Abstract
Background
The efficacy of combination chemotherapy with methotrexate (MTX) and asparaginase is not well known in relapsed and refractory acute leukemia after contemporary therapy.
Procedure
A retrospective study of pediatric patients with relapsed or refractory acute myeloid leukemia (AML) who received MTX and asparaginase as a salvage therapy at St. Jude Children Research Hospital was performed. MTX was given intravenously followed by a dose of asparaginase intramuscularly or intravenously 24 hours later. The chemotherapy cycle was repeated every 7-10 days. Response, survival, and toxicities were evaluated.
Results
Fifteen patients, median age 10.5 years (range, 1.1-18.5 years), were treated. Median number of previous therapeutic regimens was 3 (range, 1-4). Six patients responded to treatment (3 had morphologic complete remission with incomplete blood count recovery, 2 had partial remission, and 1 had stable disease for 16 months), and 4 are still alive. Three of 6 responders had monoblastic leukemia, and also developed tumor lysis syndrome. The 1- and 2-year overall survival rates are 35.6% and 17.8%, respectively. The most common adverse event was transient elevation of transaminases (9 patients). Two patients developed pancreatitis. Episodes of febrile neutropenia were rare (2 patients), and most courses (75 out of 93 total courses) were given in an outpatient setting.
Conclusions
Combination chemotherapy with MTX and asparaginase appears to be an effective salvage therapy and well tolerated in patients with relapsed or refractory childhood AML, even in those heavily pretreated with contemporary frontline or salvage therapy.
Keywords: acute myeloid leukemia, methotrexate, asparaginase, children, relapse
INTRODUCTION
Acute myeloid leukemia (AML) accounts for 17% of all childhood leukemia [1]. Recent risk-adapted therapeutic approaches, including the use of cytarabine (Ara-C), anthracyclines and etoposide, together with improved supportive care, have increased survival rates to 60-70% [2-8]. Nevertheless, up to 40% of patients still suffer from relapse or refractory disease [5,6]. After relapse, the risk of anthracycline-induced cardiomyopathy [7] and the resistance of leukemic cells to chemotherapeutic agents become the major obstacles to treatment [8], and the survival rate is dismal (around 30%) even with the use of novel agents such as deoxyadenosine analogue [3-5] and hematopoietic stem cell transplantation (HSCT) [2,9]. The combination of methotrexate (MTX) and asparaginase has been used in both relapsed acute lymphoblastic leukemia and AML [10,11]. This treatment regimen is based on an in vitro study that showed the synergistic anti-leukemic activity of time sequential combination of the medications [12]. In addition, thymidylate synthase inhibition by MTX [13] and the substantial cytotoxic effect of asparaginase in monoblastic leukemia cells [14] suggested the effectiveness in this particular subtype of AML.
In the present study, we report our recent experience of combination chemotherapy with MTX and asparaginase in children with relapsed or refractory AML after contemporary frontline or salvage chemotherapy.
METHODS
Patients
Patients with relapsed or refractory AML who had previously been treated with frontline therapy, salvage chemotherapy, or HSCT received sequential administration of MTX and asparaginase at St. Jude Children's Research Hospital from 1999 to 2012.
The diagnosis of relapsed or refractory AML was performed by morphology, molecular studies, or immunophenotyping with flow cytometry on marrow or extramedullary specimens.
Treatment
The treatment regimen included an intravenous dose of MTX followed by a dose of asparaginase (intramuscular or intravenous) 24 hours later. E. coli L-asparaginase was initially used and replaced by PEG-asparaginase after 2007. Erwinia L-asparaginase was used in patients who were allergic to E. coli L-asparaginase. The chemotherapy cycle was repeated every 7-10 days. A complete blood count, chemistries including liver, renal, and pancreatic function tests, and physical examinations were evaluated at least weekly. Triple intrathecal therapy (MTX, hydrocortisone, and cytarabine) and prophylactic antibiotics or antifungal agents were given at the treating physician's discretion [15].
