Abstract
Background
Sub-optimal levels of vitamin D have been found to be highly prevalent in all age groups, with epidemiologic studies demonstrating a link between vitamin D deficiency and disease susceptibility, such as infection and cancer, and mortality rates. In adult transplant patients, it has been suggested that the immunomodulatory properties of vitamin D may have an important role in the prevention and treatment of graft-versus-host disease.
Objective
The objective of this study was to assess serum 25-hydroxyvitamin D levels of children and adolescents submitted to allogeneic hematopoietic stem cell transplantation. Methods: Serum 25-hydroxyvitamin D levels of 66 patients, aged 4-20 years, were assessed at three stages: before hospitalization for hematopoietic stem cell transplantation and at 30 and 180 days after hematopoietic stem cell transplantation. The control group consisted of 25 healthy children.
Results
At the pre-hematopoietic stem cell transplantation stage, patients had lower levels of 25-hydroxyvitamin D compared to controls (25.7 ± 12.3 ng/mL vs. 31.9 ± 9.9 ng/mL; p-value = 0.01), and a higher prevalence of 25-hydroxyvitamin D deficiency (32% vs. 8%; p-value = 0.01). Prevalence increased significantly after hematopoietic stem cell transplantation (p-value = 0.01) with half of the patients having vitamin D deficiency at 180 days after transplantation. At this stage, mean serum 25-hydroxyvitamin D levels were 20.9 ± 10.9 ng/mL, a significant decline in relation to baseline (p-value = 0.01). No correlation was found between 25-hydroxyvitamin D levels and vitamin D intake, graft-versus-host disease, corticoid use or survival rates.
Conclusion
Low levels of 25-hydroxyvitamin D were detected even before hematopoietic stem cell transplantation and were significantly lower at 180 days after hematopoietic stem cell transplantation, thus recommending vitamin D supplementation for children and adolescents submitted to hematopoietic stem cell transplantation.
Keywords: Hematopoietic stem cell transplantation, Vitamin D, Vitamin D deficiency, Bone marrow transplantation, Child nutrition
Introduction
Hematopoietic stem cell transplantation (HSCT) is a recommended procedure for the treatment of both benign and malignant hematologic diseases. It has been increasingly possible to reduce the number and the severity of complications associated with this procedure, and consequently, a growing number of patients have achieved long-term survival.1
Sub-optimal levels of vitamin D have been found to be highly prevalent in all age groups, with epidemiologic studies demonstrating a link between vitamin D status and disease susceptibility, such as infection and cancer, and mortality rates.2 Vitamin D deficiency is known to lead to rickets, osteomalacia and secondary hyperparathyroidism, muscle weakness and myalgia, all factors that accelerate bone mass loss and increase the risk for falls and fractures.3., 4. However, besides its well-established role in the musculoskeletal physiology, activated vitamin D has been implicated in several other physiological functions in the body. These include anti-carcinogenic properties, protection against cardiovascular disease, immune response regulation and auto-immune disease suppression through the ability of 1,25(OH)2D to modulate T lymphocyte proliferation and function.5., 6., 7.
The prevalence of vitamin D deficiency has not been widely investigated in children submitted to HSCT. In adult patients receiving allogeneic HSCT, it has been suggested that the immunomodulatory properties of vitamin D may have an important role in the prevention and treatment of graft- versus-host disease (GVHD), the leading cause of morbidity and mortality in this procedure.8., 9. Patients unresponsive to corticosteroid treatment may experience milder GVHD effects after correction of vitamin D deficiency.9., 10. Thus, the objective of the present study was to assess the vitamin D status of children and adolescents before and after allogeneic HSCT, and its correlation with GVHD incidence and mortality, 180 days post HSCT.
Methods
All patients from four to 20 years of age submitted to allogeneic HSCT between August 2006 and September 2008 at the Bone Marrow Transplantation Unit of our University Hospital were included in the study. Patients with osteometabolic disorders prior to HSCT were excluded. Patients were evaluated at three stages: before hospitalization for HSCT, and at 30 and 180 days after HSCT. The control group consisted of 25 healthy children recruited among children of employees of the University Hospital, matched for gender, age, race and body mass index (BMI) with the study group.
