Abstract
While early-life estrogens are thought to play a physiologic role in prostate gland development, inappropriate estrogenic exposures either in dose, type or temporally can reprogram the prostate gland and increase susceptibility to abnormal prostate growth with aging including carcinogenesis. This review discusses the evidence for developmental estrogenic reprogramming that leads to adult prostate disease in a rat model. We propose that estrogen imprinting of the prostate is mediated through both structural reorganization of the gland early in life and epigenomic reprogramming that permits life-long memory of the inappropriate developmental exposures including heightened sensitivity to rising estradiol levels with aging. Complex interactions between early epigenetic programming and later-life experiences results in an emergence of multiple epigenomic outcomes, with some contributing to carcinogenesis with aging.
Keywords: bisphenol A, estrogen, estrogenization, prostate development, reprogramming
The prostate gland is a male accessory sex gland that functions to produce seminal plasma components. While its role in male fertility is dispensable, there is considerable interest in understanding the growth and regulation of the prostate due to its high rate of disease in aging men including prostatic adenocarcinoma. Embryologically, the prostate gland arises from the endodermal urogenital sinus (UGS) which differs from the rest of the male reproductive accessory sex glands that are derived from the mesodermal Wolffian ducts.1 Several decades ago, John McNeal proposed that this unique embryological origin for the prostate gland may be a key to the high rates of cancer in the prostate gland as compared to the low cancer incidence in the other male accessory sex structures. This fact suggests that the origins of prostate cancer may have a developmental basis.
Influence of estrogens on human prostate development
Prostate gland development commences in utero between the 10th to the 12th week of gestation in humans when buds from the UGS epithelium penetrate into the surrounding mesenchyme in response to testosterone produced by the fetal testes. During the second trimester, fetal androgens continue to rise and under their control, prostate ducts lengthen and undergo complex branching patterns. During the third trimester, as androgen levels decline and maternal estrogen levels rise, the stromal and epithelial cells undergo cytodifferentiation which is directly influenced by both androgens and estrogens.1 That estrogens influence prostate development is evidenced by the large amount of squamous metaplasia – a known marker of estrogen action – that is present in prostatic epithelium at the time of birth and sloughs during the first week of life as the maternal estrogen levels plummet in the newborn. It is in this context that we ask whether the risk of prostate cancer might be determined early in life by fetal and perinatal estrogen exposures, both natural and exogenous compounds.
Epidemiology studies have shown a strong correlation between indicators of elevated pregnancy estradiol levels, such as preeclampsia, jaundice and length of gestation and an increased risk of prostate cancer in men as they age.2 Sons of mothers who used diethylstilbestrol (DES) during pregnancy were found to have structural abnormalities of the prostatic utricle with persistent ectasia at 1 month of age as compared to unexposed newborn males.3 While DES-exposed sons have not been extensively followed as they aged, rodent models have shown that fetal or neonatal DES exposure predisposes to prostate tumors in aging animals.4 More recently, the issue of the endocrine disrupting chemicals and the long-term effects they may have on endocrine dependent organs such as the prostate gland has come to the forefront. Many potentially toxic chemicals, such as dioxins and the estrogen-mimic bisphenol A (BPA), when giving during development have been shown to affect prostate growth and disease susceptibility.
Developmental estrogenization of the prostate gland in a rodent model
To study prostate disease as a function of early life estrogen exposures, our laboratory has been working with the rat prostate model for the past 20 years. In rodents, unlike in humans, prostate morphogenesis initiates relatively late in fetal life and at the time of birth, the prostate gland is rudimentary, consisting of a few epithelial buds penetrating into a mass of mesenchyme. During the first 10 to 15 days of life, extensive branching morphogenesis and increasing structural complexity takes place along with stromal and epithelial cell differentiation.1 It is during this particular neonatal period, days 0–5, where we have identified a critical reprogramming window for toxicant exposures of the prostate gland.5 Thus we have used this neonatal model to study the structural, cellular and molecular aspects of early life estrogens and later life disease of the prostate gland (reviewed in6,7). Our initial work focused on high-dose estradiol exposures to model early life exposures to pharmaceutical estrogens (e.g. DES or ethinyl estradiol). Newborn Sprague-Dawley rats were given a brief neonatal exposure to 17 β-estradiol on days 1, 3 and 5 and their prostates are studied at various time points throughout life. As these animals matured, developmental and differentiation defects of the prostate were noted and at 1 year of age, there was extensive prostatic dysplasia and tumor formation.8 Throughout the ventral prostate lobe were adenomas, prostate intraepithelial neoplasia (PIN) lesions, areas of squamous metaplasia and inflammatory cell infiltration. Although the dorsal and lateral lobes were also affected, the most severe lesions were noted in the ventral prostate lobes. These observations led us to conclude that high-dose estrogen exposures during development can directly predispose to prostate neoplasia and the formation of tumors with aging.