Response and Toxicity Criteria
Responses to treatment were defined according to international working group response criteria [16]. The National Cancer Institute common terminology criteria for adverse events, version 3.0, were used for evaluation of toxicities.
Statistical Analyses
Overall survival was defined as the time from the date starting MTX/asparaginase to death and was analyzed by the Kaplan-Meier method. Exact Wilcoxon sum rank test was used to compare median of first remission period between responder and non-responder.
RESULTS
Patient demographics
Table 1 lists the characteristics and outcome of 15 patients treated with MTX/asparaginase. FAB M5 was the most common subtype (6 patients), and MLL rearrangement accounted for the most common genetic abnormality (7 patients). The median time from diagnosis to the first relapse was 7.3 months (range, 3.4-14.3 months). The median age of the patients was 10.5 years (range, 1.1-18.5 years) at the time of MTX/asparaginase therapy, median time from diagnosis to the initiation of MTX/asparaginase therapy was 9.4 months (range, 2.4-26.3 months), and median number of treatment regimens prior to MTX/asparaginase therapy was 3 (range, 1-4), including 5 patients who had received allogeneic HSCT. Three patients received MTX/asparaginase as the first salvage therapy. One patient (patient 1) had a partial remission after induction and consolidation therapy on the frontline protocol, and MTX/asparaginase was used as a bridging therapy to HSCT, and the second patient (patient 5) had prolonged severe pancytopenia with minimal residual disease after frontline therapy with cytarabine, daunorubicin, and etoposide. The third patient with a relapse (patient7) had previously developed severe axonal degeneration after cladribine/cytarabine. All 15 patients had either relapse or refractory disease after 1 to 4 therapeutic regimens; 3 patients had primary refractory disease, and 7 patients had prior remissions of less than 6 months. The most recent regimens before MTX treatment are listed in Table 1. Before treatment with MTX/asparaginase, 6 patients had isolated bone marrow relapses, 1 patient had combined bone marrow and extramedullary relapses (skin), 1 patient had persistent minimal residual disease, and seven patients had disease that was refractory to immediately prior courses, including 5 with bone marrow disease, 1 with combined bone marrow and extramedullary disease, and 1 with isolated extramedullary disease (skin). The median number of courses of MTX/asparaginase per patient was 2 (range, 1-54). A total of 93 courses were given to the 15 patients, and 75 courses were given in an outpatient setting. Non-escalating MTX doses of 60 to 120 mg/m2 were given to 14 patients (3 patients with 60 mg/m2, 5 patients with 100 mg/m2, and 6 patients with 120 mg/m2) while escalating doses were given to a patient who received the initial dose at 60 mg/m2 with increases of 20 mg/m2 in subsequent cycles until reaching 100 mg/m2. Six patients were treated with E. coli asparaginase (10,000 Units/m2), 7 PEG-asparaginase (3,000 Units/m2), and 2 Erwinia asparaginase (10,000 Units/m2).
Table I.