Dietary vitamin D intake was assessed through an interview conducted by a registered dietician, of a 24-hour recall and food frequency questionnaire. Dietary intakes were considered adequate when they equaled or surpassed 85% of the dietary reference intake, which recommends a daily intake of 200 IU for the 4-20 year age group.11 Patients were interviewed about their daily sun exposure and personal history of fractures and family history of osteoporosis. Sun exposure was defined as ‘low' when the child had a recreational unprotected exposure of arms and legs of less than 15 minutes per day and less than three times a week; ‘high' when the exposure was at least 30 minutes per day and five times per week; and ‘intermediate' when between the aforementioned criteria.12 This study was approved by the Ethics Committee of the Hospital das Clinicas of the Universidade Federal do Parana (UFPR) and all patients gave informed consent to participate in the study.
Serum 25-hydroxyvitamin D [25(OH)D] concentrations were measured by chemiluminescence (Liaison kit, Diasorin, Stillwater, MN) at all visits. The following values were used to classify vitamin D status: vitamin D deficiency for values of 20 ng/mL or less; vitamin D insufficiency for values between 20.1 and 29.9 ng/mL, and normal state (vitamin D sufficiency) for values of 30 ng/mL or above.3 Blood samples were frozen and stored, and serum 25(OH)D concentrations were measured at the end of the study period.
Patients were from different regions of Brazil, from places with different incidences of solar radiation, in latitudes between -30° to -5°. Hence, patients with low levels of vitamin D did not receive supplementation, since vitamin D status assessment is still not part of our center's HSCT protocol.
Statistical analysis
Values are presented as mean ± standard deviation (SD), except when otherwise stated. Differences between patients and controls for continuous variables were tested using an unpaired Student's t-test or Mann-Whitney test according to the characteristics of the data distribution. Differences for categorical variables were analyzed with the chi-square and Fisher's tests. Correlations were sought using the Pearson and Spearman coefficients for variables of normal and abnormal distributions, respectively. For all analyses, a two-sided p-value < 0.05 was considered to indicate statistical significance.
Results
Eighty children and adolescents were submitted to allogeneic HSCT in our institution during the study period. Fourteen were not included in the final analysis because they did not fulfill the inclusion criteria or they met the exclusion criteria. A total of 66 patients (38 boys and 28 girls aged 10 ± 4.1 years) were included in the study, and 15 of them (23%) died within the 6-month period following HSCT. As shown in Table 1, unrelated and related allogeneic HSCT was performed in 55% and 45% of the patients, respectively. The diagnosis was a non-malignant disease in 67% of the patients, including Fanconi anemia (35%), severe aplastic anemia (15%), Wiskott-Aldrich syndrome (9%) and adrenoleukodystrophy (8%), and a malignant disease in 33% of the group, including acute lymphocytic leukemia (14%), acute myeloid leukemia (9%), myelodysplasia (6%) and chronic myeloid leukemia (5%). Sixteen patients (25%) received a treatment scheme along with total body radiotherapy during the pre-transplantation conditioning stage, and 11 patients (17%) received corticosteroids for immunoprophylaxis of GVHD. Previous fractures were reported by eight patients (12%) and a family history of osteoporosis was identified in 14 (26%). In relation to their sun exposure before transplantation, eight (12%) patients were classified as having low sun exposure, 40 (61%) as intermediate and 18 (27%) as high exposure. Post-HSCT, all patients were instructed to refrain from sun exposure by their physician.
Table 1.
- Clinical characteristics of the patients (n = 66).
