Abstract
MRI-guided focused ultrasound (MRgFUS) surgery is a noninvasive thermal ablation method that uses magnetic resonance imaging (MRI) for target definition, treatment planning, and closed-loop control of energy deposition. Integrating FUS and MRI as a therapy delivery system allows us to localize, target, and monitor in real time, and thus to ablate targeted tissue without damaging normal structures. This precision makes MRgFUS an attractive alternative to surgical resection or radiation therapy of benign and malignant tumors. Already approved for the treatment of uterine fibroids, MRgFUS is in ongoing clinical trials for the treatment of breast, liver, prostate, and brain cancer and for the palliation of pain in bone metastasis. In addition to thermal ablation, FUS, with or without the use of microbubbles, can temporarily change vascular or cell membrane permeability and release or activate various compounds for targeted drug delivery or gene therapy. A disruptive technology, MRgFUS provides new therapeutic approaches and may cause major changes in patient management and several medical disciplines.
Keywords: focused ultrasound surgery, MRI-guided therapy, thermal ablation, targeted drug delivery
INTRODUCTION
In the past decade, three new minimally invasive procedures surfaced: thermal ablations, high-intensity focused ultrasound (HIFU), and magnetic resonance imaging (MRI)–guided interventions. Each has the potential to transform or replace invasive tumor surgeries. Thermal ablations using surgically or percutaneously inserted probes became an alternative to surgical resections (1, 2). In the early 1990s, MRI guidance for interventions and surgeries was introduced (3) and became well accepted intraoperatively in neurosurgery (3-7), primarily for maximizing the effectiveness of resections of low-grade glioma (8). MRI-based thermometry was also introduced for monitoring thermal ablations and controlling energy deposition (9). Finally, after half of a century of research and technology development efforts, HIFU or focused ultrasound (FUS) surgery became recognized as a noninvasive extracorporeal thermal ablation method and has been tested for the ablative treatment of benign lesions and malignancies (10, 11).
For guiding and monitoring HIFU ablation, MRI offers clear advantages over other imaging modalities. First, with its unparalleled soft tissue contrast, MRI provides high-resolution imaging in any orientation for planning treatment and evaluating treatment effects. In contrast, ultrasound (US) imaging, without the ability to clearly define tumor margins, may limit application of HIFU technology. Second, MRI is the only currently available technique with proven capabilities to create quantitative temperature maps. MRI thermal imaging is the only method that provides a means to ensure that, without effects to surrounding tissues, the proper US exposures are being applied for a safe and effective ablation of the target volume. Without these essential features that MRI possesses, US-guided HIFU will have a relatively narrow application area and, in most cases, will not be competitive with surgery.
Combining thermal ablation using advanced acoustic transducer technology with the anatomic, functional, and thermal guidance possible with MRI methods allows accurate targeting, real-time temperature monitoring, and closed-loop control of energy deposition. The result of this integration is arguably the most complex image-guided and controlled therapy delivery system available today: MRI-guided focused ultrasound (MRgFUS) (12-14). So far, only MRI offers the best combination of tumor margin detection, depictions of details of surrounding anatomy, and the monitoring of temperature changes during therapy (15, 16). Using intraoperative MRI, MRgFUS offers far more accurate target definition than US or a surgeon’s direct visualization.
Although the cost of integrated therapy delivery systems is high, it can be offset by the elimination of the need for an operating room, the absence of expensive hospitalization and anesthesia, and a reduced complication rate. An MRgFUS system provides safe, real-time-monitored, controlled, and repeatable treatment for benign tumors that does not necessitate an aggressive approach. For malignancies, it can be used either for complete in situ tissue destruction or for debulking prior to chemo- or radiation therapy. As such, MRgFUS is safe and effective for noninvasive surgery that can replace invasive surgery and ionizing radiation-based therapies, such as radiosurgery or brachytherapy, and can provide treatment for patients for whom invasive surgery or radiation may not be options. The feasibility and effectiveness of MRgFUS are being tested in several clinical applications, which include the ablation of benign and malignant tumors and palliative therapy of bone pain due to metastasis (12-14). Nonthermal effects of FUS are also being utilized for various novel clinical applications from targeted drug delivery to gene therapy (17, 18). Because of these multiple uses, future advances of this method will likely have a major impact on several medical fields, such as surgery, oncology, and radiation therapy. This article reviews this innovative and potentially revolutionary technology and future developments that can expand its use.
THERMAL ABLATIONS
If live tissue is heated beyond the threshold for protein denaturation (57–60°C) for a few seconds, coagulation necrosis occurs. Lower temperatures can also kill tissues if the exposure time is lengthened. Freezing below −20°C, especially if it is repeated multiple times, results in irreversible cell damage. Because heating or cooling above or below these critical levels is not selective and kills both normal and neoplastic cells, thermal ablations are comparable to surgery rather than to more selective hyperthermia. The safe and effective use of heat or cold has required improvements in energy-delivery technologies and in treatment monitoring. Technical difficulties, particularly the lack of small, percutaneously introducible probes that ablate relatively large tissue volumes, slowed down clinical introduction. The development of energy-delivery devices significantly advanced thermal ablation applications by allowing percutaneous treatment, especially with MRI guidance (19-23). Heat-conducting probes and the way they deposit and distribute energy, however, are suboptimal. Using a single source of heat or cold limits the size and shape of the resulting thermal tissue injury. This then necessitates long exposures to increase the ablation volume, which result in a relatively shallow temperature gradient within the treated volume that causes uncertainty in tissue-killing effects and ambiguity about the demarcation zone between irreversible tissue death and reversible tissue damage. The resulting geometry may not exactly correspond to the size and shape of the treated tumor. The lesion size cannot be increased indefinitely because of the developing steady state between the rate of energy deposition and heat sink effects influenced mainly by perfusion and blood flow. These limitations can cause either undertreatment with insufficient results or overtreatment with higher complication rates.
In thermal ablations, therefore—to achieve the threshold of cell destruction inside the targeted tumor volume and to remain safely away from this threshold outside that volume— spatial and temporal monitoring of temperature distribution within the targeted tumor volume is important. A temperature-sensitive imaging method is required. If image-based quantitative thermometry is available, it enables feedback or closed-loop control of energy deposition. As part of MRgFUS, this ability allows the complete ablation of the gross target volume (GTV), which includes the tumor with additional margins as deemed appropriate by the treating physician, while sparing the nontargeted tissue. As in radiosurgery, to optimize energy delivery, preoperative treatment planning is essential, but unlike radiosurgery, MRgFUS can be monitored and controlled in real time by MRI thermometry (12-16). Thermal ablation also enables immediate evaluation of the treatment response and allows repeated treatments.
