Abstract
Significance: A high bacterial load interferes with the healing process of a wound. Vacuum-assisted closure (VAC) is a wound healing therapy that utilizes a dressing system that continuously or intermittently applies a negative pressure to the wound surface.
Recent Advances: VAC stimulates wound healing, but data on changes in the bacterial load and changes in the bacterial spectrum are scarce.
Critical Issues: While VAC supposedly removes bacteria from the treated wounds and therefore reduces the risk of infection, this relationship has not yet been clinically proven. If VAC increases the bacterial load instead of decreasing it, then this may be a reason not to use VAC on certain types of wounds. Only seven small and heterogeneous studies reporting on the relationship between VAC usage and the bacterial load and type of bacteria in the treated wounds in clinical practice were found in the literature. Although there is some low quality evidence that VAC therapy does not change the bacterial load, no definite conclusions on changes in the bacterial load and type of bacteria during VAC can be drawn.
Future Directions: Prospectively monitoring changes in the bacterial load and bacterial spectrum in patients that will receive VAC treatment on indication might be an effective way to find out whether it should indeed be used on specific wounds.

Roelf S. Breederveld, MD, PhD
Scope and Significance
Vacuum-assisted closure (VAC) is a wound healing therapy that utilizes a dressing system that continuously or intermittently applies a negative pressure to the wound surface. It can be used in the management of both acute and chronic wounds, and complex wounds such as burn wounds.1 VAC stimulates wound healing through two processes called macrostrain and microstrain. Macrostrain draws the edges of the wound together, equally distributes the negative pressure, and removes exudate and infectious materials. Microstrain reduces edema, stimulates perfusion, and stimulates granulation tissue formation.2
Translational Relevance
While VAC supposedly removes bacteria from the treated wounds and therefore reduces the risk of infection, this relationship has not yet been clinically proven. A high bacterial load has a negative outcome on the healing process of a wound.
Clinical Relevance
If VAC increases the bacterial load instead of decreasing it, then this may be a reason not to use VAC on certain types of wounds. In the past two decades, several studies have been performed to find out the relationship between VAC and changes in the wounds' microbiology. Various reviews have provided an overview of the overall effectiveness of VAC and recommendations for use. Although they agree on the fact that VAC does accelerate wound healing, data on changes in the bacterial load and changes in the bacterial spectrum are scarce.3,4
Methods
This systematic review was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines.5
Data sources and search strategy
For this review, a selection of relevant articles on the relationship between VAC and changes in the VAC-treated wounds' microbiology was made with assistance of a trained medical librarian. To find these articles, the PubMed, EMBASE, Web of Science, and CINAHL databases were searched on April 23, 2013 using the following search terms.
PubMed
(“Negative-Pressure Wound Therapy”[Mesh] OR “VAC therapy” [all fields] OR “VAC treatment” [all fields] OR “Vacuum assisted” [all fields] OR “Negative pressure” [all fields] OR “NPWT” [all fields]) AND (“Bacterial Infections” [Mesh] OR “Colony Count, Microbial” [Mesh] OR “Bacterial load” [all fields] OR “Bacterial count” [all fields] OR “Bacterial burden”[all fields] OR “Bacterial infection” [all fields] OR “Bacterial infections”[all fields] OR “Wound bioburden” [all fields] OR “Pseudomonas” [Mesh] OR “Pseudomonas” [all fields] OR “Pseudomonas Infections” [Mesh]).
EMBASE
(vacuum-assisted closure/OR “VAC therapy”.mp. OR “VAC treatment”.mp. OR “Vacuum assisted”.mp. OR “Negative pressure”.mp. OR “NPWT”.mp.) AND (bacterial count/OR bacterial load/OR “Bacterial load”.mp. OR “Bacterial count”.mp. OR “Bacterial burden”.mp. OR “Bacterial infection”.mp. OR “Wound bioburden”.mp. OR Pseudomonas infection/or Pseudomonas/OR “Pseudomonas”.mp.).