Patient Characteristics
| Pt | Age at Tx (years) | Sex | FAB | Cytogenetics | No of prior regimens | Prior BMT | Response to most recent therapy | Most recent therapy | Site of disease | Doses of MTX/Asp(type) | No. of courses | Response to MTX/Asp | Post-MTX/Asp transplant | Outcome (survival period) | Remission period | Cause of death |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1* | 3.4 | M | M2 | normal | 1 | relapse | ara-C/Asp | marrow | 60/10000 (E.coli) | 3 | CRi | haplo | alive (91 mo) | 90 mo | ||
| 2 | 10.5 | M | M5 | t(10;11) | 3 | MUD | refractory | clofarabine/ara-C | skin | 120/3000 (PEG) | 2 | CRi | alive (8.5 mo) | 8 mo | ||
| 3 | 1.1 | F | M5 | t(10;11) | 3 | refractory | clofarabine/ara-C | marrow,skin | 120/3000 (PEG) | 4 | CRi | UCBT | dead (4.5 mo) | 2 mo | disease | |
| 4 | 1.8 | F | M5 | t(10;11) | 2 | relapse | clofarabine/ara-C | marrow MRD | 100/10000 (E.coli) | 11 | PR | alloCBT | alive (57 mo) | 56 mo | ||
| 5 | 4.8 | F | M0 | normal | 1 | relapse | ara-C/Dauno/Eto | marrow | 120/3000 (PEG) | 4 | PR | alloBMT | alive (8.8 mo) | 8 mo | ||
| 6 | 11.3 | F | M1 | t(6;9) | 3 | refractory | hydroxyurea | marrow | 100/10000 (Erwinia) | 54 | stable disease | dead (16 mo) | disease | |||
| 7 | 18.5 | M | M4 | t(16;16) | 1 | relapse | cladribine/ara-C | marrow | 60/10000 (E.coli) | 2 | NR | dead (8.1 mo) | disease | |||
| 8 | 18.3 | M | M1 | normal | 4 | refractory | cladribine/ara-C | marrow | 60/10000 (E.coli) | 2 | NR | haplo | dead (16.5 mo) | disease | ||
| 9 | 15.8 | M | M4 | gain 5p | 4 | MSD | relapse | mitoxantrone/ara-C/GO | marrow | 100/2500 (PEG) | 1 | NR | dead (1.5 mo) | disease | ||
| 10 | 1.1 | M | M5 | t(3;11) | 3 | refractory | cladribine/ara-C | marrow | 100/10000 (E.coli) | 1 | NR | dead (1.4 mo) | disease | |||
| 11 | 2.9 | M | M7 | t(2;21;3) | 3 | haplo | refractory | BMT | marrow | 100/2500 (PEG) | 3 | NR | haplo | dead (8.5 mo) | disease | |
| 12 | 1.2 | M | M7 | t(9;11) | 2 | refractory | clofarabine/ara-C | marrow | 60/10000 (E.coli) | 2 | NR | dead (1.4 mo) | disease | |||
| 13 | 12 | M | M5 | t(6;11) | 3 | MSD | relapse | clofarabine/ara-C | marrow,skin | 120/3000 (PEG) | 1 | NR | dead (0.6 mo) | disease | ||
| 14 | 16.1 | M | M5 | t(10;11) | 3 | relapse | ara-C/idarubicin | marrow | 120/10000 (Erwinia) | 2 | NR | UCBT | dead (2.7 mo) | TRM due to BMT | ||
| 15 | 16.7 | M | M7 | t(3;21) | 3 | haplo | relapse | BMT | marrow | 120/3000 (PEG) | 1 | NR | dead (1.0 mo) | disease |
Abbreviations: ara-C, cytarabine; Asp, asparaginase; BM, bone marrow; BMT, bone marrow transplantation; CR, complete remission; CRi, morphologic complete remission with incomplete blood count recovery; Dauno, daunorubicin; Dx, diagnosis; Eto, etoposide; F, female; FAB, The French–American–British (FAB) classification; GO, gemtuzumab ozogamicin; haplo, haplo identical transplantation; M, male; mo, months; MRD, minimal residual disease; MSD, match sibling donor transplantation; MTX, methotrexate; MUD, match unrelated donor transplantation; NR, no response; PEG, polyethylene glycol- L –asparaginase; PR, partial response; Pt, patient; TRM, treatment related mortality; Tx, treatment with methotrexate/asparaginase; UCBT, umbilical cord blood transplantation.
Patient who received escalated doses of methotrexate.
