel et al. (2001) have therefore proposed an alternative approach to assessing the central pressure waveform. Their method relies on the measurement of the distension waveform of the carotid artery through echo-tracking. By using a calibration procedure as described in (Kelly and Fitchett 1992), where the difference between the Mean Arterial Pressure (MAP) and the Diastolic Blood Pressure (DBP) is assumed constant throughout the arterial tree, and by measuring both the MAP and DBP at the reference artery (e.g., brachial), the pressure waveform can be derived at the target artery (e.g., the carotid). Such a method has been shown to be particularly reliable for the estimation of pressure waveforms at the common carotid artery. The authors have compared their method to applanation tonometry and have found better reproducibility and reliability. However, this method relies on a calibration procedure that requires measurements at both the site of interest and the brachial artery.
The objective of the present study is to propose a non-invasive, ultrasound-based method for measuring the local pulse pressure waveform at the arterial site of interest, directly, i.e., without relying on a transfer function and without any calibration procedure involving brachial or radial measurements. It is also the objective of this method to be simple of use, so that it could be implemented easily in clinical ultrasound imaging systems. The proposed method relies on the measurement of the local distension waveforms at high frame rate using raw RF ultrasound signals, combined with the measurement of the local Pulse Wave Velocity (PWV) that yields an estimate of the local stiffness. PWV is measured using the Pulse Wave Imaging method (PWI) that has been developed by our group (Fujikura et al 2007) as a high-frame rate method for both real-time qualitative visualization of the pulse wave propagation (Luo and Konofagou 2010) and quantitative estimation of the local arterial stiffness (Vappou et al 2010). Information on both the local stiffness and distension waveform is used to reconstruct the local pulse pressure waveform. Similar approaches have been previously proposed.Brands et al. (1998) measured the PWV from the distension waveforms between two M-lines, whereas Beulen et al. (2011) estimated the PWV using the ratio between the changes in flow and changes in the cross-sectional area of the vessel. Both studies proposed to estimate relative changes in pressure from distension waveforms, and tested it on arterial phantoms. In this study, we propose to measure pulse pressure waveforms in human subjects in vivo by using PWV estimates based on the PWI method, and to compare these waveforms to those estimated by arterial tonometry
2. Material and Methods
Pulse pressure estimation: Theory
The proposed Pulse Wave-based Ultrasound Manometry (PWUM) method relies on the measurement of both the distension of the imaged arterial segment and of its local elasticity. The underlying assumption is that the artery is cylindrical, and that the arterial wall behaves as a linear elastic solid. At this stage, such assumptions are inherent to the proposed method and limitations associated with them will be discussed further in this manuscript. The Laplace’s law relates an infinitesimal variation of the vessel radius dR to the variation of the internal fluid pressure dP (Fung 1996):
| (1) |