| Variable | n (%) |
|---|---|
| Diagnosis | |
| Non-malignant diseases | 44 (67) |
| Fanconi anemia | 23 (35) |
| Severe aplastic anemia | 10 (15) |
| Wiskott-Aldrich syndrome | 6 (9) |
| Adrenoleukodystrophy | 5 (8) |
| Malignant diseases | 22 (33) |
| Acute lymphocytic leukemia | 9 (14) |
| Acute myeloid leukemia | 6 (9) |
| Myelodysplasia | 4 (6) |
| Chronic myeloid leukemia | 3 (5) |
| Type of hematopoietic stem cell transplantation | |
| Non-related allogeneic | 36 (55) |
| Related allogeneic | 30 (45) |
| Conditioning | |
| Without radiotherapy | 50 (75) |
| With radiotherapy | 16 (25) |
At baseline, suboptimal 25(OH)D levels were observed in the majority of patients and controls, with vitamin D insufficiency or deficiency present in 47 (71%) of patients and in 13 (52%) controls. The mean serum 25(OH)D levels of the patients before HSCT was 25.7 ± 12.3 ng/mL, a value significantly lower than the mean levels of 31.9 ± 9.9 ng/mL found in the control group (p-value = 0.01; Figure 1). In addition, as shown in Table 2, the prevalence of vitamin D deficiency was significantly higher in patients compared to controls (32% vs. 8% p-value = 0.01). Dietary vitamin D intake was similar between the study groups (230.4 ± 110.8 IU/day in the patients vs. 196.8 ± 95.6 IU/ day in the controls); inadequate intakes were found in 18 patients (27%) and nine controls (36%).
Figure 1.
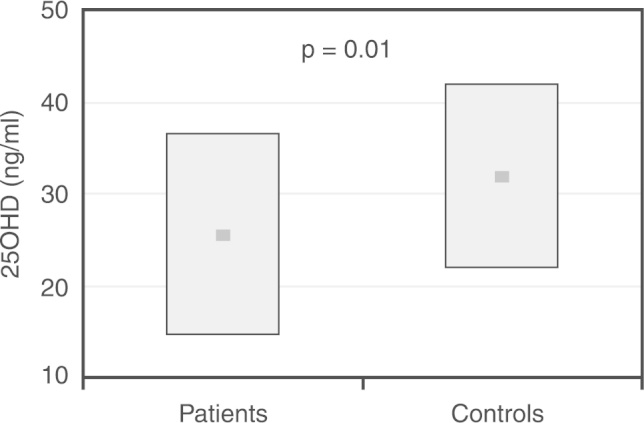
Serum 25(OH) vitamin D levels in 66 children and adolescents before hematopoietic stem cell transplantation in comparison with 25 controls matched by gender, age and BMI. The plot shows mean levels (dark squares) and standard deviations.
Table 2.
- Clinical characteristics of the patients before hematopoietic stem cell transplantation compared to controls.
| Variable | Patients (n = 66) | Controls (n = 25) | p-value |
|---|---|---|---|
| Age (years) | 10.1 ± 4.1 | 10.2 ± 3.8 | 1.00 |
| Gender (M:F) | 1.4:1 | 1.5:1 | 0.87 |
| Weight (kg) | 35.6 ± 17.3 | 39.0 ± 18.6 | 0.46 |
| BMI (kg/m2) | 18.3 ± 3.8 | 18.6 ± 4.1 | 0.61 |
| Z-Score BMI/age | 0.50 (-4.0 to 3.0) | 0.28 (-1.0 to 2.5) | 0.76 |
| Lean body mass (kg) | 28.7 ± 14.2 | 31.7 ± 15.2 | 0.49 |
| Normal vitamin D | 19 (29%) | 12 (48%) | 0.04a |
| Insufficiency vitamin D | 26 (39%) | 11 (44%) | |
| Deficiency vitamin D | 21 (32%) | 2 (8%) | |
| BMI: body mass index. a p-value = 0.04 (Fisher’s test for the prevalence of insufficiency, deficiency and normal vitamin D levels between patients and controls). | |||
After HSCT, 24 patients (36%) developed either acute or chronic forms of GVHD (20 had acute GVHD, four had chronic GVHD and eight had both acute and chronic GVHD), and 33 patients (50%) received corticosteroids at an average cumulative dose of 105.0 ± 63.5 mg/kg body weight (0.97 ± 0.42 mg/kg/day) of prednisone. There were no significant differences in serum 25(OH)D levels between patients with or without GVHD (20.2 ± 11.9 ng/mL vs. 20.5 ± 11.1 ng/mL, respectively), between those receiving or not receiving corticosteroids (21.6 ± 12.9 vs. 18.5 ± 13.0 ng/mL, respectively), or in the prevalence of vitamin D deficiency or insufficiency pre-HSCT in patients who developed GVHD. Median length of hospital stay was 36 days (range: 22 to 137 days; mean: 45.4 days), during which all patients received a daily vitamin D dose of 400 to 800 IU in accordance with the medical protocol of preventive vitamin supplementation. After discharge, 39 patients (59%) received vitamin D supplements (as part of the multivitamin supplementation scheme post HSCT) for an average of 140 ± 55 days; six patients (9%) received a daily dose of more than 1200 IU of vitamin D, and 33 (50%) received between 400 to 1200 IU of the vitamin per day. No correlation was found between 25(OH)D levels and dietary intake or supplemental use of the vitamin. The group receiving supplemental vitamin D, however, showed an increase in 25(OH)D levels at 180 days post HSCT, although this difference did not reach statistical significance (21.4 ± 10.7 vs. 18.9 ± 12.4 ng/mL, respectively).