Low-dose estrogens increase prostatic susceptibility to hormonal carcinogenesis
We next evaluated the prostatic response to neonatal low-dose 17 β-estradiol exposures in a dose–response study that examined a 7-log range of doses (0.15 µg/kg BW to 15 mg/kg BW). While a direct induction of neoplastic prostate lesions was observed following early-life high-dose estrogen exposure, we failed to observe histologic evidence for prostatic pathology at the lower doses (150 µg/kg BW or less).9 Although this indicated that low-level estrogens might not be a direct carcinogen initiator, we asked whether developmental low-dose exposures might shift the sensitivity or susceptibility of the prostate gland to adult hormone carcinogenesis. This is particularly relevant since estradiol is a classified carcinogen and has been shown to drive prostate cancer in animal models.10 Furthermore, relative estrogen levels rise in aging men due to aromatase expression in fat cells that convert testosterone to estradiol combined with declining levels of circulating androgens. To address whether developmental exposures to estrogens could heighten sensitivity of the prostate to rising adult estradiol levels, we administered a ‘first hit’ of estradiol (25 µg or 0.1 µg/kg BW) or bisphenol A at low, environmentally relevant levels (10 µg/kg BW) to neonatal rats during the developmental critical period (days 1, 3 and 5) and subsequently exposed these males to a 16-week ‘second’ hit of elevated estradiol when they reached adulthood to initiate carcinogenesis. Testosterone implants were given to maintain normal androgen levels in the males while the estradiol levels were at three times the level found in young adult males. The Sprague-Dawley rat was chosen since it has low sensitivity to adult estrogen-induced carcinogenesis in contrast to the Noble rat where adult estrogens drive severe dysplasia and PIN lesions in all males after 16 weeks, which progress to adenocarcinoma. We wanted to test whether early life exposures could shift the susceptibility of estrogen-induced carcinogenesis in the Sprague- Dawley rat to the one similar to the Noble rat.
At 7 months of age, the prostate glands were histologically assessed for pathologic lesions in a manner blinded to the treatment groups. Results revealed that while high-dose estradiol exposures resulted in prostate dysplasia with aging, low-dose BPA exposure by itself did not result in pathology.11 However, we observed that early-life BPA exposure caused a significant increase in susceptibility to adult estradiol exposures, shifting the PIN incidence from 40% in control animals to 100% in rats exposed perinatally to BPA. This was recently confirmed in a follow-up study where 10 µg/kg BW BPA was administered by either the oral route or through subcutaneous exposure and found comparable increased rates of T+E induced prostate lesions irregardless of exposure route.12 Together, these studies indicate that early-life exposures to environmentally relevant levels of xenoestrogens such as BPA reprogram the prostate such that it is more sensitive to estrogenic exposures later in life leading to increased disease susceptibility.