NONINVASIVE ENERGY DELIVERY BY FOCUSED ULTRASOUND
Among the thermal ablations, FUS is the only noninvasive technique in which the energy penetrates the body without significant absorption until the beam reaches the focal spot. If correctly targeted, a high-energy acoustic beam completely coagulates targeted tissue and causes no tissue damage outside the treated volume. In each transmission of the acoustic beam, only a very limited volume (the focal volume) is heated to coagulation level; hence, heat buildup is very short (seconds), implying negligible effect by perfusion and diffusion and steep thermal gradients. The focal volumes are tightly packed or even overlap, assuring homogeneous ablative temperatures and confluent regions of cell killing within the entire target volume. Use of short exposures also reduces, or even eliminates, the effects of perfusion and blood flow on the resulting lesion shape. Using this strategy, however, multiple locations are sequentially targeted. This results in a relatively long treatment time to ablate larger tissue volumes because of the need to wait for the tissue to cool between sonications. However, with the application of strategies to increase the heated volume and optimization of target order, large volumes of several hundred cubic centimeters can be ablated in a clinically realistic time. Common strategies to increase the focal volume per sonication include dynamically steering the focal point using positioning systems or phased-array transducers.
In FUS therapy delivery systems, acoustic energy is generated by piezoelectric transducers that, depending on the application, are typically operated between 200 kHz and 4 Mhz. Such transducers are a flexible platform for which one can produce a wide range of devices that are tailored for particular applications. The simplest focused devices use a single-element spherically curved transducer or a flat transducer with a spherically curved acoustic lens. Such transducers are moved mechanically (sometimes with robots) to ablate multiple locations, and they can either be fairly large, to allow high powers that permit focusing deep within the body, or relatively small to fit on an intracavitary (e.g., transurethral or transrectal) device.
Greater control over the acoustic field can be achieved by transducers that consist of multiple elements with individual driving signals. Such phased arrays can steer the focal point electronically by modulating the phase of the individual driving signals. They are useful to target different locations and to increase the focal volume per sonication, and could eliminate the need for mechanical motion. Another strategy to increase the focal volume with such arrays is to simultaneously create multiple focal points. Commercially available FUS systems have phased-array transducers with several hundreds to thousands of piezoelectric elements. The higher number of elements provides greater flexibility in both targeting and shaping the focus, a useful aspect for the treatment of larger or complex tumor volumes. The ability to steer the focal point in real time may also permit the treatment of moving organs without inducing imaging artifacts.
Phased arrays can be used for correcting or compensating for phase distortions due to tissue inhomogeneities or bone (24-26). An example of this is a device designed to ablate brain tumors through the intact skull. This device, now being tested, uses a hemispherical array and a lower operating frequency (<1 MHz) to penetrate the skull without overheating (24-26). The hemispheric transducer evenly distributes the acoustic energy over the skull surface, and the phases of the elements adjust to correct for the severe aberrations induced by the skull bone in addition to beam steering. The aberration corrections are achieved using acoustic models based on X-ray computed tomography (CT) scans of the skull.
Following initial research (27-28), Insightec (Haifa, Israel) developed the Exablate 2000 and later the Exablate 3000 and 4000 systems. The Exablate 2000 was the first commercial MRg-FUS therapy delivery system and the first to be approved by the U.S. Food and Drug Administration (FDA). It is approved for the treatment of uterine fibroids and is being tested for numerous other applications. Though not yet commercially available, MRgFUS systems are also being developed by Philips, Siemens, and SuperSonic Imagine (Aix en Provence, France).
IMAGE GUIDANCE AND CONTROL: MRI VERSUS ULTRASOUND
As a noninvasive procedure, FUS requires image guidance for each step of the procedure: anatomic imaging for accurate target localization, tumor margin, and surrounding anatomy definition (to plan the safe trajectory of FUS beam through acoustic windows); thermal imaging to verify the focal coordinate before ablation and to monitor the temperature elevation to ensure a sufficient thermal dose is delivered only to the focal zone; and post-treatment imaging to verify the ablation. Only if all of these conditions are met can a complete and safe treatment be performed. US imaging, which has been used for guidance, does not currently fulfill these requirements. In most cases, especially in malignancies, diagnostic US is not sensitive enough to detect exact tumor margins, and it may fail to identify essential anatomical details, such as the exact location of nerves. Bone and air cause serious artifacts that can impede visualization. The ability to localize the thermal spot at low energy levels requires thermal sensitivity that is currently possible only with MRI (29). Efforts to develop other noninvasive techniques to visualize the focus have not materialized to a useful level. Although tissue temperature is accepted as a surrogate measure of tissue viability, direct measurement of tissue viability by functional or metabolic nuclear tracers is not yet performed. MRI is able to measure tissue temperature with sensitivities of better than ± 2°C, whereas US imaging cannot measure temperature with needed accuracies and sensitivities, despite a significant research effort.
At present, without sufficient thermal sensitivity of US imaging, the focal spot cannot be localized and the temperature elevations cannot be measured. Because of the limitations of US guidance, neither the thoroughness of treatment nor the safety of normal tissue can be guaranteed.
Treatment Planning
US image-guided FUS has been applied for the treatment of benign and malignant tumors with some success in China but without widespread acceptance elsewhere (10, 11). Many advocates of US guidance believe that MRI is needed only for temperature monitoring and that a temperature-sensitive US method will eventually be developed. However, the localization of tumor margins and the three-dimensional definition of the targeted tumor volume are the most essential requirements for noninvasive image-guided thermal therapies. Localization defines the exact spatial extent of the targeted tissue and demonstrates the related critical anatomic structures around it. For both purposes, MRI is superior to US as a diagnostic imaging method. For example, in prostate cancer diagnosis, compared to transrectal US, MRI has improved the evaluation of cancer location, size, and extent, and has allowed concurrent evaluation of the details of prostate, periprostatic, and pelvic anatomy (30, 31). In determining the extent of breast cancer, contrast-enhanced diagnostic MRI was proven to be more sensitive than all other imaging techniques. In addition, MRI can detect intraductal spread more accurately than other imaging modalities. MRI appears to be indispensable for accurate tumor localization in radiation therapy or for minimizing local recurrence (32) in breast-conserving surgery. MRI can also be used to reduce high re-excision rates for lumpectomies and margin-positive partial mastectomies (33).
High-resolution MRI of the prostate and breast at the higher field strength of 3T can further improve the quality and usefulness of imaging not only for diagnosis but also for surgical and ablation therapy. Increased signal-to-noise allows imaging at higher resolution, which may improve the definition of tumor margins over 1.5 T (34, 35). Additional advantages of higher field strength, such as improved sensitivity of temperature measurements (which can enable multi-slice or three-dimensional thermometry) well justify the integration of FUS with 3-T MRIs.
Despite the ever-improving ability of MRI to detect cancerous tissue, the full extent of most malignant tumors is not revealed by MRI because of ill-defined tumor margins and the method’s limited sensitivity to relatively small amounts of any disseminated tumor cells infiltrating normal tissue. The direct consequence of this inaccurate target definition is the frequent failure of surgical and thermal therapy methods. Molecular imaging with tumor-seeking contrast agents or biomarkers may improve detection of tumors and better define their margins, resulting in better targeting and, in some cases, complete removal.