Web of Science
TS=(“VAC therapy” OR “VAC treatment” OR “Vacuum assisted” OR “Negative pressure” OR “NPWT”) AND TS=(“Bacterial load” OR “Bacterial count” OR “Bacterial burden” OR “Bacterial infection”[all fields] OR “Bacterial infections” OR “Wound bioburden” OR “Pseudomonas”)
CINAHL
(MH “Negative Pressure Wound Therapy” OR TX “VAC therapy” OR TX “VAC treatment” OR TX “Vacuum assisted” OR TX “Negative pressure” OR TX “NPWT”) AND (MH “Bacterial Infections+” OR MH “Colony Count, Microbial” OR TX “Bacterial load” OR TX “Bacterial count” OR TX “Bacterial burden” OR TX “Bacterial infection” OR TX “Bacterial infections” OR TX “Wound bioburden” OR MH “Pseudomonas” OR MH “Pseudomonas Infections” OR TX “Pseudomonas”)
Selection of studies
Potentially relevant articles identified in the databases were exported to Reference Manager 12. Using Reference Manager, all duplicates were removed from the literature database. Title and abstract of the remaining articles were independently screened by two reviewers (A.S.P.P. and R.S.B.). Articles were selected for this review if they met the following inclusion criteria: human studies describing the relationship between VAC treatment and changes in microbiology, original studies (no reviews), no case reports, articles in English.
The full-text articles of the selected studies were collected. The full-text articles were screened using the same criteria mentioned above. Articles meeting the selection criteria were included in the definite selection. Discrepancies between the reviewers were resolved by discussion.
Data extraction
A standardized extraction form was used to collect relevant data of the selected articles, including study design, number of patients per treatment group, type of wounds and infections, type of VAC system, pressure level maintained in the VAC systems, dressing change interval, and sampling method. Registered endpoints were change in bacterial load (increase or decrease) and change in type of microorganisms (with special interest in Pseudomonas and Staphylococcus aureus) at the end of VAC treatment. Results were described per study, and meta-analysis was to be performed if the selected studies included comparable study groups and outcomes.
Overview of Findings and Relevant Literature
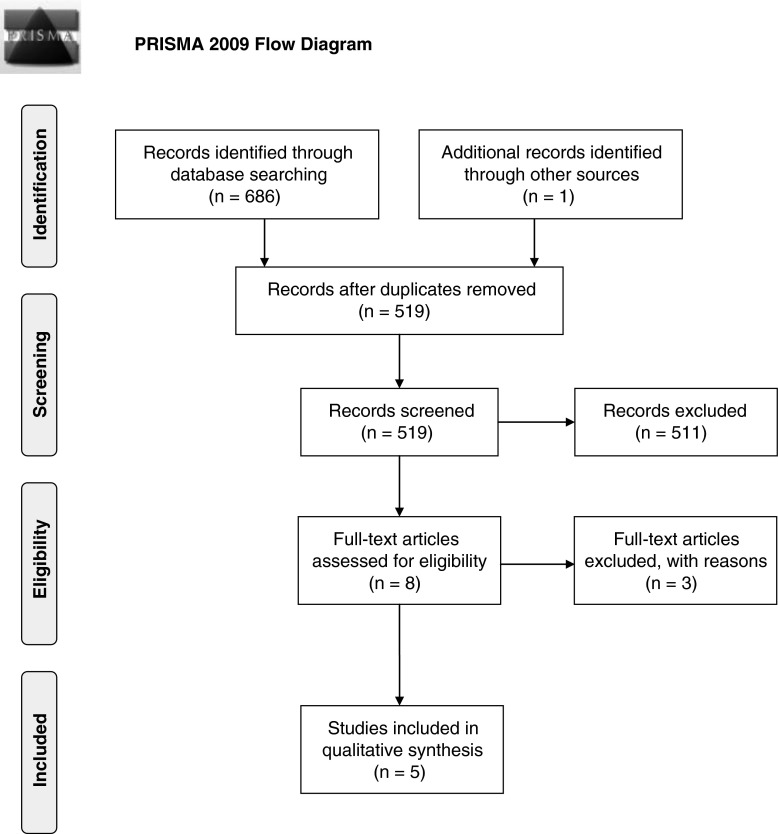
The literature search identified a total of 687 articles published between 1973 and 2013. After having removed the duplicates, 519 publications remained of which the title and abstract were screened. Eight studies met the inclusion criteria for the review (Fig. 1) and the full text articles were obtained. One full-text article did not describe outcome data on change in bacterial load or bacterial spectrum and was excluded. Another study by Gabriel et al.6 was excluded because the VAC therapy in this study was combined with silver nitrate and no neutralizer was used for the silver nitrate after taking the swab culture, thereby making the cultures useless because the silver nitrate would continue to kill bacteria on the way to the laboratory. Finally, a study by Yusuf et al.7 was excluded from our review because the dressings and not the wounds themselves were cultured, which make the results not comparable to the other studies that used wound cultures.