Mechanisms of estrogen action in the developing prostate gland
What is the cellular and molecular basis whereby a brief estrogen exposure during development can permanently reprogram the prostate gland? How does a transient estrogen encounter permanently alter the memory of prostate cells to adult estrogenic exposures? To begin to answer these questions, we first examined the developing prostate gland for expression and localization of steroid receptors that mediate estrogenic effects. During early prostatic development in rodents, the dominant estrogen receptor is ERα, localized to periacinar stromal cells that direct epithelial growth and differentiation through paracrine signaling.13 ERβ expression, although much lower in early development, is localized to epithelial cells as they begin differentiation between days 4–6.14 In studies with Ken Korach (NIEHS, Research Triangle Park, NC, USA) using ERα and ERβ knockout mice, we determined that the effects of high-dose estrogen exposures were entirely mediated through ERα and not ERβ, suggesting a paracrine effect on prostate epithelium through the stromal ERα.15 Which ER is involved in mediating early-life BPA effects on the prostate gland is unclear at present and may involve stromal ERα, epithelial ERβ and/or membrane ERs on both cell types.
Estrogen action through ERs is typically envisioned as activational, that is, a reversible response to hormone exposure that lasts as long as the hormone is present. The classic example of adult activational estrogen action would be cyclic changes in uterine receptivity. However, estrogen actions during development can also be organizational which are considered irreversible. The classic example of organizational estrogen actions is structural rearrangement of brain regions in a sex-specific manner that result in male and female-specific behaviors. Another type of organizational action that estrogens may direct is epigenomic reorganization whereby epigenetic marks are laid down within the developing structure leading to epigenetic memory in the mature organ. We have collected evidence for both of these organizational actions in the developing prostate gland in response to estrogens early in life and propose that structural reorganization and epigenomic reorganization by estrogens together provide the cellular and molecular underpinnings for prostatic reprogramming that may predispose to carcinogenesis with aging.
Structural reorganization of the developing prostate by estrogens
When newborn rats are exposed to high doses of estradiol during the developmental critical window for prostate development, branching morphogenesis is severely retarded such that fewer branch points are established in the ventral lobe while the main ducts fail to branch in the dorsal and lateral lobes.16 Vom Saal et al.17 have shown that following low-dose estrogen exposures during development, the opposite occurs with more prostatic buds forming which results in more extensive branching in the mature prostate. At the cellular level, we have observed early responses to estrogen exposures at days 3–5 of life that include excessive proliferation of periductal fibroblast cells which creates a physical barrier between acinar epithelial cells and smooth muscle cell-derived paracrine signals.18 Concomitant with these stromal changes are perturbations in normal epithelial differentiation that result in increased basal cell numbers, altered gap junctions and adhesion molecules and inappropriate secretory gene expression.19 As a consequence of this early structural reorganization in cellular composition of the gland, the adult prostate is highly disorganized with piled epithelial cells, altered vascularization and excess stromal tissue which in turn attracts inflammatory cell infiltration, including resident macrophages and T cells, that further drive prostate dysplasia in a vicious cycle.20,21
Research from our laboratory has determined that several of these permanent changes in prostate structure are a result of direct estrogen actions on prostate gene expression during the critical developmental window. In response to high-dose estradiol, androgen receptor levels are suppressed in stromal and epithelial cells due to targeted proteolytic degradation.22 At the same time, estradiol up-regulates ERα expression in the mesenchymal cells thus further amplifying estrogenic actions over the next 10 days.13 Stromal and epithelial RARs and RXRs are additionally upregulated by estrogens early in development which together leads to perturbations in steroid-mediated differentiation signals that control prostate development6 (See Fig. 1).