where E is the arterial wall Young’s elastic modulus, h its thickness and R the radius of the lumen. The Moens-Korteweg equation relates the Pulse Wave Velocity (PWV) to the elasticity of the arterial wall:
| (2) |
with ρ being the density of the arterial wall. It is important to mention that this equation relies on the assumption of a thin arterial wall (h<<R). By replacing this expression into equation 1, eq.1 becomes:
| (3) |
We propose to measure the infinitesimal variations of the lumen diameter 2*dR and to measure the local value of the PWV by measuring incremental wall displacements by ultrasound at a high frame rate. By integrating equation 3 over the cardiac cycle, the pressure waveform P(t) can be calculated as follows:
| (4) |
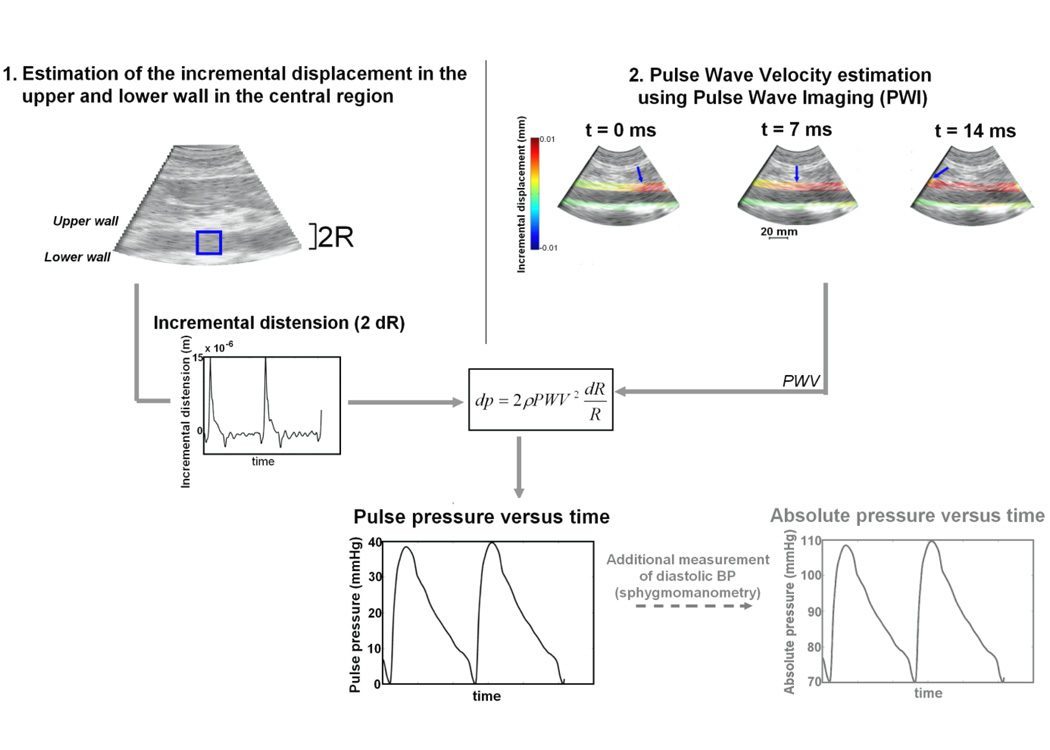
with P0 and R0 being the minimum values of the pressure and of the lumen radius, i.e., P0 being the diastolic BP. Eq. 4 illustrates that our method allows to assess the pulse pressure, and not the absolute value of the pressure since the value of P0 is unknown. However, it is well established that the diastolic pressure variations are negligible along the entire arterial tree (Nichols and O’Rourke 2005, Kelly and Fitchett 1992). A routine additional sphygmomanometric measurement can be performed at the brachial artery in order to convert pulse pressure waveform into an absolute pressure waveform by just adding the pulse pressure measured by our method to the diastolic BP. Figure 1 summarizes how the pulse pressure waveform is calculated from our ultrasonic measurements.
Figure 1.
Summary of the PWUM method for estimating the local arterial pulse pressure waveform: 1) B-mode image illustrating the central lines where the incremental distension will be measured, 2) Three consecutive frames illustrating the incremental displacement overlaid onto B-mode, the arrow depicting the onset of the wave, allowing to estimate the Pulse Wave Velocity (PWV)
Experiments were performed in a total of 12 subjects with no known history of cardiovascular disease, among which 1 was excluded due to poor image quality at the imaging location. As a consequence, 11 subjects were included in this study (age 35.7± 16, 18–66 y.o.). This study was approved by the Institutional Review Board of Columbia University. All subjects were scanned by ultrasound and, for comparison purposes, by radial applanation tonometry using a commercially available system (Sphygmocor, At-Cor medical, Sydney, Australia). The two experiments were performed consecutively in order to ensure similar conditions and to minimize the effect of blood pressure variations. For the Sphygmocor measurements, the arterial pulse was measured at the radial artery using the standard protocol as recommended by the manufacturer. Diastolic and systolic blood pressures were measured at the brachial artery by sphygmomanometry.
Ultrasound Measurements
In order to illustrate the feasibility of the PWUM method, all ultrasound measurements were performed on the mid-section of the abdominal aorta, since it is a region that allows for straightforward calculation of the PWV (Vappou et al 2010). Abdominal aortas were scanned at the level of the renal arteries. Ultrasound radiofrequency (RF) signals were acquired using a clinical ultrasound scanner (Sonix Touch, UltraSonix, BC, Canada). The incremental displacements (i.e., from one frame to the next one) of the arterial wall were estimated using a fast cross-correlation method on RF signals (Luo and Konofagou 2010), and were used for both the measurement of the PWV and for the distension waveform.