Figure 1.
Flow chart describing the selection process for the literature reviewed.
The five selected studies included two randomized and two non-randomized retrospective studies comparing VAC therapy to conventional therapy (open wound dressing or closed irrigation), and one study describing a retrospective cohort of patients treated with VAC. The studies included patients with various types of wounds: two studies included patients with infected wounds,8,9 two studies patients with acute and chronic wounds,10,11 and one study included patients with various types of wounds.12,13 In all selected studies, the number of patients was low (between 10 and 30 per treatment group). Bacterial sampling methods also differed between the studies. Five studies described the change in bacterial load during treatment, and one study reported on the change in bacterial spectrum. The results of the five selected studies are described in the next paragraphs and summarized in Table 1. Since the selected studies included heterogeneous patients groups and outcomes, no meta-analysis could be performed.
Table 1.
Characteristics of five included studies in a systematic review on (change in) bacterial load during therapy with vacuum-assisted closure or conventional therapy
| Saadi et al.8 | Steingrimsson et al.9 | Mouës et al.12,13 | Weed et al.10 | Braakenburg et al.11 | |
|---|---|---|---|---|---|
| Study design | Retrospective cohort study | Retrospective comparative cohort study | Randomized clinical trial | Retrospective cohort study | Randomized clinical trial |
| N/treatment | VAC: N=27 | VAC: N=20 CVT: N=23 |
VAC: N=29 CVT: N=25 |
VAC: N=25 with 26 uses of VAC | VAC: N=26 CVT: N=21 |
| Type of wound or infection | Infected thoracic wounds | Postoperative DSWIa | Crushed tissue, infected wounds, chronic wounds | Acute and chronic wounds | Acute and chronic wounds |
| Sampling method | Smear | n.r. | Biopsy | Swab | Swab |
| Dressing change interval | 3.9 days on average | Every 2–4 days | Every 2 days | Every 3–5 days | 3×/week |
| Pressure level during VAC (mmHg) | −50 to −75 | −125 | −125 | n.r. | −125 |
| Debridement | Yes | Yes | Yes | Yes | Yes |
| Systemic antibiotic | Yes | Yes | n.r. | n.r. | Yes |
| Change in bacterial load | n.r. | n.r. | No change in bacterial load in both groups | Increase in 43% of cases, no change in 35%, decrease in 22% | Increase in 84% of VAC vs. 58% in CVT (p=0.09) |
| Infection (% of cases) cleared or re-infection | Cleared: 33% | Re-infection: VAC: 5% vs. CVT: 35% (p=0.02) | n.r. | n.r. | n.r. |
| Change in bacterial spectrum | n.r. | n.r. | VAC: Reduction in no. of non-fermentative negative rods; Increase in no. of Staphylococcus aureus | n.r. | n.r. |
The VAC system in all studies was KCI medical.
Deep sternal wound infection.
VAC, vacuum-assisted closure therapy; CVT, conventional (non-VAC) therapy; n.r., not reported.
Ultimately, the following five studies were inserted in our review.
Saadi et al. reviewed the records of 27 patients that were treated with intrathoracic VAC therapy for thoracic infections without a comparison group.8 The median age of the patients was 64 years and all patients had multiple comorbidities. Patients belonged to one of the following three groups: post-resectional empyema (N=8), severe necrotizing pleuropulmonary infections (N=7), or intrathoracic gastrointestinal leaks with mediastinitis and empyema (N=12). A pressure of −50 to −75 mmHg was applied, and dressing changes were carried out under general anesthesia. Wound smears were taken for microbial analysis and, if necessary, surgical debridement of the wound was performed. All patients received systemic antibiotic therapy. The median time of the VAC therapy was 22 days (range 5–66 days), the median number of dressing changes was 6 (range 2–14), and the mean interval between dressing changes was 3.9 (SD 0.3) days. Two of the 27 wounds were not closed before discharge of the patient. The median hospital stay was 44 days (range 20–114).