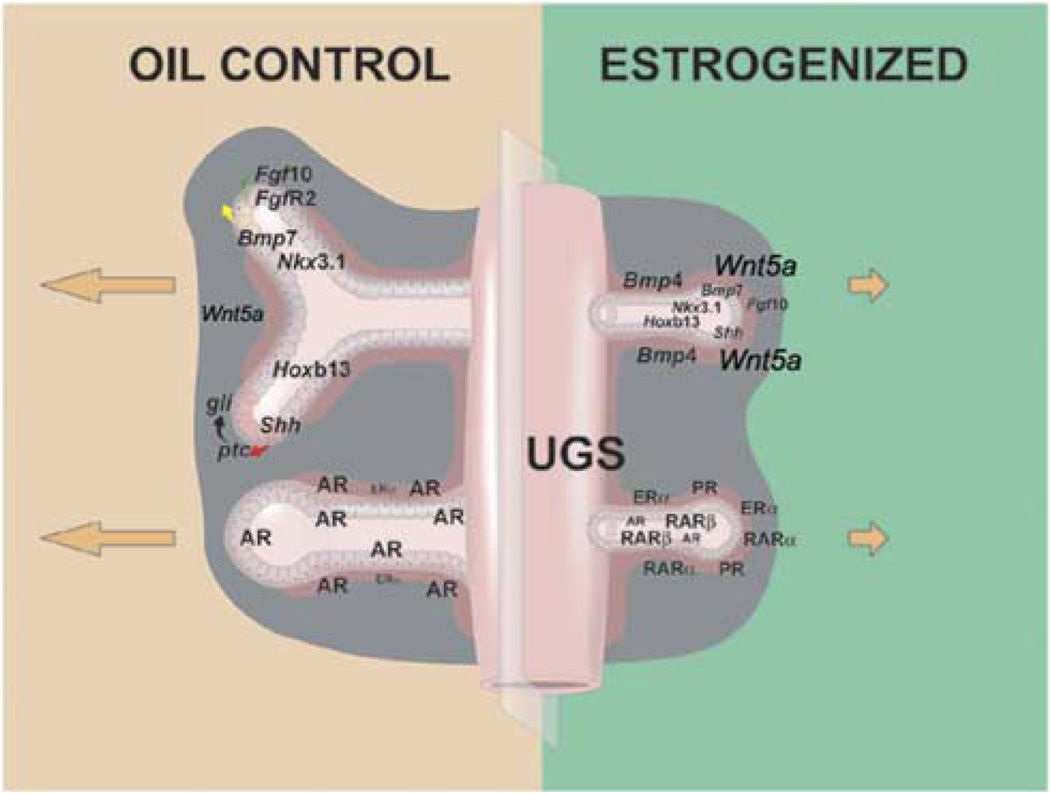
Fig. 1.
A schematic representation of the neonatal estradiol-induced organizational and gene expression changes found in the developing rat prostate lobes. Relative gene expression levels between control and treated prostates are represented by type size. In the normal prostate (left panel), androgen receptor (AR) is the dominant steroid receptor dictating morphogenesis in the branching and differentiating structure. Lower amounts of ERα are found in the proximal ducts. Together, these steroids influence the expression of multiple morphoregulatory genes that drive ductal outgrowth and branching. Mesenchymal expression of Fgf10 acts on epithelial FgfR2 receptors to stimulate branching while mesenchymal Wnt5a and Bmp4 act as localized inhibitors to restrict growth at specific sites. Epithelial cells secrete Shh, which acts on mesenchymal ptcreceptors that in turn activate the gli transcription factors to direct growth at the distal tips of emerging ducts. Transcription factors Nkx3.1 and Hoxb13 are expressed by developing epithelial cells and control early differentiation pathways. Neonatal exposure to high-dose estradiol suppresses ductal outgrowth and branching morphogenesis and perturbs differentiation through alterations in these signaling networks (right panel). The structural alterations are initiated through changes in steroid receptor expression in the developing gland including reduced AR, amplified ERα, induction of progesterone receptor (PR) and increased RARα, β and γ expression (lower right schematic). The alterations in steroidal signaling immediately redirect the expression of morphoregulatory genes (upper right schematic). Increased Bmp4 and Wnt5a combined with reduced Fgf10 expression suppress growth and branching of the ducts. Transient reduction in Nkx3.1 and permanent downregulation of Hoxb13 initiate epithelial differentiation defects that persist throughout life and predispose to neoplasia with aging.
Over the past several years, work from our laboratory and other investigators have identified key developmental genes that are expressed in a coordinate, temporal manner that effect proper development of the prostate gland.1 Importantly, many of these transcription factors and secreted morphogens are under direct or indirect regulation by androgens, estrogens and retinoids in the developing prostate gland. We now have strong evidence that inappropriate estrogen exposures during development can alter expression levels and actions of these morphoregulatory genes, which contributes to the permanent structural reorganization of the prostate gland.23 While several genes are affected by estrogens, two clear examples are mentioned here. Expression of Hoxb13, a homeobox transcription factor, is initiated in prostate epithelial cells shortly after birth and is essential for driving their early differentiation.24 Exposure to high-dose estradiol during this window blocks the temporal increase in Hoxb13 expression responsible for normal cell differentiation during development. This estrogen effect on Hoxb13 expression is not only immediate but also permanent which contributes to perturbed differentiation of the epithelial cells throughout life.