The abdominal aorta was imaged using a curvilinear array. The sector of the acquired image varied between 50 and 70 degrees, and its depth between 70 mm and 120 mm, depending on the subject. For all subjects, a line density of 32 beams per full sector was used, yielding a frame rate within the 248–392 Hz range. For each subject, several acquisitions (n=3–6) were performed at the same location, the acquisition time being of 2.5s. Therefore, there were generally 2 full cardiac cycles in one acquisition. The Pulse Wave Velocity (PWV) was calculated using the PWI method as presented previously (Vappou et al 2010, Luo et al 2008). In short, the arterial segment of interest is imaged longitudinally by using a standard curvilinear ultrasound probe. The foot of the distension wave is tracked along the arterial wall within the imaged segment. The temporal onset of this foot is represented versus the distance from the heart, and the fitting of the slope of this curve yields an estimate of the PWV within the imaged segment (see Vappou et al., 2010, fig.4 for a visual representation of how PWV is estimated from distension waveforms). More specifically, a fiduciary point was selected to be the point located at 50% of the incremental distension waveform (Luo et al 2008), which corresponds approximately to 25% of the maximum of the distension. Correction was performed in order to account for the time delay due to ultrasound beam sweeping. PWV values were averaged over several acquisitions and cardiac cycles (n=6–12 depending on the subject). The average PWV was used as the input to equation 4. Only the central lines (n=2–4) were used for the distension, as the motion of the arterial wall at this central location coincides with the axial direction of the ultrasound beam and is therefore not affected by the imaging angle artifact. For each subject, the pulse pressure waveforms were averaged within these central lines and over several acquisitions and cardiac cycles (n = 6–12 depending on the subject) to obtain the average pulse pressure waveform.
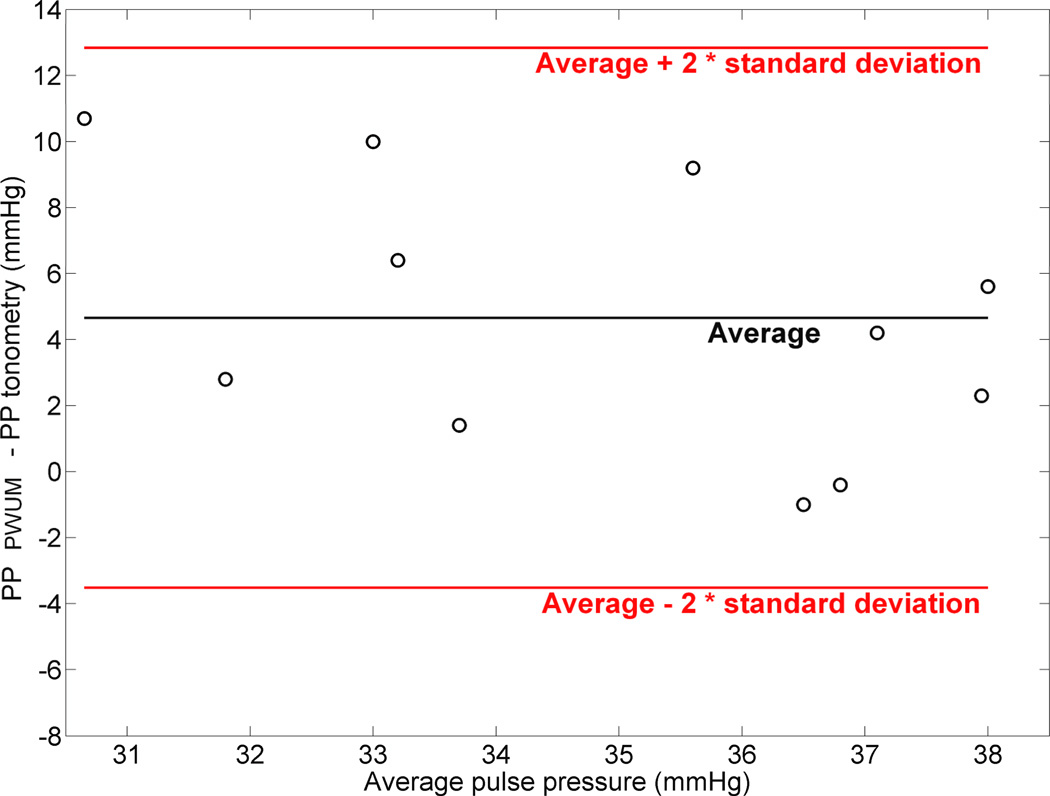
Figure 4.