Responses
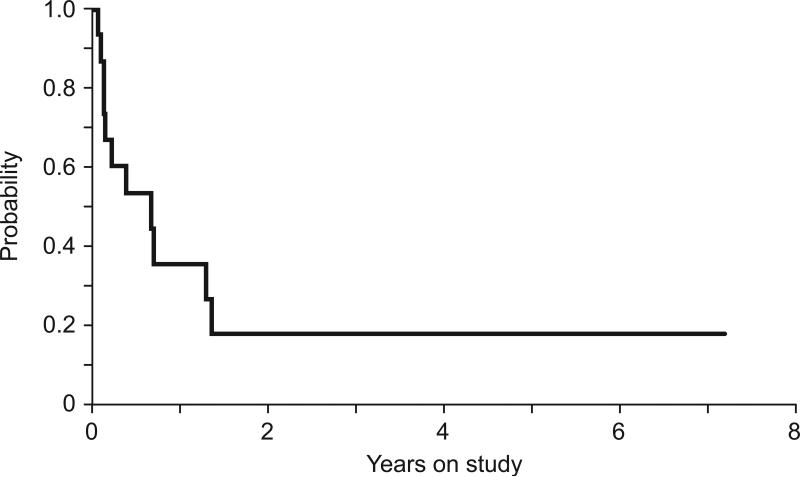
Six patients had responses. There was no difference in the duration of response between responders and non-responders (median, 164 days and 290 days, respectively, P=0.37). Three patients had morphologic complete remission with incomplete blood count recovery (CRi), 2 attained partial remission (PR), and one had stable disease, having received a total of 54 MTX/asparaginase courses in 16 months until she died of disease progression. After achieving remission, 4 patients (2 CRi and 2 PR) received HSCT, and 3 of them are alive for 90, 56, and 8 months. One patient (patient 2) who achieved CRi developed pancreatitis after the second course of MTX/asparaginase and was subsequently treated with monthly vincristine, weekly methotrexate, and daily mercaptopurine. Unfortunately, his disease relapsed, and thereafter he received MTX/asparaginase with concomitant octreotide as a prophylaxis for pancreatitis; he remains alive without disease after a total of 8.5 months. Eight of nine patients who did not respond to MTX/asparaginase died of disease and 1 of sepsis after HSCT. The 1-and 2-year overall survival were 35.6% and 17.8%, respectively.
Toxicities
Table 2 shows the toxicities from this regimen. Transient elevation of transaminase is the most common side effect (9 of 15 patients); 3 experienced grade III, and 6 had grade II toxicity. One patient also experienced transient grade II hyperbilirubinemia. Two patients had pancreatitis after the second course. Three patients had tumor lysis syndrome, and 1 had capillary leak syndrome. Febrile neutropenia was observed in only 2 patients. No evidence of asparaginase allergy was seen in our cohort including 2 patients who had history of E. coli asparaginase allergy and received Erwinia asparaginase.
Table II.
Toxicity of combination therapy with methotrexate/asparaginase
| Adverse event | Total events | Grade 1 | Grade 2 | Grade 3 |
|---|---|---|---|---|
| Gastrointestinal | ||||
| Nausea | 1 | 1 | ||
| Mucositis | 1 | 1 | ||
| Pancreatitis Metabolic/laboratory | 2 | 2 | ||
| AST elevation | 9 | 6 | 3 | |
| ALT elevation | 8 | 6 | 2 | |
| Hyperbilirubinemia Infection | 1 | 1 | ||
| Febrile neutropenia | 2 | 2 | ||
| Capillary leak syndrome | 1 | 1 | ||
| Tumor lysis syndrome | 3 | 3 |
Abbreviations: ALT, alanine transaminase; AST, aspartate transaminase.
No grade 4 or grade 5 adverse events occurred in the study.
DISCUSSION
In our study, the sequential combination of MTX and asparaginase produced objective responses in 6 of 15 patients with heavily pretreated pediatric relapsed/refractory AML and yielded 1- and 2-year overall survival rates of 35.6 % and 17.8 %, respectively. This therapy was tolerable in the majority of the patients and can be administered in an outpatient setting. Additionally, this regimen is more affordable and available than the novel nucleoside analogues regimen (e.g., clofarabine with or without cytarabine) even in countries with limited resources.