Another developmental gene important in prostate morphogenesis is Wnt5a, a member of the Wnt gene family of secreted glycoproteins that is expressed by periductal mesenchymal/stromal cells in the developing prostate.25 Our studies have shown that Wnt5a is a negative regulator of prostate growth and branching morphogenesis. Importantly, we find that early high-dose estrogen exposures results in a marked and immediate increase in stromal expression of this gene. Combined with reduced Fgf10, an inducer of epithelial proliferation and branching, and increased Bmp4, another branching inhibitor, these morphogen alterations contribute to the branching phenotypes observed in the estrogenized prostate glands as depicted in Fig. 1.7,26 It is noteworthy that emerging evidence indicates dysregulation of many of these same genes occurs during prostate carcinogenesis in humans.
Epigenomic reorganization of the developing prostate by estrogens
In addition to structural organization of the prostate by early-life estrogens, prostate cells exposed to inappropriate estrogen levels during development retain a lifelong memory of this early contact such that they are sensitized to secondary estrogen exposures later in life. Evidence is now emerging that this is mediated through alterations in the prostate epigenome that create a life-long epigenetic memory of the early-life exposures, resulting in differential gene expression and responses to later life experiences that contributes to disease onset with aging. Specifically, we have shown that developmental estrogens permanently alter DNA methylation and gene expression of multiple prostatic genes that are associated with altered susceptibility to adult pathology.
Our initial studies examined the prostates exposed neonatally to low- or high-dose estradiol or to low-dose BPA using methylation sensitive restriction fingerprinting. Examination of prostates collected on days 10, 90 and 200 of life, without or with the second exposure to elevated estradiol in adulthood, identified over 50 candidate genes that were differentially methylated in the treatment groups as compared to controls.11,27 Interestingly, we found that alterations in DNA methylation marks and resulting gene expression changes occur at different times in the animals’ life suggesting complex interactions between early exposures and later life events (see Fig. 2). A persistent epigenetic mark was observed with Nucleosome Binding Protein 1 (Nsbp1), which was found to be immediately and permanently hypomethylated by all early estrogenic exposures resulting in elevated expression of this gene throughout life. This gene is particularly significant since Nsbp1 is a nucleosome-core-particle binding protein that plays a role in chromatin remodeling, which itself underpins a higher order epigenetic processes. Nsbp1 is structurally similar to the conserved functional domains of the high-mobility group proteins, HMG 14/ 17, that interact with nucleosomes, transiently destabilize chromatin, increase access to DNA and enhance gene transcription at targeted sites. Thus, modifications in Nsbp1 methylation and expression by early estrogens have the potential to affect higher order chromatin structure, which in turn, may alter expression of many genes and possibly drive oncogenesis. A different type of methylation response was found in the phosphodiesterase type 4 variant 4 gene (Pde4d4), an enzyme that degrades cAMP. No immediate affects of early estrogen or BPA exposures were observed at day 10 of life. Rather, hypomethylation with resultant overexpression was observed at day 90 and onwards, suggesting that an adult event brings out a cryptic or dormant neonatal mark set by early-life estrogens.11 An entirely different response was observed with Hippocalcin-like 1 (Hpcal1), a gene involved in cAMP formation. Hypermethylation with loss of gene expression was found at day 10 in all estrogen/BPA exposed groups, but was slowly reversed in adult animals exposed to low-dose estradiol or BPA. However, in response to a second estrogen exposure as adults, Hpcal1 was again hypermethylated with expression lost in all treatment groups. We classify this response pattern as an alterable epigenetic mark that is susceptible to adult life modifications. These examples point to the complexities of epigenomic reprogramming and emphasize the importance of temporal changes over the lifetime and the influences of later life events that may result in multiple outcomes with regards to prostate health and disease.