At 30 days post HSCT, serum levels of 25(OH)D were 22.7 ± 10.7 ng/mL, and no statistically significant difference was found in comparison to baseline (Figure 2). Sub-optimal levels of 25(OH)D were observed in 80% of the patients, with 38.2% showing vitamin D insufficiency and 41.8% vitamin D deficiency. There was no difference in 25(OH)D levels comparing the 15 patients who died to the 51 patients who survived (19.6 ± 6.8 vs. 23.1 ± 12.2 ng/mL, respectively).
Figure 2.
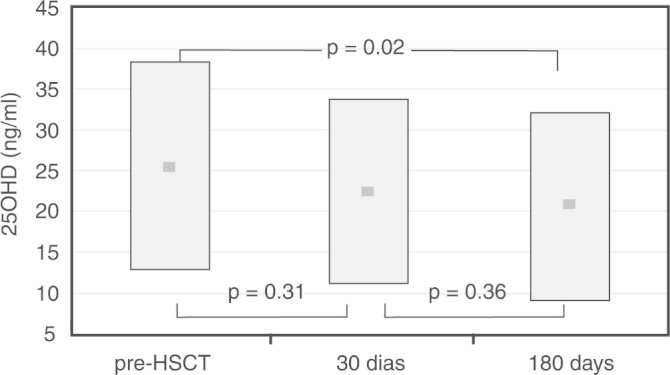
Serum 25(OH) vitamin D levels in children and adolescents before hematopoietic stem cell transplantation (n = 66) and 30 days (n = 55) and 180 days (n = 51). The plot shows mean levels (dark squares) and standard deviations.
At 180 days post-HSCT, serum levels of 25(OH)D were significantly reduced to 20.9 ± 10.9 ng/mL (p-value = 0.02) in relation to the baseline values (Figure 2). Sub-optimal levels of 25(OH)D were observed in 69% of the patients, with 18% showing vitamin D insufficiency and 51% vitamin D deficiency. Dietary vitamin D intake was not significantly different at this stage in relation to the baseline values (199.4 ± 135.4 IU/day).
By multivariate analysis, the variation of 25(OH)D levels at 180 days after HSCT was tested for dietary intake of vitamin D, the use of vitamin D supplementation, weight loss, type of conditioning, the use of corticosteroids, the presence of GVHD and number of days in the hospital, but no significant correlation was found.