Out of 27 patients, bacterial contamination was found in 23 (85%) and positive fungal cultures in 13 (48%). In 7 of the 21 patients (33%) with available microbiological analysis complete sterilization using VAC therapy was achieved. Persistent bacterial contamination remained in 14 (67%) patients with available microbiological analysis.
In Steingrimsson's population-based retrospective cohort study, 43 patients were diagnosed with culture-verified deep sternal wound infection after open heart surgery.9 These patients belonged to the nationwide population in the period January 2000 and December 2010 from Reykjavik. All 43 patients were treated with intravenous antibiotics and surgical debridement of infected and necrotic tissue. Twenty-three patients receiving conventional treatment in the period 2000–2005 were compared with 20 patients getting VAC therapy from 2005 till 2010. A negative pressure of −125 mmHg was applied using VAC. In the VAC group, wounds were reopened after 2–4 days and the foams were changed. The median length of hospital stay was 43 days in both groups and the average follow-up time was 3.9 years. The two groups were comparable because both patient groups, which were following open heart surgery, had the same mean age, gender, frequency of diabetes mellitus, and prior surgery. Only peripheral artery disease was more common in the conventional group.
In nine patients (21%) early re-infection was found: eight patients (35%) in the conventional treatment group and one (5%) in the VAC group (Fisher's exact test: p=0.02). The one patient with persistent deep sternal wound infection in the latter group was found to have a Pseudomonas aeruginosa infection. This was cultured from both the sponge and the wound. In one patient of the VAC group (5%), a late chronic sternal wound infection that required surgical treatment developed, and this occurred in six patients of the conventional treatment group (26%). However, the difference was not statistically significant (Fisher's exact test: p=0.10).
Mouës et al. described the results of 54 patients that were followed between July 1998 and October 2002 with complex wounds that could not be closed because of crushed tissue, infection, or chronic character in a randomized clinical trial. This trial was published in two publications, one describing the bacterial load12 and the other the wound condition and duration of therapy.13 Twenty-nine patients were treated with VAC and 25 with conventional gauze therapy. Randomization was performed through closed envelope assignment. A continuous pressure level of −125 mmHg was applied during VAC therapy and dressings were changed every 48 h. Debridement of necrotic tissue took place when considered clinically needed in 50 wounds. Systemic antibiotic treatment was not clearly described. The median length of hospital stay was 43 days in both groups and the average follow-up time was 3.9 years. To quantify the bacterial load in the treated wounds, tissue biopsies were taken. These were performed every 2 to 3 days from the beginning until the end of the treatment. The total number of colony forming units were counted and calculated per gram of tissue. The medical microbiologist was unaware of the allocated treatment.
In both treatment groups, the initial number of bacteria after debridement was around 105 bacteria per gram of tissue. At the end of follow-up, no significant decrease of the bacterial load was found in either group. The median bacterial count remained around the level of 105 per gram of tissue.
A significant reduction in the number of non fermentative negative rods and an increase of S. aureus was found in the VAC group but the differences with the conventional therapy were not significant. The number of Enterobacteriaceae and anaerobes did not change in both groups.
The two randomized groups were not balanced for some prognostic factors. The VAC group contained more patients with diabetes, peripheral vascular disease and osteomyelitis, and less patients with spinal cord injury. In the VAC group, three patients did not reach the endpoint and in the conventional therapy group two.
In this randomized clinical trial the allocation sequence was generated by picking a closed envelope with the description of the two therapies. The method used to conceal the allocation sequence was not described. The study participants and personnel except for the medical microbiologist were not blinded for the allocated treatment because of the visible suction marks present in the wound treated with VAC.
Weed et al. retrospectively studied the quantitative assessment and monitoring of the degree of bacterial bioburden during negative pressure wound therapy for both acute (trauma wounds) and chronic wounds (pressure and diabetic ulcer wounds) from 1999 to 2003 without a comparison group of patients.10 Quantitative culture swabs were taken during VAC treatment and were performed as a part of wound evaluation. The VAC sponges were changed every 3–5 days. Twenty-five patients met the inclusion criteria and 26 wounds were treated with VAC therapy. All necrotic material was removed from the wound bed before application of the VAC device. Systemic antibiotic treatment was not clearly described. Not all wounds were followed to complete closure.