Treatment Monitoring
While other strategies are being tested, MRgFUS clinical applications to date involve targeted tissue volumes being sonicated with the intensity of US at between 500 and 20,000 W/cm2 and a duration of 1–60 s. Shorter times are preferred to circumvent perfusion cooling effects that increase over time. After each sonication, a cooling period lasts until the temperature returns to baseline. This prevents the buildup of heat in the nontargeted tissue volume (beam path zone). Using MRI thermometry, the focal spot is visible on the images and the temperature rise and cooling are measurable. Therefore, the spatiotemporal control of the sonication by MRI guidance is precise, predictable, and reproducible. Before the actual treatment, MRI defines the target by detecting the focal spot with a noncoagulative low-power test pulse. When targeting is accomplished, the power level can be increased to achieve irreversible tissue damage by protein denaturation and capillary bed destruction. Sequential imaging steps follow the progressive tissue coagulation by overlapping focal spots. The coagulative effect is instant and the related tissue phase transitions are detectable by MRI with contrast administration immediately after the sonication ceases (27).
In addition to image guidance, it is important to be in constant contact with the patient. In most situations, the patient is awake, though sedated, during the procedure and can notify the doctor about unanticipated heating-related pain before irreversible damage is done. He or she holds a panic button that can instantly stop the treatment if discomfort or pain occurs.
CLINICAL APPLICATIONS OF MRgFUS ABLATION
Complete excision or local destruction of cancer without associated injury of the surrounding normal tissue is a preferred, though difficult to achieve, goal of invasive surgery, minimally invasive thermal ablation, and radiation therapy. Ideally, in concept, the entire tissue volume within the tumor boundaries should be completely removed by the surgeon or destroyed in situ by ionizing radiation, heat, or cold. Normal tissue outside the margins should be left intact, and its function should be preserved. Using the invasive approaches of open surgeries or probe-delivered thermal ablations, this “ideal surgery” cannot be accomplished. Radiotherapy or radiosurgery, even in its most advanced form—for example, intensity-modulated radiation therapy—cannot entirely fulfill these requirements. No real-time feedback on tissue viability exists, so the radiation dose must be set statistically on the basis of accumulated experience, which is influenced by the nonresponding (hypoxic) parts of the tumors. By contrast, MRgFUS can fulfill these requirements and has the following advantages over radiosurgery and radiotherapy: Because it provides real-time feedback on tissue viability, the treatment is personalized, avoiding overtreatment; it is not cumulative and can be repeated as many times as necessary; secondary tumor formation, a rare complication following radiosurgery and radiotherapy, is unlikely following FUS; and, owing to advanced phased-array technology that can modify the shape and size of the focal spot, the thermal gradients of FUS can be much narrower than the dose-fall curves in radiosurgery. MRgFUS is also more precise and can cause less damage to adjacent structures.
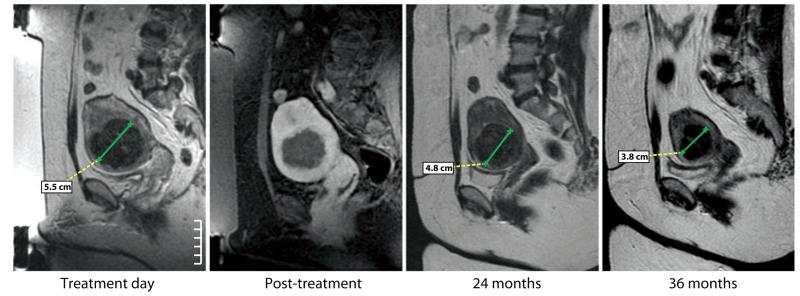
MRgFUS should be the treatment of choice for benign tumors that have well-defined boundaries and do not infiltrate normal-functioning tissues. These tumors do not necessarily require complete tissue destruction; in most cases, decreases in tumor volume are a satisfactory outcome. Repeated FUS treatments can control occasional regrowth. The first clinical application of MRgFUS was the treatment of benign breast fibroadenoma (36). After this successful feasibility trial and significant improvements in acoustic technology, MRgFUS was utilized for the treatment of benign uterine fibroids (37) (Figure 1). Without the invasiveness of hysterectomy, myomectomy, or uterine artery embolization, the procedure offers symptom relief, most likely related to tumor volume decrease and hemodynamic changes. No hospitalization and few to no days of missed work or days in bed are required. Multicenter clinical trials and commercial use of the method have confirmed its efficacy and safety (38-40). Larger fibroids can be pretreated with gonadotropin-releasing hormone analogues to cause a temporary decrease in volume and vascularity. After this pretreatment, MRgFUS produces larger nonperfused volumes of thermal ablation at a similar energy input (41). The effect of MRgFUS treatment of uterine fibroids on fertility is currently being studied.
Figure 1.
Pre- and post-MRgFUS imaging of uterine fibroid. (a) Sagittal T2W image of the uterus shows a single dominant uterine fibroid, measuring 5.5 cm in AP dimension. (b) Sagittal T1W image after intravenous administration of gadolinium shows homogeneous nonenhancing tissue in the area of treatment. This corresponds to the area of thermal necrosis. (c) Sagittal T2W image 24 months after MRgFUS shows the fibroid, now reduced in size (measuring 4.8 cm in AP dimension). (d) Sagittal T2W image at 36 months after MRgFUS shows continued shrinkage, with the AP dimension now measuring 3.8 cm. Note also the entire uterus is shrinking. MRgFUS is an effective, noninvasive, FDA-approved treatment for benign uterine fibroids.
Although MRgFUS can be the treatment of choice for benign tumors, its highest impact is anticipated in treating cancer. Like open surgeries, MRgFUS can be used for complete removal (destruction) or only for debulking, depending on how well-defined the tumor margins are on MRI. Breast cancer, in most cases, is very well seen with high-resolution contrast-enhanced MRI, making it a good application for noninvasive MRgFUS. MRI defines the extent of invasive breast cancer and can guide the FUS beams on the target. In the absence of bone or gas and complex anatomy, technically, breast cancer treatment by MRgFUS is relatively simple. A lumpectomy is not major surgery but is still invasive and can be cosmetically unappealing. MRgFUS offers a breast-conserving focal treatment with less cosmetic deformity that can be followed by adjuvant chemotherapy or radiation. Since the original work (42-44), ongoing clinical trials have demonstrated the feasibility of complete ablation of the tumors by MRgFUS in most cases (45).
For the treatment of primary and metastatic liver tumors, thermal ablations are alternatives to surgical resection. Current techniques, such as radiofrequency, microwave, and cryoablations, are still invasive. The use of FUS to ablate tumors deep in the liver offers the first completely noninvasive alternative to these techniques that has been tested with US imaging guidance, mostly in China (10, 46). The MRI-guided treatment requires general anesthesia and forced apnea to overcome liver motion during temperature measurement by MRI and to allow accurate targeting (47, 48). Because of the presence of the ribcage around the liver, acoustic windows are limited. Larger phased arrays, around the entire abdomen, may be necessary for reaching all locations, using only the intercostal spaces to deliver the energy. With a high number of phased-array elements and MRI-based tracking and gating of the liver, the acoustic beams can be synchronized with the moving organ to eventually allow treatment of freely breathing patients.