The administration of MTX followed by asparaginase results in a synergistic effect, although the exact mechanisms are unclear [17,18]. On the other hand, the inverse order of administration of asparaginase appears to attenuate the antileukemic properties of MTX [17,19]. From this in vitro evidence, it is suggested that a 10-day interval between each cycle of MTX/asparaginase is the most effective when native E. coli asparaginase is used [17,20]. However, we have observed responses with the use of PEG-asparaginase, which has at least 2 weeks of therapeutic activity [21,22]. As seen in Dana-Farber ALL protocols, which gives asparaginase prior to MTX administration, the sequence may not be important in vivo [23,24]. In vitro, the cytotoxic effect of asparaginase is higher in acute monoblastic leukemia than in other AML subtypes [14], which correlates to the presence of the lowest level of asparagine synthetase in monoblasts [25]. Further, monoblastic leukemia is highly sensitive to MTX because of the increased polyglutamylation pattern and relatively lower efflux of MTX [13,26]. We have observed 2 patients with CRi (both with extramedullary disease) and 1 with PR among 6 patients with monoblastic leukemia and MLL rearrangement, and all 3 responders developed tumor lysis syndrome. However, responses were seen in 3 other patients with non-monoblastic leukemia. Thus, this regimen can be tried in all subtypes of pediatric AML.
Transient mild elevation of transaminase was the most common complication; severe hepatitis and cholestatic jaundice were not seen. Importantly, febrile neutropenia was seen in only 2 cases, and most of the cases were managed as outpatient. There was no incidence of asparaginase allergy in our study; however, pancreatitis was seen in 2 patients. For heavily pre-treated patients, monitoring of pancreatic enzymes is important. Octreotide can be administered together with MTX/asparaginase as a possible prophylaxis for recurrence of pancreatitis [27]. As described above, we observed effective cytoreductions, especially in monoblastic leukemia with MLL rearrangement, and elevated uric acid, phosphate, and potassium at the same time (i.e., tumor lysis syndrome). Some of the patients received this regimen in a palliative care setting.
In conclusion, MTX/asparaginase has efficacy in pediatric patients with refractory or relapsed AML after contemporary intensive therapy. The regimen appeared to be well tolerated. This regimen can be a consideration for treatment of relapsed or refractory AML but needs to be prospectively studied.
Fig. 1.
The Kaplan–Meier analysis of overall survival in patients with relapsed/refractory acute myeloid leukemia treated with methotrexate and asparaginase.
ACKNOWLEDGEMENTS
This work was supported in part by Cancer Center Support (CORE) grant P30 CA021765-30 from the National Institutes of Health and by the American Lebanese Syrian Associated Charities (ALSAC). Ching-Hon Pui is an American Cancer Society Professor. The authors thank David Galloway for editorial assistance in the development of this manuscript.
Footnotes
Conflicts of interest
The authors have no competing interests.