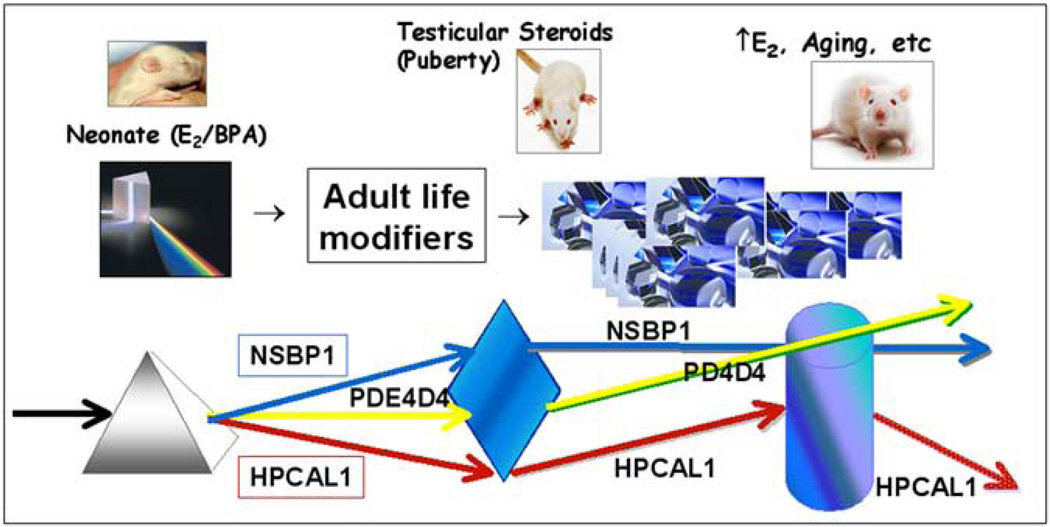
Fig. 2.
Model depicting proposed interaction between early epigenetic reprogramming by estrogens and later-life modifications that result in a spectrum of gene expression patterns and perhaps phenotypic outcomes. White light represents the genome that can be partitioned into a spectrum of epigenomes (colored light) by a prism that represents early and later life events. Together, early-life marks with subsequent modifications by later-life events can result in a wide array of phenotypes. The arrows represent a change in direction of gene expression. Nsbp1 is an example of a persistent epigenetic mark; early life exposure to estradiol or BPA leads to hypomethylation and elevated Nsbp1 expression throughout life that is not affected by later-life modifiers. Pde4d4 represents a dormant or cryptic epigentic mark that is initiated by neonatal estradiol or BPA exposure but not revealed until a secondary event occurs, either at puberty or early adulthood, and Pde4d4 becomes hypomethylated leading to gene overexpression throughout aging. Hpcal1 represents an early epigenetic mark that is alterable throughout life by interactions with adult life events. While estradiol and BPA initially suppress Hpcal1 expression via hypermethylation, this epigenetic reprogramming is reversed during adult life with a slow return to normal expression levels. Subsequent elevations in estrogen with aging can then revert the estrogen or BPA-primed prostate to hypermethylation and gene silencing with aging.
More recently, we have employed MIRA-assisted rat DNA methylation promoter arrays (Nimblegene) to globally identify genes differentially methylated at CpG islands in the 1.8 kb promoter region of all known rat genes. Preliminary analysis of day 90 prostates treated neonatally with estradiol or BPA identified several hundred gene candidates as potential methylation targets as a function of early-life exposures. LR Path analysis was applied to identify pathways enriched by chromosomal loci, transcription factors or gene ontogeny pathways within the treatment groups and interesting treatment-specific patterns have emerged. Most noticeably, we have identified differential methylation patterns and gene expression changes that are specific to the type of early-life estrogen (estradiol v. BPA) and to the dose of estradiol (high- v. low-dose exposure) as well as common gene sets for the three treatments we have examined. These findings indicate that responses to estradiol and BPA may be unique and hold promise for identifying xenoestrogen-specific DNA methylation fingerprints. This information additionally has the potential to provide molecular clues regarding the natural process of hormonal carcinogenesis of the prostate gland and to identify early-life molecular changes that predispose to adult diseases common in this organ.