Motivated by the relative success of US-guided FUS for the treatment of localized prostate cancer (49) and by the rise in the number of men diagnosed early with prostate cancer, academic and industrial preparations for MRg-FUS are under way using both endourethral and endorectal transducers. High-field MRI has a significant advantage over US in defining the tumor, a necessary step to deliver focal therapy that does not treat the entire prostate and that depicts critical structures such as the neurovascular bundle. Nevertheless, the greatest advantage of MRI guidance in the prostate is real-time thermal mapping and monitoring, which assure not only complete tissue necrosis but also the safety of the procedure (avoiding the heating of rectal mucosa, urinary sphincter, and neurovascular bundle). Focal tumor ablation is very appealing, but it is mainly based on MRI data that may be misleading about the extent of tumor involvement, since prostate cancer is most likely multifocal. As a result, MRI findings have to be confirmed and complemented by multiple biopsies targeted not only to the MRI-identified tumor but to the normal-looking prostate as well. Imaging techniques must be improved to better define the targeted focal prostate lesion. This improvement can be provided by newly developed molecular imaging methods and further advances in MRI technology (31).
For the palliation of pain caused by bone metastases (50), MRgFUS can be an effective noninvasive solution. Bone absorbs 50 times more acoustic energy than soft tissues, and the FUS treatment raises bone temperature significantly. Due to irreversible heat-induced denervation, the heating of the periosteum and the bone around the metastasis results in a loss of pain sensation. The ablation of the metastasis itself is also possible, but because of the presence of bone around the tumor, its role in heat absorption makes planning and execution of bone tumor treatment different from the sonication of other bone tumors that are surrounded only by soft tissue.
Because it is “trackless,” without an invasive trajectory to the target, FUS seems an ideal solution for brain tumor surgery (51, 52). However, penetration of US through the bony skull represents a challenge. Craniotomy makes this potentially noninvasive method invasive and, therefore, intensive research efforts have been made to resolve US penetration and focusing through the intact skull and develop a FUS system for brain treatments. Several successful attempts have been made to treat malignant brain tumors through the open skull without (51) and with MRI-guidance (53).
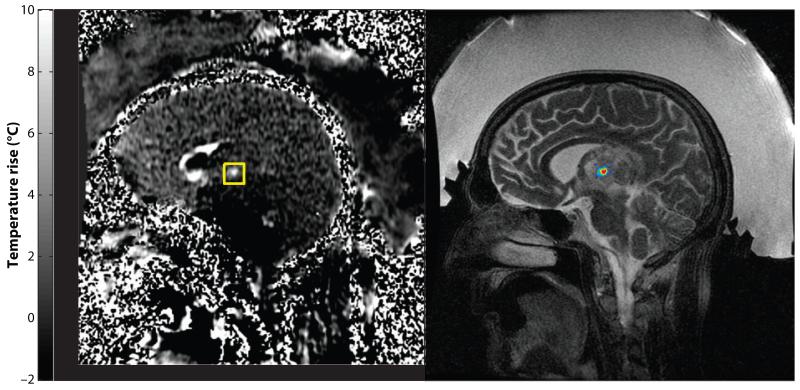
US penetrates the skull with significant loss of energy, but if focusing can be done, enough energy remains to achieve ablation. The beams, however, are distorted by the skull owing to varying irregular bone thickness. Using full hemispheric phased arrays around the head, the focus can be restored by adjusting the driving phase of each phased-array element according to the thickness of the underlying bone (24-26, 54). Both Insightec (Haifa, Israel) and SuperSonic Imagine (Aix en Provence, France) are developing an MRgFUS system for brain treatment. The Insightec system with a 1000-element phased array (Exablate 4000) is in a phase I clinical trial for the treatment of primary and metastatic malignancies of the brain (Figure 2).
Figure 2.
Commercial system for MRgFUS treatment of brain tumors (Exablate 4000, Insightec, Haifa, Israel). (a) MR thermometry image showing focal heating during a clinical MRgFUS brain tumor treatment. (b) T2-weighted MRI with the focal heating superimposed. Exablate 4000 uses a hemispherical 1000-element phased-array transducer to sonicate through the intact skull, providing a completely noninvasive ablation method. The region between the scalp and the transducer is filled with chilled, degassed water to provide acoustic coupling and cooling. The phased array is used to steer the focal point to different locations and to correct skull-induced aberrations.
Because most glioma is infiltrative and, in most cases, tumor coexists with normal-functioning brain tissue, neither conventional nor FUS surgery can completely remove or destroy the entire tumor. For well-defined metastasis or benign brain tumors, however, FUS as an ablative treatment has more potential. Real-time control of temperature provides a safe and controllable method and precise step-by-step coagulation that can be done close to nerves, cortical and subcortical structures, and other critical structures. Benign tumors that are inoperable because of inaccessible location (e.g., brainstem) or because of closeness to cranial nerves (e.g., skull base tumors) may be better approached by well-targeted and real-time-controlled MRgFUS than through the use of a GammaKnife or radiosurgery with a linear accelerator.
FUS lesions can be made at selected areas of the brain for functional neurosurgical applications such as movement disorders, epilepsy, or pain (55). MRI-based anatomical targeting will most likely represent an improvement over currently used stereotactic methods. Anatomically accurate targeting, however, in most cases, will not be sufficient for functional neurosurgery, where functional localization is necessary. Before permanent lesions are made, stimulation and/or electrophysiology are required. Through MRgFUS, however, this “functional testing” might be possible. Localized acoustic energy delivery can block nerve conduction; thus, “local acoustic anesthesia” can be used for pain control and the treatment of spasticity (56). The FUS-induced nerve block may be reversible (57; K. Hynynen, V. Colucci, G. Strichartz, et al., unpublished results). Evidence also exists for reversible stimulation at lower power levels (58). Although these are functional effects in peripheral nerves, it is possible that FUS affects the function of the central neural pathways in the brain (59). If developed in the future, this method can be applied to functional testing and mapping, once it has better localization and targeting abilities and once its ability to reach deeper subcortical regions is comparable to that of other noninvasive methods, such as transcranial magnetic stimulation.
CLINICAL USE OF NONTHERMAL MECHANISMS OF FUS
The nonthermal effects of FUS are rather complex and include multiple acoustic-mechanical mechanisms related to microbubble generation (cavitation), mechanical force in the focal zone (radiation pressure), and ultrasound-induced flow of fluids in the acoustic field (acoustic streaming) (60).
Acoustic cavitation occurs only in the presence of gas-containing microbubbles that are generated in fluid or in tissues by acoustic pressure waves or are administered intravenously in FDA-approved US imaging contrast agents. FUS can cause oscillation of the bubbles and can store energy that releases abruptly in the form of shock waves when the bubbles implode (inertial cavitation). Cavitation has less predictable effects than heating; it is a complex and nonlinear phenomenon that can lead to tissue destruction or hemorrhage.
At lower energy levels, cavitation may cause reversible increases in vascular and cell membrane permeability (61). This mechanism can be used for targeted delivery of large molecules through vascular barriers such as those separating the blood and brain or the blood and urine (62-63; K. Fischer, N. McDannold, Y. Zhang, et al., unpublished results). Cavitation can be used for tissue destruction, but unlike thermal effects, its mechanisms are difficult to control. Nevertheless, it can be used in combination with heating to enhance the size of thermal ablations and at lower power without increasing the temperature. For the MRgFUS treatment of larger uterine fibroids, this “cavitation-enhanced ablation” is currently being tested under a research protocol.