Discussion
Serum 25(OH)D is the indicator of vitamin D bioavailability in the body.13 It has been recommended that serum levels of 25(OH)D above 30 ng/mL in children and adults are optimal for gaining all the potential health benefits associated with vitamin D.13., 14. Regular sun exposure and adequate dietary intake are essential in meeting these recommendations. Dietary sources of the vitamin, however, are limited to milk and dairy products, fatty fish and egg yolk, and fortification of industrialized foods is still uncommon in many places, as in Brazil, thus prompting the need for vitamin D supplementation.13
In the present study, we found a high prevalence of vitamin D deficiency and insufficiency both in our children and adolescents submitted to HSCT and in our control group, with sub-optimal levels observed in 66% of all participants. However, this alarming percentage is not exclusive to our institution and it has been demonstrated all over the world.3., 15. For instance, the National Health and Nutrition Examination Survey 2001-2004, which involved 6275 individuals aged one to 21, found that 9% of their population (which represents 7.6
million US children and adolescents) were vitamin D deficient, whereas 61% (50.8 million US children and adolescents) had vitamin D insufficiency.16 These numbers are a warning of the poor vitamin D status of younger populations, which is mainly related to the lack of unprotected moderate sun exposure and insufficient intake through food and supplements. In addition, low 25-OHD levels have been related to adiposity, physical activity, and fitness.15 Considering the dietary reference intake from The Institute of Medicine (IOM) in 1997, which recommends 200 IU of vitamin D per day for individuals between 0 and 18 years of age, only 29.7% of the participants of our study reported a daily vitamin D intake lower than this cut-off.11 However, it is known that this recommendation is far from optimal for maintaining adequate serum 25(OH) D levels.14 If we consider the new dietary reference intake published by the IOM in 2010, which recommends at least 400 IU of vitamin D per day, all participants of our study would be classified as having inadequate vitamin D intakes.17
Given the proposed role of activated vitamin D in immune cells, vitamin D status might be of special importance to children and adolescents with malignant and non-malignant diseases treated with HSCT. In vitro studies report that due to its inhibitory effects on T cell proliferation and cytokine production, vitamin D plays an important role in controlling the immune response that occurs in GVHD.8 In fact, some studies have reported an association between low vitamin D levels and the presence or progression of GVHD or corticosteroid use. Robien et al.4 found a link between 25(OH)D levels after HSCT and prednisone and immunosuppressive drug use. Kreutz et al.8 studied 48 patients before and after allogeneic bone marrow transplantation and detected the lowest levels in patients with Grades 3 and 4 of GVHD, in comparison to patients without GVHD and Grades 1 and 2 of the disease. In our study, we could not detect a statistically significant difference in the serum 25(OH)D levels between patients who developed GVHD and those who did not, or between those who were treated or not treated with corticosteroids. Since the sampling size of our study was similar to that of the other series, our negative findings may be a result of the high prevalence of sub-optimal levels of vitamin D, both before and after HSCT. In fact, the mean serum 25(OH)D level was approximately 20 ng/mL in the whole group, regardless of GVHD or the use of corticosteroids, with 50% of the patients exhibiting vitamin D deficiency 180 days after transplantation. It is worth mentioning that this high prevalence of vitamin D deficiency and the progressive and significant decline of 25(OH)D levels up to 180 days after HSCT occurred despite the protocol of oral vitamin supplementation for these patients in our institution.
Vitamin D status has been assessed in adults receiving bone marrow transplantation, with many studies looking at the correlation with musculoskeletal health. Sproat et al. evaluated 58 adult patients after HSCT and found that 89.7% of them had low serum 25(OH)D levels.18 Schulte et al.19 assessed 81 adult patients and found that the majority had vitamin D deficiency with secondary hyperparathyroidism, and a subsequent reduction in vitamin D levels 28 days after the procedure, despite daily vitamin D supplementation. Kerschan-Schindl et al.20 found that 25% of 22 adults treated with HSCT had vitamin D deficiency six or more years after the procedure, with a correlation between 25(OH)D levels and bone mineral density of the lumbar spine. Another study assessed adult patients between 12 to 115 months after HSCT, and found a correlation between osteoporosis and osteopenia with low levels of 25(OH)D and insulin resistance.21
The literature on serum vitamin D levels in children and adolescents on HSCT is scarcer. Duncan et al. assessed the prevalence of vitamin D deficiency in 67 children after HSCT and found that 80.6% of the group had sub-optimal levels of 25(OH)D.22 Robien et al.4 followed 44 children and adolescents aged one to ten years after HSCT, and observed 34% and 5% of vitamin D insufficiency and deficiency, respectively. Moreover, the authors showed that the lower the vitamin D intake (through diet and supplementation), the lower their 25(OH)D levels were thus suggesting that an intake of 400 to 600 IU of vitamin D per day is required to maintain adequate serum levels. In the current study, all patients received 400 to 800 IU per day of vitamin D during hospitalization, and 39 (59%) received vitamin D supplements after discharge for an average of 140 days: 50% at doses ranging from 400 to 1200 IU and only 9% at a larger daily dose. Accordingly, the prevalence of vitamin D deficiency in this study should have been much lower as most of the patients received supplements according to the current recommendations. Compliance may have been a problem and this was not addressed directly in the study. Another explanation might be related to alterations in dietary intake due to gastrointestinal abnormalities, which are more common in these patients, or the use of corticosteroids, cyclosporin and anticonvulsants that may also interfere with the vitamin D metabolism.4., 18., 23., 24. Lastly, these patients received formal recommendations from their doctors for reclusion after HSCT, and to avoid any sun exposure in order to prevent GVHD and new cases of cancer. Taken together, these factors contribute to a high prevalence of vitamin D deficiency in children and adolescents treated with HSCT, which may have short- and long-term consequences for their general health. We believe there is a strong need to review the current protocols for preventing and treating vitamin D deficiency and insufficiency in these patients, in order to achieve adequate and safe vitamin D levels.