During VAC therapy, there was a statistically significant higher bacterial load compared to pre- and post-treatment measurements (p=0.000 and 0.003, respectively). The difference between pre-VAC and post-VAC means was not statistically significant (p=0.303). During VAC therapy, 43% of the treated wounds had an increase in the bacterial bioburden, 35% of the wounds did not show an overall change, and 22% of the wounds showed a decrease in bacterial load, but these percentages were not significantly different. VAC therapy failed in 12% of the wounds. This was determined by an increasing wound size or the development of necrotic tissue.
Braakenburg et al. performed a randomized controlled trial, in which 65 patients with 66 chronic, subacute, or acute wounds were assigned to conventional treatment (n=33) or VAC therapy (n=32) between March 2002 and May 2004.11 Surgical debridement was carried out in case of necrosis or infection. The patients were evaluated three times a week and photographs and bacteriologic swabs were taken once a week to monitor the progress of the wound. Conventional therapy consisted of standard dressings according to the hospital's wound protocol. During VAC, a continuous pressure of −125 mmHg was maintained. VAC dressings were changed three times per week. When surface exudate was removed, superficial bacteriologic swabs were performed for further semi-quantitative culture analysis. The primary endpoint of the study was a granulated wound or a wound ready for skin grafting or healing by secondary intention. One of the primary outcome measures was bacterial clearance. Antibiotics were given to 9 patients (28%) in the VAC group and to 15 in the conventionally treated patients (47%; p=0.20). The VAC group had a median healing time of 16 days (95% confidence interval [CI]: 9–23) and the conventional group 20 days (95% CI: 16–24; p=0.32).
No reduction in bacterial load was found. There was an increase of bacterial growth in 84% (n=21) of 25 patients with VAC-treated wounds and in 58% (n=11) of 19 patients with conventionally treated wounds (p=0.09). The bacterial species most frequently cultured were S. aureus, Enterobacteriaceae, and anaerobes.
In this randomized clinical trial the allocation sequence was generated by block randomization by closed envelopes. The exact number of patients who were excluded because of exclusion criteria, for instance osteomyelitis, is unknown. The method used to conceal the allocation sequence was not described. The study participants and personnel except for the personnel of the medical microbiology laboratory were not blinded for the allocated treatment because of marks in the wounds treated with VAC. The results from the bacterial cultures were revealed to the researchers at the end of the study. The two groups were comparable for age, diabetes, and sex. Cardiovascular disease was seen in 11 VAC group patients (34%) and in 20 conventional group patients 20 (61%; p=0.05). Twenty-three (74%) wounds in the VAC group were chronic compared with 18 (56%) in the conventional group (p=0.19).
Discussion
A high bacterial load interferes with the healing process of a wound. The goal of this review therefore was to systematically assess in the available literature whether VAC usage influences the bacterial load and type of bacteria in traumatic (acute) wounds, infected postoperative wounds and chronic wounds. Another goal of this review was to provide insight into the effectiveness of VAC therapy in the clearance of wound infections. Only five original clinical studies on this subject could be superficially compared. From these included five studies no definite conclusions could be drawn for both infected and non-infected wounds.