REFERENCES
- 1.Georgina W H. Childhood myeloid leukaemias. Best Practice & Research Clinical Haematology. 2001;14(3):573–591. doi: 10.1053/beha.2001.0155. [DOI] [PubMed] [Google Scholar]
- 2.Aladjidi N, Auvrignon A, Leblanc T, et al. Outcome in children with relapsed acute myeloid leukemia after initial treatment with the French Leucemie Aique Myeloide Enfant (LAME) 89/91 protocol of the French Society of Pediatric Hematology and Immunology. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2003;21(23):4377–4385. doi: 10.1200/JCO.2003.11.066. [DOI] [PubMed] [Google Scholar]
- 3.Martin MG, Augustin KM, Uy GL, et al. Salvage therapy for acute myeloid leukemia with fludarabine, cytarabine, and idarubicin with or without gemtuzumab ozogamicin and with concurrent or sequential G-CSF. American journal of hematology. 2009;84(11):733–737. doi: 10.1002/ajh.21545. [DOI] [PubMed] [Google Scholar]
- 4.Sarper N, Yalman N. FLAG (fludarabine, high-dose cytarabine and G-CSF) for refractory and high-risk relapsed acute leukemia in children. Med Pediatr Oncol. 2000;34(2):163. doi: 10.1002/(sici)1096-911x(200002)34:2<163::aid-mpo21>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 5.Sander A, Zimmermann M, Dworzak M, et al. Consequent and intensified relapse therapy improved survival in pediatric AML: results of relapse treatment in 379 patients of three consecutive AML-BFM trials. Leukemia : official journal of the Leukemia Society of America, Leukemia Research Fund, UK. 2010;24(8):1422–1428. doi: 10.1038/leu.2010.127. [DOI] [PubMed] [Google Scholar]
- 6.Rubnitz JE, Razzouk BI, Lensing S, et al. Prognostic factors and outcome of recurrence in childhood acute myeloid leukemia. Cancer. 2007;109(1):157–163. doi: 10.1002/cncr.22385. [DOI] [PubMed] [Google Scholar]
- 7.Temming P, Qureshi A, Hardt J, et al. Prevalence and predictors of anthracycline cardiotoxicity in children treated for acute myeloid leukaemia: Retrospective cohort study in a single centre in the United Kingdom. Pediatric blood & cancer. 2011;56(4):625–630. doi: 10.1002/pbc.22908. [DOI] [PubMed] [Google Scholar]
- 8.Styczynski J, Wysocki M, Debski R, et al. Ex Vivo Drug Resistance Profile in Childhood Acute Myelogenous Leukemia: No Drug is More Effective in Comparison to Acute Lymphoblastic Leukemia. Leukemia & lymphoma. 200243(9):1843–1848. doi: 10.1080/1042819021000006394. [DOI] [PubMed] [Google Scholar]
- 9.Rubnitz JE, Inaba H, Ribeiro RC, et al. NKAML: a pilot study to determine the safety and feasibility of haploidentical natural killer cell transplantation in childhood acute myeloid leukemia. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2010;28(6):955–959. doi: 10.1200/JCO.2009.24.4590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yap BS, Mccredie KB, Keating MJ, et al. Asparaginase and Methotrexate Combination Chemotherapy in Relapsed Acute Lymphoblastic-Leukemia in Adults. Cancer Treat Rep. 1981;65:83–87. [PubMed] [Google Scholar]
- 11.Hudson MM, Dahl GV, Kalwinsky DK, et al. Methotrexate plus L-asparaginase. An active combination for children with acute nonlymphocytic leukemia. Cancer. 1990;65(12):2615–2618. doi: 10.1002/1097-0142(19900615)65:12<2615::aid-cncr2820651202>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 12.Chłopkiewicz B, Koziorowska J. Role of Amino Acid Depletion in Combined Treatment of Neoplastic Cells with Methotrexate and L-Asparaginase. Cancer research. 1975;35(6):1524–1529. [PubMed] [Google Scholar]
- 13.Rots MG, Pieters R, Jansen G, et al. A possible role for methotrexate in the treatment of childhood acute myeloid leukaemia, in particular for acute monocytic leukaemia. European Journal of Cancer. 2001;37(4):492–498. doi: 10.1016/s0959-8049(00)00433-0. [DOI] [PubMed] [Google Scholar]