Tukey mean-difference plot representing the difference between the amplitude of the pulse pressure measured by PWUM and the one measured by tonometry, versus the average value between the two methods.
Pulse pressure waveforms obtained by the PWUM method were directly compared to pulse pressure waveforms obtained by radial tonometry. Correlation was plotted between the two curves and Tukey mean-difference plots (also known as Bland and Altman plot) were generated for the amplitude of the pulse pressure (systolic pressure – diastolic pressure).
3. Results
Reproducibility
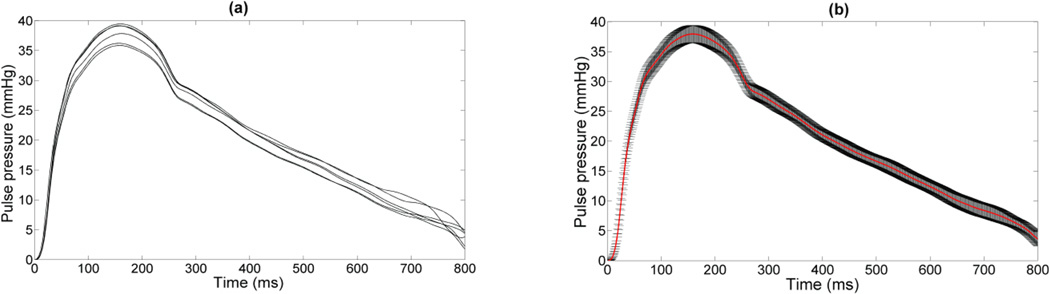
Figure 2 illustrates an example of 6 waveforms obtained on the same subject at different cardiac cycles, and the corresponding average pulse pressure curve, illustrating the reproducibility of the PWUM method. The average intra-subject standard deviation of the amplitude of the pulse pressure was found equal to 4.2 mmHg.
Figure 2.
(a) Example of waveforms obtained on the same subject at 6 different cardiac cycles; (b) the corresponding averaged waveform (red curve), as well as the standard deviation.
Comparison with applanation tonometry
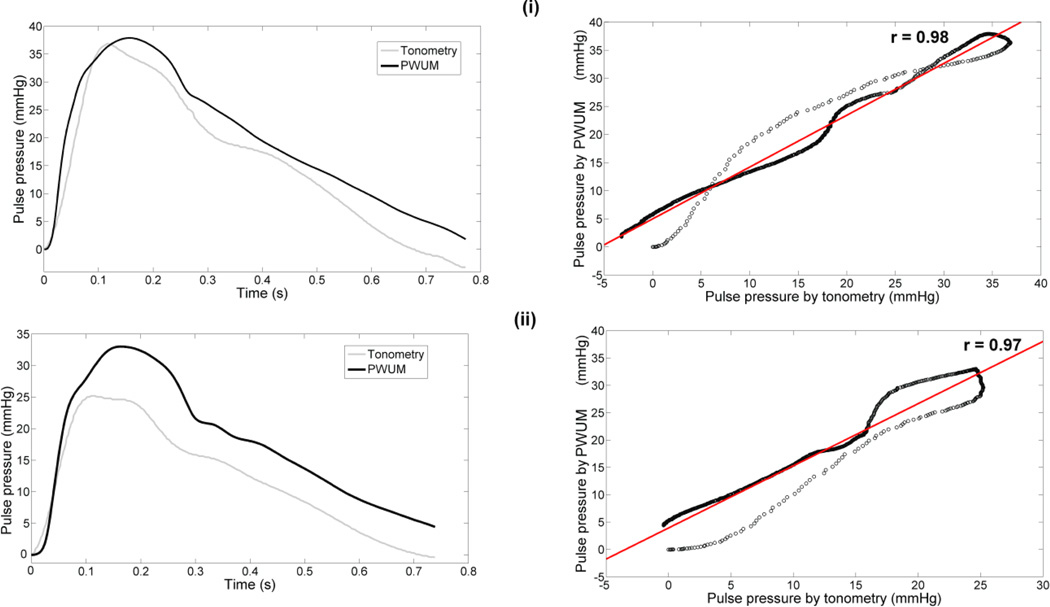
Good correlation (0.94<r<0.98) was found in all subjects between the pulse pressure waveforms obtained by tonometry and those obtained by the proposed method. Figure 3 illustrates an example of pulse pressure waveforms obtained in two human subjects by the two methods, as well as the corresponding correlation plots. Table 1 summarizes the pulse pressure results for all subjects.