One of the two studies including patients with infected wounds reported favorable outcomes of VAC usage.9 This comparative study found less wound infections after applying VAC compared with conventional therapy, and faster clearance of wound infections. However, in the third study by Saadi et al., which covered thoracic infections, the infection was not cleared after VAC therapy in the majority of patients.8
The studies that included patients with non-infected wounds reported an increase or no change in bacterial load in all or in the majority of included patients, and a higher incidence of infections during and after VAC usage.10–12
Infection was insufficiently defined, so it was not clear what was meant by infection in all of the selected studies. None of the studies systematically assessed whether a shift in the bacterial spectrum took place. However, a few remarkable cases have to be mentioned. Steingrimsson et al. reported one patient with a persistent P. aeruginosa infection in the VAC group.9 Two major complications occurred in two patients in the VAC group in the study by Mouës et al., namely sepsis and necrosis.12
The main difficulty in finding a conclusive answer lies in the fact that all of the studies that are described in this review contain heterogeneous data. In these studies, VAC was applied to a variety of types of wounds, including acute, chronic, and infected wounds. Although the maintained pressure level during VAC and the interval of dressing changes are comparable in all the selected articles, the results would have been more generalizable if there were no differences between these factors. Further, the patient numbers in the studies were low (<30 per treatment group but higher than at least 10 participants). Two studies did not include a control group.8,10 One study with a comparison group but without randomization had non-comparable treatment groups because peripheral artery disease was more common in the conventional group.9 Only two studies reported results of a randomized trial, and reported primary endpoints differed. However, both randomized trials showed no different change in bacterial load between VAC and conventional therapy. Therefore, we conclude there is some low quality evidence that VAC therapy does not change the bacterial load. Both randomized clinical trials could be subject to selection bias because the allocation concealment was not reported. These trials could also be subject to performance and detection bias because the participants and personnel except for the microbiologist were not blinded for the treatment. These two trials were also not balanced for some prognostic factors that make the treatment comparisons less reliable.
Summary
There is no clear answer to the question whether VAC can be safely used on any wound without causing or worsening wound infection. Since only a limited number of studies appeared to be relevant after the literature search, it becomes clear that there is still much not known about the relationship between VAC usage and the effect on the bacterial load. Shifts in the bacterial spectrum have only been documented by the study by Mouës et al.12 This study showed change of bacterial spectrum in biopsies, but the numbers were small.
VAC is a treatment method that has been thoroughly tested on animals, where it has a positive outcome on the bacterial load or has shown a clear shift in the bacterial spectrum.14,15 Two other reviews, which evaluated the effectiveness of VAC in general, have very briefly touched the subject of VAC and its effect on bacterial clearance, but no clear relationship was found either.3,4 More well-designed studies involving patients are needed to clarify the effects of VAC on bacteriology in both infected and non-infected wounds, and what these effects mean for patient outcome. These studies should be large randomized controlled trials with infected wounds with different types of bacteria. The VAC treatment should be started after debridement. Patients should be followed for a sufficient long time and treatment with antibiotics in the different study groups should be similar. Tissue biopsies and wound swabs should both be used for bacterial counts and examination of type of bacteria. Prospectively monitoring changes in the bacterial load and bacterial spectrum in patients that will receive VAC treatment on indication might be an effective way to find out whether it should indeed be used on specific wounds.
Take-Home Messages.
• No conclusions on changes in the bacterial load and type of bacteria during VAC can be drawn.
• There is no clear answer to the question whether VAC can be safely used on any wound without causing or worsening wound infection.
Abbreviations and Acronyms
- CI
confidence interval
- CVT
conventional (non-VAC) therapy
- PRISMA
Preferred Reporting Items for Systematic Reviews and Meta-Analyses
- VAC
vacuum-assisted closure
Author Disclosure and Ghostwriting
No competing financial interests exist. The content of this article was expressly written by the authors listed. No ghostwriters were used to write this article.
About the Authors
Aryan S.P. Patmo is a medical student at the Leiden University Medical Center. He conducted this review as part of his scientific internship. Pieta Krijnen is a clinical epidemiologist and works as a research coordinator at the department of Trauma Surgery, Leiden University Medical Center in Leiden, The Netherlands. Wim E. Tuinebreijer is a retired surgeon, epidemiologist, and clinical psychologist who is still involved in guiding research at the Burn Center in Beverwijk, The Netherlands. Roelf S. Breederveld is Professor in acute burn care at the university medical center of the Leiden university, the Netherlands. He also works as a burn and trauma surgeon in the burn center of the Red Cross Hospital in Beverwijk. Main topics of his research project is cost effectiveness in burn care. In the educational area he is the director of the Emergency Management of Severe Burns course in The Netherlands.