- 14.Okada S, Hongo T, Yamada S, et al. In vitro efficacy of l-asparaginase in childhood acute myeloid leukaemia. British journal of haematology. 2003;123(5):802–809. doi: 10.1046/j.1365-2141.2003.04703.x. [DOI] [PubMed] [Google Scholar]
- 15.Kurt B, Flynn P, Shenep JL, et al. Prophylactic antibiotics reduce morbidity due to septicemia during intensive treatment for pediatric acute myeloid leukemia. Cancer. 2008;113(2):376–382. doi: 10.1002/cncr.23563. [DOI] [PubMed] [Google Scholar]
- 16.Cheson BD, Bennett JM, Kopecky KJ, et al. Revised Recommendations of the International Working Group for Diagnosis, Standardization of Response Criteria, Treatment Outcomes, and Reporting Standards for Therapeutic Trials in Acute Myeloid Leukemia. Journal of Clinical Oncology. 2003;21(24):4642–4649. doi: 10.1200/JCO.2003.04.036. [DOI] [PubMed] [Google Scholar]
- 17.Capizzi RL. Asparaginase-methotrexate in combination chemotherapy: schedule-dependent differential effects on normal versus neoplastic cells. Cancer Treat Rep. 1981;65(Suppl 4):115–121. [PubMed] [Google Scholar]
- 18.Vadlamudi S, Krishna B, Reddy VVS, et al. Schedule-dependent Therapeutic Synergism for l-Asparaginase and Methotrexate in Leukemic (L5178Y) Mice. Cancer research. 197333(9):2014–2019. [PubMed] [Google Scholar]
- 19.Capizzi RL, Summers WP, Bertino JR. L-asparaginase induced alteration of amethopterin (methotrexate) activity in mouse leukemia L5178Y. Ann N Y Acad Sci. 1971;186:302–311. [PubMed] [Google Scholar]
- 20.Mandelli F, Amadori S, Pacilli L, et al. Preliminary results of the combination methotrexate-asparaginase in patients with acute non lymphoid leukemia. Biomedicine. 1978;29(4):116–117. [PubMed] [Google Scholar]
- 21.van den Berg H. Asparaginase revisited. Leukemia & lymphoma. 2011;52(2):168–178. doi: 10.3109/10428194.2010.537796. [DOI] [PubMed] [Google Scholar]
- 22.Silverman LB, Supko JG, Stevenson KE, et al. Intravenous PEG-asparaginase during remission induction in children and adolescents with newly diagnosed acute lymphoblastic leukemia. Blood. 2010;115(7):1351–1353. doi: 10.1182/blood-2009-09-245951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kamen BA. L'Asparaginase and Methotrexate Combinations: Clashes of Empiric Success and Laboratory Models? Journal of Pediatric Hematology/Oncology. 2007;29(9):587–588. doi: 10.1097/MPH.0b013e3181483e1b. 510.1097/MPH.1090b1013e3181483e3181481b. [DOI] [PubMed] [Google Scholar]
- 24.Moghrabi A, Levy DE, Asselin B, et al. Results of the Dana-Farber Cancer Institute ALL Consortium Protocol 95-01 for children with acute lymphoblastic leukemia. Blood. 2007;109(3):896–904. doi: 10.1182/blood-2006-06-027714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dubbers A, Wurthwein G, Muller HJ, et al. Asparagine synthetase activity in paediatric acute leukaemias: AML-M5 subtype shows lowest activity. British journal of haematology. 2000;109(2):427–429. doi: 10.1046/j.1365-2141.2000.02015.x. [DOI] [PubMed] [Google Scholar]
- 26.Goker E, Kheradpour A, Waltham M, et al. Acute monocytic leukemia: a myeloid leukemia subset that may be sensitive to methotrexate. Leukemia : official journal of the Leukemia Society of America, Leukemia Research Fund, UK. 1995;9(2):274–276. [PubMed] [Google Scholar]
- 27.Tokimasa S, Yamato K. Does octreotide prevent L-asparaginase-associated pancreatitis in children with acute lymphoblastic leukaemia? British journal of haematology. 2012;157(3):381–382. doi: 10.1111/j.1365-2141.2011.08971.x. [DOI] [PubMed] [Google Scholar]