Figure 3.
Example of pulse pressure waveforms obtained by both applanation tonometry and the PWUM method for two subjects (left column) and corresponding correlation plots (right column) (i) Subject 1 (28 y.o.) and (ii) Subject 2 (25 y.o.).
Table 1.
Pulse pressure results measured by both PWUM and tonometry for all subjects.
| Subject # | Age | Peripheral DBP (mmHg) |
Pulse pressure (PWUM), mmHg |
Pulse pressure (tonometry), mmHg |
|---|---|---|---|---|
| 1 | 28 | 64 | 39±3.1 | 37 |
| 2 | 23 | 66 | 36±4.8 | 25 |
| 3 | 66 | 48 | 40.2±4.1 | 31 |
| 4 | 18 | 58 | 33.2±3.9 | 30 |
| 5 | 24 | 52 | 40.8±5.2 | 35 |
| 6 | 38 | 64 | 34.4±5.0 | 33 |
| 7 | 25 | 64 | 38±4.5 | 28 |
| 8 | 55 | 64 | 39.2±4.9 | 35 |
| 9 | 27 | 58 | 36±3.5 | 37 |
| 10 | 31 | 54 | 36.4±3.6 | 30 |
| 11 | 58 | 62 | 36.3±3.9 | 37 |
A bias was found for the amplitude of the pulse pressure (PP) between the two methods. In average, pulse pressure amplitudes measured by PWUM were found to be 4.7 mmHg higher than those measured by applanation tonometry. Figure 4 illustrates the Tukey mean-difference plot for all the subjects. The standard deviation of (PPPWUM - PPtonometry) was equal to 4.45 mmHg.
4. Discussion
In this study, the novel method of Pulse Wave-based Ultrasound Manometry (PWUM) was presented and initial feasibility shown. Since PWUM is based on the integration of incremental pressure changes, it does not allow to directly measure the absolute values of the pulse pressure. However, it is commonly accepted that the mean arterial pressure and the Diastolic Blood Pressure (DBP) do not vary significantly along the arterial tree. The brachial DBP assessed by sphygmomanometry could therefore be used as a baseline to convert relative pressure waveforms into absolute pressure curves, as represented in fig. 1.
The pulse pressure waveforms were also compared to those measured by applanation tonometry. Very good correlation was found between the two methods. A significant bias was found, with the pulse pressure amplitudes measured by the PWUM method being on the average 4.7 mmHg higher than those found by tonometry. However, such a comparison should be considered with caution. First, the tonometry method provides the pressure at the ascending aorta, whereas in this study, PWUM measurements were performed in the abdominal aorta. Differences in profile and amplitude of the pulse pressure could be partially explained by natural variations of the pulse waveform between these two sites. Moreover, despite showing good reproducibility and despite validation studies (Pauca et al 2001, Wilkinson et al 1998), applanation tonometry combined with generalized transfer functions is still subject to debate, particularly regarding the accuracy of pressure waveforms that are measured, and because of the lack of prospective validation studies in the general population (Lehmann et al 2000, Hope et al 2008). Finally, it is also important to keep in mind that PWUM average intra-subject variability was found to 4.2 mmHg in terms of pulse pressure amplitude.