Conflicts of interest
The authors declare no conflicts of interest.
Acknowledgment
We thank the personnel of the Frischmann Aisengart Laboratories for technical support in the measurements of serum 25(OH)D levels.
REFERENCES
- 1.Frangoul H., Najjar J., Simmonsb J., Domm J. Long-term followup and management guidelines in pediatric patients after allogeneic hematopoietic stem cell transplantation. Semin Hematol. 2012;49:94–103. doi: 10.1053/j.seminhematol.2011.10.012. [DOI] [PubMed] [Google Scholar]
- 2.Wagner C.L., Greer F.R. Prevention of rickets and vitamin D deficiency in infants, children and adolescents. Pediatrics. 2008;122:1142–1152. doi: 10.1542/peds.2008-1862. [DOI] [PubMed] [Google Scholar]
- 3.Holick M.F. N Engl J Med. 2007;357:266–281. doi: 10.1056/NEJMra070553. Comment in: N Engl J Med. 2007;357:1980-1; author reply 1981-2; Int J Clin Pract. 2009;63:1265. [DOI] [PubMed] [Google Scholar]
- 4.Robien K., Strayer L.G., Majhail N., Lazovich D., Baker K.S., Smith A.R. Vitamin D status among long-term survivors of hematopoietic cell transplantation. Bone Marrow Transplant. 2011;46:1472–1479. doi: 10.1038/bmt.2010.326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Harbans L., Rajesh P., Aggarwal S.K. Vitamin D: Non-skeletal actions and effects on growth. Nutr Research. 1999;19:1683–1718. [Google Scholar]
- 6.Adams J.S., Hewison M. Unexpected actions of vitamin D: new perspectives on the regulation of innate and adaptive immunity. Nat Clin Pract Endocrinol Metab. 2008;4:80–90. doi: 10.1038/ncpendmet0716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Misra M., Pacaud D., Petryk A., Collett-Solberg P.F., Kappy M. Drug and Therapeutics Committee of the Lawson Wilkins Pediatric Endocrine Society. Vitamin D deficiency in children and its management: review of current knowledge and recommendations. Pediatrics. 2008;122:398–417. doi: 10.1542/peds.2007-1894. [DOI] [PubMed] [Google Scholar]
- 8.Kreutz M., Eissner G., Hahn J., Andreesen R., Drobnik W., Holler E. Variations in 1 alpha,25-dihydroxyvitamin D3 and 25-hydroxyvitamin D3 serum levels during allogeneic bone marrow transplantation [letter] Bone Marrow Transplant. 2004;33:871–873. doi: 10.1038/sj.bmt.1704448. [DOI] [PubMed] [Google Scholar]
- 9.Silva F., Perez-Simon J.A., Caballero-Velazquez T., Sanchez-Guijo F.M., Villanueva-Gomez F., Vazquez L. Effect of vitamin D treatment in chronic GVHD. Bone Marrow Transplant. 2011;46:1395–1397. doi: 10.1038/bmt.2010.317. [DOI] [PubMed] [Google Scholar]
- 10.Rosenblatt J., Bissonnette A., Ahmad R., Wu Z., Vasir B., Stevenson K. Immunomodulatory effects of vitamin D: implications for GVHD. Bone Marrow Transplant. 2010;45:1463–1468. doi: 10.1038/bmt.2009.366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.IOM - Institute of Medicine . National Academies Press; 1997. Dietary Reference Intake for calcium, phosphorus, magnesium, vitamin D and fluoride. [PubMed] [Google Scholar]
- 12.Maeda S.S., Kunii I.S., Hayashi L., Lazaretti-Castro M. The effect of sun exposure on 25-hydroxyvitamin D concentrations in young healthy subjects living in the city of São Paulo. Brazil. Braz J Med Biol Res. 2007;40:1653–1659. doi: 10.1590/s0100-879x2006005000162. [DOI] [PubMed] [Google Scholar]