Because current drug delivery methods are not selective, the most promising future clinical application of MRgFUS is targeted drug delivery. MRgFUS could be used to release drugs at a preferred target. Today, even when drugs are applied locally with maximal efficiency at the target tissue, systemic toxicity can result. With MRgFUS, preferred targeting can occur even with systemic administration and without toxicity because drugs can be encapsulated in gascontaining bubbles or liposomes and attached to nanoparticles before being administered systemically. The release can be induced locally by the rupture of the drug-containing bubbles or can be activated by heat or the mechanical oscillation of intravascular microbubbles. Lacking the systemic toxicity associated with current systemic drug delivery methods, MRgFUS is promising even for local targeted delivery of highly concentrated antibiotics, chemotherapeutic agents, and antibodies for multiple clinical applications (17, 18, 65-67). In addition, molecular targeting of drugs with the combination of nanotechnology and FUS can be used for cell-specific therapy (68-69).
FUS’s ability to enhance the delivery of large molecules (up to the size of DNA) through vascular and membrane barriers can also be utilized for multiple applications. Sonoporation, a method using the energy released from the collapse of microbubbles, causes transient increases of cell membrane permeability, enabling the delivery of extracellular molecules into the cell (61, 70). If FUS is applied in the presence of microbubbles, it can temporarily disrupt the blood-brain barrier (BBB) (63, 71), allowing the penetration of large molecules such as peptides, proteins, drugs, and genes to reach image-selected regions of the brain. Now that noninvasive transskull sonication is possible (71), this targeted drug delivery method may move into clinical practice relatively fast. Experimental results demonstrate the ability to drive large-molecule drugs like Herceptin and doxorubicin (72, 73) through the BBB without damage to brain tissue, which may allow the use of these drugs to treat primary and metastatic brain tumors. The enhanced delivery of antibodies through the BBB may have diagnostic or therapeutic applications in Parkinson’s and Alzheimer’s diseases (74, 75). The targeted delivery of tissue-protective drugs, growth factors, and genes may find clinical use in stroke and spinal cord injury.
Nonthermal mechanical effects of FUS can enhance thrombolysis (76) and, in combination with thrombolytic drugs, may be even more effective (77-79). Acoustic cavitation, however, can cause tissue damage, primarily hemorrhage, that might prevent clinical implementation. Stable cavitation has been proposed as a mechanism for enhancing the effect of thrombolytic agents in resolving blood clots (79). Transcranial MRgFUS has the potential to treat ischemic stroke (80), but the risk of associated cavitation-induced hemorrhage is great (81). FUS with and without microbubbles can be used to close arteries and veins noninvasively (82-84) to induce hemostasis and possibly to treat arteriovenous malformations (85). Mechanical tissue liquefaction (86) and dissolution of intracerebral hematoma into fluid are other possible uses of MRgFUS.
CONCLUSIONS
MRgFUS is an advanced image-guided and controlled therapy method for noninvasive ablation and the targeted delivery of drugs. It is an alternative to surgery or radiation therapy. A disruptive technology, MRgFUS can lead to profound changes in the management of various disease conditions and may transform several medical fields. MRgFUS is performed on an outpatient basis and, in most cases, without general anesthesia. It holds great potential for improved outcomes, shorter hospitalizations, faster recovery, and a reduced economic burden on societies for health care.
Early experiences with MRgFUS treatment give good reason to conclude that, without MRI-guided localization, targeting, and MRI thermometry, the method is inadequate, especially for treating tumors.
Today the primary use of MRgFUS is thermal ablation of benign and malignant tumors. In the future, however, nonthermal effects of US will offer new therapeutic platforms, such as targeted drug delivery and gene therapy. Further advances in acoustic technology and high-field MRI will likely result in better target definition, improved accuracy, and shorter treatment times.
At an early stage in its development, MRg–FUS’s safety and efficacy to address specific diseases must be demonstrated through rigorous clinical trials that are scrutinized by regulatory agencies including the FDA. In the future, insurance companies must accept MRgFUS as standard treatment. Physicians and patients may need to be made more aware of its advantages over conventional treatments.
Footnotes
DISCLOSURE STATEMENT
The author is a consultant to and receives research support from Insightec Ltd.
LITERATURE CITED
- 1.Haemmerich D, Laeseke PF. Thermal tumour ablation: devices, clinical applications and future directions. Int. J. Hyperthermia. 2005;21(8):755–60. doi: 10.1080/02656730500226423. [DOI] [PubMed] [Google Scholar]
- 2.Jolesz FA. Image guidance and control of thermal ablation. In: van Sonnenberg E, McMullen W, Solbiati L, editors. Tumor Ablation: Principles and Practice. Vol. 1. Springer-Verlag; New York: 2005. pp. 182–91. [Google Scholar]
- 3.Jolesz FA, Blumenfeld SM. Interventional use of magnetic resonance imaging. Magn. Reson. Q. 1994;10(2):85–96. [PubMed] [Google Scholar]
- 4.Black PM, Moriarty T, Alexander E, 3rd, et al. Development and implementation of intraoperative magnetic resonance imaging and its neurosurgical applications. Neurosurgery. 1997;41(4):831–42. doi: 10.1097/00006123-199710000-00013. discussion 842-45. [DOI] [PubMed] [Google Scholar]
- 5.Nimsky C, Ganslandt O, Kober H, et al. Intraoperative magnetic resonance imaging combined with neuronavigation: a new concept. Neurosurgery. 2001;48(5):1082–89. doi: 10.1097/00006123-200105000-00023. [DOI] [PubMed] [Google Scholar]