Substantial limitations of the proposed PWUM method should be emphasized. First, it relies on fundamental physical assumptions, namely, the cylindrical geometry and the linear elasticity of the arterial wall. Such assumptions are inherent to the validity of the Laplace’s law and the Moens-Korteweg equation used in this study. Assuming linear elasticity means that the distensibility waveform and the pressure waveform have the same temporal profile. This is a commonly accepted hypothesis (Van Bortel et al 2001) that relies on the fact that the non linear behavior of the arterial wall in vitro starts to prevail at higher deformation than the physiological one (Fung 1993). However, it is not certain how this translates in human arteries in vivo, and the nonlinear behavior of the arterial wall may infer to a more complex relationship between distension and pressure, which would also result in a non linear propagation of the PWV (Couade et al 2010, Meinders and Hoeks 2004). In our study, as in most commonly used methods for PWV measurements, the PWV was measured around the minimum distension point (foot of the wave). The assumption of cylindrical geometry limits the applicability of the method to relatively straight arterial segments, and excludes therefore cases of highly tortuous arteries or asymmetric abdominal aortic aneurysms (AAA). Further work is required to evaluate the validity of the PWUM method in such cases.
It is important to emphasize that any PWV estimation errors will be significantly amplified in the pulse pressure calculation due to the squared term in equation 3. Parameters such as low intra-subject variability and the quality of the spatio-temporal regression are good indicators of the reliability of the PWV estimate. Such indicators were found to be very good in this study while further enhanced by the averaging performed over several cardiac cycles in normal human abdominal aortas in vivo. However, factors such as poor signal-to-noise ratio or strongly non-uniform arterial properties may significantly affect the quality of these indicators, resulting in increased uncertainty of the pulse pressure estimate. Although the reliability of the PWI method has been demonstrated both in vitro (Vappou et al., 2010) and in vivo in healthy subjects (Vappou et al., 2011), further work is currently ongoing in order to evaluate the reliability of both PWV and pulse pressure estimates in subjects with vascular disease.
While PWUM can be criticized with respect to the aforementioned reasons, it should be noted that it allows for a local measurement of blood pulse pressure and it does not rely on a transfer function between the location of measurement (e.g., radial artery) and the location of interest (e.g., thoracic aorta), as opposed to transcutaneous applanation tonometry-based methods. As such, one of its main strengths is that it can be applied on several different arterial segments to measure variations of the pulse pressure waveform throughout the arterial tree.
In this feasibility study, measurements were performed only at one location, namely, the abdominal aorta at the level of the renal arteries. While this method could be easily extended to other standard regions of routine ultrasound use such as the carotid artery, its application to the ascending thoracic aorta, and therefore to the measurement of the true central pressure, is not straightforward at this stage of development. Its feasibility is currently being assessed on parasternal long-axis views of the heart where a clear aortic segment can be visualized. Transesophageal echocardiography could also be considered for this particular application.
The sensitivity of the PWUM method could not be properly evaluated in this study. Due to the limited population sample, the range of the pulse pressure amplitude was found to be narrow (7.6 mmHg, from 33.2 mmHg to 40.8 mmHg). An ongoing study is currently performed on patients with history of hypertension in order to obtain wider ranges of pulse pressure amplitudes and to evaluate the sensitivity of the proposed method. Finally, future studies include the validation of the method against invasive measurements. Such studies will be limited to patients undergoing catheterization procedures due to obvious ethical reasons.
Acknowledgments
This study was supported by NIH R01EB006042 and R01HL098830.