- 13.Holick M.F., Binkley N.C., Bischoff-Ferrari H.A., Gordon C.M., Hanley D.A., Heaney R.P. Evaluation, treatment, and prevention of vitamin D deficiency: An Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2011;96:1911–1930. doi: 10.1210/jc.2011-0385. [DOI] [PubMed] [Google Scholar]
- 14.Holick M.F., Vitamin D. Status: Measurement, Interpretation, and Clinical Application. Ann Epidemiology. 2009;19:73–78. doi: 10.1016/j.annepidem.2007.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dong Y., Pollock N., Stallmann-Jorgensen I.S., Gutin B., Lan L., Chen T.C. Low 25-hydroxyvitamin D levels in adolescents: race, season, adiposity, physical activity, and fitness. Pediatrics. 2010;125:1104–1111. doi: 10.1542/peds.2009-2055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kumar J., Muntner P., Kaskel F.J., Hailpern S.M., Melamed M.L. Prevalence and associations of 25-hydroxyvitamin D deficiency in US children: NHANES 2001-2004. Pediatrics. 2009;124:e362–e370. doi: 10.1542/peds.2009-0051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.IOM - Institute of Medicine . National Academies Press; 2010. Dietary Reference Intake for calcium and vitamin D. [PubMed] [Google Scholar]
- 18.Sproat L., Bolwell B., Rybicki L., Dean R., Sobecks R., Pohlman B. Vitamin D level after allogeneic hematopoietic stem cell transplant. Biol Blood Marrow Transplant. 2011;17:1079–1083. doi: 10.1016/j.bbmt.2010.12.704. [DOI] [PubMed] [Google Scholar]
- 19.Schulte C., Beelen D.W., Schaefer U.W., Mann K. Bone loss in long-term survivors after transplantation of hematopoietic stem cells: a prospective study. Osteoporos Int. 2000;11:344–353. doi: 10.1007/s001980070124. [DOI] [PubMed] [Google Scholar]
- 20.Kerschan-Schindl K., Mitterbauer M., Füreder W., Kudlacek S., Grampp S., Bieglmayer C. Bone metabolism in patients more than five years after bone marrow transplantation. Bone Marrow Transplant. 2004;34:491–496. doi: 10.1038/sj.bmt.1704618. [DOI] [PubMed] [Google Scholar]
- 21.Faulhaber G.A.M., Premaor M.O., Moser Filho H.L., Silla L.M., Furlanetto T.W. Low bone mineral density is associated with insulin resistance in bone marrow transplantation subjects. Bone Marrow Transplant. 2009;43:953–957. doi: 10.1038/bmt.2009.70. [DOI] [PubMed] [Google Scholar]
- 22.Duncan C.N., Vrooman L., Apfelbaum E.M., Whitley K., Bechard L., Lehmann L.E. 25-Hydroxy vitamin D deficiency following pediatric hematopoietic stem cell transplant. Biol Blood Marrow Transplant. 2011;17:749. doi: 10.1016/j.bbmt.2010.10.009. 3. [DOI] [PubMed] [Google Scholar]
- 23.Kulak C.A.M., Borba V.Z.C., Silvado C.E., Paola L., Seibel M.J., Bilezikian J.P. Bone density and bone turnover markers in patients with epilepsy on chronic antiepileptic drug therapy. Arq Bras Endocrinol Metab. 2007;51:466–471. doi: 10.1590/s0004-27302007000300016. [DOI] [PubMed] [Google Scholar]
- 24.Grenet O., Bobadilla M., Chibout S.D., Steiner S. Evidence for the impairment of the vitamin D activation pathway by cyclosporine A. Biochem Pharmacol. 2000;59:267–272. doi: 10.1016/s0006-2952(99)00321-4. [DOI] [PubMed] [Google Scholar]