- 6.Lewin JS, Metzger A, Selman WR. Intraoperative magnetic resonance image guidance in neurosurgery. J. Magn. Reson. Imaging. 2000;12(4):512–24. doi: 10.1002/1522-2586(200010)12:4<512::aid-jmri2>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 7.Hall WA, Truwit CL. Intraoperative MR-guided neurosurgery. J. Magn. Reson. Imaging. 2008;27(2):368–75. doi: 10.1002/jmri.21273. [DOI] [PubMed] [Google Scholar]
- 8.Claus EB, Horlacher A, Hsu L, et al. Survival rates in patients with low-grade glioma after intraoperative magnetic resonance image guidance. Cancer. 2005;103(6):1227–33. doi: 10.1002/cncr.20867. [DOI] [PubMed] [Google Scholar]
- 9.McDannold NJ, Jolesz FA. Magnetic resonance image-guided thermal ablations. Top. Magn. Reson. Imaging. 2000;11(3):191–202. doi: 10.1097/00002142-200006000-00005. [DOI] [PubMed] [Google Scholar]
- 10.Wu F. Extracorporeal high intensity focused ultrasound in the treatment of patients with solid malignancy. Minim. Invasive Ther. Allied Technol. 2006;15(1):26–35. doi: 10.1080/13645700500470124. [DOI] [PubMed] [Google Scholar]
- 11.Haar GT, Coussios C. High intensity focused ultrasound: past, present and future. Int. J. Hyperthermia. 2007;23(2):85–87. doi: 10.1080/02656730601185924. [DOI] [PubMed] [Google Scholar]
- 12.Jolesz FA, McDannold N. Current status and future potential of MRI-guided focused ultrasound surgery. J. Magn. Reson. Imaging. 2008;27(2):391–99. doi: 10.1002/jmri.21261. [DOI] [PubMed] [Google Scholar]
- 13.Gedroyc WM. New clinical applications of magnetic resonance-guided focused ultrasound. Top. Magn. Reson. Imaging. 2006;17(3):189–94. doi: 10.1097/RMR.0b013e318038f782. [DOI] [PubMed] [Google Scholar]
- 14.Moonen CT, Quesson B, Salomir R, et al. Thermal therapies in interventional MR imaging. Focused ultrasound. Neuroimaging Clin. N. Am. 2001;11(4):737–47. [PubMed] [Google Scholar]
- 15.Cline HE, Hynynen K, Watkins RD, et al. Focused US system for MR imaging-guided tumor ablation. Radiology. 1995;194(3):731–37. doi: 10.1148/radiology.194.3.7862971. [DOI] [PubMed] [Google Scholar]
- 16.Salomir R, Delemazure AS, Palussière J, et al. Image-based control of the magnetic resonance imaging-guided focused ultrasound thermotherapy. Top. Magn. Reson. Imaging. 2006;17(3):139–51. doi: 10.1097/RMR.0b013e31803774c1. [DOI] [PubMed] [Google Scholar]
- 17.Deckers R, Rome C, Moonen CT. The role of ultrasound and magnetic resonance in local drug delivery. J. Magn. Reson. Imaging. 2008;27(2):400–9. doi: 10.1002/jmri.21272. [DOI] [PubMed] [Google Scholar]
- 18.Moonen CT. Spatio-temporal control of gene expression and cancer treatment using magnetic resonance imaging-guided focused ultrasound. Clin. Cancer Res. 2007;13(12):3482–89. doi: 10.1158/1078-0432.CCR-07-0204. [DOI] [PubMed] [Google Scholar]
- 19.Kurumi Y, Tani T, Naka S, et al. MR-guided microwave ablation for malignancies. Int. J. Clin. Oncol. 2007;12(2):85–93. doi: 10.1007/s10147-006-0653-7. [DOI] [PubMed] [Google Scholar]
- 20.Clasen S, Pereira PL. Magnetic resonance guidance for radiofrequency ablation of liver tumors. J. Magn. Reson. Imaging. 2008;27(2):421–33. doi: 10.1002/jmri.21264. [DOI] [PubMed] [Google Scholar]
- 21.Morrison PR, Silverman SG, Tuncali K, et al. MRI-guided cryotherapy. J. Magn. Reson. Imaging. 2008;27(2):410–20. doi: 10.1002/jmri.21260. [DOI] [PubMed] [Google Scholar]
- 22.Kettenbach J, Silverman SG, Hata N, et al. Monitoring and visualization techniques for MR-guided laser ablations in an open MR system. J. Magn. Reson. Imaging. 1998;8(4):933–43. doi: 10.1002/jmri.1880080424. [DOI] [PubMed] [Google Scholar]
- 23.Schwarzmaier HJ, Eickmeyer F, von Tempelhoff W, et al. MR-guided laser irradiation of recurrent glioblastomas. J. Magn. Reson. Imaging. 2005;22(6):799–803. doi: 10.1002/jmri.20446. [DOI] [PubMed] [Google Scholar]
- 24.Clement GT, White PJ, King RL, et al. A magnetic resonance imaging-compatible, large-scale array for trans-skull ultrasound surgery and therapy. J. Ultrasound Med. 2005;24(8):1117–25. doi: 10.7863/jum.2005.24.8.1117. [DOI] [PubMed] [Google Scholar]
- 25.Clement GT, Hynynen K. A noninvasive method for focusing ultrasound through the human skull. Phys. Med. Biol. 2002;47(8):1219–36. doi: 10.1088/0031-9155/47/8/301. [DOI] [PubMed] [Google Scholar]
- 26.Hynynen K, McDannold N, Clement G, et al. Pre-clinical testing of a phased array ultrasound system for MRI-guided noninvasive surgery of the brain—a primate study. Eur. J. Radiol. 2006;59(2):149–56. doi: 10.1016/j.ejrad.2006.04.007. [DOI] [PubMed] [Google Scholar]
- 27.Cline HE, Schenck JF, Hynynen K, et al. MR-guided focused ultrasound surgery. J. Comput. Assist. Tomogr. 1992;16(6):956–65. doi: 10.1097/00004728-199211000-00024. [DOI] [PubMed] [Google Scholar]
- 28.Hynynen K, Freund WR, Cline HE, et al. A clinical, noninvasive, MR imaging-monitored ultrasound surgery method. Radiographics. 1996;16(1):185–95. doi: 10.1148/radiographics.16.1.185. [DOI] [PubMed] [Google Scholar]
- 29.McDannold N, Hynynen K, Jolesz F. MRI monitoring of the thermal ablation of tissue: effects of long exposure times. J. Magn. Reson. Imaging. 2001;13(3):421–27. doi: 10.1002/jmri.1061. [DOI] [PubMed] [Google Scholar]
- 30.Fuchsjäger M, Shukla-Dave A, Akin O, et al. Prostate cancer imaging. Acta Radiol. 2008;49(1):107–20. doi: 10.1080/02841850701545821. [DOI] [PubMed] [Google Scholar]
- 31.Hricak H, Choyke PL, Eberhardt SC, et al. Imaging prostate cancer: a multidisciplinary perspective. Radiology. 2007;243(1):28–53. doi: 10.1148/radiol.2431030580. [DOI] [PubMed] [Google Scholar]
- 32.Schnall M. MR imaging evaluation of cancer extent: Is there clinical relevance? Magn. Reson. Imaging Clin. N. Am. 2006;14(3):379–81. doi: 10.1016/j.mric.2006.07.005. [DOI] [PubMed] [Google Scholar]
- 33.Grobmyer SR, Mortellaro VE, Marshall J, et al. Is there a role for routine use of MRI in selection of patients for breast-conserving cancer therapy? J. Am. Coll. Surg. 2008;206(5):1045–50. doi: 10.1016/j.jamcollsurg.2007.12.039. [DOI] [PubMed] [Google Scholar]
- 34.Cornfeld DM, Weinreb JC. MR imaging of the prostate: 1.5T versus 3T. Magn. Reson. Imaging Clin. N. Am. 2007;15(3):433–48. doi: 10.1016/j.mric.2007.06.004. [DOI] [PubMed] [Google Scholar]