References
- World Health Organization-International Society of Hypertension Guidelines for the Management of Hypertension. Guidelines Subcommittee. J. Hypertens. 1999;17:151–183. [PubMed] [Google Scholar]
- Avolio A, Van Bortel L, Boutouyrie P, Cockcroft J, McEniery C, Protogerou A, Roman M, Safar M, Segers P, Smulyan H. Role of pulse pressure amplification in arterial hypertension: Experts' opinion and review of the data. Hypertension. 2009;54:375–383. doi: 10.1161/HYPERTENSIONAHA.109.134379. [DOI] [PubMed] [Google Scholar]
- Beulen BW, Bijnens N, Koutsouridis GG, Brands PJ, Rutten MC, van de Vosse FN. Toward noninvasive blood pressure assessment in artieries by using ultrasound. Ultrasound Med. Biol. 2011;37:788–797. doi: 10.1016/j.ultrasmedbio.2011.01.020. [DOI] [PubMed] [Google Scholar]
- Brands PJ, Willigers JM, Ledoux LAF, Reneman RS, Hoeks APG. A noninvasive method to estimate pulse wave velocity in arteries locally by means of ultrasound. Ultrasound Med. Biol. 1998;24:1325–1335. doi: 10.1016/s0301-5629(98)00126-4. [DOI] [PubMed] [Google Scholar]
- Chen C-H, Nevo E, Fetics B, Pak P, Yin F, Maughan W, Kass D. Estimation of Central aortic pressure waveform by mathematical transformation of radial tonometry pressure: Validation of generalized transfer function. Circulation. 1997;95:1827–1836. doi: 10.1161/01.cir.95.7.1827. [DOI] [PubMed] [Google Scholar]
- Couade M, Pernot M, Prada C, Messas E, Emmerich J, Bruneval P, Criton A, Fink M, Tanter M. Quantitative Assessment of Arterial Wall Biomechanical Properties Using Shear Wave Imaging. Ultrasound Med. Biol. 2010;36:1662–1676. doi: 10.1016/j.ultrasmedbio.2010.07.004. [DOI] [PubMed] [Google Scholar]
- Fetics B, Nevo E, Chen C-H, Kass D. Parametric model derivation of transfer function for noninvasive estimation of aortic pressure by radial tonometry. IEEE Trans. Biomed. Eng. 1999;46:698–706. doi: 10.1109/10.764946. [DOI] [PubMed] [Google Scholar]
- Fujikura K, Luo J, Gamarnik V, Pernot M, Fukumoto R, Tilson MD, III, Konofagou EE. A Novel, Non-Invasive Technique for Pulse-Wave Imaging and Characterization of Clinically Significant Vascular Mechanical Properties in vivo. Ultrason. Imaging. 2007;29:137–154. doi: 10.1177/016173460702900301. [DOI] [PubMed] [Google Scholar]
- Fung YC. Biomechanics: Circulation Springer. 2nd edition 1996. [Google Scholar]
- Fung YC. Biomechanics: Mechanical Properties of Living Tissues Springer. 2nd edition 1993. [Google Scholar]
- Goldberg R, Larson M, Levy D. Factors associated with survival to 75 years of age in middleaged men and women: The Framingham study. Arch. Intern. Med. 1996;156:505–509. [PubMed] [Google Scholar]
- Hope S, Tay D, Meredith I, Cameron J. Comparison of generalized and gender-specific transfer functions for the derivation of aortic waveforms. Am. J. Physiol. Heart Circ. Physiol. 2002;283:H1150–H1156. doi: 10.1152/ajpheart.00216.2002. [DOI] [PubMed] [Google Scholar]
- Hope S, Tay D, Meredith I, Cameron J. Use of arterial transfer functions for the derivation of aortic waveform characteristics. J. Hypertens. 2003;21:1299–1305. doi: 10.1097/00004872-200307000-00017. [DOI] [PubMed] [Google Scholar]
- Hope S, Meredith I, Cameron J. Arterial transfer functions and the reconstruction of central aortic waveforms: Myths, controversies and misconceptions. J. Hypertens. 2008;26:4–7. doi: 10.1097/HJH.0b013e3282f0c9f5. [DOI] [PubMed] [Google Scholar]
- Karamanoglu M, O'Rourke M, Avolio A, Kelly R. An analysis of the relationship between central aortic and peripheral upper limb pressure waves in man. Eur. Heart J. 1993;14:160–167. doi: 10.1093/eurheartj/14.2.160. [DOI] [PubMed] [Google Scholar]
- Kelly R, Fitchett D. Noninvasive determination of aortic input impedance and external left ventricular power output: A validation and repeatability study of a new technique. J. Am. Coll. Cardiol. 1992;20:952–963. doi: 10.1016/0735-1097(92)90198-v. [DOI] [PubMed] [Google Scholar]