References
- 1.Gestring M: Negative pressure wound therapy. In: UpToDate, edited by Collins KA. and Sanfey H. Philadelphia, PA: Wolters Kluwer Health [Google Scholar]
- 2.KCI Licensing: Science behind wound therapy. Available at: www.kci1.com/KCI1/sciencebehindwoundtherapy (last accessed August15, 2013)
- 3.Birke-Sorensen H, Malmsjo M, Rome P, Hudson D, Krug E, Berg L, Bruhin A, Caravaggi C, Chariker M, Depoorter M, Dowsett C, Dunn R, Duteille F, Ferreira F, Francos Martinez JM, Grudzien G, Ichioka S, Ingemansson R, Jeffery S, Lee C, Vig S, Runkel N, Martin R, and Smith J; International Expert Panel on Negative Pressure Wound Therapy [NPWT-EP]: Evidence-based recommendations for negative pressure wound therapy: treatment variables (pressure levels, wound filler and contact layer)—steps towards an international consensus. J Plast Reconstr Aesthet Surg 2011; 64:S1. [DOI] [PubMed] [Google Scholar]
- 4.Lambert KV, Hayes P, and McCarthy M: Vacuum assisted closure: a review of development and current applications. Eur J Vasc Endovasc Surg 2005; 29:219. [DOI] [PubMed] [Google Scholar]
- 5.Moher D, Liberati A, Tetzlaff J, and Altman DG; PRISMA Group: Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int J Surg 2010; 8:336. [DOI] [PubMed]
- 6.Gabriel A, Shores J, Heinrich C, Baqai W, Kalina S, Sogioka N, and Gupta S: Negative pressure wound therapy with instillation: a pilot study describing a new method for treating infected wounds. Int Wound J 2008; 5:399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yusuf E, Jordan X, Clauss M, Borens O, Mäder M, and Trampuz A: High bacterial load in negative pressure wound therapy (NPWT) foams used in the treatment of chronic wounds. Wound Repair Regen 2013; 21:677. [DOI] [PubMed] [Google Scholar]
- 8.Saadi A, Perentes JY, Gonzalez M, Tempia AC, Wang Y, Demartines N, Ris HB, and Krueger T: Vacuum-assisted closure device: a useful tool in the management of severe intrathoracic infections. Ann Thorac Surg 2011; 91:1582. [DOI] [PubMed] [Google Scholar]
- 9.Steingrimsson S, Gottfredsson M, Gudmundsdottir I, Sjögren J, and Gudbjartsson T: Negative-pressure wound therapy for deep sternal wound infections reduces the rate of surgical interventions for early re-infections. Interact Cardiovasc Thorac Surg 2012; 15:406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Weed T, Ratliff C, and Drake DB: Quantifying bacterial bioburden during negative pressure wound therapy: does the wound VAC enhance bacterial clearance? Ann Plast Surg 2004; 52:276. [DOI] [PubMed] [Google Scholar]
- 11.Braakenburg A, Obdeijn MC, Feitz R, van Rooij IA, van Griethuysen AJ, and Klinkenbijl JH: The clinical efficacy and cost effectiveness of the vacuum-assisted closure technique in the management of acute and chronic wounds: a randomized controlled trial. Plast Reconstr Surg 2006; 118:390. [DOI] [PubMed] [Google Scholar]
- 12.Mouës CM, Vos MC, van den Bemd GJ, Stijnen T, and Hovius SE: Bacterial load in relation to vacuum-assisted closure wound therapy: a prospective randomized trial. Wound Repair Regen 2004; 12:11. [DOI] [PubMed] [Google Scholar]
- 13.Mouës CM, van den Bemd GJ, Huele F, and Hovius SE: Comparing conventional gauze therapy to vacuum-assisted closure wound therapy: a prospective randomised trial. J Plast Reconstr Aesthet Surg 2007; 60:672. [DOI] [PubMed] [Google Scholar]
- 14.Stinner DJ, Waterman SM, Masini BD, and Wenke JC: Silver dressings augment the ability of negative pressure wound therapy to reduce bacteria in a contaminated open fracture model. J Trauma 2011; 71 (1 Suppl):S147. [DOI] [PubMed] [Google Scholar]
- 15.Lalliss SJ, Stinner DJ, Waterman SM, Branstetter JG, Masini BD, and Wenke JC: Negative pressure wound therapy reduces pseudomonas wound contamination more than Staphylococcus aureus. J Orthop Trauma 2010; 24:598. [DOI] [PubMed] [Google Scholar]