- 35.Kuhl CK. Breast MR imaging at 3T. Magn. Reson. Imaging Clin. N. Am. 2007;15(3):315–20. doi: 10.1016/j.mric.2007.08.003. [DOI] [PubMed] [Google Scholar]
- 36.Hynynen K, Pomeroy O, et al. MR imaging-guided focused ultrasound surgery of fibroadenomas in the breast: a feasibility study. Radiology. 2001;219(1):176–85. doi: 10.1148/radiology.219.1.r01ap02176. [DOI] [PubMed] [Google Scholar]
- 37.Tempany CM, Stewart EA, McDannold N, et al. MR imaging-guided focused ultrasound surgery of uterine leiomyomas: a feasibility study. Radiology. 2003;226(3):897–905. doi: 10.1148/radiol.2271020395. [DOI] [PubMed] [Google Scholar]
- 38.Stewart EA, Gedroyc WM, Tempany CM, et al. Focused ultrasound treatment of uterine fibroid tumors: safety and feasibility of a noninvasive thermoablative technique. Am. J. Obstet. Gynecol. 2003;189(1):48–54. doi: 10.1067/mob.2003.345. [DOI] [PubMed] [Google Scholar]
- 39.Hindley J, Gedroyc W, Regan L, et al. MRI guidance of focused ultrasound therapy of uterine fibroids: early results. Am. J. Roentgenol. 2004;183(6):1713–19. doi: 10.2214/ajr.183.6.01831713. [DOI] [PubMed] [Google Scholar]
- 40.Fennessy FM, Tempany CM, McDannold NJ, et al. Uterine leiomyomas: MR imaging-guided focused ultrasound surgery—results of different treatment protocols. Radiology. 2007;243(3):885–93. doi: 10.1148/radiol.2433060267. [DOI] [PubMed] [Google Scholar]
- 41.Smart OC, Hindley JT, Regan L, et al. Magnetic resonance guided focused ultrasound surgery of uterine fibroids—the tissue effects of GnRH agonist pretreatment. Eur. J. Radiol. 2006;59(2):163–67. doi: 10.1016/j.ejrad.2006.04.009. [DOI] [PubMed] [Google Scholar]
- 42.Gianfelice D, Khiat A, Amara M, et al. MR imaging-guided focused ultrasound surgery of breast cancer: correlation of dynamic contrast-enhanced MRI with histopathologic findings. Breast Cancer Res. Treat. 2003;82(2):93–101. doi: 10.1023/B:BREA.0000003956.11376.5b. [DOI] [PubMed] [Google Scholar]
- 43.Gianfelice D, Khiat A, Amara M, et al. MR imaging-guided focused US ablation of breast cancer: histopathologic assessment of effectiveness—initial experience. Radiology. 2003;227(3):849–55. doi: 10.1148/radiol.2281012163. [DOI] [PubMed] [Google Scholar]
- 44.Gianfelice D, Khiat A, Boulanger Y, et al. Feasibility of magnetic resonance imaging-guided focused ultrasound surgery as an adjunct to tamoxifen therapy in high-risk surgical patients with breast carcinoma. J. Vasc. Interv. Radiol. 2003;14(10):1275–82. doi: 10.1097/01.rvi.0000092900.73329.a2. [DOI] [PubMed] [Google Scholar]
- 45.Furusawa H, Namba K, Thomsen S, et al. Magnetic resonance-guided focused ultrasound surgery of breast cancer: reliability and effectiveness. J. Am. Coll. Surg. 2006;203(1):54–63. doi: 10.1016/j.jamcollsurg.2006.04.002. [DOI] [PubMed] [Google Scholar]
- 46.Wu F, Wang ZB, Chen WZ, et al. Extracorporeal high intensity focused ultrasound ablation in the treatment of patients with large hepatocellular carcinoma. Ann. Surg. Oncol. 2004;11(12):1061–69. doi: 10.1245/ASO.2004.02.026. [DOI] [PubMed] [Google Scholar]
- 47.Okada A, Murakami T, Mikami K, et al. A case of hepatocellular carcinoma treated by MR-guided focused ultrasound ablation with respiratory gating. Magn. Reson. Med. Sci. 2006;5(3):167–71. doi: 10.2463/mrms.5.167. [DOI] [PubMed] [Google Scholar]
- 48.Tokuda J, Morikawa S, Haque HA, et al. Adaptive 4D MR imaging using navigator-based respiratory signal for MRI-guided therapy. Magn. Reson. Med. 2008;59(5):1051–61. doi: 10.1002/mrm.21436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Poissonnier L, Chapelon JY, Rouviere O, et al. Control of prostate cancer by transrectal HIFU in 227 patients. Eur. Urol. 2007;51(2):381–87. doi: 10.1016/j.eururo.2006.04.012. [DOI] [PubMed] [Google Scholar]
- 50.Catane R, Beck A, Inbar Y, et al. MR-guided focused ultrasound surgery (MRgFUS) for the palliation of pain in patients with bone metastases—preliminary clinical experience. Ann. Oncol. 2007;18(1):163–67. doi: 10.1093/annonc/mdl335. [DOI] [PubMed] [Google Scholar]
- 51.Fry WJ, Fry FJ. Fundamental neurological research and human neurosurgery using intense ultrasound. IRE Trans. Med. Electron. 1960;ME-7:166–81. doi: 10.1109/iret-me.1960.5008041. [DOI] [PubMed] [Google Scholar]
- 52.Lele PP. A simple method for production of trackless focal lesions with focused ultrasound: physical factors. J. Physiol. 1962;160:494–512. doi: 10.1113/jphysiol.1962.sp006862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ram Z, Cohen ZR, Harnof S, et al. Magnetic resonance imaging-guided, high-intensity focused ultrasound for brain tumor therapy. Neurosurgery. 2006;59:949–55. doi: 10.1227/01.NEU.0000254439.02736.D8. discussion 955-46. [DOI] [PubMed] [Google Scholar]
- 54.Hynynen K, Jolesz FA. Demonstration of potential noninvasive ultrasound brain therapy through an intact skull. Ultrasound Med. Biol. 1998;24:275–83. doi: 10.1016/s0301-5629(97)00269-x. [DOI] [PubMed] [Google Scholar]
- 55.Meyers R, Fry WJ, Fry FJ, et al. Early experiences with ultrasonic irradiation of the pallidofugal and nigral complexes in hyperkinetic and hypertonic disorders. J. Neurosurg. 1959;16:32–54. doi: 10.3171/jns.1959.16.1.0032. [DOI] [PubMed] [Google Scholar]
- 56.Foley JL, Little JW, Starr FL, 3rd, et al. Image-guided HIFU neurolysis of peripheral nerves to treat spasticity and pain. Ultrasound Med. Biol. 2004;30(9):1199–207. doi: 10.1016/j.ultrasmedbio.2004.07.004. [DOI] [PubMed] [Google Scholar]
- 57.Young RR, Henneman E. Functional effects of focused ultrasound on mammalian nerves. Science. 1961;134:1521–22. doi: 10.1126/science.134.3489.1521. [DOI] [PubMed] [Google Scholar]
- 58.Currier DP, Greathouse D, Swift T. Sensory nerve conduction: effect of ultrasound. Arch. Phys. Med. Rehabil. 1978;59(4):181–85. [PubMed] [Google Scholar]
- 59.Fry FJ, Ades HW, Fry WJ. Production of reversible changes in the central nervous system by ultrasound. Science. 1958;127(3289):83–84. doi: 10.1126/science.127.3289.83. [DOI] [PubMed] [Google Scholar]
- 60.Mitragotri S. Healing sound: the use of ultrasound in drug delivery and other therapeutic applications. Nat. Rev. Drug Discov. 2005;4(3):255–60. doi: 10.1038/nrd1662. [DOI] [PubMed] [Google Scholar]