- Lehmann E, Wilkinson I, Cockcroft J, Webb D. Regarding the accuracy of generalized transfer functions for estimating central aortic blood pressure when calibrated non-invasively (multiple letters) J. Hypertens. 2000;18:347–350. doi: 10.1097/00004872-200018030-00015. [DOI] [PubMed] [Google Scholar]
- London G, Asmar R, O'Rourke M, Safar M. Mechanism(s) of Selective Systolic Blood Pressure Reduction after a Low-Dose Combination of Perindopril/Indapamide in Hypertensive Subjects: Comparison with Atenolol. J. Am. Coll. Cardiol. 2004;43:92–99. doi: 10.1016/j.jacc.2003.07.039. [DOI] [PubMed] [Google Scholar]
- Luo J, Lee W-N, Wang S, Konofagou E. Pulse wave imaging of human abdominal aortas in vivo. Proceedings - IEEE Ultrasonics Symposium. 2008:859–862. [Google Scholar]
- Luo J, Konofagou E. A fast normalized cross-correlation calculation method for motion estimation. IEEE Trans. Ultrason. Ferroelectr. Freq. Control. 2010;57:1347–1357. doi: 10.1109/TUFFC.2010.1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meinders J, Hoeks A. Simultaneous assessment of diameter and pressure waveforms in the carotid artery. Ultrasound Med. Biol. 2004;30:147–154. doi: 10.1016/j.ultrasmedbio.2003.10.014. [DOI] [PubMed] [Google Scholar]
- Nichols W, O'Rourke M. McDonald's Blood Flow in Arteries: Theoretical, Experimental and Clinical Principles. Fifth Edition 2005. [Google Scholar]
- Pauca A, O'Rourke M, Kon N. Prospective evaluation of a method for estimating ascending aortic pressure from the radial artery pressure waveform. Hypertension. 2001;38:932–937. doi: 10.1161/hy1001.096106. [DOI] [PubMed] [Google Scholar]
- Roman M, Devereux R, Kizer J, Lee E, Galloway J, Ali T, Umans J, Howard B. Central pressure more strongly relates to vascular disease and outcome than does brachial pressure: The strong heart study. Hypertension. 2007;50:197–203. doi: 10.1161/HYPERTENSIONAHA.107.089078. [DOI] [PubMed] [Google Scholar]
- Safar M, Blacher J, Pannier B, Guerin A, Marchais S, Guyonvarc'h P-M, London G. Central pulse pressure and mortality in end-stage renal disease. Hypertension. 2002;39:735–738. doi: 10.1161/hy0202.098325. [DOI] [PubMed] [Google Scholar]
- Van Bortel L, Balkestein E, Van Der Heijden-Spek J, Vanmolkot F, Staessen J, Kragten J, Vredeveld J, Safar M, Boudier H, Hoeks A. Non-invasive assessment of local arterial pulse pressure: Comparison of applanation tonometry and echo-tracking. J. Hypertens. 2001;19:1037–1044. doi: 10.1097/00004872-200106000-00007. [DOI] [PubMed] [Google Scholar]
- Vappou J, Luo J, Konofagou E. Pulse wave imaging for noninvasive and quantitative measurement of arterial stiffness in vivo. Am. J. Hypertens. 2010;23:393–398. doi: 10.1038/ajh.2009.272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vappou J, Luo J, Okajima K, Di Tullio M, Konofagou E. Aortic pulse wave velocity measured by pulse wave imaging (PWI): A comparison with applanation tonometry. Artery Research. 2011;5:65–71. doi: 10.1016/j.artres.2011.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson I, Fuchs S, Jansen I, Spratt J, Murray G, Cockcroft J, Webb D. Reproducibility of pulse wave velocity and augmentation index measured by pulse wave analysis. J. Hypertens. 1998;16:2079–2084. doi: 10.1097/00004872-199816121-00033. [DOI] [PubMed] [Google Scholar]
- Williams B, Lacy P, Thom S, Cruickshank K, Stanton A, Collier D, Hughes A, Thurston H, O'Rourke M. Differential impact of blood pressure-lowering drugs on central aortic pressure and clinical outcomes: Principal results of the Conduit Artery Function Evaluation (CAFE) study. Circulation. 2006;113:1213–1225. doi: 10.1161/CIRCULATIONAHA.105.595496. [DOI] [PubMed] [Google Scholar]