Summary
In summary, our work demonstrates that both high- and low-dose exposures to estradiol as well as an environmentally relevant level of BPA (i.e. levels found in developing and adult humans) during a developmental critical window are able to reprogram the prostate gland both structurally and through altered epigenomic memory that increase their susceptibility to prostate lesions with aging. These findings contribute to the growing body of work that supports a developmental origin for adult diseases and extends this paradigm to include endocrinology, environmental toxicants and cancer with aging.
Acknowledgements
This work was supported by NIH grants DK40890 and ES15584.
References
- 1.Prins GS, Putz O. Molecular signaling pathways that regulate prostate gland development. Differentiation. 2008;6:641–659. doi: 10.1111/j.1432-0436.2008.00277.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ekbom A, Hsieh CC, Lipworth L, et al. Perinatal characteristics in relation to incidence of and mortality from prostate cancer. Br Med J. 1996;313:337–341. doi: 10.1136/bmj.313.7053.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Driscoll SG, Taylor SH. Effects of prenatal maternal estrogen on the male urogenital system. Obstet Gynecol. 1980;56:537–542. [PubMed] [Google Scholar]
- 4.Arai Y, Chen CY, Nishizuka Y. Cancer development in male reproductive tract in rats given diethylstilbestrol at neonatal age. Gann. 1978;69:861–862. [PubMed] [Google Scholar]
- 5.Prins GS. Neonatal estrogen exposure induces lobe-specific alterations in adult rat prostate androgen receptor expression. Endocrinology. 1992;130:3703–3714. doi: 10.1210/endo.130.6.1597166. [DOI] [PubMed] [Google Scholar]
- 6.Prins GS, Birch L, Habermann H, et al. Influence of neonatal estrogens on rat prostate development. Reprod Fertil Dev. 2001;13:241–252. doi: 10.1071/rd00107. [DOI] [PubMed] [Google Scholar]
- 7.Prins GS, Huang L, Birch L, Pu Y. The role of estrogens in normal and abnormal development of the prostate gland. Annals of the New York Academy of Sciences. 2006;1089:1–13. doi: 10.1196/annals.1386.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Prins GS. Developmental estrogenization of the prostate gland. Chapter 10. In: Naz RK, editor. Prostate: Basic and Clinical Aspects. Boca Raton: C.R.C. Press; 1997. pp. 247–265. [Google Scholar]
- 9.Putz O, Schwartz CB, Kim S, LeBlanc GA, Cooper RL, Prins GS. Neonatal low- and high-dose exposure to estradiol benzoate in the male rat: I. Effects on the prostate gland. Biol Reprod. 2001;65:1496–1505. doi: 10.1095/biolreprod65.5.1496. [DOI] [PubMed] [Google Scholar]
- 10.Bosland MC. Chemical and hormonal induction of prostate cancer in animal models. Urol Oncol. 1996;2:103–110. doi: 10.1016/s1078-1439(97)82840-2. [DOI] [PubMed] [Google Scholar]
- 11.Ho SM, Tang WY, Belmonte J, Prins GS. Developmental exposure estradiol and bisphenol A (BPA) increases susceptibility to prostate carcinogenesis and epigenetically regulates phosphodiesterase type 4 variant (PDE4D4) in the rat prostate. Cancer Res. 2006;66:5624–5632. doi: 10.1158/0008-5472.CAN-06-0516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Prins GS, Ye SH, Birch L, Ho SM, Kannan K. Serum Bisphenol A pharmacokinetics and prostatic responses following oral and subcutaneous exposures in neonatal Sprague-Dawley rats. Repro Toxicology. 2010 doi: 10.1016/j.reprotox.2010.09.009. online Oct 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Prins GS, Birch L. Neonatal estrogen exposure up-regulates estrogen receptor expression in the developing and adult rat prostate lobes. Endocrinology. 1997;138:1801–1809. doi: 10.1210/endo.138.5.5106. [DOI] [PubMed] [Google Scholar]