- 61.Deng CX, Sieling F, Pan H, et al. Ultrasound-induced cell membrane porosity. Ultrasound Med. Biol. 2004;30(4):519–26. doi: 10.1016/j.ultrasmedbio.2004.01.005. [DOI] [PubMed] [Google Scholar]
- 62.Hynynen K, McDannold N, Vykhodtseva N, et al. Noninvasive MR imaging-guided focal opening of the blood-brain barrier in rabbits. Radiology. 2001;220:640–46. doi: 10.1148/radiol.2202001804. [DOI] [PubMed] [Google Scholar]
- 63.McDannold NJ, Vykhodtseva NI, Hynynen K. Microbubble contrast agent with focused ultrasound to create brain lesions at low power levels: MR imaging and histologic study in rabbits. Radiology. 2006;241(1):95–106. doi: 10.1148/radiol.2411051170. [DOI] [PubMed] [Google Scholar]
- 64.Deleted in proof
- 65.Frenkel V. Ultrasound mediated delivery of drugs and genes to solid tumors. Adv. Drug. Deliv. Rev. 2008;60(10):1193–208. doi: 10.1016/j.addr.2008.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Frenkel V, Li KC. Potential role of pulsed–high intensity focused ultrasound in gene therapy. Future. Oncol. 2006;2(1):111–19. doi: 10.2217/14796694.2.1.111. [DOI] [PubMed] [Google Scholar]
- 67.Unger EC, McCreery TP, Sweitzer RH. Ultrasound enhances gene expression of liposomal transfection. Invest. Radiol. 1997;32(12):723–27. doi: 10.1097/00004424-199712000-00001. [DOI] [PubMed] [Google Scholar]
- 68.Unger EC, Porter T, Culp W, et al. Therapeutic applications of lipid-coated microbubbles. Adv. Drug. Deliv. Rev. 2004;56(9):1291–1314. doi: 10.1016/j.addr.2003.12.006. [DOI] [PubMed] [Google Scholar]
- 69.Bednarski MD, Lee JW, Callstrom MR, et al. In vivo target-specific delivery of macromolecular agents with MR-guided focused ultrasound. Radiology. 1997;204(1):263–68. doi: 10.1148/radiology.204.1.9205257. [DOI] [PubMed] [Google Scholar]
- 70.Li YS, Reid CN, McHale AP. Enhancing ultrasound-mediated cell membrane permeabilisation (sonoporation) using a high frequency pulse regime and implications for ultrasound-aided cancer chemotherapy. Cancer Lett. 2008;266(2):156–62. doi: 10.1016/j.canlet.2008.02.041. [DOI] [PubMed] [Google Scholar]
- 71.Hynynen K, McDannold N, Sheikov NA, et al. Local and reversible blood-brain barrier disruption by noninvasive focused ultrasound at frequencies suitable for trans-skull sonications. Neuroimage. 2005;24(1):12–20. doi: 10.1016/j.neuroimage.2004.06.046. [DOI] [PubMed] [Google Scholar]
- 72.Kinoshita M, McDannold N, Jolesz FA, et al. Noninvasive localized delivery of Herceptin to the mouse brain by MRI-guided focused ultrasound-induced blood-brain barrier disruption. Proc. Natl. Acad. Sci. USA. 2006;103:11719–23. doi: 10.1073/pnas.0604318103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Treat LH, McDannold N, Vykhodtseva N, et al. Targeted delivery of doxorubicin to the rat brain at therapeutic levels using MRI-guided focused ultrasound. Int. J. Cancer. 2007;121(4):901–7. doi: 10.1002/ijc.22732. [DOI] [PubMed] [Google Scholar]
- 74.Kinoshita M, McDannold N, Jolesz FA, et al. Targeted delivery of antibodies through the blood-brain barrier by MRI-guided focused ultrasound. Biochem. Biophys. Res. Commun. 2006;340:1085–90. doi: 10.1016/j.bbrc.2005.12.112. [DOI] [PubMed] [Google Scholar]
- 75.Raymond SB, Treat LH, Dewey JD, et al. Ultrasound enhanced delivery of molecular imaging and therapeutic agents in Alzheimer’s disease mouse models. PLoS ONE. 2008;3(5):e2175. doi: 10.1371/journal.pone.0002175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Daffertshofer M, Fatar M. Therapeutic ultrasound in ischemic stroke treatment: experimental evidence. Eur. J. Ultrasound. 2002;16(1-2):121–30. doi: 10.1016/s0929-8266(02)00049-6. [DOI] [PubMed] [Google Scholar]
- 77.Alexandrov AV, Wojner AW, Grotta JC, et al. CLOTBUST: design of a randomized trial of ultrasound-enhanced thrombolysis for acute ischemic stroke. J. Neuroimaging. 2004;14(2):108–12. [PubMed] [Google Scholar]
- 78.Molina CA, Ribo M, Rubiera M, et al. Microbubble administration accelerates clot lysis during continuous 2-MHz ultrasound monitoring in stroke patients treated with intravenous tissue plasminogen activator. Stroke. 2006;37:425–29. doi: 10.1161/01.STR.0000199064.94588.39. [DOI] [PubMed] [Google Scholar]
- 79.Francis CW, Blinc A, Lee S, et al. Ultrasound accelerates transport of recombinant tissue plasminogen activator into clots. Ultrasound Med. Biol. 1995;21:419–24. doi: 10.1016/0301-5629(94)00119-x. [DOI] [PubMed] [Google Scholar]
- 80.Alexandrov AV, Molina CA, Grotta JC, et al. Ultrasound-enhanced systemic thrombolysis for acute ischemic stroke. N. Engl. J. Med. 2004;351:2170–78. doi: 10.1056/NEJMoa041175. [DOI] [PubMed] [Google Scholar]
- 81.Daffertshofer M, Gass A, Ringleb P, et al. Transcranial low-frequency ultrasound-mediated thrombolysis in brain ischemia: increased risk of hemorrhage with combined ultrasound and tissue plasminogen activator: results of a phase II clinical trial. Stroke. 2005;36:1441–46. doi: 10.1161/01.STR.0000170707.86793.1a. [DOI] [PubMed] [Google Scholar]
- 82.Hynynen K, Colucci V, Chung A, et al. Noninvasive arterial occlusion using MRI-guided focused ultrasound. Ultrasound Med. Biol. 1996;22(8):1071–77. doi: 10.1016/s0301-5629(96)00143-3. [DOI] [PubMed] [Google Scholar]
- 83.Hynynen K, Chung AH, Colucci V, et al. Potential adverse effects of high-intensity focused ultrasound exposure on blood vessels in vivo. Ultrasound Med. Biol. 1996;22(2):193–201. doi: 10.1016/0301-5629(95)02044-6. [DOI] [PubMed] [Google Scholar]
- 84.Zderic V, Keshavarzi A, Noble ML, et al. Hemorrhage control in arteries using high-intensity focused ultrasound: a survival study. Ultrasonics. 2006;44:46–53. doi: 10.1016/j.ultras.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 85.Vaezy S, Martin R, Yaziji H, et al. Hemostasis of punctured blood vessels using high-intensity focused ultrasound. Ultrasound Med. Biol. 1998;24(6):903–10. doi: 10.1016/s0301-5629(98)00050-7. [DOI] [PubMed] [Google Scholar]
- 86.Lake AM, Hall TL, Kieran K, et al. Histotripsy: minimally invasive technology for prostatic tissue ablation in an in vivo canine model. Urology. 2008;72(3):682–86. doi: 10.1016/j.urology.2008.01.037. [DOI] [PMC free article] [PubMed] [Google Scholar]