- 14.Prins GS, Marmer M, Woodham C, et al. Estrogen receptor-b messenger ribonucleic acid ontogeny in the prostate of normal and neonatally estrogenized rats. Endocrinology. 1998;139:874–883. doi: 10.1210/endo.139.3.5827. [DOI] [PubMed] [Google Scholar]
- 15.Prins GS, Birch L, Couse JF, Choi I, Katzenellenbogen B, Korach KS. Estrogen imprinting of the developing prostate gland in mediated through stromal estrogen receptor a: studies with aERKO and bERKO mice. Cancer Res. 2001;61:6089–6097. [PubMed] [Google Scholar]
- 16.Pu Y, Huang L, Prins GS. Sonic Hedgehog-patched-Gli signaling in the developing rat prostate gland: lobe-specific suppression by neonatal estrogens reduces ductal growth and branching. Dev Biol. 2004;273:257–275. doi: 10.1016/j.ydbio.2004.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Timms BG, Howdeshell KL, Barton L, Bradley S, Richter CA, vom Saal FS. Estrogenic chemicals in plastic and oral contraceptives disrupt development of the fetal mouse prostate and urethra. PNAS. 2005;102:7014–7019. doi: 10.1073/pnas.0502544102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chang WY, Wilson MJ, Birch L, Prins GS. Neonatal estrogen stimulates proliferation of periductal fibroblasts and alters the extracellular matrix composition in the rat prostate. Endocrinology. 1999;140:405–415. doi: 10.1210/endo.140.1.6401. [DOI] [PubMed] [Google Scholar]
- 19.Putz O, Schwartz CB, LeBlanc GA, Cooper RL, Prins GS. Neonatal low- and high-dose exposure to estradiol benzoate in the male rat: II. Effects on male puberty and the reproductive tract. Biol Reprod. 2001;65:1506–1517. doi: 10.1095/biolreprod65.5.1506. [DOI] [PubMed] [Google Scholar]
- 20.Prins GS, Woodham C, Lepinske M, Birch L. Effects of neonatal estrogen exposure on prostatic secretory genes and their correlation with androgen receptor expression in the separate prostate lobes of the adult rat. Endocrinology. 1993;132:2387–2398. doi: 10.1210/endo.132.6.8504743. [DOI] [PubMed] [Google Scholar]
- 21.Gilleran JP, Putz O, De Jong M, et al. The role of prolactin in the prostatic inflammatory response to neonatal estrogen. Endocrinology. 2003;144:2046–2054. doi: 10.1210/en.2002-0038. [DOI] [PubMed] [Google Scholar]
- 22.Woodham C, Birch L, Prins GS. Neonatal estrogens down regulate prostatic androgen receptor levels through a proteosome-mediated protein degradation pathway. Endocrinology. 2003;144:4841–4850. doi: 10.1210/en.2003-0035. [DOI] [PubMed] [Google Scholar]
- 23.Huang L, Pu Y, Alam S, Birch L, Prins GS. Estrogenic regulation of signaling pathways and homeobox genes during rat prostate development. J Androl. 2004;25:330–337. doi: 10.1002/j.1939-4640.2004.tb02796.x. [DOI] [PubMed] [Google Scholar]
- 24.Huang L, Pu Y, Birch L, Prins GS. Posterior Hox gene expression and differential androgen regulation in the developing and adult rat prostate lobes. Endocrinology. 2007;148:1235–1245. doi: 10.1210/en.2006-1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huang L, Pu Y, Hu WY, et al. The role of Wnt5a in prostate gland development. Dev Biol. 2009;358:188–199. doi: 10.1016/j.ydbio.2009.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huang L, Pu Y, Alam S, Birch L, Prins GS. The role of Fgf10 signaling in branching morphogenesis and gene expression in the rat prostate gland: lobe-specific supression by neonatal estrogens. Dev Biol. 2005;278:396–414. doi: 10.1016/j.ydbio.2004.11.020. [DOI] [PubMed] [Google Scholar]
- 27.Prins GS, Birch L, Tang WY, Ho SM. Developmental estrogen exposures predispose to prostate carcinogenesis with aging. Reprod Toxicol. 2007;23:374–382. doi: 10.1016/j.reprotox.2006.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]